Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Perspective
- Open access
- Published: 01 December 2022
Clinical Studies

The importance of long-term follow up of participants in clinical trials
- Jack Cuzick ORCID: orcid.org/0000-0001-7420-7512 1
British Journal of Cancer volume 128 , pages 432–438 ( 2023 ) Cite this article
7712 Accesses
6 Citations
16 Altmetric
Metrics details
- Medical research
As good health is a lifetime issue, long-term follow up is an important part of evaluating any medical condition or treatment. This is well appreciated in epidemiologic studies where exposure to a harmful substance is often long-term, and its impact on health can appear many years after first occurrence. Examples include the classic study of Doll and Peto [ 1 ] on 34,439 male British doctors, which began in 1951 and last reported in 2004 after 50 years of follow up, in which lifelong cigarette smoking was shown to reduce life expectancy by an average of 10 years, but cessation at age 60, 50, 40 or 30 years reduced this by about 3, 6, 9, or almost the full 10 years, respectively. Other classic long-term epidemiologic studies have focussed on diet and alcohol consumption [ 2 , 3 ], hormone replacement therapy [ 4 ], or have been more general and studied a wider set of risk factors, often among healthcare professionals; for example, the ACS Cancer Prevention Study [ 5 ], the Harvard-based Nurses Health Studies [ 6 ] and the Health Professionals Follow Up Study [ 7 ].
Screening trials
Treatments for clinical problems are usually directed at an immediate health issue, and obtaining data on long-term sequelae may seem less relevant and can be expensive and difficult to organise, especially in countries which do not have national repositories containing the needed information. For these reasons, long-term follow up is often not pursued. Long-term consequences are much more relevant for screening interventions and prophylactic treatments aimed at preventing future cancer, such as the value of human papilloma virus (HPV) vaccination, or the importance of screening for breast and colorectal cancer. For example, for breast cancer screening with short term follow up many cancers were found which did not appear to require treatment, leading to the belief that up to 50% of the screen detected cancers were over-diagnosis of indolent cancers that would not be clinically detected in the woman’s lifetime [ 8 ], and this was a drawback of breast screening. However, with longer follow up [ 9 ] these cancers were found to be earlier diagnosis of clinically relevant disease, and it was estimated that of the total number of screen detected cancers in the screened population, only 9.5% overall were over-diagnosed, and this was reduced to 3.7% after adjustment for self-selection. Thus, long-term follow up, even after screening has ended, is necessary to accurately assess over-diagnosis. Also, additional procedures and late side-effects need to be recorded and analysed.
Early studies clearly demonstrated the value of the cytological Papanicolaou smear test in identifying cervical lesions at a treatable stage and reducing mortality. This has been well established for many decades, and the evidence was so clear that randomised clinical trials were not needed for confirmation [ 10 ], although several long-term cohort studies have demonstrated the benefits of screening in reducing cervix cancer mortality [ 11 ]. More recently, tests have been developed for HPV, which causes cervix cancer, and trials have been conducted in which all women receive both HPV and Pap tests, and those positive for either test are referred to colposcopy to directly compare the two tests in the same woman [ 12 ]. These trials have clearly shown that HPV tests detect more cervical lesions than cytology in a range of countries and settings. Four randomised European trials with extended individual patient follow up in 176,464 women have been conducted to compare the impact of an HPV test vs. the Pap test on subsequent cancer rates. After a 6.5-year median follow up, these trials have demonstrated that HPV testing reduces cancer incidence by a further 40% overall compared with cytology, and by 70% when the HPV test was negative [ 13 ]. Longer follow up of these studies is needed to demonstrate that this reduction in cancer incidence translates into a reduction in cervix cancer mortality. However, a large four-arm cluster randomised trial of 131,746 women in rural India has also reported substantial increases in detection of precursor lesions, and, after a 20-year follow up, a significant reduction in cervix cancer deaths (34 vs. 64), which was not seen with Pap cytology or visual inspection (VIA) was also demonstrated [ 14 ]. A range of trials have also been conducted that look at the duration of protection following a negative HPV test, and after a 6-year follow up, Dillner et al. [ 15 ] have demonstrated a roughly fourfold reduction in high-grade precursor lesions and cancer (CIN3+) after a negative HPV test compared with a negative cytology test. These findings have been confirmed in a recent systematic review [ 16 ]. Several studies have also shown that HPV testing can be done on a vaginal self-sample or a urine sample, and achieves similar sensitivity for high-grade CIN, although specificity was somewhat lower [ 17 ]. This approach promises to increase acceptance of cervical screening for women, especially for those who feel uncomfortable having their sample taken by a clinician. Additional follow up of these studies will be necessary to determine which self-sampling device is best and the most appropriate screening interval when self-sampling is performed.
The Flexi-Sig screening trial of once-in-a-lifetime sigmoidoscopy for preventing colorectal cancer randomised 170,034 individuals, aged 55–64 years, in a 2:1 ratio to a single sigmoidoscopy or no screening. After 17 years of follow up it has demonstrated that the higher detection of precursor lesions found with screening was followed by a 26% reduction overall in colorectal cancer incidence, and a 30% reduction in mortality, based on 41% and 46% reductions in distal cancer incidence and mortality respectively [ 18 ]. When restricted to those actually receiving screening, larger reductions were seen: 56% and 66%, respectively [ 18 ]. Similar results have been seen for the colorectal component of the PLCO trial [ 19 ] and elsewhere [ 20 ].
Prostate-specific antigen (PSA) screening for prostate cancer is a controversial subject, where even long-term follow up of a large number of trials has not resolved the major issues. While all investigators acknowledge that screening has led to a substantial amount of over-diagnosis and over-treatment, the extent of a reduction in prostate cancer mortality, if any, remains controversial. The large European ERSPC trial has reported a 20% reduction in mortality after a maximum of 16 years of follow up in 182,160 men [ 21 ] with increasing benefit occurring with longer follow up, and the number needed to treat to prevent one prostate cancer death falling from 48 after 9 years of follow up [ 22 ] to 27 after 13 years [ 23 ] and 18 after 16 years [ 21 ]. Support for this method of screening as also been expressed [ 24 ] from North American investigators [ 24 ]. However, other studies have not been so positive, with conclusions of little or no mortality benefit in large studies and concerns about side-effects associated with treatment of indolent disease. Notable among these are the PLCO trial, with 13-year follow up in 76,685 men [ 25 ], an individual patient overview of 5 trials of 727,718 men with a 10-year follow up [ 26 ], and a large overview view of 1,904,950 patients in 63 studies [ 27 ]. These studies compared an invitation for screening with usual care, and were conducted mostly in North America, for which there was likely to be a substantial amount of opportunistic screening in the control arm. These results indicate that study size and length of follow up are not the only relevant factors. Monitoring and follow up procedures also need to be examined. Selection of who needs screening, an efficient triage algorithm to determine who to biopsy following a positive screening test, and avoidance of over-treatment of likely indolent lesions are also areas that merit further research.
Major trials have also been conducted for ovarian cancer screening, with mixed results. The most recently reported was the UKCTOCS trial, which randomised women to an annual CA125 blood test ( N = 50,640), annual ultrasound ( N = 50,639), or no screening ( N = 101,359). After a median follow up of 11.1 years, a much higher proportion of low-volume ovarian and peritoneal cancers was found in the CA125 group vs. controls (40% vs. 26%, P < 0.0001), along with a non-significant 16% lower ovarian cancer mortality ( P = 0.23) [ 28 ], leading the investigators to continue blinded follow up for mortality. After a 16.3-year median follow up, the difference in stage distribution was maintained, but no relative improvement in ovarian cancer mortality was seen in either screened arm [ 29 ] (hazard ratio for CA125 group = 0.96 (0.83–1.10), P = 0.52), leading to the conclusion that the stage shift did not translate into a mortality gain.
Screening for lung cancer provides another example of the need to evaluate cause specific mortality and not just rely on the detection of better risk cancers. An early major trial of screening by chest X-ray and sputum cytology in 9211 male smokers was conducted by the Mayo Clinic from 1971–83, and early findings based on screen detected cancer indicated that they were more likely to be resectable, postsurgical Stage I or II, and associated with better 5-year survival [ 30 ]. However, after 20.5 years of follow up, non-significantly more lung cancer deaths were seen in the screened group (337 vs. 303, relative risk 1.13) [ 31 ], indicating a lead time bias in screen cases detected that did not translate into a mortality benefit. While chest X-ray was not effective, subsequent trials using more sensitive CT scans have been shown to reduce mortality [ 32 , 33 , 34 ] and are now widely used in smokers and other high-risk individuals.
Prevention trials
Prevention trials are an example of research intermediate between epidemiologic studies and therapeutic treatment trials. Many of these studies have been done without randomisation, and in some cases this is appropriate. However, the most informative prevention trials have been randomised, and the extra information provided by this can be very valuable. As breast cancer is the commonest cancer in women in most countries, and is known to be related to hormone levels, it is an obvious candidate for preventive trials. The first major trials examined the role of tamoxifen as a preventive medicine. It was already accepted as an effective adjuvant treatment for reduction in progression of oestrogen receptor-positive breast cancer, and in those trials a reduction was seen in new contralateral tumours, which provided a strong basis for believing it could be effective for primary prevention. Four major trials have been undertaken: the largest was a North American trial known as P-1 [ 35 ], which recruited 13,388 high-risk women, but sadly was curtailed after 7 years median follow up, so no long-term information is available. An Italian trial in 5408 hysterectomised women [ 36 ], and the IBIS-I trial, which recruited 7154 women [ 37 ] and which was preceded by the Marsden ‘pilot study’ of 2494 high-risk women [ 38 ] have also been conducted. Follow up was 11 years in the Italian trial and 20 years in the Marsden trial. Long-term follow up is continuing in the IBIS-I trial, with a 16.0-year median follow up at last publication. Long-term follow up data in that trial proved very informative, and demonstrated that 5 years of tamoxifen in high-risk women without breast cancer could prevent almost 29% of cancers over a 20-year follow up period, and the preventive effect was virtually identical in the first and second 10-year follow up periods, suggesting that the preventive effect of 5 years of tamoxifen could last a lifetime (Fig. 1 ). The number needed to treat to prevent one cancer was reduced from 59 after 10 years of follow up to 22 after 20 years.
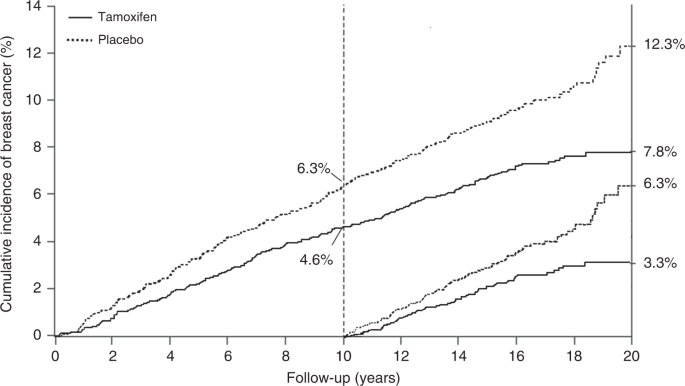
20-year incidence of breast cancer in high risk women receiving tamoxifen or placebo in the IBIS-I prevention trial. Incidence in the first 10 years and second 10 years of follow up is also shown separately.
Trials of raloxifene, another selective oestrogen receptor modulator like tamoxifen, have also evaluated its effect on breast cancer occurrence. The initial MORE trial was aimed at reducing fracture rates in 7705 osteoporotic women, and after 4 years of treatment it was extended for another 4 years in the CORE trial, which combined the two active treatment arms into a simple active arm at the lower dose, and retained the placebo arm. After 8 years of follow up in the combined trials a large 66% reduction in new breast cancer was seen, with an even larger 76% reduction in oestrogen receptor-positive cancer [ 39 ]. This led to the STAR trial, which compared raloxifene with tamoxifen in women at high risk of breast cancer, and after an 81-month median follow up it was found to be 24% less effective than tamoxifen, but had fewer side-effects [ 40 ]. The reasons for its lower efficacy remain unclear, but it might be related to the fact that women in MORE/CORE were osteoporotic.
Two trials have looked at the role of aromatase inhibitors in breast cancer prevention, again supported by strong findings, both for preventing recurrence and new cancers in the adjuvant setting, where they were more effective than tamoxifen [ 41 ]. The North American trial known as MAP.3 [ 42 ] showed a very large 65% reduction in invasive cancers in the first 3 years of follow up, but unfortunately follow up was also curtailed after 35 months median follow up, before long-term data could be obtained. The IBIS-II trial comparing anastrozole with placebo in 3864 high-risk postmenopausal women has now reported, with a median follow up of 10.9 years [ 43 ]. It found that reductions on new cancers continued after the 5-year active treatment period, although they were non-significantly smaller than those seen during treatment (49% overall, 61% years 0–5, 36% subsequently). Further follow up is planned, and is necessary to see if a continued preventive effect will be seen in the extended long-term follow up period, as with tamoxifen. This clearly has a major impact on number needed to treat to prevent one cancer, and will be important for determining optimal use of aromatase inhibitors for prevention.
Vaccine trials provide another good example of the need for long-term follow up. Early trials showed clearly that vaccination against HPV is effective in preventing HPV infection [ 44 , 45 ] and longer term follow up of these trials has now demonstrated that this protection against infection carries forward to reduce the incidence of precursor CIN lesions [ 46 , 47 ], but further follow up is needed to see if the expected reduction in cervical cancer incidence and mortality can also be achieved.
Another somewhat serendipitous set of trials has also been conducted for low-dose aspirin. These trials, of at least 4 years of aspirin or control use, were originally designed to look at its impact on cardiovascular disease, and only short term follow up, typically for less than 5 years, was reported for this. However, Peter Rothwell and colleagues [ 48 ] resurrected these 8 trials, involving 25,570 patients, and obtained information on deaths and cancers for up to 20 years of follow up, which led to the discovery of important benefits for cancer prevention, primarily for colorectal, stomach and oesophageal cancers. Curiously, very little effect on cancer was seen in the first 5 years of follow up, but large effects were seen subsequently (Fig. 2 ), leading to an ~20% reduction in cancer deaths overall, a 34% reduction in the post 5-year-period for all cancer deaths, and a 54% reduction in gastrointestinal cancers, which was still diverging after 20 years of follow up. The role of long-term follow up was vital in discovering this pronounced preventive activity. The clearest evidence was for a reduction in colorectal cancer, but gastric and oesophageal cancer were also substantially reduced. These results have now been validated in several non-randomised epidemiologic studies [ 49 , 50 ], and are summarised in Table 1 [ 51 ]. A current challenge for understanding the role of aspirin for cancer prevention comes from the results of two recent reports on studies with short follow up [ 52 , 53 ]. Given what was known from cardiovascular studies, it should have been anticipated that no benefit would be seen in the first 5 years, and only the side-effects would be apparent. This is what has happened, and has been misinterpreted by many to conclude that aspirin has no effect on cancer when, in fact, no useful efficacy information has yet to be obtained from these studies [ 54 ].
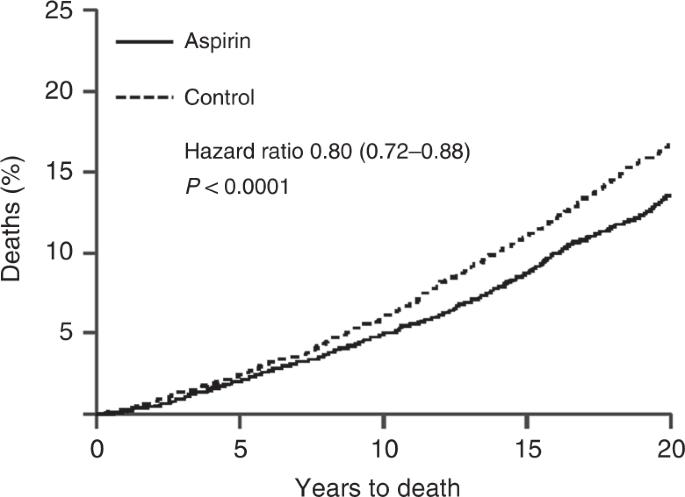
Twenty year impact of daily aspirin on deaths from all solid tumours (Rothwell et al. [ 48 ]).
The Prostate Cancer Prevention Trial (PCPT) compared the anti-androgen finasteride vs. placebo in 18,882 low to average risk men aged 55 years or over [ 55 ]. After a 7-year follow up, the trial reported a 24.8% reduction in prostate cancer cases overall (803 vs. 1147, P < 0.0001), but high-grade tumours (Gleason grade ≥7) were more common in the treated group (280 vs. 337, P = 0.005). This has led to much debate, but after further follow up for a median of 18 years, no excess in prostate cancer deaths has been observed [ 56 ]. It has been suggested that the reason for the increased detection of high-grade tumours was due to a reduction in prostate size associated with finasteride treatment, which led to more accurate biopsies being taken. This in turn may have altered the assessment of Gleason grade, again reinforcing the need for long-term follow up to discover the full impact of a clinical intervention.
Treatment trials
Long-term follow up often needs to be seen in a different context in treatment trials, as often the norm is for some form of treatment where there is considerable evidence for a short term benefit, and the ethics and safety of giving no or less treatment is a bigger concern. Thus, the main question is whether a long-term benefit exists when short-term efficacy is well established, and if it outweighs any late adverse effects.
An early example of the importance of long-term follow up in treatment trials comes from a UKCCR overview of the use of radiotherapy in early breast cancer. This overview of four early trials, initiated between 1949 and 1974, included 4148 deaths in 7842 women. No impact on deaths was seen in the first 10 years of follow up, but it was found that the regimens used at that time led to an increase in cardiac deaths for left-sided breast cancer after 10 years of follow up [ 57 , 58 ]. As a result of these findings, newer radiotherapy regimens have been developed and an Early Breast Cancer Trials Coordinating Group (EBCTCG) overview has demonstrated that these regimens have led to reductions in recurrences and deaths from breast cancer, and with minimal impact on non-breast cancer deaths [ 59 ], and minimal cardiac toxicity [ 60 , 61 ].
Another area where long-term follow up has provided important information has been for the use of adjuvant endocrine therapy in early breast cancer. Notable in this area are the overviews conducted for tamoxifen by the EBCTCG. Among other things, they showed that in 10,645 oestrogen receptor-positive tumours, 5 years of tamoxifen led to a 50% reduction in recurrence in the first 5 years of follow up, a further 30% reduction in years 5–9, but no further effect in years 10–14 [ 62 ]. This is in contrast to the prevention trials, where 5 years of tamoxifen prevented new breast cancers for at least 20 years [ 33 ]. For breast cancer mortality, a one-third reduction was seen in the overview, a reduction sustained for at least 15 years. In contrast to the recurrence data, mortality reductions were as strong in years 10–14 after treatment as in earlier years (Relative Risk = 0.71, 0.66 and 0.68 in each 5-year period). Tamoxifen had little effect on deaths from other causes, so that there was a substantial effect on overall mortality. It was also shown that tamoxifen had little effect on breast cancer recurrence or mortality for the 5984 oestrogen receptor poor/negative breast cancers [ 62 ].
Several large trials of aromatase inhibitors vs. tamoxifen for 5 years as adjuvant therapy in postmenopausal women have been conducted. In addition to straight two-arm comparisons, some have also compared trials which switch between aromatase inhibitors and tamoxifen after 2–3 years of initial treatment [ 63 ]. This analysis showed a larger effect for an aromatase inhibitor during its use, but not afterwards. A mortality benefit was also seen. Only one trial (ATAC) has reported even moderately long-term follow up, which has now been extended to a median of 10 years [ 64 ]. Serious side-effects were lower with anastrozole than tamoxifen.
Improvements in progression rates do not always translate into disease mortality reductions. One trial of radiotherapy for prostate cancer [ 65 ] reported improvements in biochemical progression-free survival with dose escalated radiotherapy vs. a conventional dose, which have been maintained for a median of 10 years, but this has not translated into improvements in overall survival. An overview of three available subsequent radiotherapy trials with a median follow up between 60 and 78 months was unable to establish a difference in event free survival between upfront or early salvage radiotherapy, and noted that the latter produced fewer side-effects [ 66 ]. Longer follow up, or a larger trial may be needed to see if there is an effect.
Longer term toxicity issues can also negate any improvements seen in progression markers. Mauch et al. [ 67 ] noted the excess mortality from cardiovascular disease compared with the general population in both arms of a trial of radiotherapy vs. radiotherapy and chemotherapy in Hodgkin’s disease. In an overview of 8 trials of more vs. less radiotherapy in 1974 patients, and 13 trials in 1688 patients of adding chemotherapy to radiotherapy, Specht et al. [ 68 ] reported a one-third reduction in recurrence with added radiotherapy, and a halving with added chemotherapy. However, after 10 years of follow up, no significant effect was seen on mortality, suggesting that less intensive primary treatment—particularly a reduction in radiotherapy field—may have achieved the same results. Endpoints that are surrogates for disease-specific survival, such as recurrence, metastatic spread, or increases in tumour size or grade can be indicators of better survival, but as noted above, these do not always translate into reduced disease-specific mortality [ 69 ].
Because of the longer number of years at risk after cancer treatment, long-term follow up is especially important for childhood and adolescent cancers. A range of different issues need to be addressed, including effects on mental development and childbearing. Partly because of the heterogeneity of these cancers, this is rarely done within a clinical trial, but efforts to establish registries to create large cohorts to document late side-effects are now being made [ 70 , 71 , 72 ]. This is only a start, and more resources and effort are needed to develop this area.
Not all treatment questions can be answered by a single long-term follow up analysis. Factors other than follow up duration include sample size, choice of study endpoints, aggressiveness of applying salvage therapies, and subgroups with differing prognoses [ 73 ]. Adequate sample size is essential and can be determined by standard power calculations. A common question is whether reducing disease recurrence or progression translates into an improvement in survival. Often, overall survival is used for the latter. While this clearly has value as a bottom line, for diseases in which only a small to moderate number of patients actually die from the disease under study, such as early breast or prostate cancer, a substantial loss of power can occur due to deaths from other causes for which no treatment effect is anticipated and deaths from the disease under study can be a more powerful and useful measure of efficacy [ 74 ]. Differences in other causes of death that might be due to treatment are important, but are usually specific to a particular site, and often are not captured in an ‘all other cause’ mortality assessment.
Another issue is comparing up-front treatment with salvage therapy, often studied for the use of radiotherapy. Here the threshold for using salvage therapy can be important. For example, in intermediate-risk or high-risk, localised or locally advanced prostate cancer, Vale et al. [ 66 ] found salvage therapy to be equally effective, and that it reduced the number of men with size effects.
In addition, treatments may be more or less effective in different subgroups. It is clearly clinically important to identify any heterogeneity in response, but the reporting of apparent subgroup differences is one of the most common errors in analysing clinical trial results [ 75 , 76 ]. Often numerous subgroups are examined, and the chance that one is truly significantly different at, say, a 5% level, is much less than the 95% suggested by this nominal two-sided P -value, due to the multiple comparisons being made. A Bonferroni or similar correction should be made. This requires specifying the number of subgroups to be investigated, and often can be problematic. Also any difference should be based on a significant interaction between a subgroup and the remaining trial population, rather than simply a significant effect in a subgroup [ 77 ].
In other reports, randomised studies are mixed with observational studies to increase sample size, but the potential for biased allocation in the observational studies still exists [ 73 ], although it may be less than for short term follow up.
Clinically important findings can arise several years after treatment is completed, and often after formal follow up is stopped. An early analysis can give a distorted view of a treatment’s value. Especially in the prevention setting, unfavourable side-effects often occur early, and well before any benefits become apparent. In addition, favourable early effects on, e.g., recurrence, may or may not be maintained in the longer term, and may or may not lead to reductions in disease-specific mortality. Late treatment-related side-effects can also be uncovered, which can be particularly important when the prognosis is good. Many countries have national databases that can usually provide cause specific mortality, although recurrence and side effect data are less common. Linking basic long-term data to earlier clinical records can lead to valuable additional findings for a randomised clinical trial, and can provide important new findings that are more reliable than non-randomised comparisons.
Data availability
No new data was created or analysed in this report
Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004;328:1519. https://doi.org/10.1136/bmj.38142.554479.AE . Epub 2004 Jun 22. PMID: 15213107; PMCID: PMC437139.
Article PubMed PubMed Central Google Scholar
Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5:1113–24. https://doi.org/10.1079/PHN2002394 . PMID: 12639222.
Article CAS PubMed Google Scholar
Ubago-Guisado E, Rodríguez-Barranco M, Ching-López A, Petrova D, Molina-Montes E, Amiano P, et al. Evidence update on the relationship between diet and the most common cancers from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: a systematic review. Nutrients. 2021;13:3582. https://doi.org/10.3390/nu13103582 . PMID: 34684583; PMCID: PMC8540388.
Article CAS PubMed PubMed Central Google Scholar
Beral V, Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–27. https://doi.org/10.1016/s0140-6736(03)14065-2 . Erratum in: Lancet. 2003 Oct 4;362:1160. PMID: 12927427.
Jacobs EJ, Newton CC, Gapstur SM, Thun MJ. Daily aspirin use and cancer mortality in a large US cohort. J Natl Cancer Inst 2012;104:1208–17. https://doi.org/10.1093/jnci/djs318 . Epub 2012 Aug 10. PMID: 22888140.
Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5:388–96. https://doi.org/10.1038/nrc1608 . PMID: 15864280.
Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404. https://doi.org/10.1056/NEJMoa1014296 . PMID: 21696306; PMCID: PMC3151731.
Beckmann KR, Lynch JW, Hiller JE, Farshid G, Houssami N, Duffy SW, et al. A novel case-control design to estimate the extent of over-diagnosis of breast cancer due to organised population-based mammography screening. Int J Cancer 2015;136:1411–21. https://doi.org/10.1002/ijc.29124 . Epub 2014 Aug 18. PMID: 25098753.
Blyuss O, Dibden A, Massat NJ, Parmar D, Cuzick J, Duffy SW, et al. A case-control study to evaluate the impact of the breast screening programme on breast cancer incidence in England. Cancer Med. 2022 Jul. https://doi.org/10.1002/cam4.5004 . Epub ahead of print. PMID: 35851849.
Tambouret RH. The evolution of the Papanicolaou smear. Clin Obstet Gynecol. 2013;56:3–9. https://doi.org/10.1097/GRF.0b013e318282b982 . PMID: 23314726.
Article PubMed Google Scholar
Jansen EEL, Zielonke N, Gini A, Anttila A, Segnan N, Vokó Z, et al. Effect of organised cervical cancer screening on cervical cancer mortality in Europe: a systematic review. Eur J Cancer 2020;127:207–23. https://doi.org/10.1016/j.ejca.2019.12.013 . Epub 2020 Jan 21. PMID: 31980322.
Cuzick J, Clavel C, Petry KU, Meijer CJ, Hoyer H, Ratnam S, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095–101. https://doi.org/10.1002/ijc.21955 . PMID: 16586444.
Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524–32. https://doi.org/10.1016/S0140-6736:62218-7 . Epub 2013 Nov 3. Erratum in: Lancet. 2015 Oct 10;386(10002):1446. PMID: 24192252.
Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, et al. HPV screening for cervical cancer in rural India. N. Engl J Med. 2009;360:1385–94. https://doi.org/10.1056/NEJMoa0808516 . PMID: 19339719.
Dillner J, Rebolj M, Birembaut P, Petry KU, Szarewski A, Munk C, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008;337:a1754. https://doi.org/10.1136/bmj.a1754 . PMID: 18852164; PMCID: PMC2658827.
Melnikow J, Henderson JT, Burda BU, Senger CA, Durbin S, Weyrich MS. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2018;320:687–705. https://doi.org/10.1001/jama.2018.10400 . PMID: 30140883.
Arbyn M, Smith SB, Temin S, Sultana F, Castle P, Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ 2018;363:k4823. https://doi.org/10.1136/bmj.k4823 . PMID: 30518635; PMCID: PMC6278587.
Atkin W, Wooldrage K, Parkin DM, Kralj-Hans I, MacRae E, Shah U, et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet. 2017;389:1299–311. https://doi.org/10.1016/S0140-6736(17)30396-3 . Epub 2017 Feb 22. PMID: 28236467; PMCID: PMC6168937.
Miller EA, Pinsky PF, Schoen RE, Prorok PC, Church TR. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: long-term follow-up of the randomised US PLCO cancer screening trial. Lancet Gastroenterol Hepatol. 2019;4:101–10. https://doi.org/10.1016/S2468-1253(18)30358-3 . Epub 2018 Nov 29. PMID: 30502933; PMCID: PMC6335177.
Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ. 2014;348:g2467. https://doi.org/10.1136/bmj.g2467 . PMID: 24922745; PMCID: PMC3980789.
Hugosson J, Roobol MJ, Månsson M, Tammela TLJ, Zappa M, Nelen V, et al. A 16-yr follow-up of the European Randomized Study of screening for prostate cancer. Eur Urol. 2019;76:43–51. https://doi.org/10.1016/j.eururo.2019.02.009 . Epub 2019 Feb 26. PMID: 30824296; PMCID: PMC7513694.
Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–8. https://doi.org/10.1056/NEJMoa0810084 . Epub 2009 Mar 18. PMID: 19297566.
Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Zappa M, Nelen V, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027–35. https://doi.org/10.1016/S0140-6736(14)60525-0 . Epub 2014 Aug 6. PMID: 25108889; PMCID: PMC4427906.
Catalona WJ. Prostate cancer screening. Med Clin North Am. 2018;102:199–214. https://doi.org/10.1016/j.mcna.2017.11.001 . PMID: 29406053; PMCID: PMC5935113.
Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR, et al. Prostate cancer screening in the randomized prostate, lung, colorectal, and ovarian cancer screening trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–32. https://doi.org/10.1093/jnci/djr500 . Epub 2012 Jan 6. PMID: 22228146; PMCID: PMC3260132.
Ilic D, Djulbegovic M, Jung JH, Hwang EC, Zhou Q, Cleves A, et al. Prostate cancer screening with prostate-specific antigen (PSA) test: a systematic review and meta-analysis. BMJ. 2018;362:k3519. https://doi.org/10.1136/bmj.k3519 . PMID: 30185521; PMCID: PMC6283370.
Fenton JJ, Weyrich MS, Durbin S, Liu Y, Bang H, Melnikow J. Prostate-specific antigen-based screening for prostate cancer: evidence report and systematic review for the US preventive services task force. JAMA. 2018;319:1914–31. https://doi.org/10.1001/jama.2018.3712 . PMID: 29801018.
Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387:945–56. https://doi.org/10.1016/S0140-6736(15)01224-6 . Epub 2015 Dec 17. Erratum in: Lancet. 2016 Mar 5;387(10022):944. Erratum in: Lancet. 2016 Mar 5;387(10022):944. PMID: 26707054; PMCID: PMC4779792.
Menon U, Gentry-Maharaj A, Burnell M, Singh N, Ryan A, Karpinskyj C, et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2021;397:2182–93. https://doi.org/10.1016/S0140-6736(21)00731-5 . Epub 2021 May 12. PMID: 33991479; PMCID: PMC8192829.
Fontana RS, Sanderson DR, Taylor WF, Woolner LB, Miller WE, Muhm JR, et al. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Mayo Clinic study. Am Rev Respir Dis. 1984;130:561–5. https://doi.org/10.1164/arrd.1984.130.4.561 . PMID: 6091507.
Marcus PM, Bergstralh EJ, Fagerstrom RM, Williams DE, Fontana R, Taylor WF, et al. Lung cancer mortality in the Mayo Lung Project: impact of extended follow-up. J Natl Cancer Inst. 2000;92:1308–16. https://doi.org/10.1093/jnci/92.16.1308 . PMID: 10944552.
National Lung Screening Trial Research Team. Lung cancer incidence and mortality with extended follow-up in the National Lung Screening Trial. J Thorac Oncol. 2019;14:1732–42. https://doi.org/10.1016/j.jtho.2019.05.044 . Epub 2019 Jun 28. PMID: 31260833; PMCID: PMC6764895.
Article Google Scholar
de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503–13. https://doi.org/10.1056/NEJMoa1911793 . Epub 2020 Jan 29. PMID: 31995683.
Jonas DE, Reuland DS, Reddy SM, Nagle M, Clark SD, Weber RP, et al. Screening for lung cancer with low-dose computed tomography: updated evidence report and systematic review for the US preventive services task force. JAMA. 2021;325:971–87. https://doi.org/10.1001/jama.2021.0377 . PMID: 33687468.
Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–62. https://doi.org/10.1093/jnci/dji372 . PMID: 16288118.
Veronesi U, Maisonneuve P, Rotmensz N, Bonanni B, Boyle P, Viale G, et al. Tamoxifen for the prevention of breast cancer: late results of the Italian Randomized Tamoxifen Prevention Trial among women with hysterectomy. J Natl Cancer Inst. 2007;99:727–37. https://doi.org/10.1093/jnci/djk154 . PMID: 17470740.
Cuzick J, Sestak I, Cawthorn S, Hamed H, Holli K, Howell A, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16:67–75. https://doi.org/10.1016/S1470-2045(14)71171-4 . Epub 2014 Dec 11. PMID: 25497694; PMCID: PMC4772450.
Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99:283–90. https://doi.org/10.1093/jnci/djk050 . PMID: 17312305.
Martino S, Cauley JA, Barrett-Connor E, Powles TJ, Mershon J, Disch D, et al. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–61. https://doi.org/10.1093/jnci/djh319 . PMID: 15572757.
Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Philos). 2010;3:696–706. https://doi.org/10.1158/1940-6207.CAPR-10-0076 . Epub 2010 Apr 19. PMID: 20404000; PMCID: PMC2935331.
Article CAS Google Scholar
Cuzick J. Aromatase inhibitors for breast cancer prevention. J Clin Oncol. 2005;23:1636–43. https://doi.org/10.1200/JCO.2005.11.027 . PMID: 15755971.
Goss PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011;364:2381–91. https://doi.org/10.1056/NEJMoa1103507 . Epub 2011 Jun 4. Erratum in: N Engl J Med. 2011 Oct 6;365(14):1361. PMID: 21639806.
Cuzick J, Sestak I, Forbes JF, Dowsett M, Cawthorn S, Mansel RE, et al. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395:117–22. https://doi.org/10.1016/S0140-6736(19)32955-1 . Epub 2019 Dec 12. Erratum in: Lancet. 2020 Feb 15;395(10223):496. Erratum in: Lancet. 2021 Feb 27;397(10276):796. PMID: 31839281; PMCID: PMC6961114.
Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–51. https://doi.org/10.1056/NEJMoa020586 . PMID: 12444178.
Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–70. https://doi.org/10.1016/S0140-6736(07)60946-5 . Erratum in: Lancet. 2007 Oct 20;370(9596):1414. PMID: 17602732.
Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet. 2011;377:2085–92. https://doi.org/10.1016/S0140-6736(11)60551-5 . PMID: 21684381.
Falcaro M, Castañon A, Ndlela B, Checchi M, Soldan K, Lopez-Bernal J, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet. 2021;398:2084–92. https://doi.org/10.1016/S0140-6736(21)02178-4 . Epub 2021 Nov 3. PMID: 34741816.
Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. https://doi.org/10.1016/S0140-6736(10)62110-1 . Epub 2010 Dec 6. PMID: 21144578.
Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and cancer risk: a quantitative review to 2011. Ann Oncol. 2012;23:1403–15. https://doi.org/10.1093/annonc/mds113 . Epub 2012 Apr 19. PMID: 22517822.
Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–27. https://doi.org/10.1016/S1470-2045(12)70112-2 . Epub 2012 Mar 21. PMID: 22440112.
Cuzick J, Thorat MA, Bosetti C, Brown PH, Burn J, Cook NR, et al. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann Oncol. 2015;26:47–57. https://doi.org/10.1093/annonc/mdu225 . Epub 2014 Aug 5. PMID: 25096604; PMCID: PMC4269341.
ASCEND Study Collaborative Group, Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529–39. https://doi.org/10.1056/NEJMoa1804988 . Epub 2018 Aug 26. PMID: 30146931.
McNeil JJ, Nelson MR, Woods RL, Lockery JE, Wolfe R, Reid CM, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519–28. https://doi.org/10.1056/NEJMoa1803955 . Epub 2018 Sep 16. PMID: 30221595; PMCID: PMC6433466.
Chan AT. Aspirin and the USPSTF-what about cancer? JAMA Oncol. 2022. https://doi.org/10.1001/jamaoncol.2022.2967 . Epub ahead of print. PMID: 35900749.
Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003;349:215–24. https://doi.org/10.1056/NEJMoa030660 . Epub 2003 Jun 24. PMID: 12824459.
Thompson IM Jr, Goodman PJ, Tangen CM, Parnes HL, Minasian LM, Godley PA, et al. Long-term survival of participants in the prostate cancer prevention trial. N Engl J Med. 2013;369:603–10. https://doi.org/10.1056/NEJMoa1215932 . PMID: 23944298; PMCID: PMC4141537.
Cuzick J, Stewart H, Peto R, Baum M, Fisher B, Host H, et al. Overview of randomized trials of postoperative adjuvant radiotherapy in breast cancer. Cancer Treat Rep. 1987;71:15–29. PMID: 2856861.
CAS PubMed Google Scholar
Cuzick J, Stewart H, Rutqvist L, Houghton J, Edwards R, Redmond C, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994;12:447–53. https://doi.org/10.1200/JCO.1994.12.3.447 . PMID: 8120544.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Darby S, McGale P, Correa C, Taylor C, Arriagada R, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–16. https://doi.org/10.1016/S0140-6736(11)61629-2 . Epub 2011 Oct 19. PMID: 22019144; PMCID: PMC3254252.
Taylor CW, Kirby AM. Cardiac side-effects from breast cancer radiotherapy. Clin Oncol (R Coll Radio). 2015;27:621–9. https://doi.org/10.1016/j.clon.2015.06.007 . Epub 2015 Jun 28. PMID: 26133462.
Giordano SH, Kuo YF, Freeman JL, Buchholz TA, Hortobagyi GN, Goodwin JS. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005;97:419–24. https://doi.org/10.1093/jnci/dji067 . PMID: 15770005; PMCID: PMC1853253.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Davies C, Godwin J, Gray R, Clarke M, Cutter D, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–84. https://doi.org/10.1016/S0140-6736(11)60993-8 . Epub 2011 Jul 28. PMID: 21802721; PMCID: PMC3163848.
Regan MM, Neven P, Giobbie-Hurder A, Goldhirsch A, Ejlertsen B, Mauriac L, et al. Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1-98 randomised clinical trial at 8·1 years median follow-up. Lancet Oncol. 2011;12:1101–8. https://doi.org/10.1016/S1470-2045(11)70270-4 . Epub 2011 Oct 20. PMID: 22018631; PMCID: PMC3235950.
Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135–41. https://doi.org/10.1016/S1470-2045(10)70257-6 . Epub 2010 Nov 17. PMID: 21087898.
Dearnaley DP, Jovic G, Syndikus I, Khoo V, Cowan RA, Graham JD, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15:464–73. https://doi.org/10.1016/S1470-2045(14)70040-3 . Epub 2014 Feb 26. PMID: 24581940.
Vale CL, Fisher D, Kneebone A, Parker C, Pearse M, Richaud P, et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: a prospectively planned systematic review and meta-analysis of aggregate data. Lancet. 2020;396:1422–31. https://doi.org/10.1016/S0140-6736(20)31952-8 . Epub 2020 Sep 28. PMID: 33002431; PMCID: PMC7611137.
Mauch PM, Kalish LA, Marcus KC, Shulman LN, Krill E, Tarbell NJ, et al. Long-term survival in Hodgkin’s disease relative impact of mortality, second tumors, infection, and cardiovascular disease. Cancer J Sci Am. 1995;1:33–42. PMID: 9166452.
Specht L, Gray RG, Clarke MJ, Peto R. Influence of more extensive radiotherapy and adjuvant chemotherapy on long-term outcome of early-stage Hodgkin’s disease: a meta-analysis of 23 randomized trials involving 3,888 patients. International Hodgkin’s Disease Collaborative Group. J Clin Oncol. 1998;16:830–43. https://doi.org/10.1200/JCO.1998.16.3.830 . PMID: 9508163.
Buyse M, Saad ED, Burzykowski T, Regan MM, Sweeney CS. Surrogacy beyond prognosis: the importance of “Trial-Level” surrogacy. Oncologist. 2022;27:266–71. https://doi.org/10.1093/oncolo/oyac006 . PMID: 35380717; PMCID: PMC8982389.
Suh E, Stratton KL, Leisenring WM, Nathan PC, Ford JS, Freyer DR, et al. Late mortality and chronic health conditions in long-term survivors of early-adolescent and young adult cancers: a retrospective cohort analysis from the Childhood Cancer Survivor Study. Lancet Oncol. 2020;21:421–35. https://doi.org/10.1016/S1470-2045(19)30800-9 . Epub 2020 Feb 14. PMID: 32066543; PMCID: PMC7392388.
Winther JF, Kenborg L, Byrne J, Hjorth L, Kaatsch P, Kremer LC, et al. Childhood cancer survivor cohorts in Europe. Acta Oncol. 2015;54:655–68. https://doi.org/10.3109/0284186X.2015.1008648 . Epub 2015 Mar 27. PMID: 25813473.
Brinkman TM, Recklitis CJ, Michel G, Grootenhuis MA, Klosky JL. Psychological symptoms, social outcomes, socioeconomic attainment, and health behaviors among survivors of childhood cancer: current state of the literature. J Clin Oncol. 2018;36:2190–7. https://doi.org/10.1200/JCO.2017.76.5552 . Epub 2018 Jun 6. PMID: 29874134; PMCID: PMC6053297.
Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976;34:585–612. https://doi.org/10.1038/bjc.1976.220 . PMID: 795448; PMCID: PMC2025229.
Cuzick J. Primary endpoints for randomised trials of cancer therapy. Lancet. 2008;371:2156–8. https://doi.org/10.1016/S0140-6736(08)60933-2 . PMID: 18586160.
Cuzick J. The assessment of subgroups in clinical trials. Experientia Suppl. 1982;41:224–35. PMID: 6958512.
Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet. 2000;355:1064–9. https://doi.org/10.1016/S0140-6736(00)02039-0 . PMID: 10744093.
Cuzick J. Forest plots and the interpretation of subgroups. Lancet. 2005;365:1308. https://doi.org/10.1016/S0140-6736(05)61026-4 . PMID: 15823379.
Download references
Acknowledgements
I thank many colleagues for useful comments on earlier drafts, and especially Professor Stephen Duffy for those related to cancer screening.
Author information
Authors and affiliations.
Wolfson Institute of Population Health, Queen Mary University of London, London, UK
Jack Cuzick
You can also search for this author in PubMed Google Scholar
Contributions
JC is the only author.
Corresponding author
Correspondence to Jack Cuzick .
Ethics declarations
Competing interests.
I have developed an algorithm for predicting future breast cancer, which is freely available, but if used commercially requires a license from Cancer Research UK, and I receive a royalty from the income generated. No other conflicts to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Cuzick, J. The importance of long-term follow up of participants in clinical trials. Br J Cancer 128 , 432–438 (2023). https://doi.org/10.1038/s41416-022-02038-4
Download citation
Received : 20 September 2022
Revised : 13 October 2022
Accepted : 18 October 2022
Published : 01 December 2022
Issue Date : 02 February 2023
DOI : https://doi.org/10.1038/s41416-022-02038-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
The comparison of functional status and health-related parameters in ovarian cancer survivors with healthy controls.
- Sukriye Cansu Gultekin
- Ahmet Burak Cakir
- Didem Karadibak
Supportive Care in Cancer (2024)
Recent advances and future perspectives in the therapeutics of prostate cancer
- Ganji Lakshmi Varaprasad
- Vivek Kumar Gupta
- Yun Suk Huh
Experimental Hematology & Oncology (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Open access
- Published: 15 December 2016
Post-trial follow-up methodology in large randomized controlled trials: a systematic review protocol
- Rebecca Llewellyn-Bennett ORCID: orcid.org/0000-0002-9708-9923 1 ,
- Louise Bowman 1 &
- Richard Bulbulia 1
Systematic Reviews volume 5 , Article number: 214 ( 2016 ) Cite this article
16k Accesses
33 Citations
Metrics details
Clinical trials typically have a relatively short follow-up period, and may both underestimate potential benefits of treatments investigated, and fail to detect hazards, which can take much longer to emerge. Prolonged follow-up of trial participants after the end of the scheduled trial period can provide important information on both efficacy and safety outcomes. This protocol describes a systematic review to qualitatively compare methods of post-trial follow-up used in large randomized controlled trials.
Methods/design
A systematic search of electronic databases and clinical trial registries will use a predefined search strategy. All large (more than 1000 adult participants) randomized controlled trials will be evaluated. Two reviewers will screen and extract data according to this protocol with the aim of 95% concordance of papers checked and discrepancies will be resolved by a third reviewer. Trial methods, participant retention rates and prevalence of missing data will be recorded and compared. The potential for bias will be evaluated using the Cochrane Risk of Bias tool (applied to the methods used during the in-trial period) with the aim of investigating whether the quality of the post-trial follow-up methodology might be predicted by the quality of the methods used for the original trial.
Post-trial follow-up can provide valuable information about the long-term benefits and hazards of medical interventions. However, it can be logistically challenging and costly. The aim of this systematic review is to describe how trial participants have been followed-up post-trial in order to inform future post-trial follow-up designs.
Systematic review registration
Not applicable for PROSPERO registration.
Peer Review reports
Randomized controlled trials (RCTs) are considered to be the gold standard for assessing the effects of a treatment. However, RCTs are costly and usually involve a relatively brief treatment period with limited follow-up. A treatment response restricted to this brief “in-trial” period can potentially underestimate the long-term benefits of treatment and also may fail to detect delayed hazards.
Post-trial follow-up (PTFU) is defined here as extended follow-up which starts after the end of the scheduled period of the original trial. Longer term follow-up of trial participants is important as persistent effects may be detected years later after treatment cessation [ 1 ] or even enhanced benefits observed decades later—a so-called “legacy-effect” [ 2 ]. Furthermore, delayed hazards may only emerge several years after exposure to certain treatments. Therefore, PTFU may add significant scientific value to the evaluation of many healthcare interventions.
There is a wide literature describing the importance of completeness of follow-up during the in-trial period of a RCT, without which the unbiased ascertainment of outcomes may be compromised and statistical power considerably reduced [ 3 ]. Many strategies to enhance follow-up during RCTs have been investigated and this remains an area of much ongoing research [ 4 ]. Without high quality in-trial follow-up, the value of post-trial follow-up will be extremely limited.
By contrast, little research has been done to evaluate methods for PTFU. Face-to-face follow-up is widely used during the initial "in-trial" period, but is costly if employed longer term. Telephone-based approaches are more practical, with the ability to contact many participants coordinated by a central trial office, and postal follow-up has been shown to be effective [ 1 ]. Web-based techniques may become more widespread as technological advances develop [ 5 ].
The use of routine health records can provide detailed information relatively inexpensively [ 6 ], but the availability of such data and rules governing access to it varies across countries. In the UK, Health Episode Statistics (HES) are held by the Health and Social Care Information Centre (HSCIC) and can be used as a streamlined method to follow-up trial participants. These routinely collected electronic health records include diagnostic codes (ICD-10) for hospital admissions and can be supplemented with mortality records and cancer registry data.
Eligibility criteria
Study designs.
All published, health-related RCTs which have recruited more than 1000 participants and implemented PTFU are to be included in this systematic review. The RCT must have reached its scheduled end before PTFU commenced. Only studies published between 2006 and 2016 will be included.
Health-related interventions will include medical (licensed or unlicensed drugs), surgical, or psychological treatments. There will be no time limit of post-trial follow-up (Table 1 ).
Participants
Trials including participants aged over 18 years old are eligible.
Interventions
Methods and incentives (monetary or by other means) used for post-trial follow-up including direct “face-to-face” follow-up and indirect follow-up, eg, medical record review, telephone and postal follow-up, and electronic follow-up including access to electronic health records will be included.
Comparators
Methodology used to follow up participants’ post trial will be compared qualitatively in a table format.
Outcome measures
Included studies must have published the total number of participants followed-up compared to the total number alive at the end of the in-trial period to calculate retention rates. Where available, secondary outcome measures of cost, incentives used for follow-up, and cost-effectiveness will be recorded and assessed. If there are missing data, an attempt to contact the study authors will be made. Further exploratory comparisons will be made depending on the information available (for example, describing the use of different approaches according to context, such as regional variations or comparisons of industry-funded trials versus those funded through other sources).
Only studies published in English will be included.
Search methods
Electronic searches.
The electronic search strategy includes the last 10 years of published articles using broad search criteria ( Appendix ). Searches for eligible studies will take place in a structured, step-wise process. A screening log will be kept. Results of searches from each electronic database and registries will be logged. The following electronic databases will be searched:
Cochrane methodology group register
Cochrane Central Register of Controlled Trials (CENTRAL)
Other sources of searches will include the following trials registry:
Trials registry: Clinical-trials.gov ( http://clinicaltrials.gov/ )
Screening for eligible studies
One reviewer will compile the titles and abstracts of all citations retrieved from the electronic database searches and order these by record number in Endnote® reference management software. Duplicates will be removed using the “deduplication tool” [ 7 ]. The screening process will involve two reviewers. The first 10% of abstracts will be screened by both reviewers independently. Concordance of 95% between both reviewers’ decisions on screening will be sought. If concordance is not reached at this point, discrepancies will be discussed and reviewed (including consultation with a third reviewer if necessary), and a further 10% of abstracts will be reviewed (Fig. 1 ). Once concordance has been reached, the remaining records for screening will be shared equally between the two reviewers and abstracts will be checked for eligibility. All records that are considered to be eligible will be confirmed by both reviewers. Full-text papers will be requested for all potential eligible papers.
Process of screening abstracts and checking for concordance between reviewers
Data collection and analysis
Data extraction and management.
Two reviewers will follow a similar step-wise process for data extraction (Fig. 2 ). A data extraction form will be used, and data extracted from all eligible studies will be compared qualitatively. All data regarding the intervention, the participants (demographics), attrition, retention, incentives used, and if specified, costs of PTFU will be extracted. If required data items are not available in the published article, the study’s corresponding authors will be contacted. If no response is received after two further attempts or from an alternative contact, the study will be excluded from the analysis but recorded on the PRISMA diagram and in an appendix.
Process of extracting data and checking for concordance between reviewers
Assessing the quality of the post-trial follow-up methodology
In order to investigate whether the quality of the post-trial follow-up methodology might be predicted by the quality of the methods used for the original trial, risk of bias will be assessed in those trials chosen for data extraction using the Cochrane Risk of Bias tool. The tool will be applied to the methods used in the main trial, (not the PTFU) focusing on incomplete data; outcome reporting; for-profit bias and other bias sources. Two reviewers will independently assess the risk of bias, and disagreements will be resolved by a third reviewer. The assessment of bias results will be taken into account as part of the assessment of quality of the PTFU methods used.
Presenting and reporting of results
The Preferred Reporting Items for Systematic Review Protocols (PRISMA-P) [ 8 ] will be followed, including a PRISMA diagram to illustrate the process of selecting eligible studies (Fig. 1 ). Using the PRISMA guidelines (Additional file 1 ), the results of this review will be presented and the outcomes tabulated with respect to the different methodologies used in a qualitative and comparative style.
Interpretation of findings
The findings of this review will be discussed and potential limitations considered.
Large randomized trials are essential for determining the magnitude of the effects of an intervention. Post-trial follow-up of large RCTs is important, not only for defining the effect of an intervention long-term but also for ascertaining the safety profile and potential hazards which might not be apparent during the relatively brief in-trial period. However, randomized trials can be very expensive, and funding is limited, hence streamlined and effective methodology for PTFU is desirable. This systematic review aims to inform the design of post-trial follow-up for a wide range of randomized trials.
Abbreviations
Cochrane Central Register of Controlled Trials
Excerpta Medica database
Grading of Recommendations Assessment, Development and Evaluation
Health Episode Statistics
Health and Social Care Information Centre
International Classification of Diseases codes
Preferred Reporting Items for Systematic Review Protocols
Post-trial follow-up
- Randomized controlled trial
Bulbulia R, Bowman L, Wallendszus K, Parish S, Armitage J, Peto R, Collins R, Collins R, Meade T, Sleight P, Armitage J, Parish S, Peto R, Youngman L, Buxton M, De Bono D, George C, Fuller J, Keech A, Mansfield A, Pentecost B, Simpson D, Warlow C, McNamara J, O’Toole L, Doll R, Wilhelmsen L, Fox KM, Hill C, Sandercock P. Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20 536 high-risk individuals: a randomised controlled trial. Lancet. 2011;378:2013–20.
Article CAS PubMed Google Scholar
Ford I, Murray H, McCowan C, Packard CJ. Long-term safety and efficacy of lowering low-density lipoprotein cholesterol with statin therapy 20-year follow-up of west of Scotland coronary prevention study. Circulation. 2016;133:1073–80.
Article CAS PubMed PubMed Central Google Scholar
Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357:1191–4.
Brueton VC, Tierney JF, Stenning S, Meredith S, Harding S, Nazareth I, Rait G. Strategies to improve retention in randomised trials: a Cochrane systematic review and meta-analysis. BMJ Open. 2014;4:e003821.
Barton J, Young A, Lay M. Introduction of electronic data capture method using participant-completed online web-based follow up questionnaire in mail-based study achieves expected benefits and positive participant feedback. Trials. 2015;16 Suppl 2:44–P44.
Article Google Scholar
Scuffham P, Chaplin SLR. Incidence and costs of unintentional falls in older people in the United Kingdom. J Epidemiol Community Heal. 2003;57:740–4.
Article CAS Google Scholar
Rathbone J, Carter M, Hoffmann T, Glasziou P. Better duplicate detection for systematic reviewers: evaluation of Systematic Review Assistant-Deduplication Module. Syst Rev. 2015;4:6.
Article PubMed PubMed Central Google Scholar
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Download references
Acknowledgements
Many thanks to Danielle Edwards (Clinical Trial Service Unit, University of Oxford) who advised on the figures for publication and Nia Roberts (Bodelian libraries, University of Oxford) who advised on the search strategy.
RLB has received funding from the Royal College of Surgeons of England Research Fellowship.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Authors’ contributions
RLB designed the protocol. RLB, LB, and RB drafted the protocol. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
RLB, LB, and RB consent for publication.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and affiliations.
Clinical Trial Service Unit, Nuffield Department of Population Health, University of Oxford, Richard Doll Building, Roosevelt Drive, Oxford, OX3 7LF, UK
Rebecca Llewellyn-Bennett, Louise Bowman & Richard Bulbulia
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Rebecca Llewellyn-Bennett .
Search strategies
Search conducted via OvidSP interface: 1946 to present in process and other non-indexed citations
Key to operators used in MEDLINE/Ovid: where.pt is publication type, (?) represents any single character, (*) is a group of characters,.mp.is multi-purpose search,/is Medical Subject Headings (MeSH), exp is explode subject heading,.sh. is subject heading, (“ “) is phrase search.
Search conducted via OvidSP interface 1974–2016, March 04
Search conducted via Cochrane Library via Wiley interface
Cochrane Central Register for Controlled Trials (Issue 2 of 12, February 2016)
Cochrane Methods Register (Issue 3 of 4, July 2012)
https://clinicaltrials.gov/
All results will be downloaded with all fields displayed and in a tab delimited format. This file will then be opened in ExCel. Duplicates will be removed. The spreadsheet sort order will be changed to enrollment A-Z and studies with fewer than 1000 enrolees will be removed.
Additional file
Additional file 1:.
PRISMA-P (Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols) 2015 checklist: recommended items to address in a systematic review protocol: recommended items to address in a systematic review protocol. (DOC 85 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Llewellyn-Bennett, R., Bowman, L. & Bulbulia, R. Post-trial follow-up methodology in large randomized controlled trials: a systematic review protocol. Syst Rev 5 , 214 (2016). https://doi.org/10.1186/s13643-016-0393-3
Download citation
Received : 17 August 2016
Accepted : 29 November 2016
Published : 15 December 2016
DOI : https://doi.org/10.1186/s13643-016-0393-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Methodology
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Search Menu
- Browse content in Arts and Humanities
- Browse content in Archaeology
- Anglo-Saxon and Medieval Archaeology
- Archaeological Methodology and Techniques
- Archaeology by Region
- Archaeology of Religion
- Archaeology of Trade and Exchange
- Biblical Archaeology
- Contemporary and Public Archaeology
- Environmental Archaeology
- Historical Archaeology
- History and Theory of Archaeology
- Industrial Archaeology
- Landscape Archaeology
- Mortuary Archaeology
- Prehistoric Archaeology
- Underwater Archaeology
- Urban Archaeology
- Zooarchaeology
- Browse content in Architecture
- Architectural Structure and Design
- History of Architecture
- Residential and Domestic Buildings
- Theory of Architecture
- Browse content in Art
- Art Subjects and Themes
- History of Art
- Industrial and Commercial Art
- Theory of Art
- Biographical Studies
- Byzantine Studies
- Browse content in Classical Studies
- Classical History
- Classical Philosophy
- Classical Mythology
- Classical Literature
- Classical Reception
- Classical Art and Architecture
- Classical Oratory and Rhetoric
- Greek and Roman Papyrology
- Greek and Roman Epigraphy
- Greek and Roman Law
- Greek and Roman Archaeology
- Late Antiquity
- Religion in the Ancient World
- Digital Humanities
- Browse content in History
- Colonialism and Imperialism
- Diplomatic History
- Environmental History
- Genealogy, Heraldry, Names, and Honours
- Genocide and Ethnic Cleansing
- Historical Geography
- History by Period
- History of Emotions
- History of Agriculture
- History of Education
- History of Gender and Sexuality
- Industrial History
- Intellectual History
- International History
- Labour History
- Legal and Constitutional History
- Local and Family History
- Maritime History
- Military History
- National Liberation and Post-Colonialism
- Oral History
- Political History
- Public History
- Regional and National History
- Revolutions and Rebellions
- Slavery and Abolition of Slavery
- Social and Cultural History
- Theory, Methods, and Historiography
- Urban History
- World History
- Browse content in Language Teaching and Learning
- Language Learning (Specific Skills)
- Language Teaching Theory and Methods
- Browse content in Linguistics
- Applied Linguistics
- Cognitive Linguistics
- Computational Linguistics
- Forensic Linguistics
- Grammar, Syntax and Morphology
- Historical and Diachronic Linguistics
- History of English
- Language Evolution
- Language Reference
- Language Acquisition
- Language Variation
- Language Families
- Lexicography
- Linguistic Anthropology
- Linguistic Theories
- Linguistic Typology
- Phonetics and Phonology
- Psycholinguistics
- Sociolinguistics
- Translation and Interpretation
- Writing Systems
- Browse content in Literature
- Bibliography
- Children's Literature Studies
- Literary Studies (Romanticism)
- Literary Studies (American)
- Literary Studies (Asian)
- Literary Studies (European)
- Literary Studies (Eco-criticism)
- Literary Studies (Modernism)
- Literary Studies - World
- Literary Studies (1500 to 1800)
- Literary Studies (19th Century)
- Literary Studies (20th Century onwards)
- Literary Studies (African American Literature)
- Literary Studies (British and Irish)
- Literary Studies (Early and Medieval)
- Literary Studies (Fiction, Novelists, and Prose Writers)
- Literary Studies (Gender Studies)
- Literary Studies (Graphic Novels)
- Literary Studies (History of the Book)
- Literary Studies (Plays and Playwrights)
- Literary Studies (Poetry and Poets)
- Literary Studies (Postcolonial Literature)
- Literary Studies (Queer Studies)
- Literary Studies (Science Fiction)
- Literary Studies (Travel Literature)
- Literary Studies (War Literature)
- Literary Studies (Women's Writing)
- Literary Theory and Cultural Studies
- Mythology and Folklore
- Shakespeare Studies and Criticism
- Browse content in Media Studies
- Browse content in Music
- Applied Music
- Dance and Music
- Ethics in Music
- Ethnomusicology
- Gender and Sexuality in Music
- Medicine and Music
- Music Cultures
- Music and Media
- Music and Religion
- Music and Culture
- Music Education and Pedagogy
- Music Theory and Analysis
- Musical Scores, Lyrics, and Libretti
- Musical Structures, Styles, and Techniques
- Musicology and Music History
- Performance Practice and Studies
- Race and Ethnicity in Music
- Sound Studies
- Browse content in Performing Arts
- Browse content in Philosophy
- Aesthetics and Philosophy of Art
- Epistemology
- Feminist Philosophy
- History of Western Philosophy
- Metaphysics
- Moral Philosophy
- Non-Western Philosophy
- Philosophy of Language
- Philosophy of Mind
- Philosophy of Perception
- Philosophy of Science
- Philosophy of Action
- Philosophy of Law
- Philosophy of Religion
- Philosophy of Mathematics and Logic
- Practical Ethics
- Social and Political Philosophy
- Browse content in Religion
- Biblical Studies
- Christianity
- East Asian Religions
- History of Religion
- Judaism and Jewish Studies
- Qumran Studies
- Religion and Education
- Religion and Health
- Religion and Politics
- Religion and Science
- Religion and Law
- Religion and Art, Literature, and Music
- Religious Studies
- Browse content in Society and Culture
- Cookery, Food, and Drink
- Cultural Studies
- Customs and Traditions
- Ethical Issues and Debates
- Hobbies, Games, Arts and Crafts
- Lifestyle, Home, and Garden
- Natural world, Country Life, and Pets
- Popular Beliefs and Controversial Knowledge
- Sports and Outdoor Recreation
- Technology and Society
- Travel and Holiday
- Visual Culture
- Browse content in Law
- Arbitration
- Browse content in Company and Commercial Law
- Commercial Law
- Company Law
- Browse content in Comparative Law
- Systems of Law
- Competition Law
- Browse content in Constitutional and Administrative Law
- Government Powers
- Judicial Review
- Local Government Law
- Military and Defence Law
- Parliamentary and Legislative Practice
- Construction Law
- Contract Law
- Browse content in Criminal Law
- Criminal Procedure
- Criminal Evidence Law
- Sentencing and Punishment
- Employment and Labour Law
- Environment and Energy Law
- Browse content in Financial Law
- Banking Law
- Insolvency Law
- History of Law
- Human Rights and Immigration
- Intellectual Property Law
- Browse content in International Law
- Private International Law and Conflict of Laws
- Public International Law
- IT and Communications Law
- Jurisprudence and Philosophy of Law
- Law and Politics
- Law and Society
- Browse content in Legal System and Practice
- Courts and Procedure
- Legal Skills and Practice
- Primary Sources of Law
- Regulation of Legal Profession
- Medical and Healthcare Law
- Browse content in Policing
- Criminal Investigation and Detection
- Police and Security Services
- Police Procedure and Law
- Police Regional Planning
- Browse content in Property Law
- Personal Property Law
- Study and Revision
- Terrorism and National Security Law
- Browse content in Trusts Law
- Wills and Probate or Succession
- Browse content in Medicine and Health
- Browse content in Allied Health Professions
- Arts Therapies
- Clinical Science
- Dietetics and Nutrition
- Occupational Therapy
- Operating Department Practice
- Physiotherapy
- Radiography
- Speech and Language Therapy
- Browse content in Anaesthetics
- General Anaesthesia
- Neuroanaesthesia
- Clinical Neuroscience
- Browse content in Clinical Medicine
- Acute Medicine
- Cardiovascular Medicine
- Clinical Genetics
- Clinical Pharmacology and Therapeutics
- Dermatology
- Endocrinology and Diabetes
- Gastroenterology
- Genito-urinary Medicine
- Geriatric Medicine
- Infectious Diseases
- Medical Toxicology
- Medical Oncology
- Pain Medicine
- Palliative Medicine
- Rehabilitation Medicine
- Respiratory Medicine and Pulmonology
- Rheumatology
- Sleep Medicine
- Sports and Exercise Medicine
- Community Medical Services
- Critical Care
- Emergency Medicine
- Forensic Medicine
- Haematology
- History of Medicine
- Browse content in Medical Skills
- Clinical Skills
- Communication Skills
- Nursing Skills
- Surgical Skills
- Browse content in Medical Dentistry
- Oral and Maxillofacial Surgery
- Paediatric Dentistry
- Restorative Dentistry and Orthodontics
- Surgical Dentistry
- Medical Ethics
- Medical Statistics and Methodology
- Browse content in Neurology
- Clinical Neurophysiology
- Neuropathology
- Nursing Studies
- Browse content in Obstetrics and Gynaecology
- Gynaecology
- Occupational Medicine
- Ophthalmology
- Otolaryngology (ENT)
- Browse content in Paediatrics
- Neonatology
- Browse content in Pathology
- Chemical Pathology
- Clinical Cytogenetics and Molecular Genetics
- Histopathology
- Medical Microbiology and Virology
- Patient Education and Information
- Browse content in Pharmacology
- Psychopharmacology
- Browse content in Popular Health
- Caring for Others
- Complementary and Alternative Medicine
- Self-help and Personal Development
- Browse content in Preclinical Medicine
- Cell Biology
- Molecular Biology and Genetics
- Reproduction, Growth and Development
- Primary Care
- Professional Development in Medicine
- Browse content in Psychiatry
- Addiction Medicine
- Child and Adolescent Psychiatry
- Forensic Psychiatry
- Learning Disabilities
- Old Age Psychiatry
- Psychotherapy
- Browse content in Public Health and Epidemiology
- Epidemiology
- Public Health
- Browse content in Radiology
- Clinical Radiology
- Interventional Radiology
- Nuclear Medicine
- Radiation Oncology
- Reproductive Medicine
- Browse content in Surgery
- Cardiothoracic Surgery
- Gastro-intestinal and Colorectal Surgery
- General Surgery
- Neurosurgery
- Paediatric Surgery
- Peri-operative Care
- Plastic and Reconstructive Surgery
- Surgical Oncology
- Transplant Surgery
- Trauma and Orthopaedic Surgery
- Vascular Surgery
- Browse content in Science and Mathematics
- Browse content in Biological Sciences
- Aquatic Biology
- Biochemistry
- Bioinformatics and Computational Biology
- Developmental Biology
- Ecology and Conservation
- Evolutionary Biology
- Genetics and Genomics
- Microbiology
- Molecular and Cell Biology
- Natural History
- Plant Sciences and Forestry
- Research Methods in Life Sciences
- Structural Biology
- Systems Biology
- Zoology and Animal Sciences
- Browse content in Chemistry
- Analytical Chemistry
- Computational Chemistry
- Crystallography
- Environmental Chemistry
- Industrial Chemistry
- Inorganic Chemistry
- Materials Chemistry
- Medicinal Chemistry
- Mineralogy and Gems
- Organic Chemistry
- Physical Chemistry
- Polymer Chemistry
- Study and Communication Skills in Chemistry
- Theoretical Chemistry
- Browse content in Computer Science
- Artificial Intelligence
- Computer Architecture and Logic Design
- Game Studies
- Human-Computer Interaction
- Mathematical Theory of Computation
- Programming Languages
- Software Engineering
- Systems Analysis and Design
- Virtual Reality
- Browse content in Computing
- Business Applications
- Computer Security
- Computer Games
- Computer Networking and Communications
- Digital Lifestyle
- Graphical and Digital Media Applications
- Operating Systems
- Browse content in Earth Sciences and Geography
- Atmospheric Sciences
- Environmental Geography
- Geology and the Lithosphere
- Maps and Map-making
- Meteorology and Climatology
- Oceanography and Hydrology
- Palaeontology
- Physical Geography and Topography
- Regional Geography
- Soil Science
- Urban Geography
- Browse content in Engineering and Technology
- Agriculture and Farming
- Biological Engineering
- Civil Engineering, Surveying, and Building
- Electronics and Communications Engineering
- Energy Technology
- Engineering (General)
- Environmental Science, Engineering, and Technology
- History of Engineering and Technology
- Mechanical Engineering and Materials
- Technology of Industrial Chemistry
- Transport Technology and Trades
- Browse content in Environmental Science
- Applied Ecology (Environmental Science)
- Conservation of the Environment (Environmental Science)
- Environmental Sustainability
- Environmentalist Thought and Ideology (Environmental Science)
- Management of Land and Natural Resources (Environmental Science)
- Natural Disasters (Environmental Science)
- Nuclear Issues (Environmental Science)
- Pollution and Threats to the Environment (Environmental Science)
- Social Impact of Environmental Issues (Environmental Science)
- History of Science and Technology
- Browse content in Materials Science
- Ceramics and Glasses
- Composite Materials
- Metals, Alloying, and Corrosion
- Nanotechnology
- Browse content in Mathematics
- Applied Mathematics
- Biomathematics and Statistics
- History of Mathematics
- Mathematical Education
- Mathematical Finance
- Mathematical Analysis
- Numerical and Computational Mathematics
- Probability and Statistics
- Pure Mathematics
- Browse content in Neuroscience
- Cognition and Behavioural Neuroscience
- Development of the Nervous System
- Disorders of the Nervous System
- History of Neuroscience
- Invertebrate Neurobiology
- Molecular and Cellular Systems
- Neuroendocrinology and Autonomic Nervous System
- Neuroscientific Techniques
- Sensory and Motor Systems
- Browse content in Physics
- Astronomy and Astrophysics
- Atomic, Molecular, and Optical Physics
- Biological and Medical Physics
- Classical Mechanics
- Computational Physics
- Condensed Matter Physics
- Electromagnetism, Optics, and Acoustics
- History of Physics
- Mathematical and Statistical Physics
- Measurement Science
- Nuclear Physics
- Particles and Fields
- Plasma Physics
- Quantum Physics
- Relativity and Gravitation
- Semiconductor and Mesoscopic Physics
- Browse content in Psychology
- Affective Sciences
- Clinical Psychology
- Cognitive Psychology
- Cognitive Neuroscience
- Criminal and Forensic Psychology
- Developmental Psychology
- Educational Psychology
- Evolutionary Psychology
- Health Psychology
- History and Systems in Psychology
- Music Psychology
- Neuropsychology
- Organizational Psychology
- Psychological Assessment and Testing
- Psychology of Human-Technology Interaction
- Psychology Professional Development and Training
- Research Methods in Psychology
- Social Psychology
- Browse content in Social Sciences
- Browse content in Anthropology
- Anthropology of Religion
- Human Evolution
- Medical Anthropology
- Physical Anthropology
- Regional Anthropology
- Social and Cultural Anthropology
- Theory and Practice of Anthropology
- Browse content in Business and Management
- Business Ethics
- Business Strategy
- Business History
- Business and Technology
- Business and Government
- Business and the Environment
- Comparative Management
- Corporate Governance
- Corporate Social Responsibility
- Entrepreneurship
- Health Management
- Human Resource Management
- Industrial and Employment Relations
- Industry Studies
- Information and Communication Technologies
- International Business
- Knowledge Management
- Management and Management Techniques
- Operations Management
- Organizational Theory and Behaviour
- Pensions and Pension Management
- Public and Nonprofit Management
- Strategic Management
- Supply Chain Management
- Browse content in Criminology and Criminal Justice
- Criminal Justice
- Criminology
- Forms of Crime
- International and Comparative Criminology
- Youth Violence and Juvenile Justice
- Development Studies
- Browse content in Economics
- Agricultural, Environmental, and Natural Resource Economics
- Asian Economics
- Behavioural Finance
- Behavioural Economics and Neuroeconomics
- Econometrics and Mathematical Economics
- Economic History
- Economic Systems
- Economic Methodology
- Economic Development and Growth
- Financial Markets
- Financial Institutions and Services
- General Economics and Teaching
- Health, Education, and Welfare
- History of Economic Thought
- International Economics
- Labour and Demographic Economics
- Law and Economics
- Macroeconomics and Monetary Economics
- Microeconomics
- Public Economics
- Urban, Rural, and Regional Economics
- Welfare Economics
- Browse content in Education
- Adult Education and Continuous Learning
- Care and Counselling of Students
- Early Childhood and Elementary Education
- Educational Equipment and Technology
- Educational Strategies and Policy
- Higher and Further Education
- Organization and Management of Education
- Philosophy and Theory of Education
- Schools Studies
- Secondary Education
- Teaching of a Specific Subject
- Teaching of Specific Groups and Special Educational Needs
- Teaching Skills and Techniques
- Browse content in Environment
- Applied Ecology (Social Science)
- Climate Change
- Conservation of the Environment (Social Science)
- Environmentalist Thought and Ideology (Social Science)
- Natural Disasters (Environment)
- Social Impact of Environmental Issues (Social Science)
- Browse content in Human Geography
- Cultural Geography
- Economic Geography
- Political Geography
- Browse content in Interdisciplinary Studies
- Communication Studies
- Museums, Libraries, and Information Sciences
- Browse content in Politics
- African Politics
- Asian Politics
- Chinese Politics
- Comparative Politics
- Conflict Politics
- Elections and Electoral Studies
- Environmental Politics
- European Union
- Foreign Policy
- Gender and Politics
- Human Rights and Politics
- Indian Politics
- International Relations
- International Organization (Politics)
- International Political Economy
- Irish Politics
- Latin American Politics
- Middle Eastern Politics
- Political Behaviour
- Political Economy
- Political Institutions
- Political Methodology
- Political Communication
- Political Philosophy
- Political Sociology
- Political Theory
- Politics and Law
- Public Policy
- Public Administration
- Quantitative Political Methodology
- Regional Political Studies
- Russian Politics
- Security Studies
- State and Local Government
- UK Politics
- US Politics
- Browse content in Regional and Area Studies
- African Studies
- Asian Studies
- East Asian Studies
- Japanese Studies
- Latin American Studies
- Middle Eastern Studies
- Native American Studies
- Scottish Studies
- Browse content in Research and Information
- Research Methods
- Browse content in Social Work
- Addictions and Substance Misuse
- Adoption and Fostering
- Care of the Elderly
- Child and Adolescent Social Work
- Couple and Family Social Work
- Developmental and Physical Disabilities Social Work
- Direct Practice and Clinical Social Work
- Emergency Services
- Human Behaviour and the Social Environment
- International and Global Issues in Social Work
- Mental and Behavioural Health
- Social Justice and Human Rights
- Social Policy and Advocacy
- Social Work and Crime and Justice
- Social Work Macro Practice
- Social Work Practice Settings
- Social Work Research and Evidence-based Practice
- Welfare and Benefit Systems
- Browse content in Sociology
- Childhood Studies
- Community Development
- Comparative and Historical Sociology
- Economic Sociology
- Gender and Sexuality
- Gerontology and Ageing
- Health, Illness, and Medicine
- Marriage and the Family
- Migration Studies
- Occupations, Professions, and Work
- Organizations
- Population and Demography
- Race and Ethnicity
- Social Theory
- Social Movements and Social Change
- Social Research and Statistics
- Social Stratification, Inequality, and Mobility
- Sociology of Religion
- Sociology of Education
- Sport and Leisure
- Urban and Rural Studies
- Browse content in Warfare and Defence
- Defence Strategy, Planning, and Research
- Land Forces and Warfare
- Military Administration
- Military Life and Institutions
- Naval Forces and Warfare
- Other Warfare and Defence Issues
- Peace Studies and Conflict Resolution
- Weapons and Equipment

- < Previous chapter
- Next chapter >
15 Patient follow-up, close-out, and post-trial follow-up
Author Webpage
- Published: September 1986
- Cite Icon Cite
- Permissions Icon Permissions
This chapter discusses the steps involved in patient follow-up, close-out, and post-trial follow-up. Topics covered include the maintenance of investigator and patient interest during follow-up, losses to the follow-up, close-out of patient follow-up, termination stage, and post-trial patient follow-up.
Signed in as
Institutional accounts.
- GoogleCrawler [DO NOT DELETE]
- Google Scholar Indexing
Personal account
- Sign in with email/username & password
- Get email alerts
- Save searches
- Purchase content
- Activate your purchase/trial code
Institutional access
- Sign in with a library card Sign in with username/password Recommend to your librarian
- Institutional account management
- Get help with access
Access to content on Oxford Academic is often provided through institutional subscriptions and purchases. If you are a member of an institution with an active account, you may be able to access content in one of the following ways:
IP based access
Typically, access is provided across an institutional network to a range of IP addresses. This authentication occurs automatically, and it is not possible to sign out of an IP authenticated account.
Sign in through your institution
Choose this option to get remote access when outside your institution. Shibboleth/Open Athens technology is used to provide single sign-on between your institution’s website and Oxford Academic.
- Click Sign in through your institution.
- Select your institution from the list provided, which will take you to your institution's website to sign in.
- When on the institution site, please use the credentials provided by your institution. Do not use an Oxford Academic personal account.
- Following successful sign in, you will be returned to Oxford Academic.
If your institution is not listed or you cannot sign in to your institution’s website, please contact your librarian or administrator.
Sign in with a library card
Enter your library card number to sign in. If you cannot sign in, please contact your librarian.
Society Members
Society member access to a journal is achieved in one of the following ways:
Sign in through society site
Many societies offer single sign-on between the society website and Oxford Academic. If you see ‘Sign in through society site’ in the sign in pane within a journal:
- Click Sign in through society site.
- When on the society site, please use the credentials provided by that society. Do not use an Oxford Academic personal account.
If you do not have a society account or have forgotten your username or password, please contact your society.
Sign in using a personal account
Some societies use Oxford Academic personal accounts to provide access to their members. See below.
A personal account can be used to get email alerts, save searches, purchase content, and activate subscriptions.
Some societies use Oxford Academic personal accounts to provide access to their members.
Viewing your signed in accounts
Click the account icon in the top right to:
- View your signed in personal account and access account management features.
- View the institutional accounts that are providing access.
Signed in but can't access content
Oxford Academic is home to a wide variety of products. The institutional subscription may not cover the content that you are trying to access. If you believe you should have access to that content, please contact your librarian.
For librarians and administrators, your personal account also provides access to institutional account management. Here you will find options to view and activate subscriptions, manage institutional settings and access options, access usage statistics, and more.
Our books are available by subscription or purchase to libraries and institutions.
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Rights and permissions
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

- About Patients at Heart
Trial Overview & Journey Sample
- History of Patients at Heart
- About AbbVie
QUICK LINKS
Trial journey overview
Patient bill of rights
Clinical trial lingo
What patients say
- About Clinical Trials
- Clinical Trials Essentials
- Preparing for My Clinical Trial
- Deep Dive Into Clinical Trials
Meet the stakeholders
- See All Trials
Can anyone participate?
Here we show a clinical trial timeline — what a participant’s trial journey looks like, from the beginning to when they get the results. Each visit ‘milestone’ is explained: just click on the visit name for all the details.
CLINICAL TRIAL VISITS
The number of your clinical visits will depend on the specifics and length of the study. There may be follow-up visits that include a brief physical exam (not generally in phase IV observational ), a review of your study medications and your symptoms, and lab tests.
- Ask any questions you may have
- Physical exam if required
- Discuss medical history change
- Depending on the trial protocol and medical condition, these could include lab tests, X-rays, CT scan, physical exam, etc.
- Discuss possible side effects
- Discuss medication you are taking
- Discuss and account for drug intakes; review your diary
- Questionnaires on health, work issues, etc.
- Pills, injections provided*, and either administered by coordinator/nurse, or patient is taught how to self-administer
* Phases I-III. In phase IV observational trials , patients get their medication at a pharmacy through prescription.
The informed consent can be periodically updated during the trial (contact info, location change, side effects, etc.). If/when there are any changes or additions, you’ll be informed by the study coordinator.
- Continue journaling: enter medications, start/stop dates, dosages, reactions, health status, mood status, symptoms, etc. Complete any questionnaires that were not already done at the study clinic.
- I'm thinking of joining a clinical trial
- I'm in a clinical trial
- I know someone in a clinical trial
- My clinical trial is completed
You are about to leave an AbbVie Canada site, a Web site maintained by AbbVie Corporation.
This link is provided for your convenience only. AbbVie Corporation takes no responsibility for the content of any Web site maintained by any third party and makes no representation as to the accuracy or completeness of any information contained on this or any subsequent link.
Do you wish to leave this site?

The Ultimate Guide to Clinical Trial Costs
- by Kunal Sampat
- January 29, 2022
- in Clinical Project Management

Have you been tasked to develop a clinical trial budget? Well, you’re in luck because I’m going to share everything you need to know about clinical trial costs.
Clinical trial budgets are often put together in haste. The focus is on getting the product to market as quickly as possible. Or revenues and profits.
Developing a clinical trial budget can be a confusing exercise for sponsors and CROs. There are too many cost variables to account for.
This post covers the key cost drivers for medical device clinical trials. If you are a researcher or financial analyst working in clinical research space or simply curious about clinical trial costs, this post will serve you well.
So let’s get started.
1. Patient Grant Costs
Patient grant costs are broken down into screening, baseline and follow-up visits and medical imaging costs.
a. Screen Failures
Clinical trial protocols have inclusion and exclusion criteria to qualify patients. Strict inclusions and exclusion criteria reduce the available patient pool for trial enrollment. Clinical sites spend physician and site coordinator time to screen for potential patients.
During the budgeting process, map out the complete patient screening workflow. Speak with a few clinical sites to understand how many patients they would have to see in order to find one qualified patient.
For example, a site may need to screen four patients to find one qualified patient. Understand how many hours the site is spending on screening activities and reimburse accordingly. Therefore it’s not unusual to reimburse sites anywhere between $50 to $250+ per screen failure.
b. Baseline/index Procedure and Follow-up Visits
Depending on the clinical trial design, data is collected at baseline or index procedures and follow-up visits. The site coordinator is generally responsible for entering the data in the case report form. Sites are reimbursed for the time spent to collect clinical trial data.
Based on number and type data fields you are collecting, you’ll want to estimate the site coordinator time needed to collect and input trial data. Then multiply the estimated coordinator time by the hourly bill rate to obtain the fair market value for each patient visit.
In some cases, sponsors may choose to reimburse patients. Reimbursement for patients can include paying for their participation, reimbursement for travel, meals or overnight hotel stays.
c. Non-standard of Care Tests
A clinical trial may require non-standard of care tests such as medical imaging scans. Insurance companies or medical care agencies generally do not reimburse non-standard of care costs. Therefore you should include them in your clinical trial budget.
d. Procedure Costs
Medical payor such as Medicare or private insurance may reimburse clinical trial procedure costs. If procedure reimbursement is available, you don’t need to budget for the procedure cost. In case a brand new procedure where no reimbursement available, budget for the procedure costs.
2. Site costs
A. site start-up fees.
Clinical sites spend significant time to initiate a new clinical trial. Sites are responsible for site-specific informed consent development, Ethics Committee (EC)/ Investigational Review Board (IRB) submissions, staff training including participation in investigator/ site coordinator meetings and site initiation visits and execute a clinical trial contract. It is typical for a sponsor to pay anywhere between $3500 – $7500+ in site start-up fees.
b. Ethics Committee (EC)/ Institutional Review Board (IRB) Fees
EC/IRB fees are in addition to site start-up fees. These fees cover the time spent by EC/IRB to plan and conduct a review of the clinical trial protocol and other associated materials. Many EC/IRBs update and publish their rates annually.
c. Close-out fees
Close-out fees include time spent by site staff to reconcile clinical trial data, finances, and regulatory documents during study closure. Not all sites require this payment but, in recent years, this cost has become a more common line item in the study budget.
d. Storage Fees
Government regulations require that clinical trial data be stored after study close-out. The duration for storage can range from 2-years to permanent storage. Thus it’s not uncommon for sites to have boxes of regulatory paperwork that need to be stored once a clinical trial ends. The storage fees vary by country and site.
Some sponsors make arrangements for the site to send trial documents to an offsite storage location. Due to country-specific regulations, a site might be unable to move documents outside their country.
e. Administrative Overhead
Clinical sites may require as much as 30% administrative overhead in addition to per patient grant amount. This cost covers management and legal resources needed to provide clinical research oversight and legal review of clinical contracts respectively.
f. Site Management Organization (SMO)
In certain countries such as Japan, data entry and collection tasks are outsourced to SMOs. For post-approval studies, sites do not research coordinator support. Thus sponsors are expected to hire SMOs to support the site or pay the sites to hire their preferred SMOs.
3. Non-patient costs
A. clinical evaluation committee (cec).
Adverse event and endpoint data is adjudicated by a non-biased, independent CEC. CEC is generally composed of 3 or more physicians. CEC members review adverse events and trial endpoints in a team setting or independently.
A sponsor can hire physicians to serve as the CEC and reimburse them at fair market value rates. It is more cost effective for the sponsor to contract with physicians directly. But the sponsor has to assign its own resources to manage the CEC.
The other option is for the sponsor to outsource management and conduct of CEC activities. However, this option is more expensive because you are hiring professionals to manage the CEC.
CEC is a very important component of medical device clinical trial. Adjudicated adverse event data is highly regarded by regulatory agencies and the physician community. In many cases, it is a requirement to have adjudicated adverse event data in order to get the product on the market.
b. Data Safety Monitoring Board (DSMB)
DSMB is sometimes known as the Data Monitoring Committee (DMC). According to IMARC research , the purpose of the DMC is to advise the sponsor on continuing safety of the trial subjects and those yet to be recruited and provide continuing validity and scientific merit of the study.
For budgeting purposes, it is important to know that DSMB is required during the trial enrollment phase. In some cases, DSMB meetings occur until all patients have reached their primary endpoint. The decision of whether or not to conduct DSMB meetings after the primary endpoint is reached is up to the sponsor.
c. Physician consulting
Physicians are consulted during all phases of a clinical trial. Physician guidance is needed to develop clinical trial strategy, enrollment plan, final data analysis, and publication plans.
Physician consulting costs can be anywhere between $150 – $600+ per hour. The billable rate varies based on the physician’s medical expertise and geographical location. If a clinical trial is interesting to the physician, he or she may be willing to provide consulting services at little or no cost.
d. Independent core lab analysis
Many medical device trials collect imaging data such as angiograms, CT scans, and X-Rays. Since this data comes from multiple sites, variability is expected. An independent core lab standardizes the collection and analysis of imaging data.
Corelab costs can add up quickly. Costs depend upon the number of images analyzed per patient, the time it takes for the core lab to analyze the data, and the duration of the trial.
Corelabs usually hire analysts to collect and calibrate data from different sites. The final analysis is usually done by a physician. Given the complexity of imaging data collection and analysis combined with the importance of core lab data to regulatory agencies, it is important that adequate and accurate budget is allocated for independent core lab analysis.
e. Medical product cost
Once you are ready to enroll patients in the clinical trial, you’ll need to ship the medical product to the sites. Most sites will expect to receive the medical product for free. The only exception is when conducting post-approval trials for commercially available medical product.
Medical device and biologics manufacturers may conduct a trial for clinical indication expansion. For example, a stent company may conduct a trial to get their heart stent approved for use in different anatomy. For such expansion trials, sponsors may need to provide commercially available medical product to sites at no cost.
Whether or not you want to provide the medical product at no cost is a business decision. When investigational medical product is provided at no cost, sites can enroll faster and have a much stronger, collaborative relationship with the Sponsor.
4. Labor Costs
In order to conduct a clinical trial, you need to hire people that have expertise in clinical research and clinical trial management. Depending on the size of the trial and the number of trials conducted, resource allocations vary. Therefore the amount of labor needed to run a study also varies.
a. Clinical Research Assistants or Associates (CRAs)
CRAs are primarily responsible for monitoring clinical trial data that is collected during the course of the study. They visit clinical research sites to ensure data is collected in a compliant manner.
b. Project Manager (also known as Clinical Trial Manager or Study Manager)
A project manager’s responsibilities can vary from one organization to another. Project managers are like “general contractors.” A project manager is responsible for managing the clinical trial budget, resources, and timelines. The core function of a project manager is to resolve or escalate issues that come up during the course of a clinical study.
c. Clinical Data Manager
A data manager’s job is to address data discrepancy issues by generating queries to sites. Data managers may also be responsible for implementing an electronic data capture system or paper case report forms needed to collect trial data.
d. Clinical Research Scientist
The scientist is primarily responsible for developing the clinical strategy for a trial. Individuals with Ph.D. or M.D. degrees are usually the right fit for this role. In some organizations, the project manager also plays the role of the scientist.
e. Biostatistician
A biostatistician is responsible for developing a statistical analysis plan (SAP). The SAP documents on the data will be analyzed during the course of the study. A statistician or statistical programmer is also responsible for programming data tables that are incorporated in the final clinical study reports.
Clinical research is a regulated industry. Quality plays an important role in ensuring sponsors, CROs, and clinical sites are conducting the trial in a compliant manner. Thus a quality associate or manager helps an organization create and implement standard operating procedures (SOPs).
Salaries for these roles can vary by geography and experience. The above list is not comprehensive. However, it should give you an idea of the core resources needed to conduct a medical device clinical trial.
5. Site Management
A. pre-study visits.
Prior to inviting any site to participate in a clinical trial, you want to conduct a pre-study visit, also known as the site assessment visit. This visit becomes even more important if you don’t have any prior experience working with the site in a clinical or commercial setting.
Although sites don’t charge for this visit, the sponsor will need to pay for travel and CRA labor costs.
b. Site Initiation Visits (SIV)
Once the site has received Institutional Review Board (IRB) or Ethics Committee (EC) approval and the trial contract has been signed, it’s time to activate the site for patient enrollment.
A SIV is conducted when you are ready to activate the site. SIV involves training the site on the clinical protocol and any other study-specific requirements.
Similar to the pre-study visit, the sponsor will need to pay for travel and CRA labor costs.
c. Monitoring – Remote, Virtual, In-person
Once patients are enrolled in the study, it is critical to collect data in compliance with regulations and the clinical study protocol. This is when monitoring comes into play.
A CRA, sometimes known as the site monitor, visits clinical sites at regular intervals to ensure compliance.
In recent years, due to the push for a reduction in clinical trial costs, several sponsors have started to monitor remotely rather than conducting an in-person monitoring trip.
d. Close-out
Once all patients at a site have completed their follow-up visits, it’s time to conduct a close-out visit. Any open items related to study conduct are addressed during the close-out visit.
Although it’s always nice to have in-person close-out visits, it’s acceptable to close trials via remote close-out calls.
6. Miscellaneous
A. investigator meetings.
Investigator Meetings serve to kick-off a new clinical trial. Site investigators and research coordinators are invited to participate in a 1-2 day meeting. These meetings serve to educate site personnel on the clinical trial protocol and any other trial specific requirements.
These meetings can be quite expensive and the sponsor pays for attendee airfare, hotel, and meals.
Plan and budget for ad hoc travel. Clinical research is highly regulated. You’ll need to visit a site to address a compliance issue or help them prepare for an audit. In other cases, you want to visit a site to motivate them to enroll patients. Whatever the case may be, it’s always good to have a bit of money set aside for travel.
c. Document Translations
Document translations cost increase significantly depending on the countries in which the clinical trial is conducted. Sites where English is not the primary language, you may receive a request for translation of key documents such as the protocol and site-specific informed consent in the local language.
Also if the adverse event source documents from non-English speaking sites are in their native language, additional costs will incur to translate documents into English for event adjudication purposes.
d. Technology solutions
To conduct clinical trials, you need systems such as Clinical Trial Management System (CTMS), Electronic Data Capture (EDC), Electronic Trial Master File (eTMF), Interactive Voice/Web Response System (IxRS). These systems manage site contact information, collect clinical data and maintain clinical trial records. Budget monthly or annual license fees associated with these systems. Additionally, you need staff to manage and maintain these systems.
e. Regulatory filing fees
Don’t overlook regulatory filing fees. These fees can run into thousands of dollars. Depending on the class of medical device, different applications are filed with regulatory agencies, competent authorities and notified bodies.
7. Other Clinical Trial Cost Factors
A. protocol amendments.
Due to unforeseen circumstances, a clinical protocol amendment may be necessary. A protocol amendment has many downstream effects that can increase the cost of a clinical trial.
A protocol amendment usually leads to additional IRB/EC fees, site costs, regulatory re-submissions and more.
b. Inflation, Value Added Tax (VAT) and Foreign Exchange
Don’t forget to factor inflation for multi-year clinical trials. Generally speaking, plan for a minimum of 3% inflation rate.
For sites in countries such as Australia and Europe, add VAT for the research services. The VAT can be upwards of 12% on all research services.
For trials conducted in multiple countries, pay attention to foreign exchange rates. At a minimum, an annual review of exchange rates is advised. Adjust clinical trial cost projections based on exchange rates.
c. Trial enrollment delays
Enrolling in trials is a tricky business. It takes longer to complete enrollment and initial projections are overly optimistic. Therefore account for these delays when you develop your clinical trial budget.

Conclusion:
We’ve covered a lot of ground in this Ultimate Guide to Clinical Trial Costs. To summarize, you should now have a solid understanding of these factors that impact clinical trial costs:
- Patient grant amounts such as screen failure costs, data entry costs, and travel reimbursement
- Site costs such as site start-up fees, EC/IRB fees, close-out and storage fees
- Non-patient costs such as core laboratory fees, clinical events committee and data safety monitoring board
- Labor costs such as clinical research employee salaries or contractor payments
- Site management costs such as pre-study, site initiation, monitoring, and close-out visits
- Miscellaneous costs such as travel, technology solutions, and regulatory filing costs
- Other factors such as value-added tax, inflation, protocol amendment and delays in enrollment
What’s your best tip to planning a clinical trial budget? Leave in the comment section below.
Clinical Research Billing for Small to Medium Sites with Kristi Etchberger
Integrating ehr and edc systems with hugh levaux, 29 thoughts on "the ultimate guide to clinical trial costs", overview of clinical research | clinical trial podcast, role of a clinical project manager | clinical trial podcast, as the cost of clinical trials climbs, here are 3 ways to save - archemedx, best practices and assumptions for clinical trial budgeting - cereblis.
Tidor Morgan
Kunal, thanks for summarising this so concisely…. a really useful read and reference point for future discussion.
Hi Tidor, I’m so glad to hear you found this post useful. This should severe as a useful guide to the clinical community when it comes to planning trial budgets. Thanks again for taking the time to read and comment.
Nice summary Kunal. The overview is very concise and you touched on all the important aspects to consider when budgeting for a clinical trial.
Hey Chris, Thanks for the positive feedback 🙂 It’s good to hear from industry professionals such as yourself who developed hundreds of budget models and scenarios. If you have any other insights or suggestions, please do let me know.
Thank you for writing the article and sharing the excel file. These are great!
Kunal Sampat
Thanks Ehsan! Let me know if you have any follow-up questions. Happy to help!
Thank you, Ehsan!
Hi Kunal, what a tremendous resource you’ve provided here, thank you! I’m looking for someone to consult on a budget for a medical device trial. Do you know anyone offering these services?
Excellent, thank you.
Thank you, Laura. Glad to hear your feedback.
Thank you, Laura
Vladimir Shnaydman
Hi Kunal, Several questions. 1. How budgeting is coordinated with site selection? 2. How did you included risk in budgeting process? 3. What are major cost drivers for a clinical trial budget? Thank you, Vladimir
Hi Vladimir,
1. budgeting in most cases would not be coordinated with site selection 2. many of the clinical trial costs are tried to patient recruitment. patient recruitment is a dynamic process. you will likely need to re-forecast your budget on a regular basis depending on how fast or slow you enroll 3. Major cost drives are generally patient grant costs, labor costs, and monitoring. Every study is different and there may be other high ticket items.
This post should be renamed Clinical Trial Budgeting for dummies, as it gives an informative yet easy to understand breakdown of the whole process. Thank you Kunal.
Hi Ivy, Thank you 🙂 Kunal
Thanks Kunal , this is helpful. I may also add that it might also be required to include the product’s manufacturing costs in a study budget, especially in budgets of small Biotech companies.
Hi Uri, Yes, I agree. Product manufacturing costs should be part of the study budget. I’ve mentioned “device costs” in this article. Will update it to include “biologics costs” as well. Thanks for the input.
Francis Akenami
Hi Kunal – thank you for the comprehensive presentation. Where do you include costs for Medical Writing Services such content development for Protocols, Clinical Study Reports, Clinical Evaluation Reports, New Drug Applications and publications in peer-reviewed journals? Can they be added to the overall cost of Clinical Trials? I should think so since a Clinical Trial cannot be considered complete if those are missing.
Gerard Abate
What is the average cost per patient for a CRO for an interventional trial?
It depends on the study design.
Really got a good understanding of the basics especially when I am in a project involving a major player in the clinical trials domain. Thanks a lot!
Kenneth Quintana
What is the average time a cro spends on final data analysis? Thank You
This average time a CRO spends time on data analysis can vary based on study design (ex: how complex are your statistics), quality of the data collected (ex: lot of missing data = more time needed), and resources (ex: do you have a team to do the work). I would say plan for 3-months but it can take more or less time based on the above factors
raveena aher
thank you for sharing your blogs
Thankyou for sharing Clinical Trial Costs with us.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Effect of single follow-up home visit on readmission in a group of frail elderly patients - a Danish randomized clinical trial
Affiliations.
- 1 Department of Internal Medicine, Nykøbing Falster Hospital, Fjordvej 15, 4800, Nykøbing Falster, Denmark. [email protected].
- 2 National Institute of Public Health, University of Southern Denmark, Copenhagen K, Denmark.
- 3 Department of Quality and Development, Sorø, Region Zealand, Denmark.
- 4 Nykøbing Falster Hospital, Nykøbing Falster, Denmark.
- 5 Department of Internal Medicine, Nykøbing Falster Hospital, Fjordvej 15, 4800, Nykøbing Falster, Denmark.
- PMID: 31653219
- PMCID: PMC6815031
- DOI: 10.1186/s12913-019-4528-9
Background: Unplanned hospital admissions are costly and prevention of these has been a focus for research for decades. With this study we aimed to determine whether discharge planning including a single follow-up home visit reduces readmission rate. The intervention is not representing a new method but contributes to the evidence concerning intensity of the intervention in this patient group.
Methods: This study was a centrally randomized single-center controlled trial comparing intervention to usual care with investigator-blinded outcome assessment. Patients above the age of 65 were discharged from a single Danish hospital during 2013-2014 serving a rural and low socioeconomic area. For intervention patients study and department nurses reviewed discharge planning the day before discharge. On the day of discharge, study nurses accompanied the patient to their home, where they met with the municipal nurse. Together with the patient they reviewed cognitive skills, medicine, nutrition, mobility, functional status, and future appointments in the health care sector and intervened if appropriate. Readmission at any hospital in Denmark within 8, 30, and 180 days after discharge is reported. Secondary outcomes were time to first readmission, number of readmissions, length of stay, and readmission with Ambulatory Care Sensitive Conditions, visits to general practitioners, municipal services, and mortality.
Results: One thousand forty-nine patients aged > 65 years discharged from medical, geriatric, emergency, surgical or orthopedic departments met inclusion criteria characteristic of frailty, e.g. low functional status, need of more personal help and multiple medications. Among 945 eligible patients, 544 were randomized. Seven patients died before discharge. 56% in the intervention group and 54% in the control group were readmitted (p = 0.71) and 23% from the intervention group and 22% from the control group died within 180 days. There were no significant differences between intervention and control groups concerning other secondary outcomes.
Conclusions: There was no effect of a single follow-up home visit on readmission in a group of frail elderly patients discharged from hospital.
Trial registration: https://clinicaltrials.gov (identifier NCT02318680 ), retrospectively registered December 11, 2014.
Keywords: Clinical trial; Discharge planning; Elderly; Frailty; Readmission.
Publication types
- Randomized Controlled Trial
- Aged, 80 and over
- Follow-Up Studies
- Frail Elderly*
- House Calls*
- Outcome Assessment, Health Care*
- Patient Discharge
- Patient Readmission / statistics & numerical data*
Associated data
- ClinicalTrials.gov/NCT02318680

Stanford Cancer Institute
Search stanford cancer institute, clinical trials.

The Stanford Cancer Institute (SCI) Clinical Trials Office (CTO) provides regulatory, administrative, research support, budget, and educational services to SCI investigators conducting cancer clinical trials.
- Clinical Trials Office
Our Services
The Stanford Cancer Institute Clinical Trials Office facilitates translational research through extensive support services.

Clinical Research Staff Oversight
SCI-CTO provides central coordination and oversight of clinical staff to ensure consistent processes, regulatory compliance, and improved efficiencies.

Research Services
SCI-CTO provides a centralized resource that assists clinical researchers with scientific review, regulatory management, pre-award financial management, quality assurance, training and education, data collection, and monitoring.

Training and Education
SCI-CTO provides staff orientation training and ongoing education, supports investigator training, and manages the development and maintenance of Standard Operating Procedures (SOPs), Practice Guidelines, and study management tools.
Monitoring Services
Quality control monitoring of SCI clinical trials are conducted by SCI-CTO quality monitors or third-party monitors. Our external quality monitoring visits are primarily conducted remotely.

Scientific Review Committee
The SCI oversees the conduct of research studies investigated by SCI investigators, ensuring the research is of scientific merit, high quality, reliable, and verifiable.
Faculty and Staff Services
Staff and faculty members can find SCI-CTO's internal secure documents accessible through our intranet.

Patient Care
Health equity, shared resources, stanford medicine, health care.

©2024 Stanford Medicine
- Open access
- Published: 04 April 2024
Sertaconazole 300 mg versus clotrimazole 500 mg vaginal suppository for treating pregnant women with acute vaginal candidiasis: a double-blinded, randomized trial
- Chenchit Chayachinda ORCID: orcid.org/0000-0002-0153-2231 1 ,
- Manopchai Thamkhantho 1 ,
- Thanapa Rekhawasin 2 &
- Chanakarn Klerdklinhom 3
BMC Pregnancy and Childbirth volume 24 , Article number: 235 ( 2024 ) Cite this article
Vaginal candidiasis (VC) commonly affects pregnant women. Traditionally, clotrimazole vaginal tablets (CLO) have been the cornerstone of management. However, sertaconazole ovules (SER) offer a novel topical antimycotic option. This double-blinded, randomized trial evaluated the efficacy of single-dose SER and CLO in treating acute VC during pregnancy.
From June 2020 to May 2021, this trial recruited pregnant women aged ≥ 18 years with VC symptoms (abnormal vaginal discharge and/or vulvar/vaginal itching) confirmed by microscopy. Participants with ≥ 4 VC episodes in the prior year, immunocompromised status, or imidazole contraindications and those who were absent at the 2-week follow-up were excluded. Participants were randomized to receive either 300 mg SER or 500 mg CLO. Evaluations 2 weeks after the initial medication administration included clinical cure (self-reported resolution of all symptoms), microscopic cure (pseudohyphal absence), patient satisfaction, side effects, and time to clinical cure. Participants with persistent VC received weekly SER doses until delivery. Assessments of recurrence and pregnancy outcomes were done.
The analysis included 96 participants (48 per group, mean age 27.4 ± 7.4 years, gestational age at diagnosis 22.9 ± 6.4 weeks). Without statistical significance, SER achieved a higher clinical cure rate (62.5% vs 50%, p = 0.217; a mean difference of 12.5%, 95%CI: -17.5% to 42.5%; and a rate ratio of 1.25, 95%CI: 0.71 to 2.23) and a lower microscopic cure (47.9% vs. 62.5%, p = 0.151; a mean difference of -14.6%, 95%CI: -44.3% to 15.1%; and a rate ratio of 0.77, 95%CI: 0.43 to 1.37). The two groups had comparable times to clinical cure (SER: 3.1 ± 1.8 days, CLO: 3.4 ± 2.7 days; p = 0.848) and substantial satisfaction rates (SER: 66.7%, CLO: 60.4%; p = 0.753). No side effects were reported. Of 60 participants who gave birth at Siriraj Hospital, there were no significant differences in pregnancy outcomes. Repeated SER dosing eradicated symptoms and enhanced the microscopic cure rate. Recurrence was observed in four SER and two CLO participants within 1–2 months.
In the treatment of acute VC during pregnancy, 300 mg SER and 500 mg CLO exhibited comparable efficacy in terms of clinical and microscopic cure rates, satisfaction, side effects, time to clinical cure, recurrence rates, and pregnancy outcomes.
Trial registration
TCTR20190308004 (registration date March 8, 2019).
Peer Review reports
Introduction
Vaginal candidiasis (VC), or less frequently referred to as vulvovaginal candidosis, presents a significant gynecologic challenge, compelling affected women to seek medical intervention [ 1 , 2 ]. Symptoms predominantly include altered discharge exhibiting curd-like properties, augmented volume, and intense vulvar/vaginal itching [ 3 , 4 , 5 , 6 ]. The principal causative agent, Candida albicans [ 2 , 7 ] , resides within the normal vaginal microbiota. Risk factors include a sedentary lifestyle, elevated estrogen levels, stress, and dietary issues, notably iron deficiency anemia and high sugar intake [ 8 , 9 ]. These conditions render pregnant women particularly vulnerable to VC, with a noted increase in recurrence as pregnancy progresses [ 2 , 10 ]. Data on the impact of VC on pregnancy outcomes, such as preterm birth and prelabor membrane rupture, remain contentious [ 11 ]. Treating VC during pregnancy might offer protective benefits [ 12 ].
Oral or vaginal imidazoles serve as the primary treatment for VC [ 7 ], with topical formulations recommended for pregnant women. For over four decades, clotrimazole (CLO) vaginal tablets have been a mainstay for treating VC in pregnant women [ 13 ], with multiple-dose regimens being favored [ 9 ]. Nonetheless, recent systematic reviews suggest that a single 500 mg dose of CLO may offer efficacy comparable to that of multiple lower-strength CLO doses [ 13 ]. However, the gravid uterus can complicate vaginal insertion, especially in the third trimester, a peak period for VC recurrence [ 10 ]. Additionally, a notable drawback of CLO vaginal tablets is their dissolution time [ 5 ]. This limitation led to the introduction of sertaconazole (SER) 300 mg vaginal ovule. This novel formulation dissolves readily at body temperature, sustains a 96-h vaginal presence, and is minimally absorbed systemically [ 14 ]. In vitro research has corroborated the antibacterial, anti-inflammatory, and antipruritic effects of SER [ 15 ].
Recent studies [ 3 , 5 , 6 , 16 ] have shown that SER is noninferior to CLO, with quicker symptom relief and similar adverse effects. Efficacy comparable to that of other topical azoles has also been noted [ 4 , 17 ]. Nonetheless, the long-term outcomes of SER usage through to delivery have not been thoroughly investigated. This study aimed to evaluate an alternative treatment for VC in pregnant Thai women in both the short and long term. Specifically, we compared the clinical and microscopic cure rates, side effects, patient satisfaction, recurrence, and pregnancy outcomes between patients treated with 300 mg of single-dose SER and those treated with 500 mg of single-dose CLO.
Materials and methods
Study design and ethical considerations.
This double-blinded, randomized trial was conducted from June 2019 to January 2021 at the Department of Obstetrics and Gynaecology, Faculty of Medicine Siriraj Hospital. In alignment with the Declaration of Helsinki principles, ethical approval was granted by the Siriraj Institutional Review Board (reference: Si-132/2019; registration date: February 11, 2019) prior to initiating participant recruitment. The trial was registered with the Thai Clinical Trial Registry (reference: TCTR20190308004; registration date: March 8, 2019).
Participants
In our protocol, every woman with abnormal vaginal discharge was subjected to a high vaginal swab for wet mount preparation. The procedure involved mixing the swab with 1 mL of 0.9% normal saline solution and examining it under a light microscope after adding 10% potassium hydroxide solution. Eligible participants were pregnant women aged ≥ 18 years with abnormal discharge or vulvar/vaginal itching, where VC was confirmed by the presence of pseudohyphae, not blastospores, under microscopy. This diagnostic criterion was based on the yeast-to-hyphal transformation characteristic of C. albicans pathogenesis [ 18 ].
Women who were beyond 36 weeks of gestation or who had a history of ≥ 4 VC episodes in the last year, insulin-dependent diabetes mellitus, human immunodeficiency virus infection, systemic lupus erythematosus, recent use of vaginal suppositories, imidazole allergy, or symptomatic liver disease were excluded. The statistical analysis was limited to participants who returned for the 2-week follow-up.
Intervention
At the Siriraj Female STD Clinic, pregnant women with abnormal vaginal discharge were briefed about the study during their wait. After comprehensive history-taking and physical, pelvic, and microscopic examinations, study nurses detailed the study to eligible women. Those who fulfilled the eligibility criteria and consented to participate were then randomized at a 1:1 ratio to receive either a CLO 500 mg tablet (Canesten® 500 mg, Bayer, Thailand) or an SER 300 mg vaginal ovule (Zalain®, Pacific Health Care, Thailand). This randomization employed a computer-generated, block-of-four method devised by a statistician.
To ensure blinding, the study drugs were concealed within opaque envelopes and dispensed according to the predetermined block-of-four randomization. Trained study team members, either gynecologists or residents, were responsible for the deep insertion of the medication into the posterior fornix without disclosing the drug to participants. Following the insertion, the participants were instructed to remain in the supine position for 5 min to facilitate drug retention.
Subsequently, participants were interviewed using a case record form, informed about potential local and systemic side effects, and scheduled for a 2-week follow-up by a study nurse. They received guidance on mitigating VC risk behaviors, including avoiding sugar-rich foods and drinks, reducing excessive genital cleansing, refraining from using intimate hygiene products, and avoiding close-fitting garments [ 8 ]. Engaging in vaginal intercourse was prohibited during the study. Participants were also asked to track the time until clinical cure of all symptoms. Before the patient left the clinic, expulsion of the vaginal suppository was checked. The entire process on the enrollment day (Visit 0) lasted approximately 40 min.
Outcomes were evaluated at a 2-week follow-up (14 ± 2 days; Visit 1), with a study nurse providing a telephone reminder the day before. At this visit, blinded staff inquired about participants’ symptoms, time to clinical cure, satisfaction levels, and any side effects experienced. A blinded gynecologist conducted pelvic examinations and wet preparations with 10% potassium hydroxide to check for pseudohyphae.
Given the Clinic’s provision of SER at no cost, participants lacking microscopic cure (the absence of pseudohyphae) at Visit 1 received two additional weekly doses of SER and were trained on self-application using a manikin. A follow-up (Visit 2) was scheduled for 2 weeks after the self-administration of the second SER dose. Should pseudohyphae persist at Visit 2, participants were provided with four more weekly SER doses. They were instructed to return 2 weeks following the self-administration of the fourth of these doses (Visit 3) for microscopic assessment. Participants with ongoing pseudohyphae at Visit 3 were prescribed weekly SER doses until delivery.
Participants who achieved microscopic cure were advised to revisit the clinic for any recurrent abnormal vaginal discharge episodes. The pregnancy outcomes of those who gave birth at Siriraj Hospital were documented.
Outcome measures
The primary endpoint was clinical cure, characterized by the resolution of all initial symptoms, particularly abnormal vaginal discharge and vulvar/vaginal itching. Given the frequent recurrence of vulvovaginal candidiasis during pregnancy and its significant impact on quality of life [ 19 ], clinical resolution was a focal concern. Equally critical was microscopic cure, identified by the absence of pseudohyphae in wet preparations with 10% potassium hydroxide. Microscopic cure has been linked to reduced preterm birth rates in asymptomatic patients [ 12 ].
The time to clinical cure was defined as the number of days from treatment initiation to the resolution of all symptoms. Participants self-assessed their treatment response on a three-point scale (1 = no/minimal improvement, 2 = moderate improvement, 3 = substantial improvement). The side effects of the study medications were categorized as local (vaginal pain, irritation, swelling) or systemic (skin rash, respiratory distress). Recurrence was defined as a new episode of symptomatic VC, microscopically confirmed by gynecologists, at any time during pregnancy after participants were classified as microscopically cured at Visit 1.
Pregnancy outcomes were documented only for participants who gave birth at Siriraj Hospital. The neonatal outcomes included birth weight and 1- and 5-min Apgar scores. Deliveries prior to 37 gestational weeks were deemed preterm, and a birth weight under 2500 g was classified as low. An Apgar score < 7 at either 1 or 5 min indicated a poor prognosis for the newborn.
Sample size calculation and statistical analysis
The sample size and statistical analyses were conducted using Stata Statistical Software, release 12.1 (StataCorp LLC, College Station, TX, USA). The calculations were informed by the findings of a study by Lutsevich, which indicated clinical cure rates of 93.4% for SER 300 mg vaginal ovules and 71.9% for a 6-day regimen of CLO 100 mg vaginal tablets among pregnant Russian women with VC [ 16 ]. With the aim of achieving 80% power and a significance level of 0.05 (for a two-sample comparison), we determined that each group required 47 participants.
Descriptive statistics are reported as the n (%) and mean ± SD. Categorical data comparisons utilized the chi-square test and Fisher’s exact test, while the Shapiro–Wilk test was used to assess continuous data distribution. Parametric data were analyzed using Student’s t test. Binary outcome effect sizes are presented as the mean difference, rate ratio, and 95% CI, with p values < 0.05 indicating statistical significance.
Out of 129 potential participants screened, 29 were excluded based on the criteria. Fifty patients were assigned to the CLO group, and another 50 were assigned to the SER group. By Visit 1, two participants from each group were lost to follow-up (Fig. 1 ), leaving 96 participants for statistical analysis.
Flow of the participants
As depicted in Table 1 , the demographic and clinical characteristics were comparable between the groups. The mean age was 27.4 ± 7.4 years, with a mean body mass index (BMI) of 23.6 ± 5.4 kg/m 2 . Diagnosis occurred at a gestational age of approximately 23 weeks. All participants had abnormal vaginal discharge, and two-thirds experienced vulvar/vaginal itching. Approximately 10% had a history of sexually transmitted infections, comprising anogenital warts ( n = 8), herpes genitalis ( n = 2), hepatitis B ( n = 1), and syphilis ( n = 1). Almost 40% reported abnormal vaginal discharge in the past month, and 57.3% had experienced 1–2 VC episodes in the previous year.
At the first follow-up (Visit 1), compared with CLO recipients, SER recipients exhibited a nominally greater clinical cure rate of 62.5%, with a p value of 0.217. This resulted in a mean difference of 12.5% (95% CI: -17.5% to 42.5%) and a rate ratio of 1.25 (95% CI: 0.71 to 2.23). However, the microscopic cure rate was marginally lower at 47.9% for SER versus 62.5% for CLO, with a p value of 0.151. This showed a mean difference of -14.6% (95% CI: -44.3% to 15.1%) and a rate ratio of 0.77 (95% CI: 0.43 to 1.37). Neither difference reached statistical significance. The time to clinical cure was similar across groups among those who achieved microscopic cure (3.4 ± 2.7 days for CLO vs. 3.1 ± 1.8 days for SER, p = 0.848). Neither group reported any local or systemic side effects. Substantial self-rated satisfaction levels were comparable (60.4% for SER vs. 66.7% for CLO, p = 0.753; Table 2 ).
Among the participants who achieved microscopic cure at the initial follow-up, four patients in the SER group experienced recurrence within 1 to 2 months, while two patients in the CLO group experienced recurrence at 2 months. Among those without initial microscopic cure, 4/37 (10.8%) achieved it within the following 2 weeks. Of the 33 participants who continued without microscopic cure, 28 attended the subsequent follow-up, with 6 (21.4%) achieving cure.
Pregnancy outcomes did not significantly differ between the CLO and SER groups (Table 3 ). All instances of preterm birth were post-34 weeks gestation, and infants classified as having low birth weights all exceeded 2200 g. Two newborns with initial Apgar scores below 7 improved following brief oxygen therapy.
This study is the first direct comparison of two single-dose vaginal antimycotic suppositories for treating VC in pregnant women. Echoing findings from a previous study of pregnant Russian women treated with a single SER 300 mg dose versus a 7-day CLO 100 mg regimen [ 16 ], SER achieved a 12.5% higher clinical cure rate than than that of CLO, but the difference was not statistically significant. The onset of symptom improvement was similar, occurring at approximately three days. In contrast, earlier research comparing the older formulation of SER 500 mg vaginal tablet to multiple lower-strength CLO doses presented mixed outcomes. Specifically, two studies involving nonpregnant Indian women [ 3 , 6 ] noted better outcomes with SER, whereas a study involving nonpregnant Thai women [ 5 ] observed higher cure rates with CLO and reported notably lower overall cure rates. This issue warrants further exploration, as fungal culture was not performed in the present study.
The increased colonization of Candida albicans , elevated estrogen levels, altered immune function, and disrupted glucose metabolism during pregnancy [ 20 ] justify the extended regimen of topical VC treatments for expectant mothers [ 9 , 20 ]. Nevertheless, administering multiple intravaginal doses can be impractical for those with a gravid uterus, particularly in the third trimester, a time at which a heightened recurrence risk is noted [ 10 ]. Additionally, the practice of intravaginal insertion can be particularly challenging for Asian populations, including Thais [ 21 ]. A prior study among pregnant Russian women, who were treated twice with one vaginal suppository of 500 mg SER every 7 days, showed a 90% cure rate [ 22 ] which was comparable to that of multiple-dose clotrimazole regimens in pregnant women of various ethnicities [ 13 ], our study revealed improved outcomes with added weekly doses of 300 mg SER ovules. However, our investigation lacked a crossover design and a no-treatment control group, highlighting the need for further research to substantiate the efficacy of repeated single-dose antimycotic vaginal suppositories.
Concerns about the safety of SER during pregnancy have been notable. With more than 45 years of use and extensive evidence supporting its safety in pregnancy (category B) [ 13 ], CLO sets a high standard. Sertaconazole, an imidazole similar to CLO, has shown minimal systemic absorption in in vitro studies [ 14 ]. The current investigation corroborates findings from a large-scale French study involving 16 222 pregnant women exposed to SER and 91 976 controls, indicating no significant difference in the risk of congenital anomalies and adverse pregnancy outcomes [ 23 ].
The impact of VC on the vaginal environment can contribute to adverse pregnancy outcomes [ 11 ], including a marginal increase in preterm births among untreated symptomatic women [ 24 ]. Although evidence is sparse, SER is known for its anti-inflammatory effects and ability to combat Gardnerella spp., a bacterial vaginosis-associated bacterium [ 25 ]. Despite the careful monitoring of participants in the current study until delivery, the incidence of preterm births was consistent with the general rates observed in the broader population at our facility [ 26 ]. This finding parallels the suspected association between bacterial vaginosis and preterm birth, where inflammation might commence before intervention [ 27 ]. A recent systematic review and meta-analysis highlighted the lack of conclusive data supporting routine vulvovaginal candidiasis screening in pregnant women [ 28 ].
Despite recurrence typically being more prevalent during late gestation [ 10 ], our study noted a low recurrence rate. Unlike prior research [ 5 , 6 ], we identified only six instances of recurrence. Our methodology might have contributed to this variance. Earlier studies assessed cure rates 1 week after the SER dose and identified recurrences at 4–6 weeks. However, our analysis was conducted during Visit 1, which was 2 weeks after the initial SER dose. Additionally, our analysis only recognized recurrences among participants who had achieved microscopic cure by Visit 1. Another explanation for the differences between our study results and those of earlier studies is that the influence of lifestyle modifications on treatment outcomes was emphasized for all of our participants [ 7 ]. Generally recognized lifestyle changes include avoiding sugar intake, stress, and close-fitting clothing; abstaining from sexual activity during treatment; and limiting genital cleansing [ 8 ]. Furthermore, providing information on the nature of VC and encouraging open discussion can alleviate participants’ anxiety and reinforce lifestyle modifications [ 8 ]. We encourage all healthcare settings to provide comprehensive counseling in addition to standard treatment.
The study’s primary strength lies in its double-blinded, randomized design, ensuring that participants and outcome evaluators were blinded. Furthermore, the inaugural head-to-head comparison of two single-dose azole treatments in pregnant women is important. Both study drugs required a single administration and were properly applied by a well-trained study team member. However, the limitations of this study include the absence of fungal cultures and the lack of a control group for following up patients who lacked microscopic cure at Visit 1. Fungal cultures would provide deeper insights into the modest treatment efficacy observed, especially given the rising global concern over antifungal resistance. Moreover, while the presence or absence of microscopic cure signals a disrupted vaginal ecosystem, treatment based on this finding remains controversial.
Conclusions
In summary, patients with SER exhibited a greater clinical cure rate but a lower microscopic cure rate than patients with CLO; however, neither of these differences reached statistical significance. Both treatments showed comparable times to clinical cure, levels of self-rated satisfaction, side effect profiles, and pregnancy outcomes. Future research involving larger cohorts is essential to fully ascertain the clinical efficacy and safety of SER for treating VC in both pregnant and nonpregnant populations.
Availability of data and materials
The study’s dataset is accessible as part of the submission process and is available for public access. For inquiries, contact Associate Professor Chenchit Chayachinda at [email protected].
Abbreviations
Clotrimazole
Sertaconazole
- Vaginal candidiasis
Chayachinda C, Thamkhantho M, Chalermchockcharoenkit A, Nuengton C, Thipmontree W. Characteristics of clients at the Siriraj Female STD Clinic during 2011–2015. Siriraj Medical Bulletin. 2018;11:182–9.
Google Scholar
Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961–71.
Article PubMed Google Scholar
Verma K, Bhat M, Baniya G. A comparative study of antifungal activity of topical per vaginal application of tablet Sertaconazole and Clotrimazole in cases of vulvovaginal candidiasis. Int J Health Sci Res. 2015;5:111–5.
Wang P, Chao H, Chen C, Yuan C. Single-dose sertaconazole vaginal tablet treatment of vulvovaginal candidiasis. J Chin Med Assoc. 2006;69:259–63.
Article CAS PubMed Google Scholar
Roongpisuthipong A, Chalermchockcharoenkit A, Sirimai K, Wanitpongpan P, Jaishuen A, Foongladda S, et al. Safety and efficacy of a new imidazole fungicide, Sertaconazole, in the treatment of fungal vulvo-vaginitis: a comparative study using Fluconazole and Clotrimazole. Asian Biomedicine. 2010;4:443–8.
Article Google Scholar
Desai K, Sambarey P. Comparison of single dose sertaconazole versus three dose clotrimazole regime in treatment of uncomplicated vulvovaginal candidiasis- A Prospective Study. J Clin and Diagn Res. 2019;13:QC12–4.
CAS Google Scholar
Chayachinda C, Thamkhantho M, Ngamsakulrungroj P, Leeyaphan C, Tulyaprawat O. Effect of intravaginal gentian violet for acute vaginal candidiasis treated with a single dose oral fluconazole: a randomised controlled trial. J Obstet Gynaecol. 2022;7:1–7.
Rachapromma P, Chayachinda C, Kerdklinhom C, Iamvijarn W, Thanasakthitiku T. Nursing Care for Women with Acute Vaginal Candidiasis. Siriraj Medical Bulletin. 2022;15:107–13.
Saxon C, Edwards A, Rautemaa-Richardson R, Owen C, Nathan B, Palmer B, et al. British Association for Sexual Health and HIV national guideline for the management of vulvovaginal candidiasis (2019). Int J STD AIDS. 2020;31:1124–44.
Fardiazar Z, Ronaci F, Torab R, Goldust M. Vulvovaginal candidiasis recurrence during pregnancy. Pak J Biol Sci. 2012;15:399–402.
Ilkit M, Guzel A. The epidemiology, pathogenesis, and diagnosis of vulvovaginal candidosis: a mycological perspective. Crit Rev Microbiol. 2011;37:250–61.
Roberts C, Rickard K, Kotsiou G, Morris J. Treatment of asymptomatic vaginal candidiasis in pregnancy to prevent preterm birth: an open label pilot randomized controlled trial. BMC Pregnancy Childbirth. 2011;11:1–8.
Mendling W, Shazly M, Zhang L. Clotrimazole for vulvovaginal candidosis: more than 45 years of clinical experience. Pharmaceuticals. 2020;13:274.
Article PubMed PubMed Central Google Scholar
Guerin V, Delance V, Papalexious P, Duchene P, Houin G, Thomas J. Systemic absorption of C-radiolabelled sertaconazole administered in 300 MG prolonged-liberation vaginal suppository form to four healthy post-menopausal women. Journal de Mycologie Medicale. 1996;6:63–7.
Croxtall J, Plosker G. Sertaconazole: a review of its use in the management of superficial mycoses in dermatology and gynaecology. Drugs. 2009;69:339–59.
Lutsevich K, Reshetko O, Rogozhina I, Lutsevich N. Clinical efficacy of Sertaconazole (Zalain) in the local treatment of vulvovaginal candidiasis during pregnancy. Russian Bull Obstetrician-Gynaecologist. 2008;3:77–80.
Dellenbach P, Thomas J, Guerin V, Ochsenbein E, Contet-Audonneau N. Topical treatment of vaginal candidosis with sertaconazole and econazole sustained-release suppositories. Int J Gynaecol Obstet. 2000;71(Suppl 1):S47-52.
Willems H, Ahmed S, Liu J, Xu Z, Peters B. Vulvovaginal candidiasis: A current understanding and burning questions. J Fungi. 2020;6:27.
Lietz A, Eckel F, Kiss H, Noe-Letschnig M, Farr A. Quality of life in women with chronic recurrent vulvovaginal candidosis: A sub-analysis of the prospective multicentre phase IIb/III Prof-001 study. Mycoses. 2023;66:767–73.
Aguin TJ, Sobel JD. Vulvovaginal candidiasis in pregnancy. Curr Infect Dis Rep. 2015;17:462.
Hosiriphon K, Chayachinda C, Keawpoonsub K, Taibowornpitak K, Tuangrattanasirikun D. A survey of daily genital care practices among reproductive-aged female personnel at Siriraj Hospital. Siriraj Medical Journal. 2023;75:259–65.
Ergorova A, Bazina M, Savitskaya E. The experience of treatment of Candida vulvovaginitis in pregnant women with ‘zalain.’ Russian Bull Obstetrician-Gynaecologist. 2010;4:35–8.
Araujo M, Hurault-Delarue C, Sommet A, Damase-Michel C, Lacroix I. Topical sertaconazole during pregnancy and risk of adverse pregnancy outcome and major congenital anomalies: comparative study in the EFEMERIS database. Mycoses. 2022;65:481–9.
Roberts C, Algert C, Rickard K, Morris J. Treatment of vaginal candidiasis for the prevention of preterm birth: a systematic review and meta-analysis. Syst Rev. 2015;4:31.
Carrillo-Muñoz A, Tur-Tur C, Giusiano G, Marcos-Arias C, Eraso E, Jauregizar N, et al. Sertaconazole: an antifungal agent for the topical treatment of superficial candidiasis. Expert Rev Anti Infect Ther. 2013;11:347–58.
Rekhawasin T, Chayachinda C, Thamkhantho M, Thuwasee T, Munn A. Perinatal Outcomes of Pregnancy with Anogenital Warts at the Time of Delivery. J Med Assoc Thai. 2020;103:270–5.
Mohanty T, Doke P, Khuroo S. Effect of bacterial vaginosis on preterm birth: a meta-analysis. Arch Gynecol Obstet. 2023;308:1247–55.
Gigi R, Buitrago-Garcia D, Taghavi K, Dunaiski C, van de Wijgert J, Peters R, et al. Vulvovaginal yeast infections during pregnancy and perinatal outcomes: systematic review and meta-analysis. BMC Womens Health. 2023;23:116.
Download references
Acknowledgements
We thank the Faculty of Medicine Siriraj Hospital, Mahidol University, for their intellectual support and services. Special thanks to Pacific Health Care, Thailand, and Bayer, Thailand, for supplying the medications used in this study.
Open access funding provided by Mahidol University Both study drugs were provided free of charge by Bayer, Thailand, and Pacific Health Care, Thailand. Pacific Health Care, Thailand, also financially supported the participants’ transportation fees and the project’s administrative expenses (total amount = 150 000 Baht).
Author information
Authors and affiliations.
Unit of Infectious Diseases, Department of Obstetrics and Gynaecology, Faculty of Medicine Siriraj Hospital, Mahidol University, 2 Wanglang Road, Bangkok Noi, Bangkok, 10700, Thailand
Chenchit Chayachinda & Manopchai Thamkhantho
Division of Materno-Fetal Medicine, Department of Obstetrics and Gynaecology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Thanapa Rekhawasin
Department of Nursing, Department of Obstetrics and Gynaecology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Chanakarn Klerdklinhom
You can also search for this author in PubMed Google Scholar
Contributions
All the authors were involved in the study’s conception and design. CC and CK handled the material preparation and data collection. CC conducted the statistical analyses. CC, MT, and TR drafted the manuscript. All authors reviewed and approved the final version.
Not applicable.
Corresponding author
Correspondence to Chenchit Chayachinda .
Ethics declarations
Ethics approval and consent to participate.
This study adhered to the Declaration of Helsinki principles. Approval was granted by the Siriraj Institutional Review Board (Si-32/2019 on February 11, 2019) , and the study was registered with the Thai Clinical Trials Registry (TCTR20190308004 on March 8, 2019). Eligible women provided written informed consent before participation.
Consent for publication
Competing interests.
Bayer, Thailand, and Pacific Health Care, Thailand, donated the study medications and supported some project expenses, including participants’ transportation (totaling 150,000 Baht). C.C. and M.T. received honoraria from these companies for speaking at academic events. However, the companies had no role in the data analysis or manuscript preparation. T.R. and C.K. report no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Chayachinda, C., Thamkhantho, M., Rekhawasin, T. et al. Sertaconazole 300 mg versus clotrimazole 500 mg vaginal suppository for treating pregnant women with acute vaginal candidiasis: a double-blinded, randomized trial. BMC Pregnancy Childbirth 24 , 235 (2024). https://doi.org/10.1186/s12884-024-06440-z
Download citation
Received : 07 July 2023
Accepted : 24 March 2024
Published : 04 April 2024
DOI : https://doi.org/10.1186/s12884-024-06440-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Clotrimazole; Sertaconazole
- Single dose
- Pregnancy outcomes
- Pregnant women

BMC Pregnancy and Childbirth
ISSN: 1471-2393
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 08 April 2024
Clinical outcomes of liver transplantation in human immunodeficiency virus/hepatitis B virus coinfected patients in China
- Jianxin Tang 1 na1 ,
- Ruihui Weng 2 na1 ,
- Taishi Fang 1 ,
- Kangjun Zhang 1 ,
- Xin Jin 1 ,
- Linjie Xie 1 &
- Dong Zhao 1
BMC Infectious Diseases volume 24 , Article number: 383 ( 2024 ) Cite this article
Metrics details
Highly active antiretroviral therapy (HAART) has been able to improve the immune system function and survival of human immunodeficiency virus (HIV) patients. However, Patients coinfected with HIV and hepatitis B virus (HBV) are more likely to develop end-stage liver disease (ESLD) than those infected with HBV alone. Consequently, liver transplantation is often required for these patients. This study evaluates the outcomes of orthotopic liver transplantation (OLT) of HIV-HBV coinfected patients in China.
We conducted a retrospective analysis on all HIV-HBV coinfected patients that underwent OLT from April 1, 2019 to December 31, 2021 and their outcomes were compared to all HBV monoinfected patients undergoing OLT during the same period. Patient outcomes were determined, including cumulative survival, viral load, CD4 T-cell count and postoperative complications.
The median follow-up of HIV recipients was 36 months after OLT (interquartile range 12–39 months). Almost all patients had stable CD4 T-cell count (> 200 copies/ul), undetectable HBV DNA levels, and undetectable HIV RNA load during follow-up. The 1-, 2-, and 3-year posttransplant survival rates were 85.7% for the HIV group (unchanged from 1 to 3 years) versus 82.2%, 81.2%, and 78.8% for the non-HIV group. Cumulative survival among HIV-HBV coinfected recipients was not significantly different from the HBV monoinfected recipients (log-rank test P = 0.692). The percentage of deaths attributed to infection was comparable between the HIV and non-HIV groups (14.3% vs. 9.32%, P = 0.665). Post OLT, there was no significant difference in acute rejection, cytomegalovirus infection, bacteremia, pulmonary infection, acute kidney injury, de novo tumor and vascular and biliary complications.
Conclusions
Liver transplantation in patients with HIV-HBV coinfection yields excellent outcomes in terms of intermediate- or long-term survival rate and low incidence of postoperative complications in China. These findings suggest that OLT is safe and feasible for HIV-HBV coinfected patients with ESLD.
Trial registration
Chinese Clinical Trial Registry (ChiCTR2300067631), registered 11 January 2023.
Peer Review reports
Highly active antiretroviral therapy (HAART), introduced in 1996, has significantly improved the survival of patients infected with the human immunodeficiency virus (HIV) [ 1 ]. It is well-established that HAART can suppress HIV replication, enhance immune function, and reduce opportunistic infections. With effective antiretroviral therapy, HIV infection has become a chronic disease, and the clinical comorbidities are increasing [ 2 ]. Among HIV-infected patients in China, HBV coinfection rates range between 9.5 and 14.5%, and the prevalence of (hepatitis B virus) HBV surface antigen (HBsAg) is estimated to be 13.7% [ 3 , 4 ]. Liver-related mortality in HIV patients with viral hepatitis has become a major cause of death in many countries. Thus, end-stage liver disease (ESLD) has become the leading cause of death in HIV patients induced by HBV coinfection [ 5 ]. Besides, it is widely thought that HIV coinfection accelerates the course of liver disease and increases mortality. Despite recent advances in treating chronic hepatitis B, liver transplantation (LT) remains the last resort for patients with ESLD [ 6 ].
HIV infection has long been considered an absolute contraindication to liver transplantation due to this patient population’s relatively shorter life expectancy. Although the past decade has witnessed significant research progress, the clinical efficacy of LT has not been established. HIV infection and rejection-resistant immunosuppression after LT expose HIV recipients to serious complications, especially opportunistic infections. However, the advent of HAART has improved the prognosis for HIV-infected patients and encouraged many transplant centers to accept HIV-positive candidates. Several studies on outcomes of HIV-positive patients after LT have demonstrated stable HIV infection, survival, and complication rates comparable to HIV-negative patients [ 7 , 8 , 9 ].
To our knowledge, this is the first retrospective analysis of HIV-infected Chinese patients with HBV-related ESLD who underwent liver transplantation in the HAART era. Importantly, we evaluated the outcomes of all liver transplantations in HIV-positive patients and compared them with HIV-negative ones.
Materials and methods
Study design and participants.
A retrospective, analytical and unicentric study was performed, and the design scheme is shown in Fig. 1 . The study protocol followed the principles of the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of Shenzhen Third People’s Hospital (No. 2022-038-02). The study has been registered in Chinese Clinical Trial Registry (ChiCTR2300067631). All patients signed an informed consent form before liver transplantation.
The design flow chart of the study
We identified all patients ( n = 293) who underwent a total orthotopic liver transplantation (OLT) for ESLD between April 1, 2019 to December 31, 2021. We excluded patients younger than 18 years, and those underwent combined transplantation (liver-kidney) or transplantation for primary liver cancer, alcoholic liver cirrhosis, HCV, autoimmune liver disease, other benign diseases or without known ESLD. Clinical data were collected, including sex, age, etiology of liver disease, HIV viral load, CD4 T cell counts, and model of end-stage liver disease (MELD) score. HIV patients that were placed on the waiting list for OLT had similar characteristics to non-HIV ones. In addition, the criteria for efficacy in HIV patients included a stable CD4 T-cell count and serum HIV RNA levels < 500 copies/mL. Our multidisciplinary team, including infectious disease specialists, hepatologists and transplant surgeons, was in charge of selecting patients on the waiting list.
Liver transplantation and immunosuppressive therapy
Liver grafts were obtained from cadaveric donors. All donors were HIV and HBV/HCV-negative. The surgical technique of LT was a modified piggyback technique with triangulation of the hepatic veins. The liver-transplantation procedure and immunosuppressive regimen have been described in our previous published articles [ 10 ]. Besides, 2 patients with ABO-incompatible liver transplantation received additional induction therapy, including plasmapheresis twice, and rituximab 375 mg/m2 in two divided doses was administered intravenously before surgery. The immunosuppressive regimen used after liver transplantation was documented in all HIV patients.
HAART Therapy
All patients receiving HAART had documented treatment before and after LT. Instead of using a standard antiretroviral regimen, each patient received an individualized treatment regimen pattern based on tolerability, genotypic sensitivity of the HIV, and physician preference. However, treating HBV infection in a coinfected patient with lamivudine or tenofovir alone can result in HIV resistance to these drugs, which may affect anti-HIV treatment options. All changes in HAART treatment after LT were recorded. Subsequently, some post-LT patients with HIV were switched to albuvirtide and dolutegravir, which have low hepatorenal toxicity and are not CYP3A4 enzyme inhibitors, reducing the impact of calcineurin inhibitor-type immunosuppressive drugs [ 11 – 12 ].
Infection prophylaxis
Given the immunodeficiency from HIV and the antirejection medication, infection prophylaxis is critical. Preoperative prophylaxis was based on a regimen of third-generation cephalosporin plus lactamase inhibitor (3 g/day) from induction to POD 14. Caspofungin (50 mg/day), which has a low effect on tacrolimus concentrations, was administered as an antifungal prophylaxis on the first postoperative day until 2 weeks after surgery. Oral co-trimoxazole for the first 3 months to prevent Pneumocystis jirovecii prophylaxis. For cytomegalovirus prophylaxis, ganciclovir 5 mg/kg daily was given for 2 weeks after liver transplantation. When cytomegalovirus antigen level > 10 was positive, ganciclovir 10 mg/kg/day was administered intravenously (IV) until cytomegalovirus antigen negativity. The antibiotic treatment plan was changed according to the postoperative infection etiological detection and drug susceptibility test.
Prophylaxis against hepatitis B virus recurrence
All patients received long-term passive immunization to prevent hepatitis B virus recurrence after LT. The regimen for prevention of HBV recurrence after LT was as follows: intraoperative administration of 4,000 IU of hepatitis B immune globulin (HBIG) intravenously during the anhepatic phase of liver transplantation, followed by 2,000 IU daily for the first 7 postoperative days. Subsequently, the titer of hepatitis B surface antibody (HBsAb) was maintained above 500 IU/ml within the first postoperative month. Immunoprophylaxis was continued subsequently and throughout follow-up, with monthly intramuscular injections of HBIG 400 to 600 IU to keep the titer of HBsAb greater than 100 IU/ml. From 2019, all patients who underwent LT due to HBV infection received dual immunization prophylaxis with HBIG and nucleoside (acid) analogs. Resumption of oral antiretroviral drugs occurred on day 2 after LT.
Monitoring of graft liver
Regular graft liver biopsies were performed in all patients after intraoperative donor liver blood recirculation, 6 months after liver transplantation, and then annually after liver transplantation. If the result of the liver function examination was abnormal, an additional liver biopsy was required. Each liver biopsy tissue was fixed and paraffin-embedded for histological examination. Diagnosis of acute or chronic rejection was based on the Banff classification.
Postoperative follow-up
Postoperative follow-up schedule: Patients were followed 3 times weekly for the first 2 weeks, then once a week for the 1st month, every 2 weeks for the 3rd month, monthly for the 6th month, every 2 months at the end of the 1st year, and every 3 months for the second and third years. The follow-up plan was adjusted according to the patient’s condition. Post-transplant data collection included liver and kidney function tests, blood cell analysis, blood coagulation function, CD4 T cell counts, HIV-RNA, HBV-DNA, HBsAb levels, immunosuppressive doses and all clinically relevant events such as rejection and infectious complications.
Statistical analysis
All statistical analyses were performed using SPSS 24.0 statistical software. Categorical variables were displayed as frequency (%), continuous variables as mean ± SD or median (interquartile range) used for the descriptive statistics, as appropriate. Group comparisons for categorical variables were performed using the χ²-test and for metric variables using the Mann-Whitney U test. Survival curves were drawn using the Kaplan-Meier method and compared with the log-rank test. A two-sided p-value < 0.05 was statistically significant.
General clinical data
The study included 125 patients with HBV cirrhosis decompensation or liver failure who underwent OLT between April 1, 2019 to December 31, 2021. Seven were HIV/HBV coinfected patients (all male) with a median age of 52 years (interquartile range 46–61 years), including 2 patients with ABO-incompatible liver transplantation. 118 patients without HIV infection that underwent OLT during the same period were also included, and the clinical data are summarized in Table 1 . There was no statistically significant difference in charateristic variables (age, sex, indication, HBV DNA load, and MELD score) between the HIV and non-HIV groups.
Patient and graft survival
All HIV patients survived beyond 30 days following LT. The median follow-up of HIV recipients was 36 months after LT (interquartile range 12–39 months). As shown in Fig. 2 A, the actual 1-, 2-, and 3-year survival rates were 85.7%, 85.7%, and 85.7% for the HIV group versus 82.2%, 81.2%, and 78.8% for the non-HIV group, respectively (log-rank test P > 0.05). The graft 1-, 2-, and 3-year survival rates were 85.7%, 85.7%, and 85.7% for the HIV group versus 80.8%, 79.8%, and 77.5% for the non-HIV group, respectively ( P > 0.05, log-rank test) (Fig. 2 B). The observed all-cause mortality was 14.3% ( n = 1/7) in the HIV group compared to 19.5% in the non-HIV group ( n = 23/118). One HIV recipient developed severe septicemia with progressive multiorgan failure and died 2 months after LT. In the non-HIV group, 23 deaths were due to septicemia (34.78%, n = 8), severe pneumonia (13.04%, n = 3), cardiovascular events (8.70%, n = 2), neurological events (13.04%, n = 3), gastrointestinal bleeding (13.04%, n = 3), de novo lung cancer (4.35%, n = 1), graft versus host disease (4.35%, n = 1), early allograft dysfunction (4.35%, n = 1) and disseminated intravascular coagulation (4.35%, n = 1). Of the 23 deaths in the non-HIV group, 17 (73.91%) occurred within 2 months after OLT.
Kaplan-Meier curves illustrating ( A ) patient survival and ( B ) allograft survival in the liver transplant cohort, comparing HIV-HBV coinfected group with HBV monoinfected group. Log rank test P = 0.692 for patient survival and P = 0.636 for allograft survival. HBV, hepatitis B virus; HIV, human immunodeficiency virus
Rejection and immunosuppression
As shown in Tables 1 and 2 of 7 HIV-infected patients (14.29%) experienced acute allograft rejection. Acute rejection was observed in patient 1 at 2 weeks after liver transplantation. The Banff rejection activity index scores were 4 for patient 1, which suggested mild acute rejection. No patient had histological evidence of chronic rejection. A further course of methylprednisolone and an increased dose of tacrolimus and MMF to enhance baseline immunosuppression was required until graft liver function returned to normal. The only HIV-infected patient who died experienced severe pneumonia and infection post-LT. The infection in patient 7, with obvious bone marrow depression and leukopenia, was thought to result from excessive immunosuppression, and the tacrolimus dose was reduced or even stopped. This episode was unrelated to his demise, which occurred 2 months post-LT. The rejection rate was 14.29% in the HIV group and 4.24% in the non-HIV group, which was not statistically significant ( P = 0.227).
All 7 HIV-infected patients received immunosuppression with steroids, tacrolimus, and mycophenolate mofetil. During the follow-up, patients 1, 2 and 3 were switched from tacrolimus to sirolimus due to impaired renal function and patient 6 due to neurologic toxicity. As described above, patient 1 received steroid pulses therapy (80 mg methylprednisolone for 3 days, then tapered) and 200 mg rituximab twice approximately 2 weeks after ABO-incompatible LT due to high anti-A IgG and IgM titers (1:64 and 1:16, respectively) and acute cellular rejection. Liver enzymes decreased and subsequently remained in the normal range.
Patient outcomes following HAART
Of the 7 patients with HIV infection, 3 received more than 12 months of HAART prior to LT. Patients 6 and 7, receiving only 1 month of HAART, developed acute liver failure secondary to HBV and were unaware of HIV infection prior to their hospitalization. All patients receiving HAART had documented treatment before and after LT. Instead of using a standard antiretroviral regimen, each patient received an individualized treatment regimen pattern based on tolerability, genotypic sensitivity of the HIV, and physician preference.
All HIV-infected patients continued to receive HAART treatment on the second day after transplantation. Table 2 shows the antiretroviral and CD4 T-cell count and HIV load for each patient after OLT. Patient 1 had a low CD4 T-cell count (42 cells/ul) with an undetectable HIV load before OLT. However, with HAART, the HIV load was undetectable (< 50 copies/ ml), and the CD4 T-cell count was stable (> 200 cells/ ul) 43 months after OLT. Patients 2, 3 and 4 had stable CD4 T-cell count (> 200 cells/ul) and an undetectable HIV load (< 250 copies/ml) 3 years after OLT. The pretransplant antiretroviral therapy was continued until OLT. The HAART regimen was altered to completely suppress persistent low-level HIV replication, depending on the patient’s condition. Patient 1 was switched from FTC/TDF and DTG to 3TC, TAF and DTG 6 months before OLT. For antiretroviral therapy in patients with a low CD4 T-cell count and an undetectable HIV load, TAF combination therapy continued to be added after liver transplantation. Although HIV load could not be detected after liver transplantation, patient 5 had a low CD4 T-cell count, which remained above 200 cells/ul after switching to B/F/TAF. Patient 7 had an extremely low CD4 T cell count, with an undetectable HIV load after OLT. On the second day, the patient was switched to dolutegravir, albuvirtide, and tenofovir alafenamide. Patient 7 developed severe pneumonia 10 days after OLT. At the time of death, the patient had an HIV viral load of < 500 copies/ml and a CD4 T-cell count of 3 cells/ul. The patient exhibited a progressive course of progression on HAART and low-dose immunosuppressive therapy until he died of sepsis and multiple organ failure 66 days after OLT.
Incidence of infection after OLT
CMV viremia was detected in 5 patients and treated with intravenous ganciclovir. There was no significant difference in the percentage of patients with CMV viremia infection between the HIV group (71.43%, 5 of 7) and the non-HIV group (39.83%, 47 of 118). One HIV recipient developed severe septicemia and severe pneumonia 10 days after OLT. Pathogenic culture and next-generation sequencing of alveolar lavage fluid and blood samples yielded carbapenem-resistant Pseudomonas aeruginosa and Aspergillus. According to drug sensitivity results, voriconazole, amphotericin B liposome, ceftazidime and amikacin were used for combined treatment. Unfortunately, the patient died of sepsis with progressive multiorgan failure 2 months after transplantation. The observed percentage of HIV-infected patients who died of infection was 14.3% ( n = 1/7) versus only 9.32% ( n = 11/118) in the non-HIV group. The mortality rate due to infectious causes was comparable between the two groups ( P = 0.665).
Recurrence of viral hepatitis
Four HIV-infected patients had detectable HBV viral loads at the time of transplant. All four patients that underwent OLT for HBV-related liver disease had undetectable HBV DNA by polymerase chain reaction after surgery. The HBV-DNA levels were less than 100 copies/ml in serum samples from all 7 patients after liver transplantation and remained undetectable during the follow-up period. HBsAg became undetectable within 2 days of liver transplantation. All patients were HBeAg negative after liver transplantation to the end of follow-up. Details of HBV prophylaxis with hepatitis B immunoglobulin in combination with nucleoside or nucleotide analogs are shown in Table 3 .
In the HAART era, liver transplantation for HIV-infected patients is considered a reasonable choice. However, in the past, most transplant centers refused to accept HIV- infected patients for liver transplantation due to organ shortage and insufficient data. Liver transplantation has been performed in HIV-infected patients in several transplant centers in recent years [ 13 ]. Consistent with the literature, we substantiated that liver transplantation is feasible in this patient population; indeed, postoperative HAART therapy can suppress the viral load, stabilize CD4 T-cell count, and lead to no significant increase in opportunistic infections [ 14 , 15 , 16 ]. Few reports have involved liver transplantation outcomes in HIV/HBV coinfected patients. About 74 million people in China are carriers of the hepatitis B virus, which represents a serious public health issue [ 17 ]. Herein, we summarize the results of liver transplantation in HIV-positive patients with HBV from China.
The overall mortality rate for liver transplantation in HIV/HBV coinfected patients at our transplant center was 14.3% (1/7), lower than the mortality rate reported by a Spanish transplant center (38%) and in HIV-infected patients transplanted in Germany (41%) [ 18 , 19 ]. Our study found 85.7% survival of both patient and graft after a mean follow-up of 36 months (with a maximum follow-up of 43 months), demonstrating the applicability of liver transplantation in this subgroup of patients. Compared with previous studies, the lower mortality rate in HIV/HBV coinfected patients is closely related to the development of efficient antiretroviral therapy, which has low drug resistance, high efficacy, and minimal interaction with commonly used immunosuppressive drugs. In our study, HIV patients were treated with INSTIs after OLT, especially second-generation INSTIs. In addition, the low mortality rate is also influenced by factors such as improvements in liver transplant surgery techniques, perioperative management, and antibiotic upgrades. HIV monoinfection or opportunistic infection does not appear to be a significant risk factor for patient survival after transplantation [ 20 ]. Current evidence suggests bacterial infection and sepsis are the leading causes of death after liver transplantation, especially in the early post-transplantation period [ 21 , 22 ]. Patient 7, who had an HIV load < 50 copies/ml but a low CD4 T-cell count before LT, died of multiple organ failure from bacterial infection and sepsis 66 days after LT. In contrast, the CD4 T-cell count of patient 1 was less than 50 cells/ ul before LT, but the HIV disease was stable, and the patient remained alive after LT. He had an undetectable HIV load (< 50 copies/ ml) and a stable CD4 T-cell count (> 200 cells/ ul), with no opportunistic infection 43 months after LT. This indicates that a T-cell count of fewer than 100 cells/ ul is not an absolute contraindication of liver transplantation without definite infection. These discrepancies may reflect differences in postoperative infection prophylaxis and immunosuppressive management and underline the importance of aggressive infection-prevention therapy and avoidance of excessive immunosuppression early after transplantation in HIV/HBV coinfected patients.
HCV coinfection remains a key factor in the mortality of HIV-positive patients after liver transplantation in European and American HIV-positive patients [ 23 , 24 ]. HCV recurrence in HIV patients is more aggressive, and liver fibrosis is more rapid due to HAART toxicity [ 25 ]. An increasing body of evidence suggests that treatment for HCV recurrence positively affects graft survival and mortality [ 26 , 27 ]. Unlike HCV coinfection, HIV-infected patients with HBV appear to have better outcomes after OLT when HBV reinfection prophylaxis is properly provided [ 28 ]. Notably, there was no significant difference in survival between HIV/HBV coinfected patients and HBV monoinfected patients after liver transplantation. Anadol et al. showed that the 5-year survival rate of HIV/HBV-coinfected patients was 80%, and none of the HIV/HBV-coinfected patients developed clinically relevant HBV-related end-stage liver disease after liver transplantation [ 19 ]. The best option for preventing recurrent HBV infection in HIV/HBV coinfected patients appears to be combining pretransplantation and posttransplantation antiviral therapy with HBIG administration [ 29 ]. In our study, all patients received prophylaxis in combination with HBIG and antiviral therapy. Consistent with findings reported by Tateo et al. [ 30 ], all HBV/HIV coinfected patients were HBsAg-negative and HBV-DNA below 100 IU/ml. Interestingly, despite the successful prevention of recurrent hepatitis B, low levels of HBV-DNA were detected in approximately 50% of HBV/HIV coinfected patients treated with this combination regimen, and an 85% patient survival rate was achieved at 4 years of follow-up [ 31 ].
In the early posttransplant period, graft function and rejection prevention are major determinants of the outcomes of HBV/HIV coinfected patients instead of HIV infection. In addition, although HIV patients are considered immunosuppressed, there is an additional issue in managing these patients that may lead to higher rejection rates [ 32 ]. In the present study, 1 case of mild acute rejection was identified in HIV patients (14.3%) compared to 4.24% in non-HIV patients with no significant difference. All rejection episodes were easily treated according to routine protocols as previously described. Patient 7 was transferred to the intensive care unit due to severe pulmonary infection 10 days after the operation, and the concentration of tacrolimus and the dose of MMF were reduced accordingly. Two weeks after the operation, the liver function gradually deteriorated, and a liver biopsy showed acute cholestatic hepatitis with inflammation and necrosis equivalent to G3, moderate intrahepatic cholestasis, and no indication of acute rejection. Due to sepsis and multiple organ dysfunction, the patient was treated with low-dose methylprednisolone antirejection therapy. This episode was unrelated to his demise, which occurred 2 months post-LT. Coffin et al. showed that the acute rejection rates in HIV/HBV coinfected patients and monoinfected HBV patients were comparable (22.7% ( n = 5/22) vs. 10% ( n = 2/20), p > 0.05) [ 31 ].
Postoperative infection of HIV patients is also a key concern in this patient population. Although the prevalence of CMV viremia in patients with advanced HIV infection remains high, good immune recovery by antiretroviral treatment is sufficient to suppress CMV viral levels without increasing the risk of CMV end-organ disease [ 33 ]. Based on the medical literature, there has been no increase in the incidence rate of transplant or HIV-related opportunistic infections. The incidence of CMV and other opportunistic infections was not different between the HIV-positive and HIV-negative groups. More importantly, HIV did not appear to progress after liver transplantation in the post-HAART era. CD4 T-cell counts and HIV viral loads were stable in most patients as long as HAART could be administered [ 34 ]. In our study, there was no significant difference in the incidence of CMV viremia, bacteremia, and pulmonary infection between the HIV group and the non-HIV group. The comparable mortality rates attributed to infection between the HIV and non-HIV groups suggest that the risk of opportunistic infection after liver transplantation is not increased under HAART treatment.
The optimal management of the HAART regimen after OLT has not yet been determined. Pharmacological interactions between calcineurin inhibitors and HAART regimens containing protease inhibitors have been documented [ 35 , 36 ]. Potential drug interactions must be considered when considering a specific antiretroviral regimen. Protease inhibitors (PI) are a part of most HAART regimens [ 37 ]. It is well known that PIs inhibit CYP3A, a component of the cytochrome P450, which results in markedly prolonged half-lives of the calcineurin inhibitors and sirolimus [ 38 ]. Accordingly, we must consider the optimal timing of HAART initiation, drug interactions between HAART and immunosuppressive regimen, and the control of disease recurrence after transplantation. All 7 Chinese patients with HIV/HBV coinfection began to receive HAART treatment early, within 2 days after OLT. The choice of drugs for immunosuppression and antiretroviral therapy is another key factor. Potential hepatotoxicity must be considered in the selection of HAART regimens to reduce liver-related mortality, such as stavudine (D4T) [ 39 ], azidothymidine (AZT) [ 40 ] or didanosine (ddI) [ 41 ]. All Chinese patients did not receive the above HAART drugs. In addition, HAART drugs with minimal interactions with other drugs must be considered. Subsequently, some post-LT patients with HIV were switched to albuvirtide and dolutegravir, which have low hepatorenal toxicity and are not CYP450 enzyme inhibitors, reducing the impact of calcineurin inhibitor-type immunosuppressive drugs. In this study, the HIV RNA load could not be detected during follow-up after LT. However, the adverse effects of HAART combined with immunosuppressive drugs on graft survival, the right time and dose of HAART after LT and the frequency of other secondary complications need to be further evaluated.
In conclusion, our data suggest that liver transplantation for patients with HIV/HBV coinfection represents the only way to survive from decompensated cirrhosis or liver failure. The outcome of these recipients is highly dependent on the patient’s state at the time of transplantation. Patient 1 in this study had a CD4 T-cell count of less than 100 cells/ul, which is not an absolute contraindication of liver transplantation without an established infection. The acceptable survival rate and control of HIV/HBV replication corroborate that the strategy of providing liver transplantation for HIV/HBV-coinfected patients with acute liver failure and end-stage liver disease is reasonable in China. Additional studies must be performed to determine medium- or long-term survival and improve post-transplant management to balance complex interaction factors, such as HAART and immunosuppressive drug selection, optimal treatment timing and dose adjustment.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available as they contain information that could compromise participant privacy and consent but are available from the corresponding author on reasonable request.
Abbreviations
Acute-on-chronic liver failure
Bictegravir/emtricitabine/tenofovir alafenamide
Cytomegalovirus
Decompensated liver cirrhosis
- End-stage liver disease
Emtricitabine
Highly active antiretroviral therapy
- Hepatitis B virus
Hepatitis B e-antigen
Hepatitis B immune globulin
Hepatitis B surface antigen
- Human immunodeficiency virus
Model for end stage liver disease
Mycophenolate mofetil
Orthotopic liver transplantation
Postoperative day
Tenofovir alafenamide
Tenofovir disoproxil fumarate
Survival. of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017; 4: e349-e356.
Rowell-Cunsolo TL, Hu G, Bellerose M, Liu J. Trends in Comorbidities among Human Immunodeficiency Virus-Infected Hospital Admissions in New York City from 2006–2016. Clin Infect Dis. 2021;73:e1957–63.
Article PubMed Google Scholar
Xie J, Han Y, Qiu Z, Li Y, Li Y, Song X, et al. Prevalence of hepatitis B and C viruses in HIV-positive patients in China: a cross-sectional study. J Int AIDS Soc. 2016;19:20659.
Article PubMed PubMed Central Google Scholar
Yu S, Yu C, Li J, Liu S, Wang H, Deng M. Hepatitis B and Hepatitis C prevalence among people living with HIV/AIDS in China: a systematic review and Meta-analysis. Virol J. 2020;17:127.
Yang R, Gui X, Xiong Y, Gao SC, Yan Y. Impact of hepatitis B virus infection on HIV response to antiretroviral therapy in a Chinese antiretroviral therapy center. Int J Infect Dis. 2014;28:29–34.
Lacombe K, Rockstroh J. HIV and viral Hepatitis coinfections: advances and challenges. Gut. 2012;61:i47–58.
Article CAS PubMed Google Scholar
Agüero F, Forner A, Manzardo C, Valdivieso A, Blanes M, Barcena R. Human immunodeficiency virus infection does not worsen prognosis of liver transplantation for hepatocellular carcinoma. Hepatology. 2016;63:488–98.
Jacob JS, Shaikh A, Goli K, Rich NE, Benhammou JN, Ahmed A, et al. Improved survival after liver transplantation for patients with HIV and HIV/HCV Coinfection in the INSTI and DAA eras. Clin Infect Dis. 2023;76:592–9.
Peters MG, Kottilil S, Terrault N, Amara D, Husson J, Huprikar S. Retrospective-prospective study of safety and efficacy of sofosbuvir-based direct-acting antivirals in HIV/HCV-coinfected participants with decompensated liver disease pre- or post-liver transplant. Am J Transpl. 2021;21:1780–8.
Article CAS Google Scholar
Tang J, Zhang K, Fang T, Weng R, Liang Z, Yan X, et al. Outcomes of ABO-incompatible liver transplantation in end-stage liver disease patients co-infected with hepatitis B and human immunodeficiency virus. World J Gastroenterol. 2023;29:1745–56.
Yang W, Xiao Q, Wang D, Yao C, Yang J. Evaluation of pharmacokinetic interactions between long-acting HIV-1 fusion inhibitor albuvirtide and lopinavir/ritonavir, in HIV-infected subjects, combined with clinical study and simulation results. Xenobiotica. 2017;47:133–43.
Miller MM, Liedtke MD, Lockhart SM, Rathbun RC. The role of dolutegravir in the management of HIV infection. Infect Drug Resist. 2015;8:19–29.
Eman P, Chacon E, Gupta M, Berger JC, Shah MB, El Haddad HE, et al. Long term outcomes of patients transplanted for hepatocellular carcinoma with human immunodeficiency virus infection. HPB (Oxford). 2019;21:1009–16.
Baccarani U, Righi E, Adani GL, Lorenzin D, Pasqualucci A, Bassetti M, et al. Pros and cons of liver transplantation in human immunodeficiency virus infected recipients. World J Gastroenterol. 2014;20:5353–62.
Article CAS PubMed PubMed Central Google Scholar
Schreibman I, Gaynor JJ, Jayaweera D, Pyrsopoulos N, Weppler D, Tzakis A, et al. Outcomes after orthotopic liver transplantation in 15 HIV-infected patients. Transplantation. 2007;84:697–705.
Vernadakis S, Sotiropoulos GC, Brokalaki EI, Esser S, Kaiser GM, Cicinnati VR, et al. Long-term outcomes of liver transplant patients with human immunodeficiency virus infection and end-stage-liver-disease: single center experience. Eur J Med Res. 2011;16:342–8.
Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–55.
Anadol E, Beckebaum S, Radecke K, Paul A, Zoufaly A, Bickel M, et al. Orthotopic liver transplantation in human-immunodeficiency-virus-positive patients in Germany. AIDS Res Treat. 2012;2012:197501.
CAS PubMed PubMed Central Google Scholar
Testillano M, Fernandez JR, Suarez MJ, Gastaca M, Bustamante J, Pijoan JI, et al. Survival and hepatitis C virus recurrence after liver transplantation in HIV- and hepatitis C virus-coinfected patients: experience in a single center. Transpl Proc. 2009;41:1041–3.
Teicher E, Boufassa F, Vittecoq D, Antonini TM, Tateo MG, Coilly A, et al. Infectious complications after liver transplantation in human immunodeficiency virus-infected recipients. Transpl Infect Dis. 2015;17:662–70.
Miro JM, Agüero F, Duclos-Vallée JC, Mueller NJ, Grossi P, Moreno A. Infections in solid organ transplant HIV-infected patients. Clin Microbiol Infect. 2014;20:119–30.
Moreno A, Cervera C, Fortún J, Blanes M, Montejo E, Abradelo M. Epidemiology and outcome of infections in human immunodeficiency virus/hepatitis C virus-coinfected liver transplant recipients: a FIPSE/GESIDA prospective cohort study. Liver Transpl. 2012;18:70–81.
Campos-Varela I, Dodge JL, Berenguer M, Adam R, Samuel D, Di Benedetto F, et al. Temporal trends and outcomes in Liver Transplantation for recipients with HIV infection in Europe and United States. Transplantation. 2020;104:2078–86.
Dalla E, Bulfoni M, Cesselli D, Pravisani R, Hidaka M, Eguchi S, et al. Reinfection of transplanted livers in HCV- and HCV/HIV-Infected patients is characterized by a different MicroRNA expression Profile. Cells. 2022;11:690.
Gobran ST, Ancuta P, Shoukry NH. A tale of two viruses: immunological insights into HCV/HIV coinfection. Front Immunol. 2021;12:726419.
Agüero F, Forner A, Valdivieso A, Blanes M, Barcena R, Manzardo C. Human immunodeficiency virus-infected liver transplant recipients with incidental hepatocellular carcinoma: a prospective multicenter nationwide cohort study. Liver Transpl. 2017;23:645–51.
Agüero F, Rimola A, Stock P, Grossi P, Rockstroh JK, Agarwal K. Liver retransplantation in patients with HIV-1 infection: an International Multicenter Cohort Study. Am J Transpl. 2016;16:679–87.
Article Google Scholar
Congly SE, Doucette KE, Coffin CS. Outcomes and management of viral hepatitis and human immunodeficiency virus co-infection in liver transplantation. World J Gastroenterol. 2014;20:414–24.
Roche B, Roque-Afonso AM, Nevens F, Samuel D. Rational basis for optimizing short and long-term Hepatitis B Virus Prophylaxis Post Liver transplantation: role of Hepatitis B Immune Globulin. Transplantation. 2015;99:1321–34.
Tateo M, Roque-Afonso AM, Antonini TM, Medja F, Lombes A, Jardel C. Long-term follow-up of liver transplanted HIV/hepatitis B virus coinfected patients: perfect control of hepatitis B virus replication and absence of mitochondrial toxicity. Aids. 2009;23:1069–76.
Coffin CS, Stock PG, Dove LM, Berg CL, Nissen NN, Curry MP, et al. Virologic and clinical outcomes of hepatitis B virus infection in HIV-HBV coinfected transplant recipients. Am J Transpl. 2010;10:1268–75.
Righi E, Ivaldi F, La Rosa A, Carnelutti A, Londero A, Bassetti M. Immunological profiles of HIV-positive recipients of liver transplant. Transpl Immunol. 2019;57:101208.
Albasanz-Puig A, Suanzes P, Esperalba J, Fernández C, Sellarès-Nadal J, Torrella A. Low frequency of cytomegalovirus (CMV) disease despite high prevalence of CMV viraemia in patients with advanced HIV infection: a clinical and immunological 48-week follow-up study. HIV Med. 2021;22:682–9.
Neff GW, Sherman KE, Eghtesad B, Fung J. Review article: current status of liver transplantation in HIV-infected patients. Aliment Pharmacol Ther. 2004;20:993–1000.
Elliot E, Chirwa M, Boffito M. How recent findings on the pharmacokinetics and pharmacodynamics of integrase inhibitors can inform clinical use. Curr Opin Infect Dis. 2017;30:58–73.
Sparkes T, Manitpisitkul W, Masters B, Bartlett ST, Davis C, Husson J, et al. Impact of antiretroviral regimen on renal transplant outcomes in HIV-infected recipients. Transpl Infect Dis. 2018;20:e12992.
Voshavar C. Protease inhibitors for the treatment of HIV/AIDS: recent advances and Future challenges. Curr Top Med Chem. 2019;19:1571–98.
Podany AT, Scarsi KK, Fletcher CV. Comparative clinical pharmacokinetics and pharmacodynamics of HIV-1 integrase strand transfer inhibitors. Clin Pharmacokinet. 2017;56:25–40.
Su S, Fairley CK, Sasadeusz J, He J, Wei X, Zeng H, et al. HBV, HCV, and HBV/HCV co-infection among HIV-positive patients in Hunan province, China: regimen selection, hepatotoxicity, and antiretroviral therapy outcome. J Med Virol. 2018;90:518–25.
Ghare SS, Donde H, Chen WY, Barker DF, Gobejishvilli L, McClain CJ, et al. Acrolein enhances epigenetic modifications, FasL expression and hepatocyte toxicity induced by anti-HIV drug Zidovudine. Toxicol Vitro. 2016;35:66–76.
Ryom L, Lundgren JD, De Wit S, Kovari H, Reiss P, Law M, et al. Use of antiretroviral therapy and risk of end-stage liver disease and hepatocellular carcinoma in HIV-positive persons. Aids. 2016;30:1731–43.
Download references
Acknowledgements
Not applicable.
This study was supported by Shenzhen Third People’s Hospital Scientific Research Project (G2021008, G2022008), Shenzhen Key Medical Discipline Construction Fund (SZXK079), Shenzhen Science and Technology Research and Development Fund (JCYJ20210324131809027, JCYJ20220530163011026).
Author information
Jianxin Tang and Ruihui Weng contributed equally to this work.
Authors and Affiliations
Department of Liver Surgery & Organ Transplantation Center, Shenzhen Third People’s Hospital, The Second Affiliated Hospital of Southern University of Science and Technology, National Clinical Research Center for Infectious Disease, Longgang District, Bulan Road 29#, 518000, Shenzhen, China
Jianxin Tang, Taishi Fang, Kangjun Zhang, Xu Yan, Xin Jin, Linjie Xie & Dong Zhao
Department of Neurology, Shenzhen Third People’s Hospital, The Second Affiliated Hospital of Southern, University of Science and Technology, 518000, Shenzhen, China
Ruihui Weng
You can also search for this author in PubMed Google Scholar
Contributions
Conception and design: Dong Zhao, Jianxin Tang and Ruihui Weng; Administrative support: Dong Zhao; Provision of study materials or patients: Jianxin Tang, Kangjun Zhang and Taishi Fang; Collection and assembly of data: Ruihui Weng, Xu Yan and Xin Jin; Data analysis and interpretation: Ruihui Weng, Linjie Xie; Manuscript writing: Dong Zhao, Jianxin Tang and Ruihui Weng; Final approval of manuscript: All authors. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Correspondence to Dong Zhao .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocol followed the principles of the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of Shenzhen Third People’s Hospital (No. 2022-038-02). The study has been registered in Chinese Clinical Trial Registry (ChiCTR2300067631). All patients signed an informed consent form before liver transplantation.
Consent for publication
Additional information, publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Tang, J., Weng, R., Fang, T. et al. Clinical outcomes of liver transplantation in human immunodeficiency virus/hepatitis B virus coinfected patients in China. BMC Infect Dis 24 , 383 (2024). https://doi.org/10.1186/s12879-024-09284-2
Download citation
Received : 27 October 2023
Accepted : 03 April 2024
Published : 08 April 2024
DOI : https://doi.org/10.1186/s12879-024-09284-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Liver transplantation
BMC Infectious Diseases
ISSN: 1471-2334
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Study protocol
- Open access
- Published: 08 April 2024
Clinical effects of atorvastatin combined with conbercept in the treatment of patients with macular edema secondary to retinal vein occlusion and carotid plaque: study protocol for a prospective randomized controlled trial
- Bangtao Yao ORCID: orcid.org/0000-0003-4036-7440 1 na1 ,
- Bei Wang 1 na1 ,
- Jun Yang 2 ,
- Yan Geng 3 ,
- Yuhui Liu 1 ,
- Gang Liu 1 &
- Xiuying Wang 4
Trials volume 25 , Article number: 244 ( 2024 ) Cite this article
Metrics details
Introduction
Intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) drugs have been widely used in patients with macular edema (ME) secondary to retinal vein occlusion (RVO); however, recurrence is a major concern. This study aims to observe the clinical effects of atorvastatin and intravitreal therapy in the treatment of patients with branch or central RVO-ME and coexistent carotid plaques (CP).
Methods and analysis
A prospective randomized controlled clinical trial will be conducted. Sixty-four patients diagnosed with branch or central RVO-ME and coexistent CP will be enrolled and randomly allocated in a 1:1 ratio to the control and experimental groups. The control group will be treated with intravitreal conbercept monthly for 3 months, followed by monthly evaluation and injection of pro re nata (PRN) for 12 months, while the experimental group will be treated with oral atorvastatin 20 mg daily combined with the control group treatment. If a drop of best-corrected visual acuity (BCVA) is more than five Early Treatment Diabetic Retinopathy Study (ETDRS) letters (one line) or an increment in central subfield thickness (CSFT) of 100 μm (or a 10% increment from the previous visit), intravitreal re-treatment will be performed. Outcome measurements include CSFT, BCVA, number of injections, and incidence of adverse events during the 12-month follow-up period. Differences between groups will be evaluated using Student’s t -test, and comparisons between groups will be evaluated using repeated-measures analysis of variance.
Ethics and dissemination
The study has been approved by the Institutional Review Board of Nanjing Lishui People’s Hospital, Nanjing, China (approval number 2023KY0418-12, dated 18 April 2023), and has been registered on chictr.org.cn. Written informed consent will be collected from each patient and the results of this trial will be submitted to a peer-reviewed journal.
Trial registration
Chinese Clinical Trial Registry ChiCTR2300071359. Registered on 12 May 2023.
Peer Review reports
Retinal vein occlusion (RVO) is the second most common retinal vascular disease and is characterized by a sudden painless visual reduction [ 1 , 2 ]. However, the exact mechanisms underlying RVO remain unclear. Recent evidence has demonstrated that RVO is closely associated with carotid plaques (CP), an important risk factor for stroke [ 3 ]. Evidence has confirmed that CP is observed in 54.3% and 76.7% of patients with RVO and branch RVO, respectively [ 3 , 4 ]. Retinal arteriosclerosis and thrombosis caused by CP can result in retinal venous reflux disorders and secondary RVO [ 3 , 4 , 5 ]. RVO can be a predictor of CP and should be evaluated using carotid Doppler ultrasound [ 1 ]. Carotid Doppler ultrasound is non-invasive, useful for screening and diagnosing CP, and can accurately evaluate carotid intima-media thickness [ 3 ]. However, it is not commonly used for the clinical treatment of RVO.
Macular edema (ME) is the most severe and frequent complication of RVO [ 2 ]. Intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) drugs (e.g., ranibizumab, aflibercept, and conbercept) have been widely used as first-line treatment for patients with RVO-ME [ 2 , 6 , 7 , 8 ]. However, they have several limitations. First, the clinical effect of anti-VEGF drugs is not long-lasting, and the recurrence of ME indicates repeated injections. Hunt et al. reported that the median number of injections of ranibizumab and aflibercept for branch RVO-ME was seven over 12 months [ 2 ]. Sun et al. used conbercept to treat RVO-ME and the mean number of injections was 7.14 ± 1.90 in branch RVO and 7.59 ± 1.39 in central RVO, over 9 months [ 8 ]. Second, frequent injections increase surgical risks, including endophthalmitis and secondary glaucoma [ 6 ]. Third, anti-VEGF drugs are expensive, and reinjections lead to an economic burden for patients. Therefore, recurrence during follow-up is a major concern for ophthalmologists [ 7 ]. Risk factors such as retinal structures (e.g., elevated central retinal thickness and disorganization of retinal inner layers) have been described; however, to the best of our knowledge, investigations of systemic factors such as CP in RVO-ME have not been discussed in the “EURETINA Guidelines for the Diagnosis and Treatment of Retinal Vein Occlusion” [ 9 ].
Clinical trials have shown that regular statin use plays a significant role in preventing cerebrovascular atherosclerosis and reducing the risk of cerebrovascular accidents [ 10 ]. Atorvastatin, a statin (lipid-lowering drug), has proven to be effective in stabilizing and reversing plaques in patients with carotid atherosclerosis in combination with antiplatelet agents [ 11 , 12 , 13 ]. Furthermore, a multicentre study showed that atorvastatin significantly reduced the risk of cardiovascular and cerebrovascular accidents in patients with CP, and the risks of stroke and coronary artery accidents decreased by 33% and 43%, respectively [ 14 ].
Recently, atorvastatin has been proven useful in reducing retinal VEGF expression and inhibiting inflammation [ 15 , 16 ]. Oral atorvastatin was effective in patients with diabetic ME and age-related macular degeneration [ 17 , 18 ]. Similarly, intravitreal statins can help reduce the development of proliferative vitreoretinopathy [ 19 ]. However, clinical investigations of atorvastatin in the treatment of RVO-ME and CP in the current studies are lacking.
Study objectives
This study aims to observe the clinical effects of atorvastatin and intravitreal therapy in the treatment of patients with branch or central RVO-ME and coexistent CP.
Trial design
In this trial, 64 patients diagnosed with branch or central RVO-ME and coexistent CP will be enrolled and randomly allocated in a 1:1 ratio to the control and experimental groups. The control group will be treated with intravitreal conbercept (Chengdu Kanghong Pharmaceutical; National Drug Approval No. S20130012), using the 3+ pro re nata (PRN) scheme (monthly for 3 months, followed by monthly evaluation and injection PRN for 12 months) and the experimental group will be treated with oral atorvastatin (Beijing Jialin Pharmaceutical Co., Ltd., GYZZH20093819) 20 mg daily combined with the control group treatment.
Eligibility criteria
Patients diagnosed with RVO-ME and CP are based on indirect ophthalmoscopy, fundus fluorescein angiography (FFA), spectral-domain optical coherence tomography (SD-OCT), and carotid Doppler ultrasound.
Inclusion criteria
The inclusion criteria are as follows: (1) patients with a branch or central RVO, aged between 45 and 75 years old; (2) central sub-field thickness (CSFT) > 250 μm, best corrected visual acuity (BCVA) < 0.5; and (3) body mass index < 28 kg/m 2 , carotid stenosis without recanalization surgery.
Exclusion criteria
The exclusion criteria are as follows: (1) patients with severe cataract, macular membrane, optic neuropathy, glaucoma, and uveitis; (2) patients with a branch or central RVO-ME in both eyes; (3) patients with macular edema secondary to age-related macular degeneration, diabetic retinopathy, Coats disease, Eales disease, or high myopia; (4) history of intravitreal injection, retinal surgery, laser photocoagulation, or ocular trauma; (5) history of taking lipid-lowering drugs in the past month; (6) patients with severe cardiovascular, cerebrovascular, liver, and kidney dysfunction; and (7) patients who cannot tolerate surgery or have poor compliance.
Withdrawal criteria
The withdrawal criteria are as follows: (1) the follow-up was lost; (2) informed consent was withdrawn; and (3) decision to withdraw for severe adverse events.
Methodology
A prospective, randomized controlled clinical trial will be conducted at Nanjing Lishui People’s Hospital. Patients diagnosed with branch or central RVO-ME and CP will be enrolled in this study. They will be randomly allocated in a 1:1 ratio to the control and experimental groups. The study design is illustrated in Fig. 1 .
Study design. PRN, pro re nata; RVO, retinal vein occlusion; ME, macular edema; CP, carotid plaque; BCVA, best corrected visual acuity; CSFT, central subfield thickness
Randomization
Patients will be randomly divided into two groups in a 1:1 ratio using SPSS (Statistical Package for Social Sciences, version 26.0, IBM, Armonk, NY, USA). An independent investigator will generate a random sequence and allocate interventions.
Patients and clinicians will be blinded after assignment. The implementation and maintenance of the randomization and masking methods will be completely validated. To avoid evaluation bias, the treatment groups will be hidden from the ophthalmologist who collects the clinical data during each follow-up period. Analysts dealing with the data will also be masked.
Interventions
All patients will undergo preoperative and postoperative investigations. Systemic examinations include routine blood tests, blood biochemistry, infection measurements (HIV, hepatitis B and C, and syphilis), chest computed tomography (CT), electrocardiography, and carotid Doppler ultrasound. Ophthalmic examinations include BCVA, slit-lamp, intraocular pressure, indirect ophthalmoscopy, FFA, and SD-OCT. The timeline of data collection is shown in Table 1 .
Outcome measurements
Monthly follow-ups will be performed after surgery in both groups. The necessary investigations will be conducted at each follow-up visit.
The outcome measurements include CSFT, BCVA, number of injections, and incidence of adverse events.
Primary outcomes
The primary outcome is the average change in CSFT from baseline to 12 months.
Secondary outcomes
Secondary outcomes are BCVA, average number of injections, and incidence of adverse events at each visit during the 12-month follow-up visit.
Safety and combined operation-related adverse events
Possible operation-related complications include elevated intraocular pressure, endophthalmitis, traumatic cataract, vitreous hemorrhage, retinal hemorrhage, retinal detachment, and drug-related adverse events (e.g., liver function damage and gastrointestinal discomfort). In this trial, all the above adverse events and possible causes will be recorded and managed accordingly.
Follow-up plan
Monthly follow-ups and necessary re-examinations after surgery will be conducted. BCVA, slit lamp, intraocular pressure, indirect ophthalmoscopy, and SD-OCT will be performed at each visit. Blood biochemistry and carotid Doppler ultrasonography will be performed every 3 months. Routine blood tests, FFA, and electrocardiograms will be performed every 6 months. Infection measurements (HIV, hepatitis B and C, and syphilis) and chest CT will be performed during the last visit. The number of injections and the incidence of complications will be recorded and evaluated at each follow-up.
Re-injection criteria
If the BCVA drops by more than five Early Treatment Diabetic Retinopathy Study (ETDRS) letters (one line) or an increment in CSFT of 100 μm (or a 10% increment from the previous visit), intravitreal re-treatment will be performed.
Sample size calculation
The sample size is estimated as follows: according to the results from our pre-experiment, the average CSFT in the control and experimental groups was 263.72 ± 30.63 μm and 241.17 ± 18.54 μm, respectively. The type I error rate ( β ) was set at 0.05, with 80% power at 5% significance and 1:1 randomization. Zα = 1.96, Zβ = 0.84, standard deviation ( σ ) in experimental group = 30.63, difference in means ( δ ) = 263.72 − 241.17 = 22.55.
Sample size \(n=\frac{2{\left({z}_a+{z}_{\beta}\right)}^{2\ast }{\sigma}^2}{\delta^2}=2\times {\left(1.96+0.84\right)}^2\times 30.{63}^2/22.{55}^2=29.\)
Thus, 29 patients will be required in each group. Considering a loss ratio of 10%, the total sample size will increase to 32 patients in each group.
Statistical analysis
Data from the patients’ clinical records will be processed using SPSS. For normally distributed data, continuous variables will be expressed as mean ± standard deviation, difference between groups will be evaluated by the Student’s t -test, and comparisons inter-group at each endpoint will be evaluated by repeated measures analysis of variance. The Bonferroni method will be used to adjust the overall significance level for multiplicity. For abnormally distributed data, continuous variables will be expressed as M (Q1, Q3), differences between groups will be evaluated using the Mann–Whitney U test, and comparisons between groups at each endpoint will be evaluated using a generalized linear mixed model. Categorical variables will be expressed as counts (%), and the chi-square test will be performed. The effect of missing data on the trial results will be assessed using sensitivity analyses of the augmented datasets. Dropouts will be included in the analysis by using modern imputation methods for missing data. The BCVA will be converted to the minimum resolution angle in logarithmic form before data analysis. The clinical data of the branch and central RVO will be sub-analyzed. Two-tailed tests of significance will be performed, and P -values < 0.05 will be considered statistically significant.
Provisions for post-trial care
There is no anticipated harm and compensation for trial participation.
The study has been approved by the institutional review board of Nanjing Lishui People’s Hospital, Nanjing, China (approval number 2023KY0418-12, dated 18 April 2023) and registered with the Chinese clinical trial registry ( http://www.chictr.org.cn/ , No. ChiCTR2300071359). Written informed consent will be collected from each patient. They will be informed about the study procedures, possible treatment risks, and their right to withdraw from the trial. The results of this trial will be submitted to a peer-reviewed journal.
Access to the full protocol, participant-level dataset and statistical code
The datasets analyzed in the current study and the statistical code are available from the corresponding author upon reasonable request, as is the full protocol.
Patient public involvement
The patients and the public were not involved in the design, conduct, reporting, or dissemination of our research plans.
This is a prospective, randomized controlled clinical trial investigating the clinical effect of atorvastatin and intravitreal conbercept in the treatment of ME in patients with a branch or central RVO-ME and coexistent CP. We speculate that the treatments in the experimental group may have significantly improved the carotid intima-media thickness and inhibited VEGF and inflammation in the retina. This study may provide an opportunity to reduce the recurrence rate of RVO-ME. Further studies with larger sample sizes and more data are required to better understand the effects of combined treatments.
Oversight and monitoring
Composition of the coordinating center and trial steering committee.
The trial steering committee will be formed by the principal investigator and the co-investigators. The committee will manage the entire project and submit a report for publication at the end of the study. The committee will appoint inspectors of the Project Management Group once a month. The project management group will oversee standardization in line with clinical practice requirements and submit their reports to the trial steering committee.
Composition of the data monitoring committee, its role and reporting structure
A member will be designated by the principal investigator to handle the data. Electronic paper case report forms and management tools will be used to collect the data. The trial steering committee managed the trial under the supervision of the project management group.
Frequency and plans for auditing trial conduct
The project management group will meet every 2 weeks to review the trial. The Trial Steering Group and Ethics Committee will monitor throughout the trial period. Owing to the low-risk nature of the intervention, an independent Data Monitoring Committee will not be considered.
Protocol amendments
If there are any changes to the protocol, the sponsor and funder will be notified first, the principal investigator will notify the centers, and a copy of the revised protocol will be sent to the principal investigator to be added to the investigator-site file. Any deviations from the protocol will be fully documented using a breach report. This protocol will be updated in the clinical trial registry.
Trial status
At the time of manuscript submission, this trial has recruited 14 patients. This trial will be completed by December 2024. The current protocol (Code: ChiCTR2300071359) is version 1.0, dated 18 April 2023.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Best corrected visual acuity
Intraocular pressure
Spectral-domain optical coherence tomography
Fluorescence fundus angiography
Computed tomography
- Retinal vein occlusion
- Macular edema
Vascular endothelial growth factor
Carotid plaque
- Central subfield thickness
Early Treatment Diabetic Retinopathy Study
Bozkurt E, Cetin T, Rencuzogullari I. Evaluation of Systemic Endothelial Dysfunction in Retinal Vein Occlusions. Beyoglu Eye J. 2022;7:126–33.
PubMed PubMed Central Google Scholar
Hunt AR, Nguyen V, Creuzot-Garcher CP, Alforja S, Gabrielle PH, Zarranz-Ventura J, et al. Twelve-month outcomes of ranibizumab versus aflibercept for macular oedema in branch retinal vein occlusion: data from the FRB! registry. Br J Ophthalmol. 2022;106:1178–84.
PubMed Google Scholar
Yu L, Duan Z, Tao T, Xu S, Mo N, Jia Y, et al. The correlation between carotid stenosis detected by neck vascular ultrasound and branch retinal vein occlusion. Zhonghua Yan Ke Za Zhi. 2014;50:804–7 Chinese.
Lyu M, Lee Y, Kim BS, Kim HJ, Hong R, Shin YU, et al. Clinical significance of subclinical atherosclerosis in retinal vein occlusion. Sci Rep. 2021;11:11905.
Article CAS PubMed PubMed Central Google Scholar
Sanlés González I, Napal Lecumberri JJ, Pérez-Montes R, Cerveró Varona A, Casado Rojo A, Hernández Hernández JL. Retinal vein occlusion in patients under 50 years. Analysis of vascular risk factors, thrombophilia, carotid ultrasound findings and uncommon aetiologies. Arch Soc Esp Oftalmol (Engl Ed). 2022;97:443–9.
Article PubMed Google Scholar
Corazza P, D’Alterio FM, Savastano MC, Kabbani J, Duguid G, Savastano A, et al. Long-term outcomes of anti-VEGF treatment of macular oedema due to retinal vein occlusions. Eur J Ophthalmol. 2022;32:3536–46.
Xing Q, Dai YN, Huang XB, Peng L. Comparison of efficacy of conbercept, aflibercept, and ranibizumab ophthalmic injection in the treatment of macular edema caused by retinal vein occlusion: a Meta-analysis. Int J Ophthalmol. 2023;16:1145–54.
Article PubMed PubMed Central Google Scholar
Sun Z, Zhou H, Lin B, Jiao X, Luo Y, Zhang F, et al. Efficacy and safety of intravitreal conbercept injections in macular edema secondary to retinal vein occlusion. Retina. 2017;37:1723–30.
Schmidt-Erfurth U, Garcia-Arumi J, Gerendas BS, Midena E, Sivaprasad S, Tadayoni R, et al. Guidelines for the Management of Retinal Vein Occlusion by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2019;242:123–62.
Article CAS PubMed Google Scholar
Yoon M, Lee CJ, Park S, Lee SH. Statins and Clinical Outcomes in Patients With Low to Moderate Risk but With Non-obstructive Carotid Plaques: The SCOPE-CP Study. Korean Circ J. 2022;52:890–900.
Deng Y, Zou W, Chen G, Shangguan S, Zhou F, Jiang W, et al. Comparative studies on the effects of different doses of atorvastatin combined with aspirin on inflammatory cytokines and carotid plaques in patients with ischemic cerebrovascular disease. Int J Neurosci. 2019;129:1133–8.
Liping Z, Xiufang L, Tao Y, Baomin Z, Houshuai T. Efficacy comparison of rosuvastatin and atorvastatin in the treatment of atherosclerosis and drug safety analysis. Pak J Pharm Sci. 2018;31:2203–8.
Zhu YC, Jiang XZ, Bai QK, et al. Evaluating the Efficacy of Atorvastatin on Patients with Carotid Plaque by an Innovative Ultrasonography. J Stroke Cerebrovasc Dis. 2019;28:830–7.
Sillesen H, Amarenco P, Hennerici MG, Callahan A, Goldstein LB, Zivin J, et al. Stroke Prevention by Aggressive Reduction in Cholesterol Levels Investigators. Atorvastatin reduces the risk of cardiovascular events in patients with carotid atherosclerosis: a secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke. 2008;39:3297–302.
Tsujinaka H, Itaya-Hironaka A, Yamauchi A, Sakuramoto-Tsuchida S, Shobatake R, Makino M, et al. Statins decrease vascular epithelial growth factor expression via down-regulation of receptor for advanced glycation end-products. Heliyon. 2017;3:e00401.
Tian B, Al-Moujahed A, Bouzika P, Hu Y, Notomi S, Tsoka P, et al. Atorvastatin promotes phagocytosis and attenuates pro-inflammatory response in human retinal pigment epithelial cells. Sci Rep. 2017;7:2329.
Gupta A, Gupta V, Thapar S, Bhansali A. Lipid-lowering drug atorvastatin as an adjunct in the management of diabetic macular edema. Am J Ophthalmol. 2004;137:675–82.
CAS PubMed Google Scholar
Ozkiris A, Erkiliç K, Koç A, Mistik S. Effect of atorvastatin on ocular blood flow velocities in patients with diabetic retinopathy. Br J Ophthalmol. 2007;91:69–73.
Mysore Y, Del Amo EM, Loukovaara S, Hagström M, Urtti A, Kauppinen A. Statins for the prevention of proliferative vitreoretinopathy: cellular responses in cultured cells and clinical statin concentrations in the vitreous. Sci Rep. 2021;11:980.
Download references
Acknowledgements
Not applicable.
Strengths and limitations of this study
➢ This is a prospective, randomized controlled clinical trial investigating the clinical effects of atorvastatin and intravitreal conbercept in the treatment of patients with RVO-ME and coexistent CP.
➢ This trial may provide an opportunity to control ME recurrence in patients with RVO and concomitant CP.
➢ Data on carotid intima-media thickness in this trial were not collected or analyzed.
➢ Further studies with larger sample sizes and more data are required to better understand the effects of combined treatments.
Role of sponsor
The sponsor played no part in the study design, collection, management, analysis, interpretation of data, writing of the report, or decision to submit the report for publication.
Author information
Bangtao Yao and Bei Wang contributed equally to this work and should be regarded as co-first authors.
Authors and Affiliations
Department of Ophthalmology, Nanjing Lishui People’s Hospital, Zhongda Hospital Lishui branch, Southeast University, Nanjing, Jiangsu Province, China
Bangtao Yao, Bei Wang, Hao Yu, Yuhui Liu & Gang Liu
Department of Neurology, Nanjing Lishui People’s Hospital, Zhongda Hospital Lishui branch, Southeast University, Nanjing, Jiangsu Province, China
Department of Endocrinology, Nanjing Lishui People’s Hospital, Zhongda Hospital Lishui branch, Southeast University, Nanjing, Jiangsu Province, China
Department of Ophthalmology, Jiangsu Province Hospital, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu Province, China
Xiuying Wang
You can also search for this author in PubMed Google Scholar
Contributions
BTY wrote the manuscript; BW edited the manuscript; JY contributed to the supervision; YG was responsible for the analysis; HY contributed to the study design, YHL and GL consulted literature and dealt with the table; BTY and XYW established the diagnosis and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Bangtao Yao or Xiuying Wang .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the Institutional Review Board of Nanjing Lishui People’s Hospital (Approval number 2023KY0418-12, dated 18 April 2023). All participants will sign informed consent before the study, and all methods will be performed in accordance with the Declaration of Helsinki.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Yao, B., Wang, B., Yang, J. et al. Clinical effects of atorvastatin combined with conbercept in the treatment of patients with macular edema secondary to retinal vein occlusion and carotid plaque: study protocol for a prospective randomized controlled trial. Trials 25 , 244 (2024). https://doi.org/10.1186/s13063-024-08082-0
Download citation
Received : 27 September 2023
Accepted : 01 April 2024
Published : 08 April 2024
DOI : https://doi.org/10.1186/s13063-024-08082-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Atorvastatin
- Anti-vascular endothelial growth factor
- Best-corrected visual acuity
- Carotid plaques
- Intravitreal conbercept
ISSN: 1745-6215
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Clinical Evaluation After Discontinuation of Galcanezumab in Japanese Patients with Episodic and Chronic Migraine: Analysis of a Randomized, Placebo-Controlled Trial and Open-label Extension Study
- ORIGINAL RESEARCH
- Open access
- Published: 06 April 2024
Cite this article
You have full access to this open access article
- Takao Takeshima ORCID: orcid.org/0000-0003-2678-6956 1 ,
- Hikaru Doi 2 ,
- Satomi Ooba 3 ,
- Yuka Tanji ORCID: orcid.org/0000-0001-7850-1946 4 ,
- Akichika Ozeki ORCID: orcid.org/0000-0001-5510-4219 4 &
- Mika Komori ORCID: orcid.org/0000-0003-1887-1296 4
Introduction
This analysis of two Japanese clinical trials evaluated efficacy and safety after galcanezumab (GMB) discontinuation in patients with episodic migraine (EM) and chronic migraine (CM).
Data were from a 6-month, randomized, double-blind, placebo [PBO]-controlled primary trial (patients with EM) and a 12-month open-label extension trial (patients with EM/CM). Patients received 6 months’ (primary) or 12/18 months’ (extension) treatment with GMB 120 mg (GMB120) plus 240-mg loading dose or 240 mg (GMB240) with 4 months’ post-treatment follow-up. Efficacy was assessed as number of monthly migraine headache days during post-treatment. Safety was assessed via post-treatment-emergent adverse events (PTEAEs).
The analysis population included 186 patients from the primary trial (PBO N = 93; GMB120 N = 45; GMB240 N = 48), 220 patients with EM from the extension trial (PBO/GMB120 N = 57; PBO/GMB240 N = 55; GMB120/GMB120 N = 55; GMB240/GMB240 N = 53), and 55 patients with CM (GMB120 N = 28; GMB240 N = 27). In patients with EM receiving 6 months’ GMB120, mean standard deviation (SD) monthly migraine headache days increased from 5.69 (4.64) at treatment end to 6.24 (4.37) at end of follow-up but did not return to pre-treatment levels (8.80 [2.96]). In the extension trial, mean monthly migraine headache days in patients with EM receiving GMB120 were 4.13 (3.85) after 12 months and 4.45 (3.78) at end of follow-up, and 3.59 (3.48) after 18 months and 3.91 (3.57) at end of follow-up. Monthly migraine headache days in patients with CM (12 months’ GMB120) were 10.71 (4.61) at treatment end and 11.17 (5.64) at end of follow-up (pre-treatment 20.15 [4.65]). Similar results were seen for patients receiving GMB240. The most observed PTEAE after GMB discontinuation was nasopharyngitis.
Galcanezumab exhibited post-treatment efficacy for up to 4 months in Japanese patients with EM and with CM. No unexpected safety signals were observed.
Clinical Trial Registration
ClinicalTrials.gov, NCT02959177 and NCT02959190.
Avoid common mistakes on your manuscript.
Migraine is a debilitating chronic neurological condition, characterized by pulsating or pounding headaches and associated symptoms including nausea, photophobia, and phonophobia [ 1 ]. Pharmacological treatment of migraine includes both acute management during attacks and preventive therapy. However, traditional migraine preventive treatments have high discontinuation rates due to intolerance and lack of efficacy [ 2 , 3 , 4 ]. Some newer preventive treatments target the neuropeptide calcitonin gene-related peptide (CGRP) or its receptor. CGRP is implicated in the underlying pathophysiology of migraine and has been demonstrated to contribute to neurogenic inflammation, vasodilation, and transmission of painful stimuli [ 5 ]. Galcanezumab is a humanized monoclonal antibody that inhibits CGRP-mediated effects by binding to CGRP, thus preventing it from binding to its receptor [ 6 ]. The efficacy and safety of galcanezumab have been demonstrated in phase 2 and 3 randomized controlled trials for the prevention of migraine, including treatment-resistant migraine [ 7 , 8 , 9 , 10 , 11 ].
Anti-CGRP antibody treatments for migraine have demonstrated sustained response even after discontinuation in some people with migraine [ 12 , 13 , 14 , 15 ]. Results from two global randomized phase 3 trials of galcanezumab showed reduced effect in post-treatment phases, but monthly migraine headache days did not return to baseline levels [ 12 ]. In Japanese people with migraine, the efficacy and safety of galcanezumab have been demonstrated for episodic migraine (EM) in a phase 2 randomized controlled trial [ 9 ], and for EM and chronic migraine (CM) in a long-term, 12-month, open-label extension study [ 16 ]. However, continued efficacy of galcanezumab after discontinuation in Japanese patients has not yet been demonstrated. Furthermore, there is a need to identify clinical factors associated with post-treatment efficacy of galcanezumab, which could assist clinicians and patients when selecting the best treatment option and treatment duration for the individual.
The objectives of this analysis of the Japanese randomized controlled clinical trial and open-label extension were to evaluate efficacy after discontinuation of galcanezumab, both in patients with EM and in patients with CM, and to identify clinical factors associated with changes in monthly migraine headache days after discontinuation of galcanezumab.
Study Design
The primary trial was a 6-month, randomized, double-blind, placebo-controlled study of galcanezumab in Japanese people with EM (ClinicalTrials.gov, NCT02959177), conducted across 40 sites from December 2016 to January 2019 [ 9 ]. The open-label extension study was a 12-month safety study of galcanezumab in Japanese people with either EM or CM (ClinicalTrials.gov, NCT02959190), conducted at 44 sites from March 2017 to August 2019 [ 16 ]. The protocols for both trials were reviewed and approved by local ethics review boards, and written informed consent was obtained from all patients before participation. Both studies were conducted in accordance with the Helsinki Declaration of 1964 and its later amendments. A list of the institutional ethics review boards is provided in Table S1 .
Study Populations and Treatment Protocols
The inclusion and exclusion criteria for both trials have been published previously [ 9 , 16 ]. Briefly, patients eligible for the primary trial were adults with a migraine diagnosis, had migraine for ≥ 1 year prior to the study, and had 4–14 migraine headache days and ≥ 2 migraine attacks per month (i.e., EM) [ 9 ]. Patients eligible for the open-label extension trial included those with EM who completed the treatment period of the primary trial and newly recruited patients with CM. Patients with CM were eligible if they were adults with a migraine diagnosis, had ≥ 1 headache-free day per month in the 3 months prior to treatment and during the baseline period, and had ≥ 15 headache days per month (of which ≥ 8 had features of migraine) during the baseline period [ 16 ].
In the primary trial, patients were randomized (2:1:1) to monthly subcutaneous injections of placebo, 120 mg of galcanezumab, or 240 mg of galcanezumab [ 9 ]. Patients randomized to galcanezumab 120 mg received a single 240-mg loading dose at the start of treatment [ 9 ]. Patients with EM who received galcanezumab 120 mg or 240 mg in the primary trial remained on the same dose in the open-label extension study [ 16 ]. Patients with EM who received placebo in the primary trial and patients with CM who were newly recruited were randomized (1:1) to galcanezumab 120 mg or 240 mg in the open-label extension study [ 16 ]. All patients randomized to galcanezumab 120 mg received a single 240-mg loading dose at the start of open-label treatment [ 16 ]. In the primary trial, all injections were performed by trained study personnel [ 9 ]; in the open-label study, after 6 months, patients were given the option to self-administer the injection under the supervision of study personnel [ 16 ].
This analysis included three patient groups. The first group of patients, from the primary trial, had EM, received 6 months of treatment with either placebo or galcanezumab, and were followed up for 4 months post-treatment. The second group of patients had EM, rolled over to the open-label extension study from the primary trial, received 12 months of open-label treatment with galcanezumab, and were followed up for 4 months post-treatment. Patients who rolled over into the open-label extension trial from the primary trial were not included in the post-treatment follow-up of the primary trial. In this group, patients who received placebo in the primary trial received a total of 12 months of galcanezumab treatment, and patients who received galcanezumab in the primary trial received a total of 18 months of galcanezumab treatment. The third group of patients comprised newly recruited patients with CM who entered the extension study, received 12 months of open-label galcanezumab treatment, and were followed up for 4 months post-treatment.
Outcome Measures
The primary and key secondary outcomes of both the primary and open-label extension trials have been reported previously [ 9 , 16 ]. In this prespecified analysis, the efficacy outcome was the number of monthly migraine headache days during the post-treatment phase after discontinuation of galcanezumab. A migraine headache day was defined as a calendar day on which a migraine or probable migraine headache occurred. Each month was defined as a 30-day period with migraine or headache measures, normalized from the intervals between visits. Participants in both studies used daily migraine diaries to record the frequency of headaches, migraine headaches, and medications used for headache during the treatment and post-treatment phases.
Prespecified safety assessments included frequency of adverse events (AEs), serious AEs, treatment-emergent AEs (TEAEs), and post-TEAEs (PTEAEs). A PTEAE was defined as an event that first occurred or worsened during the post-treatment phase when compared with baseline. In this analysis, we report safety during the post-treatment phase of the primary trial; safety during the post-treatment phase of the open-label study has been reported previously [ 16 ]. AEs were coded using the Medical Dictionary for Regulatory Activities Version 21.1.
This analysis of data from the primary and extension trials also includes a post hoc exploratory analysis to identify clinical factors of migraine associated with changes in monthly migraine headache days during the post-treatment phases of both trials.
Statistical Analysis
The efficacy analysis population consisted of patients in the intent-to-treat (ITT) population of the primary trial who entered the post-treatment phase of the trial and who had migraine headache day data recorded both (1) at the final month of the treatment period and (2) at the end of the post-treatment period. The ITT population of the primary trial was defined as all randomized patients who received ≥ 1 dose of placebo or galcanezumab [ 9 ]. The efficacy population of the open-label extension trial was the same as the primary trial [ 16 ].
Baseline (pre-treatment) demographic and clinical characteristics are reported as mean (SD) for continuous variables and n (%) for categorical variables, for each of the treatment groups and for 50% responders and non-responders to galcanezumab. In each population, a 50% responder to galcanezumab was defined as any patient who had a ≥ 50% reduction in the total number of migraine headache days (relative to baseline) during the last month of the treatment period.
Clinical factors associated with changes in monthly migraine headache days during post-treatment phases were determined using a three-step process. The clinical factor variables used in the post hoc analyses are listed in Table S2 . Firstly, Hall’s method [ 17 ] was used to select important (continuous) covariates with a high correlation with the response variable, but lower correlations among the selected covariates. Here the response variable was the change in migraine headache days during the post-treatment phase (migraine headache days in the last month of the follow-up period minus migraine headache days in the last month of the treatment period). This was based on Eq. 4.16 in [ 17 ]. In the original algorithm, the selection stops at the maximum point in the merit function Ms. Because we had a next step to eliminate variables, we wanted to avoid too strict a selection in this step. Therefore, we stopped the selection when the merit function decreased slightly (0.01 decrease) after reaching the maximum point (in Ms) as the number of selected covariates (k in Eq. 4.16) increased. We used the non-parametric method, Kendall’s correlation in the Hall’s algorithm. Secondly, a random forest procedure [ 18 ] was used to refine the explanatory variables selected by Hall’s method, plus one binary variable sex (R package randomForest, “randomForest” function). The response variable was the same as the Hall’s method. Thirdly, after constructing 500 random forest trees, we computed important scores by decreases in mean squared errors (MSE). We selected variables with ≥ 30% of the maximum decrease in MSE among the input variables (R package randomForest, “varImpPlot” function). Note that there is no standard cutoff to select variables, so we used the relatively conservative cutoff of 30%. Finally, linear regression without interaction terms was used with the variables selected in the previous steps. As a cutoff value, we used a p value < 0.05. This process was conducted separately for all primary trial patients who received galcanezumab, all extension trial patients with EM, all extension trial patients with CM, 50% responders in the primary trial, 50% responders with EM in the extension trial, and 50% responders with CM in the extension trial. For all these post hoc analyses, there was no multiplicity adjustment.
Statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and R, Version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria) [ 19 ].
Patient Disposition and Baseline Characteristics
As previously described for the primary trial [ 9 ], 459 patients with EM were randomized to placebo ( N = 230), galcanezumab 120 mg ( N = 115), and galcanezumab 240 mg ( N = 114) (Fig. 1 ). Some patients who completed the treatment period in the primary trial shifted into the open-label extension trial and thus were not included in the post-treatment population of the primary trial. This included 58 patients on galcanezumab 120 mg and 62 patients on galcanezumab 240 mg who continued on the same dose in the extension trial, and 126 patients who received placebo in the primary trial and were re-randomized to galcanezumab 120 mg ( n = 62) or galcanezumab 240 mg ( n = 64) in the extension trial. Thus, in the primary trial, 204 patients entered the post-treatment phase (placebo N = 100; galcanezumab 120 mg N = 52; galcanezumab 240 mg N = 52), and 186 patients completed the post-treatment phase and were included in the analysis population (placebo N = 93; galcanezumab 120 mg N = 45; galcanezumab 240 mg N = 48). In the extension trial, 237 patients with EM entered the post-treatment phase (galcanezumab 120 mg N = 117; galcanezumab 240 mg N = 120), and 220 patients completed the post-treatment phase and were included in the analysis population (galcanezumab 120 mg N = 112; galcanezumab 240 mg N = 108). The disposition of patients with EM in the analysis population by primary trial dose and extension trial dose was 57 patients receiving placebo then galcanezumab 120 mg, 55 receiving placebo then galcanezumab 240 mg, 55 receiving galcanezumab 120 mg then 120 mg, and 53 receiving galcanezumab 240 mg then 240 mg (Table 1 ).
Patient flow for the primary and extension trials, including patients who transferred from the primary trial into the open-label extension study. a Patients included in the analysis population had migraine headache days recorded for the last month of treatment and the last month of the post-treatment phase. b Disposition by primary trial dose/extension trial dose: PBO/GMB120 N = 57; GMB120/GMB120 N = 55. c Disposition by primary trial dose/extension trial dose: PBO/GMB240 N = 55; GMB240/GMB240 N = 53. GMB galcanezumab, LTE long-term extension, PBO placebo
Patients with CM were also included in the extension trial ( N = 65) and randomized to galcanezumab 120 mg ( N = 32) or 240 mg ( N = 33). Of these, 64 patients entered the post-treatment phase (galcanezumab 120 mg N = 32; galcanezumab 240 mg N = 32), and 55 patients completed the post-treatment phase and were included in the analysis population (galcanezumab 120 mg N = 28; galcanezumab 240 mg N = 27).
Demographic and clinical characteristics were mostly well balanced across treatment groups in the analysis population (Table 1 ). The percentage of female patients was around 82–85% in most treatment groups, although 90.9% of the placebo/galcanezumab 240-mg group with EM and 100% of the galcanezumab 120-mg group with CM in the extension trial were female. Some expected differences were seen between patients with EM and those with CM. In patients with EM, the percentage who had previously failed ≥ 1 preventive migraine treatment ranged from 28.9% to 50.9%, whereas it was slightly higher in patients with CM (55.6–64.3%; consistent with previous reports for Japanese patients with CM [ 3 ]).
The demographic and clinical characteristics among 50% responders to galcanezumab in each trial were mostly similar across the trials (Table S3 ).
Efficacy After Discontinuation of Galcanezumab
In patients with EM who received 6 months of treatment with galcanezumab in the primary trial, the number of monthly migraine headache days increased during the 4-month post-treatment phase compared with the end of the treatment period but remained lower than baseline (Fig. 2 ). Patients in the galcanezumab 120-mg group experienced a mean (SD) of 5.69 (4.64) monthly migraine headache days in the final month of treatment (month 6) and 6.24 (4.37) monthly migraine headache days at the end of follow-up (month 10) compared with 8.80 (2.96) at baseline (before treatment). In the galcanezumab 240-mg group, mean monthly migraine headache days were 9.04 (3.15) at baseline (before treatment), 4.58 (4.49) at month 6 (end of treatment), and 6.25 (4.27) at month 10 (end of follow-up).
Mean number of monthly migraine headache days during the treatment and post-treatment phase of the primary study for patients with EM. EM episodic migraine, GMB galcanezumab, PBO placebo
After 12 or 18 months of galcanezumab treatment, patients with EM maintained a similar frequency of monthly migraine headache days during the 4-month post-treatment phase and during treatment (Fig. 3 ). In patients who received placebo for 6 months followed by galcanezumab 120 mg for 12 months, the mean (SD) number of monthly migraine headache days was 4.13 (3.85) in the final month of treatment and 4.45 (3.78) at the end of follow-up compared with 8.53 (2.95) at baseline (before treatment). Patients who received galcanezumab 120 mg for 18 months had mean (SD) monthly migraine headache days of 8.37 (2.54) at baseline (before treatment), 3.59 (3.48) at month 18 (end of treatment), and 3.91 (3.57) at the end of follow-up. In patients who received placebo for 6 months followed by galcanezumab 240 mg for 12 months, the mean (SD) number of monthly migraine headache days was 5.06 (5.17) in the final month of treatment and 4.96 (4.65) at the end of follow-up compared with 8.67 (3.10) at baseline (before treatment). Patients who received galcanezumab 240 mg for 18 months had mean monthly migraine headache days of 9.07 (2.82) at baseline (before treatment), 4.19 (3.99) at month 18 (end of treatment), and 3.98 (3.21) at the end of follow-up.
Mean number of monthly migraine headache days during the treatment and post-treatment phase of the extension study for patients with EM. EM episodic migraine, GMB galcanezumab, PBO placebo
By pooling the data for patients with EM from the primary and extension trials, we were able to visualize the change in migraine headache days from the end of treatment to the end of the post-treatment phase across treatment durations and galcanezumab dose groups (Table 2 ). There was a numerical trend toward a smaller change in monthly migraine headache days after treatment discontinuation as the treatment duration increased. Furthermore, the percentage of patients with EM who experienced an increase in monthly migraine headache days (i.e., a worsening of their condition) during the post-treatment phase was greatest for patients with the shortest treatment period (Fig. 4 ). For patients receiving galcanezumab 120 mg, the percentage who experienced a worsening of their condition during the post-treatment phase was 57.8% (26/45) after 6 months, 52.6% (30/57) after 12 months, and 47.3% (26/55) after 18 months of treatment. For patients receiving galcanezumab 240 mg, the percentage who experienced a worsening of their condition during the post-treatment phase was 64.6% (31/48) after 6 months, 49.1% (27/55) after 12 months, and 47.2% (25/58) after 18 months of treatment.
Percentage of patients with EM who experienced an increase in monthly migraine headache days during the post-treatment phase (i.e., worsened condition after the end of treatment). Data for 6 months of treatment are from the primary trial and data for 12 and 18 months of treatment are from the extension trial. EM episodic migraine, GMB galcanezumab
Patients with CM who received 12 months of galcanezumab treatment also demonstrated continued efficacy of galcanezumab for 4 months following treatment, with almost no change in migraine headache days during the follow-up period (Fig. 5 ). Patients receiving galcanezumab 120 mg had a mean (SD) baseline of 20.15 (4.65) monthly migraine headache days (before treatment), which decreased to 10.71 (4.61) at the end of treatment and was 11.17 (5.64) at the end of the 4-month follow-up. For patients in the galcanezumab 240-mg group, monthly migraine headache days were 10.62 (7.33) at the end of treatment and 11.43 (7.89) at the end of follow-up compared with 19.59 (5.57) at baseline (before treatment).
Mean number of monthly migraine headache days during the treatment and post-treatment phase of the extension study for patients with CM. CM chronic migraine, GMB galcanezumab
Safety After Discontinuation of Galcanezumab
No deaths were reported during the follow-up period for the primary trial. The only serious AE reported during the post-treatment phase occurred in one patient in the placebo group (moderate pneumonia, considered by the investigator as unrelated to the study drug). In the galcanezumab 240-mg group, there was a single discontinuation from the study during the post-treatment phase due to AEs. Overall, 30 patients (30%) in the placebo group, 21 patients (40.4%) in the galcanezumab 120-mg group, and 20 patients (38.5%) in the galcanezumab 240-mg group reported ≥ 1 PTEAE. The PTEAEs that occurred in ≥ 2% of patients who received any dose of galcanezumab were nasopharyngitis (7.7% of each of the 120-mg and 240-mg dose groups), back pain (1.9% and 3.9% of the 120-mg and 240-mg groups, respectively), gastroenteritis (5.8% and 0% of the 120-mg and 240-mg groups, respectively), influenza (3.9% and 1.9% of the 120-mg and 240-mg groups, respectively), migraine without aura (0% and 3.9% of the 120-mg and 240-mg groups, respectively), and oral herpes (3.9% and 0% of the 120-mg and 240-mg groups, respectively).
AEs that occurred during the post-treatment phase of the open-label extension study have been previously reported [ 16 ].
Clinical Factors Associated with Efficacy After Discontinuation of Galcanezumab
For the primary trial data (patients with EM after 6 months of treatment), after the random forest selection procedure, the variables selected were headache days per month at baseline (HD), migraine headache days per month at baseline (MHD), years since migraine diagnosis (DIAGyear), age, number of migraine headache days in the final month of treatment (BASEend), and the percentage change in monthly migraine headache days at treatment end (PCHGend) (Table S4 ). In the linear regression with no interaction terms, an increase in monthly migraine headache days after discontinuation of galcanezumab was significantly associated with a lower number of headache days per month at baseline (HD variable, p = 0.012) and longer disease duration (DIAGyear variable, p = 0.040) (Table 3 ).
In patients with EM in the extension trial (after 12 or 18 months of treatment), the variables selected for linear regression were the number of days with aura per 30-day period at baseline (AURA), number of migraine headache days in the final month of treatment (BASEend), and the percentage change in monthly migraine headache days at treatment end (PCHGend) (Table S5 ). An increase in monthly migraine headache days after discontinuation of galcanezumab was significantly associated with a higher number of days with aura per 30-day period (at baseline) (AURA variable, p = 0.004) and a lower number of monthly migraine headache days at treatment end (BASEend variable, p < 0.001) (Table 3 ).
In patients with CM in the extension trial (after 12 months of treatment), the variables selected by the random forest procedure were headache days per 30-day period at baseline (HD), number of days with acute medication use per 30-day period (NUMMU), body mass index at baseline (BMI), number of migraine headache days in the final month of treatment (BASEend), and the percentage change in migraine headache days at treatment end (PCHGend) (Table S6 ). However, in the linear regression, none of these clinical factors were significantly associated with an increase in monthly migraine headache days after discontinuation of treatment.
When the same analyses were performed on the 50% responder groups, only the patients with EM after 12 or 18 months of treatment showed any clinical factors significantly associated with changes in monthly migraine headache days after discontinuation of galcanezumab (Table 3 ). Specifically, in the 50% responder group, an increase in monthly migraine headache days after galcanezumab discontinuation was associated with the number of monthly migraine headache days with aura at baseline (AURA variable, p = 0.044) and a higher percentage change in monthly migraine headache days at treatment end (PCHGend variable, p = 0.012). A similar, although not statistically significant, trend was seen in the relationship between changes in monthly headache days and disease duration (Fig. 6 ).
Relationship between change in monthly migraine headache days during the post-treatment phase and years since migraine diagnosis in the 50% responder group. DIAGyear years since migraine diagnosis, GMB All all patients who received galcanezumab
This analysis of data from both a randomized controlled trial and an open-label extension study of galcanezumab in Japanese patients with migraine provides the first description of post-treatment effects of galcanezumab in this population. After 12 or 18 months of treatment with galcanezumab, Japanese people with EM and with CM maintained reduced monthly migraine headache days for 4 months. This confirms previous reports from global studies of a post-treatment effect of galcanezumab in people with migraine [ 12 ]. This analysis also identified clinical factors associated with changes in migraine headache days after discontinuation of galcanezumab. These results may assist clinicians in identifying patients who may be likely to experience prolonged post-treatment efficacy. The demonstrated post-treatment effect of galcanezumab is also important for patients with migraine who may need to temporarily cease preventive medication or switch to another medication.
In the primary trial, the number of monthly migraine headache days increased slightly during the post-treatment phase but did not return to baseline levels in the galcanezumab-treated patients with EM. This pattern was seen in patients receiving both 120 mg and 240 mg of galcanezumab. This is consistent with the results of the EVOLVE-1 and -2 global phase 3 clinical trials of galcanezumab in patients with EM [ 12 ]. In both EVOLVE-1 and -2, the reduction in monthly migraine headache days declined during the post-treatment phase, and the number of monthly migraine headache days remained significantly different from baseline for all treatments at all time points [ 12 ]. In the open-label extension study, in both patients with EM and patients with CM, the number of monthly migraine headache days during the post-treatment phase remained significantly different from baseline in patients receiving galcanezumab, with very little change in migraine headache days from end of treatment to the end of the follow-up period. A prolonged difference from baseline monthly migraine headache days was reported in the 4-month period after 9 months of open-label extension of the REGAIN global clinical trial of galcanezumab in patients with CM [ 20 ].
The post-treatment effects of galcanezumab observed in the current analysis are generally consistent with previous reports for other migraine medications targeting CGRP or its receptor, although direct comparisons between studies are difficult owing to differences in methodology, duration of post-treatment period, and reported patient outcomes. For example, in patients with EM and with CM in a real-world clinical setting, there was a significant reduction in monthly migraine days compared with baseline during weeks 1–4 after discontinuation of erenumab [ 13 ]. Post-treatment effects have also been demonstrated in a small cohort study of patients with CM who were treated with erenumab or galcanezumab after previous failure of ≥ 3 preventive treatments [ 14 ]. In these patients, a significant reduction in monthly migraine days compared with baseline was observed at 1, 2, and 3 months post-discontinuation, although with a significant increase at 2 and 3 months compared with the last month of treatment [ 14 ].
In the present study, a numerical trend was observed of a smaller change in monthly migraine headache days as the treatment duration increased (in patients with EM). After 6 months of treatment and 4 months of follow-up, migraine headache days increased by 0.55–1.68 days per month from the end of treatment; after 12 or 18 months of treatment, and 4 months of follow-up the change was − 0.21 to + 0.32 days per month, depending on galcanezumab dose. We also observed that the percentage of patients with EM who experienced an increase in monthly migraine headache days during the post-treatment phase was greatest for patients treated for 6 months, and was relatively similar for patients who received 12 or 18 months of treatment. It is therefore likely that a longer treatment period may be associated with a prolonged post-treatment effect and a lower proportion of patients with a worsened condition.
In this analysis, we identified clinical factors associated with changes in monthly migraine headache days after discontinuation of galcanezumab by a random forest procedure and subsequently by linear regression without interaction effects. Although some variables appeared to have a similar clinical meaning, we kept them in the random forest and linear regression analyses because our first step (Hall’s algorithm) would have eliminated highly correlated non-important variables (e.g., HD and MHD, BASEend and PCHGend, for the primary trial analysis).
After 6 months of treatment with galcanezumab in patients with EM, random forest analysis identified the variables of headache days per month at baseline, migraine headache days per month at baseline, age, migraine headache days in the final month of treatment, percentage change in monthly migraine headache days at treatment end, and disease duration (years since diagnosis) as being associated with changes in monthly migraine headache days during the post-treatment phase. In the linear regression analysis, an increase in monthly migraine headache days during the post-treatment phase was only associated with a lower number of headache days per month at baseline and longer disease duration. It is possible that the association with longer disease duration is related to central sensitization, which is known to be associated with longer disease duration [ 21 ]; 6 months of treatment may be insufficient for patients with a longer disease duration to develop improved central sensitization.
After 12 or 18 months of treatment with galcanezumab in patients with EM, changes in monthly migraine headache days in the post-treatment phase were associated in the random forest analysis with the number of migraine headache days with aura at baseline, the number of migraine headache days in the final month of treatment, and the percentage change in monthly migraine headache days at treatment end. The linear regression identified a significant association of increasing number of monthly migraine headache days post-treatment with a higher number of migraine headache days per month with aura (at baseline) and a lower number of migraine headache days in the final month of treatment. It is possible that patients with a lower number of monthly migraine headache days at the end of treatment experienced an increase in migraine headache days during the post-treatment phase simply because the lower headache frequency at treatment end could not be maintained without additional doses of galcanezumab. It is also possible that the post-treatment effect of galcanezumab may not persist in patients with more frequent baseline aura because galcanezumab acts primarily peripherally, whereas the primary cause of aura is thought to be a central mechanism (depolarization within the cerebral cortex [ 22 ]). It is not clear why the clinical factors associated with post-treatment changes in monthly migraine headache days differed depending on the treatment period, and further research is needed.
For patients with CM, after 12 months of treatment, changes in monthly migraine headache days in the post-treatment phase were associated in the random forest analysis with headache days per month at baseline, the number of days per month with acute medication use, BMI, the number of migraine headache days in the final month of treatment, and the percentage change in monthly migraine headache days at treatment end. However, there were no significant associations between the change in monthly migraine headache days post-treatment and any variables in the linear regression.
The safety profile of galcanezumab during the post-treatment phase in Japanese patients with migraine was consistent with previous local and global studies. PTEAEs in the primary trial were reported by 30–40% of patients, consistent with the open-label extension trial [ 16 ]. The most common PTEAE in the primary trial was nasopharyngitis, which was also the most commonly reported PTEAE in the open-label extension trial [ 16 ]. These safety parameters are similar to the global EVOLVE-1 and -2 trials, in which PTEAEs were reported in around 25% of patients, and the most common PTEAEs were upper respiratory tract infections [ 12 ].
Limitations
Limitations of this analysis include the small sample size for some treatment groups, especially the 50% responder groups, and that restrictions in enrollment criteria for the two trials may have limited the generalizability of the results [ 12 ]. Our analyses used data from company-sponsored clinical trials and may not reflect the situation for patients in real-world clinical settings. There were no patients who received placebo treatment throughout the open-label extension, and so we were unable to investigate any impact of placebo responses on the results of the open-label extension trial, including in patients with CM. Participants were permitted to use preventive medication during the post-galcanezumab treatment period, which could have affected the results; however, only a minority of participants (5.9–7.7% of patients with EM; 23.4% of patients with CM) actually took such medication. A further potential limitation is that the post-treatment duration was 4 months, and our results may not be generalizable for longer durations; however, we note that the post-treatment duration was no shorter than previous post-treatment studies [ 12 , 13 , 14 ]. Investigating in more detail the variables selected by the random forest procedure may be a future analysis approach because the random forest algorithm can account for interaction effects that were omitted by the simple linear regressions in the last step of our analysis. We also note that in our analyses, patients were considered to have worsened if they had any increase in monthly migraine headache days post-treatment, even if this was only one migraine day per month; in practice, patients may not consider a small increase to be a worsened outcome, although this cannot be confirmed without patient-reported satisfaction or quality-of-life data. The strengths of our analysis included the use of data from two robustly conducted studies: one randomized, placebo-controlled, multicenter phase 2 trial and one randomized, multicenter, open-label extension study. Both the primary and extension trials included a prolonged post-treatment phase (4 months) in the study design in order to assess post-treatment effects described in this analysis. In addition, this is the first study to evaluate post-treatment effectiveness of galcanezumab in Japanese people with migraine, and the study population included patients both with EM and with CM.
Galcanezumab exhibited post-treatment efficacy for up to 4 months in Japanese patients with EM and with CM. Clinical factors that were associated with post-treatment increases in monthly migraine headache days in patients with EM included fewer headache days per month at baseline, longer disease duration, higher aura frequency at baseline, and a higher percentage change in monthly migraine headache days at treatment end.
Data Availability
The datasets generated during and/or analyzed during the current study are available in the Eli Lilly repository at www.vivli.org . Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org .
International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24 Suppl 1:9–160.
Meyers JL, Davis KL, Lenz RA, Sakai F, Xue F. Treatment patterns and characteristics of patients with migraine in Japan: a retrospective analysis of health insurance claims data. Cephalalgia. 2019;39(12):1518–34.
Article PubMed Google Scholar
Ueda K, Ye W, Lombard L, et al. Real-world treatment patterns and patient-reported outcomes in episodic and chronic migraine in Japan: analysis of data from the Adelphi migraine disease specific programme. J Headache Pain. 2019;20(1):68.
Article PubMed PubMed Central Google Scholar
Vécsei L, Majláth Z, Szok D, Csáti A, Tajti J. Drug safety and tolerability in prophylactic migraine treatment. Expert Opin Drug Saf. 2015;14(5):667–81.
Durham PL. CGRP-receptor antagonists–a fresh approach to migraine therapy? N Engl J Med. 2004;350(11):1073–5.
Article CAS PubMed Google Scholar
Dodick DW, Goadsby PJ, Spierings ELH, Scherer JC, Sweeney SP, Grayzel DS. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2014;13(9):885–92.
Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75(9):1080–8.
Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38(8):1442–54.
Sakai F, Ozeki A, Skljarevski V. Efficacy and safety of galcanezumab for prevention of migraine headache in Japanese patients with episodic migraine: a phase 2 randomized controlled clinical trial. Cephalalgia Rep. 2020;3:1–10.
Google Scholar
Detke HC, Goadsby PJ, Wang S, Friedman DI, Selzler KJ, Aurora SK. Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology. 2018;91(24):e2211–21.
Article CAS PubMed PubMed Central Google Scholar
Mulleners WM, Kim BK, Láinez MJA, et al. Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol. 2020;19(10):814–25.
Stauffer VL, Wang S, Voulgaropoulos M, Skljarevski V, Kovacik A, Aurora SK. Effect of galcanezumab following treatment cessation in patients with migraine: results from 2 randomized phase 3 trials. Headache. 2019;59(6):834–47.
De Matteis E, Affaitati G, Frattale I, et al. Early outcomes of migraine after erenumab discontinuation: data from a real-life setting. Neurol Sci. 2021;42(8):3297–303.
Iannone LF, Fattori D, Benemei S, Chiarugi A, Geppetti P, De Cesaris F. Predictors of sustained response and effects of the discontinuation of anti-calcitonin gene related peptide antibodies and reinitiation in resistant chronic migraine. Eur J Neurol. 2022;29(5):1505–13.
Vernieri F, Brunelli N, Guerzoni S, et al. Retreating migraine patients in the second year with monoclonal antibodies anti-CGRP pathway: the multicenter prospective cohort RE-DO study. J Neurol. 2023;270(11):5436–48.
Hirata K, Takeshima T, Sakai F, et al. A long-term open-label safety study of galcanezumab in Japanese patients with migraine. Expert Opin Drug Saf. 2021;20(6):721–33.
Hall MA. Correlation-based feature selection for machine learning [PhD thesis]. Hamilton, New Zealand: The University of Waikato; 1999.
Breiman L. Random forests. Mach Learn. 2001;45(1):5–32.
Article Google Scholar
R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2019.
Kuruppu DK, North JM, Kovacik AJ, Dong Y, Pearlman EM, Hutchinson SL. Onset, maintenance, and cessation of effect of galcanezumab for prevention of migraine: a narrative review of three randomized placebo-controlled trials. Adv Ther. 2021;38(3):1614–26.
Güven H, Çilliler AE, Çomoğlu SS. Cutaneous allodynia in patients with episodic migraine. Neurol Sci. 2013;34(8):1397–402.
Fraser CL, Hepschke JL, Jenkins B, Prasad S. Migraine aura: pathophysiology, mimics, and treatment options. Semin Neurol. 2019;39(6):739–48.
Download references
Acknowledgements
The authors would like to thank all study participants.
Medical Writing/Editorial Assistance.
Medical writing assistance was provided by Koa Webster, PhD, CMPP, and Rebecca Lew, PhD, CMPP, of ProScribe – Envision Pharma Group, and was funded by Eli Lilly Japan K.K. and Daiichi Sankyo Company, Limited. ProScribe’s services complied with international guidelines for Good Publication Practice.
This study was funded by Eli Lilly Japan K.K., Kobe, Japan, manufacturer/licensee of galcanezumab in Japan. Eli Lilly Japan K.K. was involved in the study design, data collection, data analysis, and preparation of the manuscript. The study sponsor also funded the journal’s Rapid Service and Open Access Fees.
Author information
Authors and affiliations.
Headache Center, Department of Neurology, Tominaga Hospital, Osaka, Japan
Takao Takeshima
Doi Clinic Internal Medicine/Neurology, Hiroshima, Japan
Department of Neurosurgery and Headache, Ooba Clinic, Oita, Japan
Satomi Ooba
Japan Drug Development and Medical Affairs, Eli Lilly Japan K.K., 5-1-28, Isogamidori, Chuo-ku, Kobe, 651-0086, Japan
Yuka Tanji, Akichika Ozeki & Mika Komori
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: Mika Komori, Akichika Ozeki, and Yuka Tanji; Funding acquisition and supervision: Mika Komori; Project administration: Mika Komori and Yuka Tanji; Data curation, formal analysis, investigation, methodology, software, validation, and visualization: Akichika Ozeki; Writing—review and editing: Takao Takeshima, Hikaru Doi, Satomi Ooba, Yuka Tanji, Akichika Ozeki, and Mika Komori.
Corresponding author
Correspondence to Mika Komori .
Ethics declarations
Conflict of interest.
Takao Takeshima received research funding/collaborative research expenses from Biohaven, Ltd., Eisai Co., Ltd., Eli Lilly Japan K.K., Lundbeck Japan K.K., and Shionogi & Co., Ltd., and reports personal fees from Amgen K.K., Daiichi Sankyo Company, Limited, Eli Lilly Japan K.K., and Otsuka Pharmaceutical Co., Ltd. Takao Takeshima also acted as an advisor to Hedgehog MedTech, Inc. and Sawai Pharmaceutical Co., Ltd. Hikaru Doi acted as an advisor to Amgen K.K. and Otsuka Pharmaceutical Co., Ltd.; reports research funding from AbbVie GK, Allergan, Inc., Amgen K.K., Biohaven, Ltd., Eli Lilly Japan K.K., Lundbeck Japan K.K., Otsuka Pharmaceutical Co., Ltd., and Pfizer Japan Inc.; and received personal fees from Amgen K.K., Daiichi Sankyo Company, Limited, Eli Lilly Japan K.K., and Otsuka Pharmaceutical Co., Ltd. Satomi Ooba reports personal fees from Amgen K.K., Daiichi Sankyo Company, Limited, Eli Lilly Japan K.K., and Otsuka Pharmaceutical Co., Ltd. Yuka Tanji, Akichika Ozeki, and Mika Komori are employees of Eli Lilly Japan K.K. and are shareholders in Eli Lilly and Company.
Ethical Approval
The protocols for both trials were reviewed and approved by local ethics review boards, and written informed consent was obtained from all patients before participation. Both studies were conducted in accordance with the Helsinki Declaration of 1964 and its later amendments. A list of the institutional ethics review boards is provided in Table S1 .
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 209 KB)
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/ .
Reprints and permissions
About this article
Takeshima, T., Doi, H., Ooba, S. et al. Clinical Evaluation After Discontinuation of Galcanezumab in Japanese Patients with Episodic and Chronic Migraine: Analysis of a Randomized, Placebo-Controlled Trial and Open-label Extension Study. Neurol Ther (2024). https://doi.org/10.1007/s40120-024-00602-z
Download citation
Received : 29 November 2023
Accepted : 08 March 2024
Published : 06 April 2024
DOI : https://doi.org/10.1007/s40120-024-00602-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Anti-CGRP mAb
- Clinical factors
- Drug discontinuation
- Galcanezumab
- Migraine disorders
- Migraine headache days
- Open-label extension study
- Post-treatment
- Randomized controlled trial
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Simple steps to develop trial follow-up procedures
Ona mccarthy.
Department of Population Health, London School of Hygiene and Tropical Medicine, Keppel St, London, WC1E 7HT United Kingdom
Rebecca S. French
Department of Social and Environmental Health Research, London School of Hygiene and Tropical Medicine, 15-17 Tavistock Place, London, WC1H 9SH UK
Ian Roberts
Caroline free.
Loss to follow-up in randomised controlled trials reduces statistical power and increases the potential for bias. Almost half of all trials fail to achieve their follow-up target. Statistical methods have been described for handling losses to follow-up and systematic reviews have identified interventions that increase follow-up. However, there is little guidance on how to develop practical follow-up procedures. This paper describes the development of follow-up procedures in a pilot randomised controlled trial of a sexual health intervention that required participants to provide and return questionnaires and chlamydia test samples in the post. We identified effective methods to increase follow-up from systematic reviews. We developed and tested prototype procedures to identify barriers to follow-up completion. We asked trial participants about their views on our follow-up procedures and revised the methods accordingly.
We identified 17 strategies to increase follow-up and employed all but five. We found that some postal test kits do not fit through letterboxes and that that the test instructions were complicated. After identifying the appropriate sized test kit and simplifying the instructions, we obtained user opinions. Users wanted kits to be sent in coloured envelopes (so that they could identify them easily), with simple instructions and questionnaires and wanted to be notified before we sent the kits. We achieved 92 % (183/200) overall follow-up for the postal questionnaire at 1 month and 82 % (163/200) at 12 months. We achieved 86 % (171/200) overall follow-up for the postal chlamydia test at 3 months and 80 % (160/200) at 12 months.
Conclusions
By using established methods to increase follow-up, testing prototype procedures and seeking user opinions, we achieved higher follow-up than previous sexual health trials. However, it is not possible to determine if the increase in response was due to our follow-up procedures.
Trial registration
Current Controlled Trials ISRCTN02304709 Date of registration: 27 March 2013.
Electronic supplementary material
The online version of this article (doi:10.1186/s13063-016-1155-1) contains supplementary material, which is available to authorized users.
Loss to follow-up in randomised controlled trials (RCTs) reduces statistical power and increases the potential for bias. Bias can occur when loss to follow-up is associated with the outcomes, such as when those lost have poorer outcomes then those retained. In order to explore the potential bias introduced, a range of plausible assumptions about outcomes in those lost to follow-up can be made [ 1 ]. A systematic review of trials reported in top medical journals found that when different plausible assumptions are made, the interpretation of trial results could change [ 1 ].
Almost half of all trials fail to achieve their follow-up target [ 2 ] and achieving high follow-up when collecting data on sensitive topics such as sexual health is particularly challenging. In sexual health research, response rates for self-reported data and test kits have been relatively low in both RCTs and surveys. The National Survey of Sexual Attitudes and Lifestyles (Natsal) study in the UK, a probability sample household survey, achieved 57.7 % response rate for face-to-face interviews and 60 % response for urine samples requested (not all responders were asked to provide a sample) [ 3 ]. A UK cross-sectional population-based study reported an uptake of chlamydia postal screening of 31.5 % in people aged 16–24 (the chlamydia screening studies ‘ClaSS’ project) [ 4 , 5 ]. A pilot trial of a sexual health website intervention for young people (‘Sexunzipped’) achieved 45 % follow-up using chlamydia postal test kits and 72 % for self-reported data at 3 months [ 6 ].
Effective interventions for increasing follow-up have been developed and evaluated [ 7 – 12 ]. However, there is little guidance on how to develop practical follow-up procedures. Specifically, there is no guidance for developing follow-up procedures for RCTs on sensitive topics.
Intervention development and pilot trial
The National Institute for Health Research Health Technology Assessment Programme (NIHR HTA) commissioned us to develop a mobile phone-based intervention to promote safer sex behaviour in young people aged 16–24 in the UK and to conduct a pilot RCT of the intervention. Key parameters for judging the success of the research were the acceptability of the intervention and the feasibility of recruitment and follow-up in a RCT. We conducted formative research, which included focus group discussions (FGD) to inform the intervention content.
In the pilot trial we recruited participants from seven sexual health services in London, Manchester, Cambridgeshire, Norfolk, Maidstone and Hull. People aged 16–24 who had had a recent positive chlamydia test result or had reported unsafe sex in the last year (defined as more than one partner and at least one occasion of unprotected sex) were eligible to take part. Recruitment staff invited eligible participants to take part in the study either at the site or by telephone. Participants provided informed consent either written or through the secure online database. Participants were randomised by a remote randomisation service, ensuring allocation concealment. Intervention or control group messages were sent from our automated system according to allocation. The intervention consisted of up to 63 text messages and employed 12 behaviour change techniques [ 13 ]. The messages were tailored according to infection status at enrolment and gender. Control participants received monthly messages about the importance of trial participation. We collected self-reported sexual health outcome data using postal questionnaires at 1 month and 12 months and chlamydia postal testing kits at 3 months and 12 months. Participants had the option of completing follow-up by attending the clinic or completing the questionnaire online, by text message or email.
We randomised 200 people between 9 September 2013 and 26 November 2013. Sixty-six percent of eligible participants joined the trial. The intervention development and pilot trial details are reported elsewhere [ 14 ].
In order to minimise bias in our trial we aimed to maximise follow-up. In this paper we describe the approach we used to develop the pilot trial follow-up procedures.
Ethical approval for the intervention development phase of the project was granted by NRES Committee London-Bentham on 6 November 2012 (REC reference number 12/LO/1329). Ethical approval for the pilot trial was granted by NRES Committee South East Coast-Surrey on 26 July 2013 (REC reference number 13/LO/1001).
We developed our trial follow-up procedures in three steps.
Step 1. Identifying evidence-based effective strategies to increase follow-up in trials
We searched the Cochrane Library for systematic reviews of trials of interventions designed to increase follow-up in research. We identified methods for which there was there was evidence of success in increasing response to postal follow-up requests in trials. We developed prototype follow-up procedures incorporating the effective strategies identified.
Step 2. Testing prototype follow-up procedures and materials
We obtained sample postal test kits routinely used in our trial recruitment sites (Cambridgeshire, Manchester and London) and test kits used in the NHS chlamydia postal testing services. We measured London letterboxes on central London streets and measured the test kits to identify those that would fit through the smallest letter boxes.
We attempted to follow the instructions that were included in the postal test kit that would fit through the smallest letter box. Based on this experience, we generated ideas on how to make the follow-up process easier. We generated prototype test kits including combinations of the original and simplified materials. We gave test kits to volunteers from the Clinical Trials Unit (CTU) and asked them to provide feedback on the original and simplified materials. We also consulted with experts in sexual health regarding the questionnaire design and follow-up procedures.
Step 3. Consulting with users
We asked young people aged 16–-24 for their views regarding the questionnaires and follow-up procedures in four of the eight FGD convened to inform the development of the intervention. We recruited FGD participants from community sexual and reproductive health services in South-east London, Greater Manchester and rural Cambridgeshire. Clinic staff used convenience and snowball sampling methods to recruit participants. The facilitators (OM, CF, and RF) provided verbal and written study information and obtained informed written consent from participants. All discussions were audio-recorded. We explored the participants’ preferences regarding intervention content and trial follow-up procedures.
As part of the pilot trial, we conducted phone interviews with participants [ 15 ]. The pilot trial consent form included an optional tick box where the participant could consent to be contacted regarding participation in the interview study. One hundred and sixty-seven participants consented to be contacted for an interview. We purposively sampled pilot trial participants 2 to 3 weeks after enrolment so that the interview sample varied according to age, gender, sexually-transmitted infection (STI) test result at enrolment, location (urban/rural) and whether they had been allocated to the intervention or control group. We gave participants verbal and written information about the study and obtained informed written consent either by email or text message. All the participants that we contacted agreed to participate ( n = 20). We conducted follow-up phone interviews with the same participants after sending the 3-month test kits. We asked participants for their views on the trial materials and follow-up procedures. All interviews were audio-recorded. We conducted a descriptive analysis after taking notes on the recordings.
Step 1. Effective strategies to increase postal follow-up
The follow-up strategies where there is evidence of increasing the odds of response are reported in Additional file 1 [ 7 – 12 , 16 ]. We employed all but five of the 17 strategies.
Step 2. Testing the prototype follow-up procedures
Only one test kit would fit through the smallest London letterbox (approximately 19 cm × 2.5 cm). This kit was provided by a laboratory diagnostic company, which contained pre-packaged components. Contents of the test kit included nine items (see Additional file 2 ). The components in the test kit included non-essential items, which we removed. The instructions for women could be confusing because the kit contained both the swab and the urine tube but they were required to provide only one sample.
Of the 12 CTU volunteers who were asked to provide a urine sample in the pouch, only one was able to use it successfully. Female volunteers did not express a preference for providing urine or vaginal swab samples. They preferred the simplified instructions and suggested that we include a statement in the postal letters about the importance of their participation so that they would feel ‘proud’ about doing something good.
Experts in sexual health questionnaire design suggested that we order the key questions, those on treatment and sexual health behaviour, first.
Step 3. User views
We conducted eight FGD with 82 participants. The median age of FGD participants was 17 years with 32 men and 50 women; 39 were from London, eight from Manchester and 35 from rural Cambridgeshire (see Additional file 3 ). Participants wanted the questionnaires to be as short as possible. They had no objections to the prototype questionnaire design and content. They reported that the study materials should be identifiable only to them and suggested using a coloured postal envelope. They thought that the simplified instructions were clear. Participants asked to receive a text or phone call before we sent the materials so they would know to look out for them. They were concerned that sending materials by recorded delivery could call attention to the post and that parents would ask questions. Women approved of the vaginal swab only, rather than providing a choice between swab and urine sample.
Final follow-up procedures
The results of steps 1–3 informed the final follow-up procedures.
Questionnaires
Our follow-up questionnaire was two pages long. Research evidence and feedback from the target group suggests that questionnaires should be as short as possible [ 7 , 9 – 11 , 16 ]. In accordance with guidance from our consultation with experts and evidence from Edwards et al. (2009), we ordered the key questions on treatment and sexual behaviour first [ 7 ]. We did not include personal details on the questionnaires and included a statement about confidentiality [ 7 ]. We offered an online questionnaire as an alternative to postal completion. Participants had the opportunity to reply to key questions by text and email if they had not responded after the final paper mailing.
Postal test kit
We selected a postal test kit that would fit through the smallest letterbox that we measured. The kits contained only essential components (see Additional file 4 ). We included a swab only for women. We used the simplified instruction slips. We used a pared-down laboratory slip that only required participants to write the date the sample was collected. Participants had the option of providing their test sample at the clinic.
The letters were as short as possible [ 7 , 9 – 11 , 16 ] (see Additional file 5 for a sample follow-up letter). The template was formal but the tone was casual [ 16 ]. The letters included a statement saying that the recipient was helping to improve the health of young people, a National Health Service/National Institute of Heath Research logo and the Trial Coordinator’s University address [ 7 ].
Envelopes and postage
We sent all correspondences in blue envelopes and used first class outward and incoming postage [ 7 , 16 ]. We did not send the post by recorded delivery or add a ‘teaser’ on the envelope (a ‘teaser’ is a statement indicating that that there may be a benefit to opening) because of its potential to call attention to participants’ participation in the study, which could compromise confidentiality [ 7 ].
Mailings and incentives
We notified all participants by text message before the initial mailing of the questionnaire and test kit [ 7 , 16 ]. All initial mailings of the questionnaire and test kit included £5 unconditional cash incentive [ 7 , 10 , 12 ]. We sent £20 cash to all participants who returned the chlamydia test sample [ 7 , 8 , 12 , 16 ]. While there is no evidence from systematic reviews for sending additional cash after receipt, our experience in the txt2stop trial was that additional participants did respond to this [ 17 ]. We contacted non-responders by phone, text messages and email, unless they opted out of further follow-up at any stage [ 7 – 9 , 16 ].
Month 1 questionnaire
The initial questionnaire posting included a £5 cash unconditional incentive [ 7 , 10 , 12 ]. We sent an email message, which included a link to the online questionnaire, within a week after the initial questionnaire posting [ 7 – 9 , 16 ]. We posted the questionnaire again, 2 to 3 weeks later, and sent a second email within a week after this [ 7 , 16 ]. The third paper mailing included a statement in the letter saying that we would send an additional £10 if we received it within 2 weeks [ 7 , 8 , 12 , 16 ]. The fourth paper mailing included a statement in the letter that we would enter participants into a £50 prize draw if they returned the questionnaire within 2 weeks [ 7 , 8 , 12 , 16 ]. All responders were eligible for the prize draw. Finally, we emailed, texted and posted key outcome questions to non-responders [ 7 – 9 , 16 ].
Month 3 chlamydia test
The initial postal test kit included a £5 cash unconditional incentive [ 7 , 10 , 12 ]. All letters mentioned that they would receive £20 if they returned the sample [ 7 , 8 , 12 , 16 ]. We sent the test kit to non-responders a further three times [ 7 , 16 ]. The fourth mailing included a statement in the letter saying that they would be entered into a prize draw for £50 if we received it within 2 weeks [ 7 , 8 , 12 , 16 ]. At each mailing, we followed up with participants by phone and email [ 7 – 9 ]. We sent the test kit to non-responders once a month [ 7 , 16 ].
Month 12 questionnaire and chlamydia test
We sent the initial 12-month questionnaire and test kit together with a £10 cash unconditional incentive [ 7 , 10 , 12 ]. All letters mentioned that they would receive £20 if they returned the sample [ 7 , 8 , 12 , 16 ]. The initial letter included a statement saying that we would enter participants into a £50 prize draw if they returned both the questionnaire and test [ 7 , 8 , 12 , 16 ]. We phoned and sent an email message, which included a link to the online questionnaire, around 3 weeks after the initial mailing [ 7 – 9 , 16 ]. We sent the questionnaire and test kit to non-responders a further three times [ 7 – 9 , 16 ]. At each mailing, we followed up with participants by phone and email [ 7 – 9 ]. We sent the test kit to non-responders once a month [ 7 , 16 ]. We emailed, texted and posted one or two key outcome questions to questionnaire non-responders (according to their chlamydia status at enrolment) [ 7 – 9 , 16 ].
Ninety-two percent (183/200) provided questionnaire outcome data at 1 month, 86 % (171/200) provided a chlamydia test sample at 3 months, 82 % (163/200) provided questionnaire outcome data and 80 % (160/200) provided a chlamydia test sample at 12 months (see Additional file 6 ).
User views of the final follow-up procedures
We interviewed 17 of the original 20 main pilot trial interview participants within 2 months of sending the 3-month postal STI testing kits (see Additional file 7 ). Nine follow-up interview participants tested positive at enrolment. None of the participants had any criticisms of the procedures. They thought that the pre-notification served as a reminder to look in the post. Participants mentioned that the blue envelope helped them recognise that it was study material. One said that the letters were polite in that we were not telling them that they had to send it back and another appreciated that they were short and to the point. Most participants thought that the instructions were easy to follow and most did not have any criticisms of the postal test kit. One participant said that initially they were not clear whether they should post the box on its own or inside an envelope. Another suggested including a urine collection pouch. One participant said that she initially had difficulty opening the swab. Another suggested that we include a condom in the kit.
Most participants said that they would have returned the questionnaire and chlamydia sample even if they were not offered an incentive. Some indicated that the motivating factor was their health rather than the money. A few participants mentioned that the unconditional £5 motivated them to return it and another wanted to return it because we ‘treated’ them and said they would have procrastinated without it. Women preferred the swab sample collection method and no participants mentioned that they would rather have had a choice (swab or urine).
Summary of the main findings
This paper describes how we developed follow-up procedures for the pilot RCT of our sexual health intervention delivered by text message. We used evidence-based methods, tested prototype procedures and consulted with users. We achieved 92 % (183/200) overall follow-up for the self-reported data at 1 month and 82 % (163/200) at 12 months. We achieved 86 % (171/200) follow-up for the chlamydia test at 3 months and 80 % (160/200) at 12 months.
Comparisons with other studies
This pilot trial’s follow-up response for return of postal chlamydia test samples is high when compared to similar trials, screening initiatives and collection of self-reported sexual health data [ 4 , 6 ]. The chlamydia screening studies (‘ClaSS’) project [ 4 , 5 ] used steps described in this paper such as choosing a test kit that would fit through a ‘standard’ letterbox and testing the kit with the target group [ 18 ]. However, the researchers evaluated interventions to increase follow-up after the project. Our response may be higher than the ‘ClaSS’ project because our participants agreed to provide follow-up data when they were recruited, we offered unconditional incentives and included only essential test kit components. The ‘Sexunzipped’ trial used evidence-based methods to increase postal follow-up response [ 6 ]. Response in this trial may have benefitted from testing all the trial procedures and consulting with the target group [ 17 ].
We developed follow-up procedures in a similar way in the smoking cessation txt2stop pilot and main trial [ 17 , 19 ]. The pilot trial achieved 96 % response for self-reported data at 1 month and 92 % at 6 months [ 19 ]. In the main trial, we achieved 95 % (5524/5800) response for self-reported data and 81 % (542/666) response for postal salivary cotinine tests at 6 months [ 17 ]. An earlier trial that did not use a similar approach to develop follow-up procedures only achieved 74 % response for self-reported data collected at 6 months [ 20 ].
Limitations
We describe a single case study using three steps to develop follow-up procedures. The steps are not guaranteed to produce useful information. For example, researchers may identify barriers in step 2, but it may not be possible to overcome the barriers when resources are limited. Consulting with the target group could be challenging when administering a multi-site international trial from a central location. We did not test the kits with young people in step 2, which could have been beneficial. Our search for strategies to increase postal follow-up involved searching the Cochrane Library only. Researchers who adopt our method could benefit from searching a range of databases.
Implications of findings
Our case study suggests that our approach could increase follow-up in trials. Previous work has highlighted the problems with missing data, and its reporting and handling [ 1 , 21 ]. Our approach aims to boost the number of outcome events recorded, minimising bias and increasing statistical power. An advantage of this approach is that researchers can make fewer post-hoc assumptions about outcomes in participants lost to follow-up. Some of the specific follow-up procedures that we used could be relevant to other trials on sensitive topics with young people (such as using coloured envelopes). By designing acceptable and effective follow-up procedures at the outset of trials, researchers could avoid spending time and resources deploying less effective follow-up procedures.
The approach described in this paper gives researchers an additional tool to minimise losses to follow-up in trials. Our results show that a high follow-up to self-reported data in questionnaires and postal test kits can be achieved, even in sensitive areas such as sexual health.
Acknowledgements
We would like to thank LSHTM’s Clinical Trials Unit and young people in Cambridgeshire, London and Manchester for their valuable suggestions regarding the follow-up methods and the National Institute for Health Research Health Technology Assessment Programme for funding the research.
Abbreviations
Additional files.
Key findings and implications for follow-up design. Details of follow-up strategies where there is evidence of increasing the odds of response. (DOCX 35 kb)
Lab diagnostic company’s postal test kit components. List of components of the lab diagnostic company’s postal test kit. (DOCX 16 kb)
Focus group discussion participant characteristics. Characteristics of the participants who took part in the focus group discussions. (DOCX 12 kb)
Final postal test kit components. List of the final components of the postal test kit used in the pilot trial. (DOCX 15 kb)
Sample follow-up letter. A sample of a follow-up letter sent to pilot trial participants. (DOCX 53 kb)
Follow-up response. Cumulative response at each mailing and response by mode. (DOCX 16 kb)
Follow-up interview participant characteristics. Characteristics of the participants who took part in the follow-up interview study. (DOCX 13 kb)
Competing interests
The authors declare that they have no completing interests.
Authors’ contributions
OM contributed to the design of the follow-up materials and procedure, managed the trial, conducted the follow-up, co-facilitated the focus group discussions, conducted the follow-up interviews and wrote the manuscript. RF co-facilitated the FGD and contributed to later versions of the manuscript. IR contributed to the design of the pilot trial, to later versions of the manuscript and contributed to a similar approach used to design the follow-up procedures in the txt2stop trial. CF conceived the project, designed and took overall responsibility for the conduct of the pilot trial, contributed to the design of the follow-up materials and procedures, co-facilitated FGD and provided guidance on all versions of the manuscript. All authors read and approved the final manuscript.
Authors’ information
OM, BA MSc, Research Fellow, the London School of Hygiene and Tropical Medicine
RF, MSc PhD, Senior Lecturer of Sexual and Reproductive Health, the London School of Hygiene and Tropical Medicine
IR, MBBCh FRCP FPH, Professor of Epidemiology and Public Heath, the London School of Hygiene and Tropical Medicine
CF, MBBCh MSc PhD MRCGP , Clinical Senior Lecturer in Epidemiology, the London School of Hygiene and Tropical Medicine
Contributor Information
Ona McCarthy, Phone: +44 0207 927 2581, Email: [email protected] .
Rebecca S. French, Phone: +44 0207 927 2047, Email: [email protected] .
Ian Roberts, Phone: +44 0207 958 8128, Email: [email protected] .
Caroline Free, Phone: +44 0207 958 8109, Email: [email protected] .

- Latest news
- UCL in the media
- Services for media
- Student news
- Tell us your story

Shorter scan to diagnose prostate cancer will increase availability and reduce cost
8 April 2024
Removing one step from a three-part MRI scan, which could make them quicker, cheaper and more accessible, had no negative impact on diagnostic accuracy, according to clinical trial results led by UCL and UCLH.
The results, presented at the European Association of Urology (EAU) conference in Paris, confirmed the viability of a two-part MRI scan to diagnose prostate cancer and are likely to lead to changes in clinical practice. The trial was funded by Prostate Cancer UK and the John Black Charitable Foundation
Prostate cancer is the most common cancer in men, with around 52,000 diagnoses and 12,000 deaths each year in the UK. The introduction of MRI scans over the last decade, led by work from UCL researchers, has been the biggest change in how prostate cancer is diagnosed for the past 30 years.
A three-part ‘multiparametric’ MRI of the prostate is standard of care in the UK for patients suspected of having prostate cancer, which currently includes a dye injection as its third part. Abnormalities seen on the MRI scan allow targeted tissue biopsies to be taken that can improve cancer detection. A normal MRI result, which occurs in around a third of patients, is reassuring and allows men to avoid an unnecessary biopsy.
Increased demand for prostate MRI meant that in 2019, the most recent year for when data is available, only 62% of men in England and Wales who needed a prostate MRI received one. The situation is even worse in many other countries around the world.
A large prostate cancer screening trial involving MRI is expected to be announced later this year, which would likely be offered to healthy men aged 50-65 and if successful, could lead to the introduction of full national screening program, thus further increasing the demand for MRI scans.
In this study, called PRIME, cancer specialists from 22 hospitals in 12 countries across the world recruited 555 patients to see whether a shorter, streamlined two-part ‘biparametric’ MRI without the dye injection could detect cancer at the same rate as the full three-part MRI.
All patients underwent the full three-part multiparametric scan. Radiologists then assessed the two-part biparametric scan without the dye, and separately assessed the three-part multiparametric scan with the dye for every patient. A targeted prostate biopsy was done when required to confirm whether or not the diagnosis was correct.
Researchers from UCL and UCLH analysed the results and confirmed that the two-stage scan was just as effective at diagnosing prostate cancer. They found that 29% of the patients had important prostate cancer diagnosed by the shorter two-part biparametric scan, which was just as effective as the longer three-part multiparametric scan that also detected 29%.
Dr Clare Allen, a Lead Radiologist on the trial from UCLH, said: “The results of the trial indicate that in most patients we are unlikely to miss significant prostate cancer if we stop doing the contrast scan. The scans will be quicker, cheaper and can be offered to more men. It is critical to emphasise that dropping the third part of the MRI scan is dependent on the first two parts of the scan being of high quality. MRI scanners in this study were optimised before they were used, so we would advise centres wishing to change to the two-part scan to ensure MRI scanners and image quality are optimised first.”
Dr Francesco Giganti, a Lead Radiologist on the trial from UCL Surgery & Interventional Science and UCLH said: “The three-part multiparametric MRI scan has been a game-changer for the diagnosis of prostate cancer, sparing thousands of patients unnecessary biopsies. The third stage of this procedure involves injecting a dye into the patient which can show up brightly on the MRI scan when a cancer is there, but this step takes time, involves an injection and can rarely cause some mild side effects.
“Being able to make accurate diagnoses without the contrast stage will reduce scan time by around a third, meaning we can offer scans to more men using the same number of scanners and operators. However, it is vital that the scans are of optimal diagnostic quality.”
As well as time savings, a two-stage MRI would generate significant cost savings per scan. In the NHS currently, a three-phase MRI scan costs on average, £273. At £145, a two-phase scan is 47% cheaper. In countries like the US where healthcare costs tend to be much higher, the savings could be even greater.
Associate Professor Veeru Kasivisvanathan, Lead researcher and Chief Investigator on the trial from UCL Surgery & Interventional Science and UCLH, said: “Since multiparametric MRI became standard of care for diagnosing prostate cancer, an important question has been whether we could streamline the scan further in order to make it more accessible, not just in the UK but in a wide range of healthcare settings and models.
“These results suggest that, providing that MRI image quality is good, we can adopt a shorter two-part biparametric MRI as the new standard of care for prostate cancer diagnosis. These results also make a strong case for prioritising work to optimise MRI quality nationally and internationally.
“I’m really proud of the team for delivering a complex, international multi-centre trial so quickly. I hope our results will soon be incorporated into clinical practice so that as many men with suspected prostate cancer as possible can benefit. Our vision is that every man who needs a prostate MRI should be able to get a high-quality one.”
Dr Matthew Hobbs, Director of Research at Prostate Cancer UK, said: “It’s rare to see results like this that deliver impact for men so quickly after funding the research. MRI has already revolutionised prostate cancer diagnosis, and we’re thrilled to have funded this study that could further improve diagnosis for men across the globe.
“These results mean that men could now be given quicker scans, that are just as good, don’t require an injection and are cheaper to perform. This will allow more men to benefit from a better, more accurate diagnosis at a lower cost to healthcare systems not only in the UK, but worldwide. Crucially, this team has also produced guidelines to help hospitals improve the quality of their scans to such an extent that these new, quicker scans can be just as effective as the old ones.
“It also highlights just how important it is not to rest on our laurels when we make progress, which is why Prostate Cancer UK will continue working to improve new treatments and technologies through investment in excellent research like this.”
- Associate Professor Veeru Kasivisvanathan's academic profile
- Associate Professor Francesco Giganti's academic profile
- UCL Division on Surgery and Interventional Science
- UCL Faculty of Medical Sciences
- PRIME trial
Credit: Smederevac on iStock.
Media contact
Dr matt midgley.
E: m.midgley [at] ucl.ac.uk
We couldn’t find any results matching your search.
Please try using other words for your search or explore other sections of the website for relevant information.
We’re sorry, we are currently experiencing some issues, please try again later.
Our team is working diligently to resolve the issue. Thank you for your patience and understanding.
InspireMD Announces Abstract of One-Year Follow-Up Results from the C-GUARDIANS U.S. Investigational Device Exemption (IDE) Clinical Trial Accepted for Presentation at LINC 2024
TEL AVIV, Israel and MIAMI , March 26, 2024 (GLOBE NEWSWIRE) -- InspireMD, Inc. (Nasdaq: NSPR), developer of the CGuard™ Embolic Prevention Stent System (EPS) for the prevention of stroke, today announced that an abstract of the one-year outcomes from its C-GUARDIANS IDE clinical trial of the CGuard™ Prime Carotid Stent System for the treatment of carotid artery stenosis has been accepted for presentation at the Leipzig Interventional Course (LINC) 2024, which is being held May 28-31 , in Leipzig, Germany .
Presentation details:
Marvin Slosman , chief executive officer of InspireMD , stated, “At last year’s VIVA and VEITH conferences in November, Dr. Metzger , principal investigator for the C-GUARDIANS trial, presented positive 30-day follow-up data from the trial which demonstrated a DSMI1 rate of just 0.95% in the Intent-To-Treat (ITT) analysis population, and 0.63% in the per-protocol (PP) analysis population. We are very excited to announce today that an abstract of the 12-month outcomes data from this important trial has been accepted for presentation at the upcoming LINC 2024 conference, which is among the most influential and widely attended meetings focused on vascular intervention. We are optimistic that the results will show a similar level of neuroprotection, which may support a Premarket Approval Application (PMA) later this year and allow us to prepare for a commercial launch of CGuard Prime in the U.S. in the first half of 2025, if approved.”
About C-GUARDIANS The C-GUARDIANS clinical trial evaluated the safety and efficacy of the CGuard™ Carotid Stent System for the treatment of carotid artery stenosis. The study enrolled 316 patients across 24 trial sites in the U.S. and Europe .
In This Story
To add symbols:
- Type a symbol or company name. When the symbol you want to add appears, add it to My Quotes by selecting it and pressing Enter/Return.
- Copy and paste multiple symbols separated by spaces.
These symbols will be available throughout the site during your session.
Your symbols have been updated
Edit watchlist.
- Type a symbol or company name. When the symbol you want to add appears, add it to Watchlist by selecting it and pressing Enter/Return.
Opt in to Smart Portfolio
Smart Portfolio is supported by our partner TipRanks. By connecting my portfolio to TipRanks Smart Portfolio I agree to their Terms of Use .

IMAGES
VIDEO
COMMENTS
Follow-Up Time Periods. Example: If a study includes one cycle of therapy, and 3 follow up visits that are 3 months apart, after the subjects third follow-up visit at month 9, they will then be considered "off study". EXAMPLE: Subjects will be followed for toxicity for 30 days after treatment has been discontinued or until death, whichever ...
Abstract. After clinical trial participants have consented, provided baseline data, and been randomized, each participant begins study treatment and follow-up. This chapter covers administering a participant's randomly assigned treatment regimen and collecting the participant's trial data through the end of their time in the study, along ...
Background Evidence-based establishment and implementation of best principles, laws and ordinances that regulate clinical research depend on the consultation and involvement of trial participants. Yet, guidance on methodological approaches to obtain trial participants' perspectives is currently missing. This scoping review therefore aimed at identifying, describing and evaluating research ...
Notable among these are the PLCO trial, with 13-year follow up in 76,685 men , an individual patient overview of 5 trials of 727,718 men with a 10-year follow up , and a large overview view of ...
A Comprehensive and Practical Guide to Clinical Trials. 2017, Pages 133-154. Chapter 14 - Screening, Treatment, and Safety Follow-up Visit. Author links open ... Treatment, and Safety follow-up visit (end of treatment) as per the case study (see Chapter 7: Planning). The Informed Consent process is discussed and an example of sequence of events ...
Background Clinical trials typically have a relatively short follow-up period, and may both underestimate potential benefits of treatments investigated, and fail to detect hazards, which can take much longer to emerge. Prolonged follow-up of trial participants after the end of the scheduled trial period can provide important information on both efficacy and safety outcomes. This protocol ...
The term 'follow-up' in clinical trials can have different interpretations dependent on the role of the researcher. Please provide your definition of the term 'follow-up' in relation to cancer clinical trials. ... and continue on the trial for follow-up visits and are less likely to withdraw from studies. Strongly agree (7) 80: 6.21:
Survey of cancer clinical trials. To investigate how follow-up time is currently being reported in cancer clinical trials, in which overall survival is a common endpoint, we conducted a search of the Original Reports in the Journal of Clinical Oncology between January 2013 and June 2013 for articles containing the phrase "median follow up." Of the 60 articles (37% of the 161 Original ...
Therefore, when designing a post-authorisation patients' follow-up system, it is always necessary to take into account any existing requirements and guidelines for follow-up of subjects in clinical trials, as well as the follow-up system that was, or still is, in place for subjects of clinical trials with the particular ATMP.
A Comprehensive and Practical Guide to Clinical Trials. 2017, Pages 133-154. Chapter 14 - Screening, Treatment, and Safety Follow-up Visit. Author links open ... Treatment, and Safety follow-up visit (end of treatment) as per the case study (see Chapter 7: Planning). The Informed Consent process is discussed and an example of sequence of events ...
This chapter discusses the steps involved in patient follow-up, close-out, and post-trial follow-up. Topics covered include the maintenance of investigator and patient interest during follow-up, losses to the follow-up, close-out of patient follow-up, termination stage, and post-trial patient follow-up.
The 5-year follow-up assessed sustainability of this blood pressure reduction, after health worker intervention ceased at 12 months. The authors from their systematic review of effectiveness of community health workers in reducing blood pressure and controlling hypertension noted that just five (15%) of 34 completed randomised trials had an intervention lasting more than 1 year, and post ...
OVERVIEW OF A TYPICAL CLINICAL TRIAL VISIT WINDOW ALGORITHM Below is a snapshot example of the Schedule of Assessment in a clinical protocol. A visit's target study ... windows, or assigned to 'Follow-up' visit regardless of the nominal visits. Baseline visits or the visits when the treatment first starts (Day 0 or Day 1) can either be ...
5. The Follow-Up Visit. The clinical study volunteer follow-up visit may take place weeks, months, or rarely, years after the last clinical trial visit. It is important to note that the follow-up visits for a clinical trial are just as important as the clinical trial visits that took place during the study.
b) follow-up rates of SAEs reporting (SDV during IMV if differs from other sites, re-training of PI/SN on dose modifications, SAE registration, documentation, and reporting); c) ICFs and recruitment strategies should be verified on-site for all patients if some ICFs not sent to the Clinical Trials Unit/ recruitment is too slow/ any issues are ...
Site Visit Follow-Up Communications. Following a site visit, the monitor normally writes a "site visit" or "follow-up" letter to the site that clearly describes visit findings, including any items requiring follow up. Timeliness is important, so monitors normally transmit site visit letters within 10 to 20 business days after visits ...
Clinical trials typically have a relatively short follow-up period, and may both underestimate potential benefits of treatments investigated, and fail to detect hazards, which can take much longer to emerge. Prolonged follow-up of trial participants after the end of the scheduled trial period can provide important information on both efficacy ...
CLINICAL TRIAL VISITS. The number of your clinical visits will depend on the specifics and length of the study. There may be follow-up visits that include a brief physical exam (not generally in phase IV observational ), a review of your study medications and your symptoms, and lab tests. Meet with the specialist. Ask any questions you may have.
Therefore it's not unusual to reimburse sites anywhere between $50 to $250+ per screen failure. b. Baseline/index Procedure and Follow-up Visits. Depending on the clinical trial design, data is collected at baseline or index procedures and follow-up visits.
Travel reimbursement €20 per visit: €3,000,000: €413.60: Clinical appointment + telephone ... Post-trial follow-up of large RCTs can contribute significantly to the scientific value of a trial by determining the longer-term magnitude of the effects of an intervention. PTFU is valuable to ensure that there are no long-term hazards or ...
Effect of single follow-up home visit on readmission in a group of frail elderly patients - a Danish randomized clinical trial BMC Health Serv Res . 2019 Oct 25;19(1):751. doi: 10.1186/s12913-019-4528-9.
Clinical trials are scientific studies that involve people in research and are the only way to advance cancer treatment. Before people are given a new intervention, it is carefully studied in the laboratory. Studies with the most promising results are then moved into clinical trials with people. Clinical trials are used to evaluate new and ...
The Stanford Cancer Institute (SCI) Clinical Trials Office (CTO) provides regulatory, administrative, research support, budget, and educational services to SCI investigators conducting cancer clinical trials. Secured: Faculty and Staff Resources.
A follow-up (Visit 2) was scheduled for 2 weeks after the self-administration of the second SER dose. Should pseudohyphae persist at Visit 2, participants were provided with four more weekly SER doses. ... 2019), and the study was registered with the Thai Clinical Trials Registry (TCTR20190308004 on March 8, 2019). Eligible women provided ...
Chinese Clinical Trial Registry (ChiCTR2300067631), registered 11 January 2023. ... determined, including cumulative survival, viral load, CD4 T-cell count and postoperative complications. The median follow-up of HIV recipients was 36 months after OLT (interquartile range 12-39 months). ... To view a copy of this licence, visit http ...
The withdrawal criteria are as follows: (1) the follow-up was lost; (2) informed consent was withdrawn; and (3) decision to withdraw for severe adverse events. Methodology. A prospective, randomized controlled clinical trial will be conducted at Nanjing Lishui People's Hospital.
Introduction This analysis of two Japanese clinical trials evaluated efficacy and safety after galcanezumab (GMB) discontinuation in patients with episodic migraine (EM) and chronic migraine (CM). Methods Data were from a 6-month, randomized, double-blind, placebo [PBO]-controlled primary trial (patients with EM) and a 12-month open-label extension trial (patients with EM/CM). Patients ...
This pilot trial's follow-up response for return of postal chlamydia test samples is high when compared to similar trials, ... Frost C. Follow-up by mail in clinical trials: does questionnaire length matter? Control Clin Trials. 2004; 25 (1):31-52. doi: 10.1016/j.cct.2003.08.013. [Google Scholar] 12. Edwards P, Cooper R, Roberts I, Frost C ...
"I'm really proud of the team for delivering a complex, international multi-centre trial so quickly. I hope our results will soon be incorporated into clinical practice so that as many men with suspected prostate cancer as possible can benefit. Our vision is that every man who needs a prostate MRI should be able to get a high-quality one."
One-Year Follow-Up Results from the C-GUARDIANS Pivotal Trial of the CGuard™ Carotid Stent System Presenter: Chris Metzger , M.D., System Vascular Chief at Ohio Health , and lead investigator of ...