- Robotic Assisted UKA
- Robotic Assisted TKA
- Robotic Assisted Revision Knee
- Computer Guided THA
- Data Visualization
- Partial Knee
- Primary Knee
- Revision Knee
- Oxinium™ in Knee
- VISIONAIRE™ Technology
- Primary Hip
- Revision Hip
- Oxinium™ in Hip
- PICO™
- JOURNEY JOURNEY II JOURNEY UNI
- LEGION LEGION TOTAL KNEE
- SURGEON TESTIMONIALS

JOURNEY™ II
Journey II Total Knee System
For orthopaedic surgeons seeking treatment solutions beyond traditional knee replacements, JOURNEY II Active Knee Solutions has been engineered to empower patients with a renewed right to an active lifestyle by breaking through traditional knee replacement barriers and delivering Function, Motion, and Durability through PHYSIOLOGICAL MATCHING
View our latest videos on JOURNEY II below and learn more about this product.
Live Surgery: Bicruciate Ligament Sparing TKA with JOURNEY™ II XR Featuring CORI™ Surgical System
Published: April 5, 2023
NAVIO™ 7 JOURNEY™ II TKA
Published: March 30, 2020
JOURNEY™ II BCS with the NAVIO Surgical System featuring the Bur All Technique
Published: April 11, 2019
NAVIO™ Robotic-Assisted TKA: Accuracy, Gap Balancing & Journey™ II for Optimum Performance!
Published: November 19, 2018
JOURNEY™ II Bi-Cruciate Stabilized (BCS) Knee featuring the Distal Bur Technique using the NAVIO Surgical System
Journey II Total Knee system
For orthopaedic surgeons seeking treatment solutions beyond traditional knee replacements, JOURNEY? II Active Knee Solutions has been engineered to empower patients with a renewed right to an active lifestyle by breaking through traditional knee replacement barriers and delivering Function, Motion, and Durability through PHYSIOLOGICAL MATCHING
JOURNEY II Total Knee
Latest Videos

Dr. Jimmy Chow demonstrates how to perform a JOURNEY™ II XR procedure using CORI™ Surgical System.
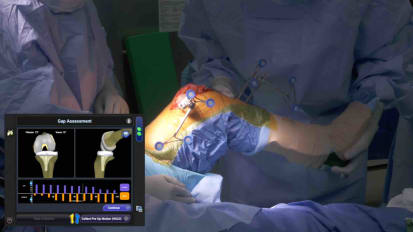
Orthopaedic Surgeon, James "Chip" Comadoll, MD, performs a total knee arthroplasty using a JOURNEY™ II and NAVIO™ 7 robotic assistance.
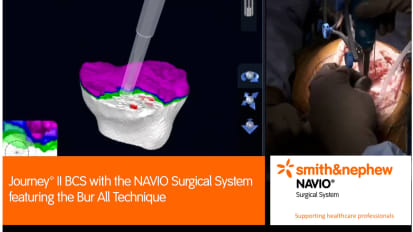
David Rovinsky, MD, performs a robotic-assisted JOURNEY™ II Bi-Cruciate Stabilized (BCS) knee surgery with NAVIO™ Surgical System featuring the bur all technique.

Smith & Nephew's Orthopaedic Surgeon David Rovinsky, MD discusses the benefits of NAVIO™ robotic-assisted TKA with the JOURNEY™ II for accuracy and gap-balancing during knee surgeries.
David Rovinsky, MD, performs a robotic-assisted JOURNEY™ II Bi-Cruciate Stabilized (BCS) knee surgery with NAVIO™ Surgical System featuring the distal bur technique.

Surgical Demonstration: JOURNEY™ II XR with NAVIO™ Bi-cruciate Retaining Robotic-Assisted TKA procedure featuring the NAVIO™ Surgical System
Dr. Jimmy Chow performs a total knee arthroplasty on a 52 year old competitive cyclist using the JOURNEY II XR knee with robotic-assistance from the NAVIO™ Surgical System

Live surgery from HSS featuring JOURNEY II BCS
Live surgery from HSS featuring JOURNEY II BCS - Performed by Dr. Steven Haas and moderated by Dr. David Mayman
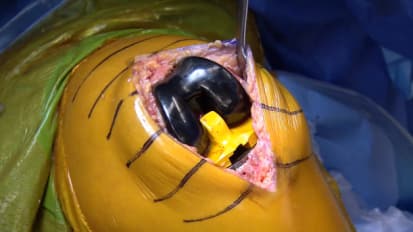
The JOURNEY II Bi-Cruciate Stabilized (BCS) Total Knee System
Steven Haas, MD - Operating Surgeon Dave Mayman, MD - Narrating Surgeon
Smith & Nephew improves the lives of their patients, whether through extending the life and functionality of implants, improving operating room efficiency, or promoting faster healing and other clinical outcomes.
Advanced Surgical Devices (Orthopaedic Reconstruction, Advanced Wound Management, Sports Medicine and Trauma)
Smith & Nephew improves the lives of their patients, whether through extending the life and functionality of implants, improving operating room efficiency, or promoting faster healing and other clinical outcomes.
The information on this website is intended for healthcare professionals only
- @smithNephewpic |
- Privacy & Cookies |
- Terms of Use
You are using an outdated browser. Please upgrade your browser to improve your experience.

The Journey II Total Knee System: A Step Ahead – An Evolutionary New Design
Richard “alex” sweet ii, md, kate s. hamilton, pa-c, richard a. sweet, m.d. (retired 2022).
HOW THE NORMAL KNEE WORKS: It is a common misconception that the human knee functions as a simple hinge joint, with straight up and down flexion and extension. In reality the motion of the knee is much more complex, with six degrees of motion (not just the two of a hinge). As the knee bends and straightens, it also rotates internally and externally and slides front to back. Traditional total knee replacement designs have never been able to recreate the complex movement necessary for the knee to feel and function normally, especially in the high demand situations of a physical job or performing athletics.
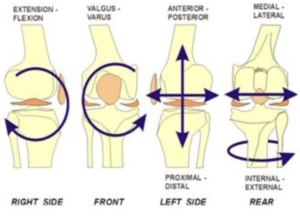
The Journey II Total Knee Medial Pivot Design The “ Normal Feeling ” Knee Replacement
The Journey II Knee is a revolutionary new knee replacement design. It incorporates several new design changes, the most significant of which is Medial Pivot design. These design changes include:
MEDIAL PIVOT DESIGN: A revolutionary new concept. It is the first true major design advancement in knee replacement surgery in decades. The Medial Pivot Design is the first TKR design to incorporate all 6 degrees of motion of the natural knee. It does so by creating a “cupped” almost ball-in-socket articulation between the plastic and the femur on the medial side of the joint (blue arrow) and a flat articulation between the plastic and the femur on the lateral side joint (red arrow). The resultant kinematics (mechanics) are such that the “ball in socket” articula-tion on the inside of the joint keeps the femur centered in one spot as the knee bends, while the “flat” articulation on the outside of the knee allows lateral femur to glide backwards and rotate like the normal knee. The result: normal kinematics are restored in the Medial Pivot Design TKR.
GREATER STABILITY: An added inherent benefit of the medial ball-in-socket “cupping” of the Medial Pivot design is that it improves front-to-back knee stability vs that of the conventional TKR. Given that the ACL is sacrificed in all TKR surgery, the added front-to-back stability of the Medial Pivot design is a crucial improvement.
NORMAL ANATOMY RESTORED: The Journey II re-establishes the subtleties of normal joint line anatomy (which are altered by the arthritis process and not corrected by conventional TKR surgery.

IMPROVED QUADRICEPS STRENGTH: The Journey II, as opposed to other TKR designs, moves the contact point between the femur and the tibia forward to its normal position (blue arrow right as compared to orange arrow of traditional TKA designs). Reestablishing this forward contact point results in increased quadriceps strength (like being on the long end of a teeter-totter) GREATER RANGE OF MOTION: The Journey II design changes the anatomy of the back of the femoral component to mimic that of the normal knee (blue arrow left). The result is to provide for an extra 15 degrees of flexion versus what is expected in a conventional TKR. The cumulative result of these design changes of the Journey II TKR is that patients experience a knee replacement with improved kinematics, speedier recovery, better ultimate range of motion, enhanced stability, and ultimate functional ability for high demand situations at full recovery.
Design Limitations of Past Conventional TKRs Conventional knee replacements work well at what they are designed to do: rid the patient of arthritis and provide a new artificial knee that will function adequately. As TKR surgery has expanded into younger more active age groups who place a higher demand on their new knee, the goal of only eliminating arthritic pain is no longer sufficient. Design limitations of the conventional knee replacement that can inhibit the return to normal functioning and can lead to patient dissatisfaction include: 1. ABNORMAL KINEMATICS: The knee is NOT a hinge. A conventional knee replacement forces the knee to flex and extend like a hinge on a door, with a straight simple up and down motion. The normal knee, however, does not function like a hinge. Instead, as it bends and straightens, there is flowing rotation and front to back sliding to its kinematics. Only the Journey II TKA recreates these kinematic movements. 2. ALTERED ANATOMY: With conventional knee replacement surgery, anatomy of the knee is altered in several ways. When looking at a normal knee from the front, the joint line is not perpendicular to the tibia as it is with conventional TKR surgery, but instead is at a slight angle with the inside of the joint being slightly lower than the outside. To compensate, the femur must be correspondingly rotated to keep the ligaments balanced. This can lead to abnormal forces on the knee, creating sensations of instability, dissatisfaction, and potential early failure of the knee replacement. 3. DIMINISHED QUADRICEPS STRENGTH: In conventional TKR surgery the contact point between the femur and tibia is shifted towards the back of the knee. This shift causes abnormal (called “paradoxical”) motion to occur when the knee starts to bend. The mechanical effect is that the strength of the quadriceps muscle is weakened. This is of clinical importance as a patient with a conventional TKR attempts to squat, climb steps, or perform other high demand activities.
Results of Conventional TKR Surgery Design Limitations: The result of the limitations noted above is that the ligaments and capsule of the knee see an altered knee anatomy, altered knee kinematics, and a weakened quadriceps muscle. This can cause a range of problems from:
- A patient perceiving a subtle sensation that the knee does not “feel normal”
- More significant problems such as less range of motion, reduced stability, a weak knee/quadriceps complex, intermittent soreness/swelling, problems with stair climbing, and difficulties engaging in recreational activities and vigorous work.
Conclusion The Journey II TKA is truly a revolutionary step forward in the design of total knee implants. With proper surgical technique and in the hands of a well-trained surgeon, the Journey II knee can produce results superior to the traditional total knee design. It is our goal as your surgical team to continue to improve our surgical techniques and to look for new advances in technology that allow us to give you the best result possible.
Louisville Orthopaedic Clinic
4130 Dutchman's Lane, Suite 300,Louisville 40207 (502) 897-1794
1425 State St., ,New Albany 47150 (812) 920-0408
- Elbow and Shoulder
- Foot and Ankle
- Hand and Wrist
- Neck and Back
- Center for Orthopedic Spine & Pain
- Hand Specialists
- Sports Medicine
- Total Joint Center
- Our Providers
- Physical Therapy
- Privacy Policy
- Areas Served
- Educational Videos
- Publications
- Patient Forms

JOURNEY ◊ II Active Knee Solutions
Aren't all total knee implants the same?

In theory, yes, all total knee implants are intended for the same purpose: the elimination of painful bone-on-bone contact, and the restoration of motion and function to the joint. In reality, however, the way each implant is designed, built and implanted not only makes them different, but can have an impact on how well they perform for each patient.
How your knees move
Commonly described as a hinge joint, your knees actually do much more than simply swing back and forth. In fact, every time your knee bends, forces in and around the joint work together to produce a subtle and complex rotational movement that you don't even realize is there. However, if this rotational movement is removed, the change can be felt in the muscles and ligaments through the entire leg.
The JOURNEY ◊ II AKS difference

One of the most remarkable breakthroughs in design of total knee replacements has been the creation of the JOURNEY II Active Knee Solutions. Designed using the latest in human simulation software, and built using some of the most wear-resistant materials available, this unique implant was designed to address two of the most common concerns associated with knee replacement implants: implant wear and implant feel.
All information provided on this website is for information purposes only. Every patient's case is unique and each patient should follow his or her doctor's specific instructions. Please discuss nutrition, medication and treatment options with your doctor to make sure you are getting the proper care for your particular situation. If you are seeking this information in an emergency situation, please call 911 and seek emergency help.
All materials copyright © 2020 Smith & Nephew, All Rights Reserved.

◊ Trademark of Smith+Nephew. The information on this site is intended for US residents only © 2024 Smith+Nephew
Smith+nephew facebook page | follow smith & nephew on twitter | privacy & cookies | terms of use.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.13(1); 2023

Original research
Comparison of the journey ii bicruciate stabilised (jii-bcs) and genesis ii total knee arthroplasty for functional ability and motor impairment: the capability, blinded, randomised controlled trial, iain mcnamara.
1 Norfolk and Norwich University Hospital, Norwich, UK
2 University of East Anglia, Norwich, UK
Valerie Pomeroy
Allan b clark.
3 Norwich Medical School, University of East Anglia, Norwich, UK
Graham Creelman
4 Mental Health Act Review Panels, Norfolk and Suffolk, UK
Celia Whitehouse
5 Department of clinical neurosciences, University of Cambridge, Cambridge, UK
Toby O Smith
6 Faculty of Medicine and Health Sciences, University of East Anglia, Norwich, UK
Juliet High
7 Norwich Clinical Trials Unit, Norwich, UK
Ann Marie Swart
8 Health Sciences, University of East Anglia, Norwich, UK
Celia Clarke
Associated data.
bmjopen-2022-061648supp001.pdf
Data are available on reasonable request. Reasonable requests for data will be considered by the trial team.
To determine if a newer design of total knee replacement (TKR) (Journey II BCS) produces superior patient-reported outcomes scores and biomechanical outcomes than the older, more established design (Genesis II).
Patients were recruited from an NHS University Hospital between July 2018 and October 2019 with surgery at two sites. Biomechanical and functional capacity measurements were at a University Movement and Exercise Laboratory.
Participants
80 participants undergoing single-stage TKR.
Interventions
Patients were randomised to receive either the Journey II BCS (JII-BCS) or Genesis II TKR.
Primary and secondary outcome measures
Primary outcome was the Oxford Knee Score (OKS), at 6 months. Secondary outcomes were: OKS Activity and Participation Questionnaire, EQ-5D-5L and UCLA Activity scores, Timed Up and Go Test, 6 min walk test, lower limb kinematics and lower limb muscle activity during walking and balance.
This study found no difference in the OKS between groups. The OKS scores for the JII-BCS and Genesis II groups were mean (SD) 42.97 (5.21) and 43.13 (5.20) respectively, adjusted effect size 0.35 (-2.01,2.71) p=0.771
In secondary outcome measures, the Genesis II group demonstrated a significantly greater walking range-of-movement (50.62 (7.33) vs 46.07 (7.71) degrees, adjusted effect size, 3.14 (0.61,5.68) p=0.02) and higher peak knee flexion angular velocity during walking (mean (SD) 307.69 (38.96) vs 330.38 (41.40) degrees/second, adjusted effect size was 21.75 (4.54,38.96), p=0.01) and better postural control (smaller resultant centre of path length) during quiet standing than the JII-BCS group (mean (SD) 158.14 (65.40) vs 235.48 (176.94) mm, adjusted effect size, 59.91 (–105.98, –13.85) p=0.01.).
Conclusions
In this study population, the findings do not support the hypothesis that the Journey II BCS produces a better outcome than the Genesis II for the primary outcome of the OKS at 6 months after surgery.
Trial registration number
ISRCTN32315753 .
STRENGTHS AND LIMITATIONS OF THIS STUDY
- This is a two arm, superiority, observer-blind, participant-blind and clinical staff-blind, randomised control trial.
- It uses a wide variety of patient reported outcomes measures and biomechanical measurements to determine if one implant is superior to the other
- The required sample size was achieved with only one person lost to follow-up.
- A potential limitation is the relatively large number of secondary outcomes.
- The surgeons all had a much greater familiarity with the implantations of Genesis II implants.
Original protocol for the study is mentioned here: https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-020-4143-4 .
Introduction
Despite total knee replacement (TKR) being a recommended surgical treatment for end-stage knee osteoarthritis, 1 up to 34% of all patients following TKR have poor functional outcomes. 2–6 With estimates of osteoarthritis of the knee affecting one in eight people in the USA 7 and 250 million individuals worldwide 8 the number of patients with intrusive symptoms after surgery is significant.
Multiple changes in implant design have been introduced to try to improve patient outcomes and while some implant design alterations have led to improvements in patient-reported outcome measures (PROMS) 9–11 and kinematics 12 13 not all have led to differences. 14–20
The Genesis II (Smith & Nephew, Memphis, Tennessee, USA) TKR has been reported to have good survivorship and patient satisfaction 13 21 and is commonly used in the UK 22 An evolutionary design, the Journey II BCS (JII-BCS; Smith & Nephew), also manufactured by Smith and Nephew, has been developed to improve kinematic outcome compared with the Genesis II by using a bicruciate design. 23 This design change has been supported by encouraging fluoroscopic studies. However, to date, no randomised controlled trials (RCTs) have been conducted to assess if there is a difference in the outcome compared with its predicate design. 24
This trial aimed to assess whether the JII-BCS would produce better patient reported and movement outcomes than the Genesis II.
The published protocol included the aims for investigating: the rotational profile around the native knee and following TKR; and patients’ experiences and surgeons’ experiences. 25 These findings will be reported in subsequent manuscripts.

Trial design, randomisation, blinding to intervention allocation, ethics and registration
A two-arm, superiority RCT comparing the JII-BCS knee implant (experimental intervention) to the Genesis II knee implant (control intervention) was performed. The trial was observer-blind, participant-blind and clinical staff-blind. Only the operating surgeon and theatre team knew which implant was used for an individual participant.
Trial participants were assigned to either the JII-BCS or Genesis II group using a computer-generated, 1:1 randomisation schedule stratified by site and age (<60 years = younger; ≥60 years = older). 26 27 Group allocation was revealed using REDCap, 28 29 the interactive web-randomisation system, to a member of the research team who was not involved in either the clinical care or assessments of any participant. Allocation was concealed from the surgical team until after the preoperation baseline measures were completed.
Sample size
The sample size was calculated from the Oxford Knee Score (OKS, primary outcome measure). 30 The RCT was powered at 80% with a 5% significance level to detect a minimally important clinical difference of five points 31 32 with an SD of 7.4 points. 33 Accounting for an estimated attrition rate of 10% at 6 months postsurgery the estimated sample size was 80 participants (40 per group).
Participants, setting and recruitment
Full eligibility criteria are provided in the published protocol. 25 In brief, participants were aged at least 18 years and met the clinical and radiological criteria for a single-stage TKR. People were excluded if they: had a fixed-flexion deformity of at least 15° or non-correctable varus/valgus deformity of at least 15°; had inflammatory arthritis or previous septic arthritis; had previous surgery to the collateral ligaments of the affected knee; had a contralateral TKR implanted less than 1 year earlier; had severe comorbidity that could present an unacceptable safety risk or were pregnant; were a private patient; were likely to be living outside the clinical centre catchment area at 6 months postsurgery or were enrolled on another clinical trial.
Patients were recruited at a university teaching hospital with surgery conducted at two sites. Outpatient physiotherapy was conducted in a single hospital. The Movement and Exercise Laboratory at the associated University (MoveExLab) was the setting for measures of functional capacity and biomechanics.
All participants received routine NHS care for people with TKR irrespective of the implant received. This included following a standard postoperative rehabilitation of outpatient physiotherapy centred on knee strength and range of motion (ROM) exercises within the first 6 weeks after surgery. Patients received the same physiotherapy protocols and classes.
Experimental intervention
Participants in the experimental group received the JII-BCS. The JII-BCS is a dual-cam post designed to substitute for both the anterior cruciate ligament and posterior cruciate ligament. In addition the femoral and tibial components are asymmetric and the polyethylene insert is a medially concave and laterally convex shape. The device is designed to provide guided motion, and thus improve knee kinematics, and increase anteroposterior stability throughout knee flexion.
Control intervention
- Participants in the control group received the Genesis II (Smith and Nephew), posterior stabilised (PS) TKR. The design features specific to the implant and a lateralised trochlear groove to improve patellar contact and tracking, an externally rotated femoral implant design and an anatomically shaped tibial baseplates.
Surgical techniques
All four surgeons had extensive experience, at least 5 years, of the Genesis II implant. All undertook cadaveric training on the JII-BCS and declared that they were competent in the surgical technique having completed their operative learning curve before starting the trial. Both implants are uncoated, cemented implants. The surgical procedure followed the standard manual surgical approach and technique through a medial parapatellar approach in all cases with intramedullary femoral and tibial rods to provide the alignment of the components. Patella resurfacing was used in both groups.
Data collection schedule
Data collection time points for the primary outcome measure were: at least 1 day before surgery (baseline), 7±2 days after surgery (1 week postoperatively), 6–8±2 weeks after surgery (2 months), 6 months±4 weeks after surgery (outcome, primary time point). Secondary outcomes were collected at baseline, 2 months and 6 months. Any differences from these time points are provided in the outcome measures section.
Outcome measures
Primary outcome measure.
The OKS was the primary outcome measure. This is a 12-question patient self-assessment of knee function and pain 30 with values ranging from 0 (worst outcome) to 48 (best outcome).
Secondary outcome measures
- The Oxford Knee Score Activity and Participation Questionnaire (OKS-APQ), which complements the OKS by assessing everyday activity and social participation. 34 The overall score is from 12 to 60 with 12 being the best outcome.
- The EQ-5D-5L is a self-report questionnaire consisting of five questions and a Visual Analogue Scale. Higher values indicate better quality of life. 35
- The UCLA Activity score (UCLA) to assess physical activity self-rating scale ranged from 0 (complete inactivity) to 10 (participation in impact sport).
- Timed Up and Go Test (TUG)—seconds to rise from chair, walk 3 m and return to sitting; mean of three trials. 36 The reported minimal detectable change after TKR is 2.27 s. 37 A lower value indicates better function.
- Six min walk test—metres walked in 6 min around a 20 m circuit. 38 39 The reported minimal detectable change from baseline after TKR is 26 metres. 40 A higher value indicates greater function.
- Modified Star-Excursion Test 41 (cm/leg length) where larger values indicate better balance.
For these simultaneous measures, participants wore shorts and were bare-footed. Reflective sensors were placed in accordance with the Plug-In Gait model (Vicon) for the lower limb and three-dimensional motion data were collected, at 100 HZ, with eight wall-mounted infrared cameras (Vicon Motion System, Oxford, UK). Three embedded force plates (BERTEC, Ohio, USA) were used to collect kinetic data at 2000 Hz for walking tasks and 100 Hz for balance tasks. Surface electromyographic sensors (EMG: Delsys) were placed bilaterally on the Vastus Medialis, Vastus Lateralis, Tibialis Anterior, Bicep Femoris and lateral head of the Gastrocnemius following SENIAM guidance. EMG data were collected at 2000 Hz.
For walking tasks, participants were asked to walk in a straight line along a 10 m walkway at their self-selected speed. For double stance balance activities, participants were instructed to stand with their feet shoulder-width apart. For single stance balance activities, participants were instructed to stand on one leg with hands-on-hips. Three trials of 10 s were recorded for each activity.
For the stair ambulation task, participants were asked to complete six ascents and six descents all unaided, leading with the operated limb for three trials and the non-operated limb for the remainder. The stairs had four steps. The first step was 16.5 cm, and the others were 15 cm high. Handrails were available if participants needed support.
Movement data were processed in accordance with the Vicon Plug-in Gait Model (Oxford Metrics, Oxford, UK). Raw EMG was filtered with pass bands at 10 and 500 Hz, rectified and low pass filtered using a fourth order Butterworth with a 10 Hz cut-off. Walking data were normalised to 101 data points for the gait cycle. Three trials of tasks were used to create a mean for each measure per participant. Values were extracted using a purpose-built MATLAB script. Data were processed by motion analysis experts in the research team.
The JII-BCS is expected to provide more normal kinematics during knee movement than Genesis II due to the design changes discussed earlier. Other authors have indicated that the femo-tibial relationship may be more normal during deep knee bend 42 and more stable during walking 43 Accordingly, people with the Journey prosthesis may 44 45 or may 43 have greater knee ROM, may walk faster, 46 47 and may have a longer stride length 46 47 than people receiving a comparison knee replacement. In addition, greater stability of the femur on the tibia could produce greater knee flexion angular velocity as dynamic knee loading could be more normal. However, there is only one non randomised study of 18 patients comparing the JII-BCS directly with the Genesis II. 45 Based on the available literature, the hypothesis driving the kinematic investigation was that people receiving the JII-BCS compared with those receiving the Genesis II would have greater walking velocity, step-length symmetry (resulting from longer stride length), knee ROM and peak knee flexion angular velocity.
- Walking speed (metres/second). A higher value indicates better performance
- Step length symmetry during walking. Step length ratio was calculated as ((2xOp)/Op+NOp))−1); where Op is the step length of the operated leg and NOp is the step length of the non-operated leg. Zero indicates perfect symmetry and best performance.
- Knee ROM during walking (degrees). Higher values indicate better performance.
- Peak knee flexion angular velocity during walking (degrees per second). This was inadvertently omitted from the statistical analysis plan (SAP). Higher value indicates better performance.
- Double stance support (% of gait cycle). It was planned to measure cadence, (steps/min), step length (m) and stride length (m). However, there is redundancy with the temporal-spatial gait parameters of walking speed and step length symmetry which are included in the primary movement performance measures.
- Peak extension and flexion moments of operated knee during the gait cycle (Nm/kg).
- Hip and ankle ROM during walking.
- Peak knee flexion angular velocity during stepping up onto a stair.
- Percentage of gait cycle for peak activation of Vastus Medialis, Vastus Lateralis, Tibialis Anterior, Biceps Femoris and Lateral head of Gastrocnemius (% of gait cycle).
- Balance measures were derived from kinetic data (from force plates) during standing still: single stance on the operated lower limb for 10 s with eyes open (yes/no) and duration maintained; resultant centre of pressure path length (COP cm) in double stance with eyes closed; and resultant COP velocity (cm/s) in double stance with eyes closed.
Clinical context and adverse events
Data on length of hospital stay and complications related to the surgery (eg, anaesthesia-related problems, bleeding, morbidities) were collected from a notes review. At each visit, participants were asked about their pain medication and if they had received additional treatment since their surgery/previous visit and what this entailed. Any need for revision surgery was recorded. All adverse events identified were tracked until resolution.
The SAP was finalised and agreed prior to database lock and analysis was completed blinded to group allocation ( online supplemental file ). For all outcomes the hypothesis tests and 95% CIs were two sided; and a p<0.05 was considered significant. An intention-to-treat analysis was conducted that is, all randomised participants regardless of their eligibility or adherence were analysed according to the treatment they were randomised to receive. The analysis was undertaken by the Trial Statistician using Stata V.16.
Supplementary data
For the primary outcome, the mean OKS at 6 months was compared between the control and experimental groups using a general linear model adjusting for site and age (<60 years/≥60 years). An adjusted analysis was conducted using the same model but adjusting for the OKS at baseline. The model assumptions were checked graphically, and sensitivity analysis done using a non-parametric bootstrap using 5000 repetitions.
All the other outcomes were analysed separately at 2 months and 6 months using the same general linear model specified above and a corresponding adjusted analysis. The exception was ability to balance for 10 s. This was analysed using a logistic regression model adjusting for site and age.
Patient and public involvement
A patient representative, who had previously undergone knee replacement surgery, was involved in the protocol development, assessment of the burden of the intervention and time taken to participate in the research and oversight of the trial as a member the trial management group. The representative also contributed to the planning and writing of research dissemination materials.
Participants were recruited between July 2018 and October 2019. Last follow-up visits were in October 2020 with some impact and delayed visits due to COVID-19.
In the published protocol, 25 the analysis plan included a per-protocol and safety analysis. This was not undertaken as the implants were used as intended so these populations would be the same as the intention-to-treat population.
Flow of participants through the trial
In total, 105 of 153 people screened were eligible to take part, 16 declined participation and eight were excluded for other reasons. Therefore, 81 of 153 people (53%) were recruited. All participants in the Genesis II group (n=40) received their allocated intervention. In the JII-BCS group (n=41), one participant withdrew prior to surgery (postrandomisation exclusion). Full details are in the Consolidated Standards of Reporting Trials (CONSORT) flow chart ( figure 1 ).

Consolidated Standards of Reporting Trials (CONSORT) diagram.
Participant characteristics
There were no discernible baseline differences between the groups ( table 1 ).
The baseline characteristics of participants
EQ-5D-5L is a measure of health-related quality of life, in the range of −0.109 (worst possible state) and 1.0 (perfect health), anchored at 0 (death).
EQ-VAS is a health state assessment ranging between 0 and 100, in which 0 is worst imaginable health state and 100 is best imaginable health state.
OKS is a 12-item knee function assessment, ranging from 0 (worst score) to 48 (best score).
Timed Up and Go Test—seconds to rise from chair, walk 3 m and return to sitting; mean of three trials. A lower value indicates better function.
Six min walk test—metres walked in 6 min around a 20 m circuit A higher value indicates greater function.
The UCLA Activity score to assess physical activity self-rating scale ranged from 0 (complete inactivity) to 10.
*Thirt-nine participants.
†Thirt-eight participants.
Primary outcome comparison: 6 months postoperatively
The OKS scores for the JII-BCS and Genesis II groups were mean (SD) 42.97 (5.21) and 43.13 (5.20), respectively. There was no significant difference between the groups: adjusted effect size 0.35 (−2.01,2.71) p=0.771 ( table 2 ).
Oxford Knee Scores (OKS, primary outcome), from baseline to 6 months after surgery (primary time point)
Journey II BCS (JII-BCS)
*Adjusted for strata used in randomisation and for baseline scores.
APQ, Activity and Participation Questionnaire; VAS, Visual Analogue Scale.
Secondary outcome comparisons: 6 months postoperatively
Patient-reported outcome questionnaires.
There were no differences between the two groups for any of the secondary patient reported outcomes ( online supplemental tables S1 ).
Walking and balance functional ability
There was no difference between the JII-BCS and Genesis II groups in the time to complete the TUG Test or the distance covered in the 6 min walk test ( online supplemental table S2 ). The Star-Excursion Test was attempted by all participants but 59% of participants at baseline, 59% at follow-up and 63% at outcome were unable to complete it ( online supplemental table S3 ). Therefore, statistical analysis was not undertaken.
Movement performance during walking and balance
The primary movement performance measures are reported in table 3 . In summary at 6 months postsurgery, the Genesis II group had a significant advantage for knee ROM and peak knee flexion angular velocity during walking. There were no differences between the groups for walking speed or peak flexion angular knee velocity on stair climbing.
Movement performance primary measures during walking from baseline to 6 months postsurgery (primary time point): walk speed, step length symmetry, knee range of motion (ROM) and peak knee flexion angular velocity
Step length symmetry—step length ratio calculated as ((2xOp)/Op+NOP))−1); where Op is the step length of the operated leg and NOP is the step length of the non-operated leg. Zero indicates perfect symmetry and best performance.
Bold text is used to denote values achieving statisitcal significance
Data for all secondary movement performance measures are provided in online supplemental tables S4–S8 . The only difference between groups that reached statistical significance was for COP path length in double stance with eyes closed ( online supplemental table S7 ). The mean (SD) values for the Genesis II and JII-BCS groups were 158.14 (65.40) mm and 235.48 (176.94) mm, respectively. Adjusted effect size was −59.91 (–105.98, –13.85) p=0.01 in favour of the Genesis II group.
Postoperative clinical context
There were no between-group significant differences for: length of stay, change in pain medication from randomisation or physiotherapy received ( online supplemental tables S9 and S10 ).
Adverse events
One patient with a JII-BCS developed acute swelling and pain in the knee and was systemically unwell at 4 months postoperatively. The joint aspiration demonstrated turbid fluid and an exchange of the polyethylene spacer and retention of the femoral and tibial components (Debridement And Implant Retention) was performed with postoperative antibiotic treatment. Subsequent microbiology was negative so infection was never conclusively demonstrated. The numbers and type of complications are reported in online supplemental table S11 .
The findings do not support the hypothesis that the JII-BCS produces a better outcome than the Genesis II for the primary outcome of the OKS at 6 months after surgery. No differences between groups were also found for: other patient-reported outcomes; measures of balance and walking function; hip and ankle ROM; knee moments during walking; double support time during walking and percentage of gait cycle for peak muscle activation. However, significant advantages for the control group (Genesis II) were found for: operated knee range-of-movement and peak knee flexion angular velocity during walking, and postural control (COP path length).
While some investigators have demonstrated differences between generations of knee designs 12 not all modern generation TKR designs have demonstrated an improvement in outcomes when compared with their predecessors. 15–20 48 One possible reason for this is that the predecessor is already producing good results and therefore is difficult to improve on. Regarding the JII-BCS, at the time of writing, only Bialy et al 45 have directly compared the Genesis II and the JII-BCS. Their study was non randomised and consisted of 18 patients between the two groups. They reported a greater supine range of movement of the JII-BCS compared with the Genesis II when measured with a long arm goniometer. They also reported an improvement in functional knee scores and stability when balancing. Their conclusions were that the JII-BCS restores more normal anatomy and kinematics which is correlates into the improvements that they found. None of the other papers reporting outcomes of the JII-BCS compared the JII-BCS to the Genesis II, none used a randomised design and none used methodology or outcomes that could be compared with the methodology used in this trial. 42–46 However, on the basis of the available literature, we measured outcomes that would be expected to be difference on the basis of the available literature, walking velocity, step-length symmetry (resulting from longer stride length), knee ROM and peak knee angular velocity.
Within our trial, we found differences in some biomechanical measures of motor impairment but not for others; patient-reported outcomes; and, walking and balance function. It is possible that knee range-of-movement during walking, walking symmetry, peak knee flexion angular velocity during walking and postural control (COP path length) are detecting motor impairment improvement for the Genesis II group and/or because statistical significance was a result of testing multiple outcomes. The latter explanation is clearly possible but knee range-of-movement is greater for people reporting good outcome after knee replacement than for those reporting poor outcome. 49 Moreover, knee range-of-movement has been found to be the main biomechanical effect of TKR 50 and to improve over time while other biomechanical measures do not. 50 51 Likewise, postural control improves over time 52 53 and approaches healthy control values. 52 Importantly, gait symmetry is an indicator of walking control 54 and, while of borderline statistical significance (p=0.05) can possibly detect differences following insertion of different prostheses. Peak knee angular velocity during walking is also an indicator of walking control 55 and has been found to change beneficially after insertion of the Genesis II prosthesis. 50 These findings indicate that secondary, in-depth, analysis of the biomechanical data should be undertaken.
A potential limitation is the relatively large number of secondary outcomes. However, this is also a strength as it ensured comprehensive examination of the potential impact of TKR on functional ability, motor impairment and health-related quality of life. Another potential limitation is that the surgeons all had a much greater familiarity with the Genesis II implants. However, all surgeons were very experienced with the Genesis II implant with at least 10 years of experience implanting the device. All surgeons received thorough training with the JII-BCS and the surgical technique and instrumentation are similar for both devices with only one additional femoral cut being necessary for the JII-BCS compared with the Genesis II. A key strength of this trial is that the required sample size was achieved with only one person lost to follow-up. Other strengths include minimisation of selection bias through a robust randomisation procedure and use of double blinding to minimise interpretation bias.
The lack of difference between implant designs is important for patients, surgeons, healthcare providers and implant companies. For the patient and surgeons, reassurance can be gained that older designs, with proven track record of function and survivorship, can provide the same patient reported and functional outcome as more modern designs. For the healthcare providers, older implants are often less expensive and, in the absence of clinical benefit with and demonstrable longevity, if the additional expenditure on more modern designs is avoided for the hundreds of thousands of patients undergoing surgery worldwide the cost savings are potentially significant. Finally, for the implant companies, it is more likely than not than implant design has reached a point when non-implant-related factors play a more important role in patient outcome. The future of design and innovation may come in the form of more modern surgical techniques such as robotic assisted implantation to assist in placing the knee in a more kinematically sympathetic position which in turn may allow the newer design philosophies to positively influence outcome. It is possible, only then in combination with modern surgical techniques, that improvements in patient outcomes can be realised but well-constructed surgical trials will need to answer such questions.
This study demonstrated no difference between the Genesis II and its successor the JII-BCS for PROMS, walking function, temporal-spatial gait parameters, balance ability and lower limb kinematic results at 6 months follow-up. However, significant advantages were seen in for the Genesis II in the operated knee range-of-movement, peak knee flexion angular velocity during walking and postural control.
Supplementary Material
Acknowledgments.
The team would like to thank all the participants and families who gave their time to be part of this study; Antony Colles, Martin Pond and the NCTU data management team; Estelle Payerne; Amanda Thacker; NNUH sponsorship team and the safety monitoring committee members, Prof Marcus Flather and Prof Simon Donell. Also: Mr Charles Mann, Mr Nish Chirodian, Mr David Calder, Dr Nicola Hancock, Nursing and clinic staff at the Spire Hospital and NNUH, Prof Andoni Toms and the Radiology department at NNUH and Addenbrooke’s hospital, Cambridge, Dr Simon Horton and Dr Anne Killett
Twitter: @tobyosmith
Contributors: IM and VP drafted this paper. IM is the guarantor. All authors (IM, VP, ABC, GC, CW, JW, BH, TOS, JH and AMS) contributed to revisions of the manuscript, read and approved the final manuscript. All authors (IM, VP, ABC, GC, CW, JW, BH, TO, JH and AMS) contributed to the development of the trial protocol as well as conception or design of the work; the acquisition, analysis or interpretation of data for the work.
Funding: This work was supported by an investigator initiated grant from Smith and Nephew, with both types of knee replacements supplied at the same cost.
Disclaimer: The funders had no role in the design of the study, the data collection, the data analysis, interpretation of data, or writing of the manuscript.
Competing interests: The trial was funded by Smith and Nephew via an unrestricted grant, administered by the Sponsor NNUH. Funding was used within NNUH for running the trial. Funds were provided via NNUH to UEA for the members of the trial team based in the movement and Exercise Laboratory (MoveExLab) at UEA and the clinical trials unit (CTU) based at UEA for statistics, and trial and data management.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Ethics statements, patient consent for publication.
Not applicable.
Ethics approval
This study involves human participants and was approved by East of England – Cambridge Central Research Ethics Committee (reference 16/EE/0230). Participants gave informed consent to participate in the study before taking part.
1 November 2022
Smith+nephew expands procedural innovation with introduction of journey◊ ii rox◊ total knee solution.
Smith+Nephew (LSE:SN, NYSE:SNN), the global medical technology company, today announces the introduction of its JOURNEY II ROX Total Knee Solution – a reverse hybrid construct for total knee arthroplasty. This new procedural product solution aims to provide surgeons with the clinical advantage of an advanced bearing material and anatomic design combined with the efficiency 1 and potential long-term tibia fixation of a cementless knee.

The JOURNEY II ROX Total Knee Solution combines several of Smith+Nephew’s high performance technologies in one construct - the characteristic kinematics 2-8 of JOURNEY II TKA, the clinical history *9,10 of CONCELOC ◊ Advanced Porous Titanium and the wear resistance of OXINIUM ◊ Oxidized Zirconium. 11-13 The JOURNEY II ROX Total Knee Solution is also compatible with Smith+Nephew’s CORI ◊ Surgical System enabling surgeons to perform the procedure using next generation robotics-assisted technology.
“The reverse hybrid combination provides my patients with a powerful collection of knee arthroplasty technologies. The kinematic functionality of JOURNEY II, the biocompatibility and longevity benefits of an oxidized zirconium alloy femoral component in OXINIUM and now CONCELOC,” said Dr. Steven B. Haas, Chief, the Knee Service and John N. Insall Chair, Knee Surgery at the Hospital for Special Surgery in New York. “CONCELOC offers the potential to achieve osseointegration where it is needed – the tibia and patellae. It’s an evidence-based approach to knee arthroplasty that utilizes high performance technologies delivered with the operative time savings recognized in a complete cementless procedure.”
“The reverse hybrid technique works very well in my hands and I’m able to ensure my patients have the opportunity to harness the JOURNEY II knee mechanics for a more natural knee function,” said Dr. Matthew Bullock, Associate Professor of Orthopaedic Surgery, Marshall University, Joan C. Edwards School of Medicine. “The hybrid approach does not add time to my cases - in fact, I find that using the porous tibia and patella components reduces my operative time by approximately 15 minutes instead of waiting for cement to cure.”
“Driving procedural innovation to help surgeons treat patients and provide better outcomes is the core of our purpose - Life Unlimited,” said Randy Kilburn, Executive Vice President and General Manager, Global Joint Reconstruction and Robotics at Smith+Nephew. “The JOURNEY II ROX Total Knee Solution underscores this by bringing together high performance components to deliver a custom procedural solution that no other company offers.”
Smith+Nephew will preview the JOURNEY II ROX Total Knee Solution in its booth (#501) at the 2022 Annual Meeting of the American Association of Hip and Knee Surgeons (AAHKS) from November 3-6 in Dallas, TX.
Smith+Nephew will also showcase at AAHKS, its ‘Power Of One’ mobile exhibit - an interactive experience focused on the capabilities of handheld robotics and computer-guided surgery using the CORI ◊ Surgical System. It is small, portable, and does not require CT or MRI, making it a well-placed solution for hospitals, Ambulatory Surgical Centres, and Hospital Outpatient Departments.
*Clinical history based on Smith+Nephew's REDAPT◊ Revision Hip System
About Smith+Nephew
Smith+Nephew is a portfolio medical technology business focused on the repair, regeneration and replacement of soft and hard tissue. We exist to restore people’s bodies and their self-belief by using technology to take the limits off living. We call this purpose ‘Life Unlimited’. Our 18,000 employees deliver this mission every day, making a difference to patients’ lives through the excellence of our product portfolio, and the invention and application of new technologies across our three global franchises of Orthopaedics, Sports Medicine & ENT and Advanced Wound Management.
Founded in Hull, UK, in 1856, we now operate in more than 100 countries, and generated annual sales of $5.2 billion in 2021. Smith+Nephew is a constituent of the FTSE100 (LSE:SN, NYSE:SNN). The terms ‘Group’ and ‘Smith+Nephew’ are used to refer to Smith & Nephew plc and its consolidated subsidiaries, unless the context requires otherwise.
For more information about Smith+Nephew, please visit www.smith-nephew.com and follow us on Twitter , LinkedIn , Instagram or Facebook .
Forward-looking Statements
This document may contain forward-looking statements that may or may not prove accurate. For example, statements regarding expected revenue growth and trading margins, market trends and our product pipeline are forward-looking statements. Phrases such as "aim", "plan", "intend", "anticipate", "well-placed", "believe", "estimate", "expect", "target", "consider" and similar expressions are generally intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause actual results to differ materially from what is expressed or implied by the statements. For Smith+Nephew, these factors include: risks related to the impact of COVID-19, such as the depth and longevity of its impact, government actions and other restrictive measures taken in response, material delays and cancellations of elective procedures, reduced procedure capacity at medical facilities, restricted access for sales representatives to medical facilities, or our ability to execute business continuity plans as a result of COVID-19; economic and financial conditions in the markets we serve, especially those affecting health care providers, payers and customers (including, without limitation, as a result of COVID-19); price levels for established and innovative medical devices; developments in medical technology; regulatory approvals, reimbursement decisions or other government actions; product defects or recalls or other problems with quality management systems or failure to comply with related regulations; litigation relating to patent or other claims; legal compliance risks and related investigative, remedial or enforcement actions; disruption to our supply chain or operations or those of our suppliers (including, without limitation, as a result of COVID-19); competition for qualified personnel; strategic actions, including acquisitions and dispositions, our success in performing due diligence, valuing and integrating acquired businesses; disruption that may result from transactions or other changes we make in our business plans or organisation to adapt to market developments; and numerous other matters that affect us or our markets, including those of a political, economic, business, competitive or reputational nature. Please refer to the documents that Smith+Nephew has filed with the U.S. Securities and Exchange Commission under the U.S. Securities Exchange Act of 1934, as amended, including Smith+Nephew's most recent annual report on Form 20-F, for a discussion of certain of these factors. Any forward-looking statement is based on information available to Smith+Nephew as of the date of the statement. All written or oral forward-looking statements attributable to Smith+Nephew are qualified by this caution. Smith+Nephew does not undertake any obligation to update or revise any forward-looking statement to reflect any change in circumstances or in Smith+Nephew's expectations.
◊ Trademark of Smith+Nephew. Certain marks registered US Patent and Trademark Office.
1. Yayac M, Harrer S, Hozack WJ, Parvizi J, Courtney M. The use of cementless components does not significantly increase procedural costs in total
knee arthroplasty. J Arthroplasty. 2020;35:407-712
2. Grieco TF, Sharma A, Dessinger GM, Cates HE, Komistek RD. In Vivo Kinematic Comparison of a Bicruciate Stabilized Total Knee Arthroplasty and the Normal Knee Using Fluoroscopy. J Arthroplasty. 2018;33(2):565-571.
3. Iriuchishima T, Ryu K. A Comparision of Rollback Ratio between Bicruciate Substituting Total Knee Arthroplasty and Oxford Unicompartmental Knee Arthroplasty. J Knee Surg. 2018;31(6):568-572.
4. Murakami K, Hamai S, Okazaki K, et al. Knee kinematics in bi-cruciate stabilized total knee arthroplasty during squatting and stair-climbing activities. J Orthop. 2018;15(2):650-654.
5. Murakami K, Hamai S, Okazaki K, et al. In vivo kinematics of gait in posterior-stabilized and bicruciate-stabilized total knee arthroplasties using image-matching techniques. Int Orthop. 2018;42(11):2573-2581.
6. Smith LA, Nachtrab J, LaCour M, et al. In Vivo Knee Kinematics: How Important Are the Roles of Femoral Geometry and the Cruciate Ligaments? J Arthroplasty. 2021;36:1445-1454.
7. Carpenter RD, Brilhault J, Majumdar S, Ries MD. Magnetic resonance imaging of in vivo patellofemoral kinematics after total knee arthroplasty. Knee. 2009;16(5):332-336.
8. Catani F, Ensini A, Belvedere C, et al. In vivo kinematics and kinetics of a bi-cruciate substituting total knee arthroplasty: a combined fluoroscopic and gait analysis study. J Orthop Res. 2009;27(12):1569-1575
9. Moriarty P, Vles G, Haddad F, Konan S. Early clinical and radiological outcomes of a new tapered fluted titanium monobloc revision stem in hip arthroplasty. Act Orthop Trauma Surg. 2021;141:1065-1071.
10. Smith+Nephew 2015. Orthopaedic Research Report OR-14-091A.
Technology for THA. Poster presented at: 2013 Annual Meeting of the Orthopaedic Research Society. Poster no. 1028.
11. Papannagari R, Hines G, Sprague J, Morrison M. Long-term wear performance of an advanced bearing technology for TKA. Poster presented at: 2011 Annual Meeting of the Orthopaedic Research Society. Poster no. 1141.
12. DesJardins J, Burnikel B, LaBerge M. UHMWPE wear against roughened oxidized zirconium and CoCr femoral knee components during force-controlled simulation. Wear. 2008;264:245-256
13. Ries MD, Salehi A, Widding K, Hunter G. Polyethylene wear performance of oxidized zirconium and cobalt-chromium knee components under abrasive conditions. J Bone Joint Surg Am. 2002;84-A Suppl 2:129-135
Orthopedics
The JOURNEY II Bi-Cruciate Stabilized (BCS) Total Knee System
Watch a live surgical webcast featuring the Smith+Nephew JOURNEY II Bi-Cruciate (BCS) Total Knee System. On December 11, 2013, at 6:30pm EST, Steven Haas, MD, from the Hospital for Special Surgery, will perform the procedure. Fred Cushner, MD, Director of ISK, and designer of the JOURNEY II BCS will moderate the surgery, and Dave Mayman, MD, from the Hospital for Special Surgery will also participate from CCJR in Orlando, FL. The goal of the JOURNEY II BCS is to enable a higher level of function for total knee replacement patients – to not only relieve pain, but help them regain active lifestyles. Function, motion and durability are achieved through the unique features of the JOURNEY II BCS system-anatomic alignment, kinematics and advanced bearings.
Related Presenters

Fred Cushner, MD
Orthopaedic Surgeon
Fred D. Cushner, M.D. completed a fellowship in adult reconstruction and sports medicine at the Insall Scott Kelly Institute. Dr. Cushner remained on staff and is currently Director of this prestigious Institute.Dr. Cushner's practice ...
View full profile

Steven B. Haas, MD
Dr. Steven B. Haas received his education and training at Harvard, Cornell and the University of Rochester. He is Chief of the Knee Service at Hospital for Special Surgery. Dr. Haas speaks extensively both nationally and internationally ...

David Mayman, MD
Dr. David Mayman is one of a handful of national experts in computer navigation in hip and knee replacement surgery. He performs over 700 computer-navigated joint replacement surgeries a year. "I believe that in the future all joint replacement ...

Gastroenterology
- chevron_left Back
BroadcastMed News
Dermatology
Diabetes & Endocrinology
Infectious Diseases
Ophthalmology
Otolaryngology
Pulmonology
person Sign In / Create Account

IMAGES
VIDEO
COMMENTS
Total knee arthroplasty patients report unmet levels of satisfaction, particularly for more active or demanding activities. 1,2 The JOURNEY II System is designed to help patients rediscover their normal through a smoother recovery, *3,4 improved function *4-8 and higher patient satisfaction *2,4-6. Normal shapes. Normal position.
JOURNEY™ II. Journey II Total Knee System. For orthopaedic surgeons seeking treatment solutions beyond traditional knee replacements, JOURNEY II Active Knee Solutions has been engineered to empower patients with a renewed right to an active lifestyle by breaking through traditional knee replacement barriers and delivering Function, ...
The opening act for JOURNEY II ROX Solution has been shown to restore the anatomical shapes, position and motion of a normal knee;6-10providing improved clinical outcomes and higher patient satisfaction*10,18-22. Normal shapes: Featuring an anatomic, asymmetric femur/tibia,8,10,23 concave medial tibial surface8,9,23and a convex lateral tibial ...
Conclusion. The Journey II TKA is truly a revolutionary step forward in the design of total knee implants. With proper surgical technique and in the hands of a well-trained surgeon, the Journey II knee can produce results superior to the traditional total knee design. It is our goal as your surgical team to continue to improve our surgical ...
ORTHOPAEDICS. JOURNEY II XR Bi-Cruciate Retaining Knee System. While JOURNEY II TKA has been shown to restore anatomical shape, position and motion, 1-4 the JOURNEY II XR system goes a step further by allowing the ACL to be retained. An anatomical design is intended to help a smoother recovery, improved function and higher patient satisfaction ...
The JOURNEY II BCS Knee. Recent advances in biomedical engineering software have opened a new chapter on high performance knee implants. One remarkable breakthrough has been the creation of the JOURNEY II BCS knee, a second-generation knee replacement that combines the stability and natural motion of the human knee with new low-friction materials that may extend the life of the implant.
Partial knees treat only the affected part of the knee, while allowing the patient to keep their healthy ligaments. JOURNEY II UK System combines clinically successful features to present a third-generation, unicompartmental knee platform featuring: Intraoperative sizing flexibility. OXINIUM Technology bearing surface. Tissue-conscious design.
The JOURNEY II AKS difference. One of the most remarkable breakthroughs in design of total knee replacements has been the creation of the JOURNEY II Active Knee Solutions. Designed using the latest in human simulation software, and built using some of the most wear-resistant materials available, this unique implant was designed to address two ...
The JOURNEY II AKS difference. One of the most remarkable breakthroughs in design of total knee replacements has been the creation of the JOURNEY II Active Knee Solutions. Designed using the latest in human simulation software, and built using some of the most wear-resistant materials available, this unique implant was designed to address two ...
JOURNEY£ II Total Knee System. JOURNEY II BCS contributing surgeons: Johan Bellemans, MD, PhD Professor of Orthopaedic Surgery Chairman of the Department of Orthoaedic Surgery and Traumatology Catholic University Hospitals Leuven and Pellenberg, Belgium Fred D Cushner, MD
The JOURNEY™ II Total Knee System was designed to help patients rediscover their normal through a smoother recovery, improved function and higher patient satisfaction.1-7* These improvements are made possible by designing JOURNEY II TKA to replicate the shapes, position and motion of the normal healthy knee.8-10**
Background and rationale. Osteoarthritis of the knee is a common musculoskeletal condition. The surgical management of painful, end-stage osteoarthritis is by total knee replacement (TKR) which should be considered before there is prolonged and established functional limitation and severe pain [].Over 100,000 TKRs were performed in the UK in 2019 [].
JOURNEY II Active Knee Solutions total knee arthroplasty animation for knee replacement.For more information, please visit our website: http://www.smith-nep...
A comparison of patient reported outcomes between total knee arthroplasty patients receiving the Journey II bi-cruciate stabilizing knee system and total hip arthroplasty patients. Ortop Travmatol ...
The JOURNEY™ II BCS provides more mobility in the lateral compartment than other total knee systems provide, to replicate normal knee kinematics. For patients who presented with significant varus or valgus deformities (> 15°), morbid obesity, or deficient collateral ligaments, the surgeon decided if additional implant constraint was appropriate.
Introduction. Despite total knee replacement (TKR) being a recommended surgical treatment for end-stage knee osteoarthritis, 1 up to 34% of all patients following TKR have poor functional outcomes. 2-6 With estimates of osteoarthritis of the knee affecting one in eight people in the USA 7 and 250 million individuals worldwide 8 the number of patients with intrusive symptoms after surgery is ...
The Journey II total knee replacement (Smith and Nephew, Watford, UK) was introduced in 2012 as a replacement for the Journey BCS, for which a number of complications had been reported including dislocation between the tibial insert peg and the femoral component which was believed to occur as a consequence of high flexion angles.The Journey II successfully addressed this issue with changes to ...
The JOURNEY II ROX Total Knee Solution combines several of Smith+Nephew's high performance technologies in one construct - the characteristic kinematics 2-8 of JOURNEY II TKA, the clinical history *9,10 of CONCELOC Advanced Porous Titanium and the wear resistance of OXINIUM Oxidized Zirconium. 11-13 The JOURNEY II ROX Total Knee Solution is ...
Smith+Nephew will preview the JOURNEY II ROX Total Knee Solution in its booth (#501) at the 2022 Annual Meeting of the American Association of Hip and Knee Surgeons (AAHKS) from November 3-6 in ...
Smith+Nephew (LSE:SN, NYSE:SNN), the global medical technology company, today announces the introduction of its JOURNEY II ROX Total Knee Solution - a reverse hybrid construct for total knee arthroplasty. This new procedural product solution aims to provide surgeons with the clinical advantage of an advanced bearing material and anatomic design combined with the efficiency 1 and potential ...
Watch a live surgical webcast featuring the Smith+Nephew JOURNEY II Bi-Cruciate (BCS) Total Knee System. On December 11, 2013, at 6:30pm EST, Steven Haas, MD, from the Hospital for Special Surgery, will perform the procedure. Fred Cushner, MD, Director of ISK, and designer of the JOURNEY II BCS will moderate the surgery, and Dave Mayman, MD ...
In Vivo Kinematic Comparison of a Bicruciate Stabilized Total Knee Arthroplasty and the Normal Knee Using Fluoroscopy. J Arthroplasty. 2018;33(2):565-571. Murakami K, Hamai S, Okazaki K, et al.
Detailed Description: This is a prospective, non-randomized, single cohort, multicenter study to evaluate the clinical outcomes of TKA using the JOURNEY™ II CR Total Knee System in subjects with degenerative joint disease (DJD) requiring primary total knee replacement. One hundred and seventy (170) subjects will be enrolled at up to 18 ...