
Official websites use .mass.gov
Secure websites use HTTPS certificate
A lock icon ( ) or https:// means you’ve safely connected to the official website. Share sensitive information only on official, secure websites.
- search across the entire site
- search in Executive Office of Health and Human Services
- search in Department of Public Health
- This page, Free telehealth for COVID-19 treatment with Paxlovid, is offered by
- Executive Office of Health and Human Services
- Department of Public Health

Free telehealth for COVID-19 treatment with Paxlovid
Table of contents, who can take paxlovid.
Paxlovid is for individuals age 12 and older who have tested positive for COVID-19, are at increased risk of severe disease, and have developed COVID-19 symptoms in the last 5 days, even mild ones (such as runny nose, sore throat, or cough). Qualifying conditions that put you at higher risk for severe disease include being over age 50, a current or former smoker, cancer, diabetes, and obesity, among others.
Get a free telehealth consultation
Free telehealth consultations are available for eligible individuals 12 or older who are currently living in Massachusetts and insurance is not required. If you’re eligible, you’ll be connected with a clinician for a phone or video consultation within 30 minutes.
Watch this video for an explanation of how this service works, interpreted in American Sign Language (ASL).
- Virtual consultations are available in English , Spanish , Haitian Creole , Portuguese , Mandarin Chinese (simplified) , Traditional Chinese , Tagalog , Arabic , Russian , Somali , French , Korean , Vietnamese , Amharic , Hmong , Hindi , and Marshallese .
- Call (833) 450-3461 (compatible with Video Relay Services)
Accessibility accommodations for telehealth visits, including American Sign Language (ASL) interpretation and captioning (CART or automatic), are now available. Note that these visits may take up to 48 hours to be scheduled either online or by phone.
This service is not for medical emergencies. If you are experiencing a medical emergency like having trouble breathing, please call 911.
Before getting started, make sure you have:
- A list of medications or vitamins you are taking
- If you have kidney disease, a copy of your lab results from the last 3 months because the dose may need to be adjusted
Clinicians are available every day from 8 a.m. to 10 p.m. EST.
This treatment is not a substitute for vaccination, which can prevent serious illness, hospitalization, and death. Learn more about getting vaccinated .
Learn more about COVID-19 treatments at mass.gov/CovidTreatments
Cost savings options for Paxlovid are available
Depending on your insurance status, you may need to pay a co-pay when picking up your Paxlovid prescription. If you need financial assistance to pay your co-pay, there are options available. Learn more below.
Individuals with MassHealth, Medicare, OR do not have health insurance:
- The U.S. Government Patient Assistance Program provides a voucher for free Paxlovid. You can learn more at paxlovid.iassist.com . Plus, overnight delivery may be available.
- Enroll by calling 1-877-219-7225 or by visiting paxlovid.iassist.com .
Individuals with a commercial or private health insurance plan:
- Pfizer’s Co-Pay Savings Program can help you save on the prescription cost. You can learn more at www.paxlovid.com/enroll-in-co-pay-program .
- Enroll by calling 1-877-219-7225 or by visiting www.paxlovid.com/enroll-in-co-pay-program .
Telehealth service is offered in collaboration with Color Health, Inc. If you need help, contact the Color Support Team at 844-352-6567 or email [email protected] from 9 a.m. to 8 p.m. EST.
Also, review Frequently Asked Telehealth Questions to learn more.
Covid-19 Treatment Flyers
The Covid-19 telehealth treatment flyers are available in English, Haitian Creole, Portuguese, Spanish, Simplified Chinese, Traditional Chinese, Russian, Korean, Arabic, French, Somali, Khmer, and Vietnamese.
- English (PDF) | Text-only (Docx)
- Haitian Creole (PDF) | Text-only (Docx)
- Portuguese (PDF) | Text-only (Docx)
- Spanish (PDF) | Text-only (Docx)
- Simplified Chinese (PDF) | Text-only (Docx)
- Traditional Chinese (PDF) | Text-only (Docx)
- Russian (PDF) | Text-only (Docx)
- Korean (PDF) | Text-only (Docx)
- Arabic (PDF) | Text-only (Docx)
- French (PDF) | Text-only (Docx)
- Somali (PDF) | Text-only (Docx)
- Khmer (PDF) | Text-only (Docx)
- Vietnamese (PDF) | Text-only (Docx)
Additional Resources
Contact for free telehealth for covid-19 treatment with paxlovid, color support team.
Available 9 a.m.–8 p.m. EST
- Begin your telehealth consult
- Information for providers
- COVID-19 vaccines
- COVID-19 testing
Help Us Improve Mass.gov with your feedback
The feedback will only be used for improving the website. If you need assistance, please contact the Executive Office of Health and Human Services . Please limit your input to 500 characters.
Thank you for your website feedback! We will use this information to improve this page.
If you would like to continue helping us improve Mass.gov, join our user panel to test new features for the site.
About Us | Contact Us | Newsroom
- Spanish - Español
Free Telehealth Appointments for COVID-19 Treatment
Telehealth is a free and easy way to see if COVID-19 oral antivirals such as Paxlovid , are right for you. Oral antivirals, which are COVID-19 treatment pills taken by mouth, are available by prescription only. They must be taken within the first five days of experiencing COVID-19 symptoms or testing positive for COVID-19.
If you have tested positive for COVID-19 and want to schedule a virtual telehealth appointment with a health care provider to determine treatment eligibility, learn more about this service below and how to book a free virtual appointment. This service is currently provided by the Washington State Department of Health (DOH) in collaboration with Color Health.
UPDATE: Oral antiviral medications are often used in the treatment of COVID-19. Previously, the federal government provided oral antivirals like Paxlovid and Lagevrio at no cost to health care providers and the general public. As of November 2023, most pharmacies and clinics are required to purchase this medication. This change may now affect how much you pay for the medication. DOH COVID-19 telehealth consultations remain free.
Who Should Take Paxlovid and When?
- You have mild to moderate COVID-19 symptoms.
- Treatment must begin as soon as possible after testing positive and within five days of symptom onset.
- You should be 12 years of age or older.
- You should also be at higher risk for severe disease, such as being older than 50 years of age or having certain medical conditions , such as chronic heart or lung disease, obesity, diabetes or a weakened immune response.
How to Set Up a Telehealth Appointment
If you think you might be eligible for oral antiviral treatment, begin your free consultation below and complete a brief intake form.
Completing the intake form to request a consultation should take you approximately 5 minutes. If the information provided indicates you may be eligible for treatment, you will be given a link for a video consultation with a health care provider. After joining the video call, a health care provider will join you within 5–30 minutes to complete the evaluation.
Begin Your Free Consultation
After receiving a telehealth consultation, if treatment is appropriate for you, you will receive a prescription for pharmacy pickup.
Telehealth providers are available every day from 8 a.m. to 8 p.m. Pacific standard time (PST). Language assistance is available. If you do not have access to a computer or smart phone, you can call 1-833-273-6330 to complete the questionnaire over the phone with a support agent.
Note: If you need additional languages assistance, or other COVID-19 services please reach out to the DOH information line at 1-800-525-0127. You can also learn more about other COVID-19 services and programs that might be available to support you.
Additional Information
You might have to pay more for these medications depending on your insurance. If you're uninsured or on Medicare, Medicaid, Veterans Affairs, or Indian Health Services, oral antiviral prescriptions will still be available at no cost though the U.S. Government Patient Assistance Program. Call 1-877-219-7225 or visit the PAXCESS Patient Portal to enroll. Individuals with private insurance can apply for the Co-Pay Savings program at Paxlovid.com to access reduced or no-cost medication.
The Washington State Department of Health (DOH) offers free consultations through their COVID-19 Telehealth program for individuals with COVID-19 symptoms. It’s possible you could obtain a prescription for oral antiviral medication through this program that you then could pick-up at a pharmacy or have delivered to your home.
To qualify for the free COVID-19 Telehealth program, you should have mild to moderate COVID-19 symptoms, be 12 years or older, and at higher risk for severe disease or certain medication conditions.
You can start your free, video consultation by clicking on “Begin Your Free Consultation” on the DOH website to register online or by calling 1-833-273-6330 to complete a questionnaire with a support agent over the phone.
Remember, DOH telehealth consultations are free, but the cost and distribution method of the oral antiviral medication to treat COVID-19 might change based on your insurance coverage.
Learn more about COVID-19 treatments .
Our COVID-19 Therapies Quick Guide (PDF) provides an overview of treatments | Available in additional languages
Learn more about oral antivirals at What Are Oral Antivirals information sheet (PDF) | Available in additional languages
Isolation and quarantine are key strategies to reduce the spread of COVID-19. If you test positive for COVID-19, have symptoms, or are identified as a close contact of someone who has COVID-19, you should isolate or quarantine as appropriate.
For more details on isolation and quarantine, including activities you should avoid during your isolation or quarantine period, please see DOH’s guidance What to do if you test positive for COVID-19 and What to do if you were potentially exposed to someone with COVID-19 . You can also visit the CDC’s Quarantine and Isolation page .
For general inquiries on the telehealth initiative, other COVID-19 related guidance, or if you need food or other assistance while you isolate, please call the DOH information line at 1-800-525-0127.
- Care-a-Van Mobile Health Services
- Disability Organizations
- Drug User Health
- Food Safety
- Healthy Aging
- Healthy Home
- Illness and Disease
- Immunization
- Infants and Children
- Injury and Violence Prevention
- Men's Health
- Nutrition and Physical Activity
- Oral Health
- Poisoning and Drug Overdose
- Sexual and Reproductive Health
- Adolescents & Young Adults
- Women's Health
- Air Quality
- Climate and Health
- Contaminants
- Drinking Water
- Essentials for Childhood Initiative
- Health Equity
- Healthy Communities Washington
- Wastewater Management
- Water Recreation
- Worksite Wellness
- Facilities - New, Renew or Update
- File Complaint About Provider or Facility
- Healthcare Enforcement and Licensing Management System (HELMS)
- Healthcare Professional Credentialing Requirements
- Medical Commission
- Board of Nursing
- Professions - New, Renew or Update
- Provider Credential Search
- Veterans, Service Members and their Families
- Vital Records
- Data Guidelines
- Data Systems
- Data Topics A-Z
- Diseases and Chronic Conditions
- Environmental Health
- Health Behaviors
- Health Statistics
- Healthcare in Washington
- Injury Violence and Poisoning
- State Health Assessment
- Washington Tracking Network (WTN)
- Bioterrorism and Terrorism
- Emergency Information for Specific Groups
- Get Ready for an Emergency
- Severe Weather and Natural Disasters
- Health and Safety Alerts
- Emergency Contacts and Numbers
- Clinical Laboratory Reporting
- Emergency Medical Services (EMS) Systems
- Emergency Preparedness
- Healthcare Professions and Facilities
- Lactation and Infant Feeding-Friendly Environments
- Notifiable Conditions
- Public Health Laboratories
- Public Health System Resources and Services
- Rural Health
- Tribal Public Health and Relations
Online Treatment for COVID-19
Diagnosed with COVID-19 and need a Paxlovid prescription? See a doctor now to get started!

Online COVID-19 Treatment
Book an online visit with one of our board-certified doctors or nurse practitioners for fast relief from COVID-19. Paxlovid prescriptions available for those who qualify.
For: Men, Women, All Ages Estimated wait time: 5 minutes or less
Who is this right for?
If you're not feeling well, and are looking for relief from Coronavirus (COVID-19) symptoms, including high fever, body aches, or difficulty breathing, schedule a visit now with an online doctor.
The CDC declared a new COVID subvariant as "Eris". So while COVID is no longer considered a global health emergency, new strains of coronavirus are still evolving and you may benefit from seeking online medical attention.
You have COVID-19 symptoms
You’re currently experiencing symptoms of COVID-19 such as a fever under 102°F, cough and cold.
You need convenience
You’re looking for convenient and cost-effective treatment advice and prefer to stay at home.
You tested positive
Your COVID-19 symptoms started at least 3 days ago and/or you have already tested positive for COVID-19.
You do not have severe underlying conditions
You do not have underlying conditions that may put you at an elevated risk for COVID-19 complications.
Doctor On Demand is a covered benefit for over 98 million Americans. If you’re covered by your employer or insurance, then you’ll pay $0. No insurance, no problem. You can use Doctor On Demand starting at $89 per visit.

Commonly prescribed medications for COVID-19 include:
- Over-the-counter pain relievers, such as acetaminophen or ibuprofen
- Prescription antiviral drugs, such as Paxlovid
- Steroids, such as dexamethasone
- Antibiotics
- Blood thinners

During a visit for COVID-19, patients can expect the clinician to:
Ask about your symptoms, exposure to COVID-19, COVID-19 testing, and medical history.
Refer you to a clinic for additional testing if you haven’t been tested yet.
Offer advice on how to manage symptoms and speed up your recovery process.
Let you know if you’re eligible to receive a same-day prescription. However, your doctor will meet with you to determine if additional testing is needed.
Offer counseling or refer you to a mental health specialist for support.

Getting ready for your COVID telehealth visit with Doctor On Demand:
- Find a comfortable and quiet space where you can speak freely about your symptoms.
- Pull all of your insurance information together (if you don’t have insurance, you can skip this step).
- Find a photo ID, like your driver’s license or passport.
- Set aside five to 10 minutes, so you can answer questions about your symptoms and health history.

Set up your account.
Once you finish registering, you’ll be able to see the cost of your visit.

See the next available provider or schedule your appointment.
Select your concern and answer a few questions to provide the doctor with some context.

Start your live virtual visit.
Meet with one of our board-certified doctors who will diagnose your symptoms and offer a custom treatment plan. If needed, you can get prescriptions delivered to you or sent to your local pharmacy.
" Wow, Dr. Karimi performed above and beyond! He actually called my pharmacy to address a COVID prescription issue my wife was having. Dr. Karimi was direct, informative, and personable. It was the first time my wife and I used Doctor On Demand, and we'll definitely use it again."
—Paul

"I tested positive for Covid & was feeling horrible. Last thing I wanted to do was get in a car & go to urgent care. My niece told me about Doctor On Demand. What a blessing in time of need. I was very impressed with the care I received as the Dr. listened & was very informative. Prescriptions were easily filled at my pharmacy. Thank you!"
—Janet
"I'm stuck at home, positive for COVID, which turned into a sinus infection. Doctor On Demand saved me a trip to urgent care and infecting others with COVID. Thank you!"
"Despite 5 COVID vaccinations, I got COVID and felt terrible. I did a home testing kit and it confirmed I had COVID. It was a Saturday night, and I knew I needed Paxlovid because I have multiple sclerosis. I couldn't find any urgent care open and didn't want to go to an ER and infect everybody and wait hours and hours. My local ER nurse advisor told me about Doctor On Demand, and I downloaded the iPhone app and filled out all the history and medication information. Within half an hour I was talking to Dr. Kerry Eley on my iPhone face time, and she agreed that I needed Paxlovid. She was able to immediately send a prescription to a 24-hour pharmacy near my house. I started the medication immediately and felt much better the next day. If not for Doctor On Demand, I would have had to delay treatment at least 12 hours and go in person to urgent care because no one in my city is doing telephone consultations."
—Noemi

Top-rated providers
Get care from a team of doctors rated 4.9 out of 5 stars by patients just like you.

Same-day appointments, 24/7/365
Why wait to be seen? Connect to care in as little as 5 minutes.

Custom treatment plan
Get the right treatment option and prescription for your unique care needs.

Care at home or on the go
Connect with a provider from anywhere you have internet.
What is COVID-19?
Coronaviruses are a family of viruses, such as the common cold, that can cause respiratory illness in humans. They are called “corona” due to the crown-like spikes on the surface of the virus. The latest strain of coronavirus (SARS-CoV-2) was reported in December 2019 and is also known as COVID-19.
SARS-CoV-2 has consistently mutated over the course of the pandemic, resulting in variants that are different from the original COVID-19 virus, which is why COVID-19 has not yet disappeared. For example, the latest covid subvariant is called EG.5.1 (AKA “Eris”) and remains the world’s most prevalent coronavirus strain. For more info, check out the Centers for Disease Control and Prevention .
How can I get Paxlovid for COVID?
The FDA has issued emergency approval of Paxlovid for anyone over 12 years old and weighs at least 88 pounds, making it widely available for most. However, there are some restrictions and eligibility requirements: you need to have tested positive for COVID-19 and be at high risk of serious complications from COVID-19 . In order to get access to Paxlovid, you must get a prescription from your doctor or physician. Start an online visit now with Doctor On Demand to find out if you’re eligible and get access to a prescription.
What are the symptoms of COVID-19?
The most common symptoms of COVID-19 include fever, chills, cough, shortness of breath, tiredness, muscle or body aches, headaches, new loss of taste or smell, sore throat, congestion or runny nose, nausea, vomiting, and diarrhea.
Will I need to be seen for in-person care for my COVID symptoms?
If your cough or breathing is extremely severe, your doctor may recommend in-person care so that they can use a stethoscope for their exam.
Is there anything I need to know in order to prepare for my online COVID doctor visit?
Your doctor may ask to perform a telehealth lung exam. This should be done in a quiet setting to help the doctor properly assess your breathing.
Do I need any devices for my online COVID visit?
It could be helpful (but not necessary) to have a pulse oximeter device for your online visit. This device checks how much oxygen your blood is carrying and can help your doctor with diagnosis.

What is Paxlovid? Can this antiviral medication treat COVID-19?

How to Protect Yourself and Your Family From Coronavirus

Is It a Cold, the Flu, RSV or Coronavirus? What Your Symptoms May Mean

Tips to Prevent a Cold: Minimize Your Risk of Colds, Flu and COVID-19 During Flu Season

Diagnosing the Cold and Flu Over Video

Cold vs. Flu: What’s the difference?
- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
- Health Inc.
- Public Health
3 ways to get COVID pills, if you've just tested positive

The Pfizer COVID treatment Paxlovid can be a challenge to get quickly. Fabian Sommer/Picture Alliance via Getty Images hide caption
The Pfizer COVID treatment Paxlovid can be a challenge to get quickly.
If you've just tested positive for COVID-19, and you have common risk factors for serious illness, there are now ample treatments available – generally at little or no cost – that could help you avoid the worst and recover more quickly from a mild or moderate case of COVID.
Paxlovid, a five-day course of pills from Pfizer, is at the top of the list of recommended treatments . Studies from the drugmaker showed that – in unvaccinated people at risk of serious COVID medical risk factors – Paxlovid was nearly 90% effective at cutting the risks of getting hospitalized or dying because of COVID.
People who are vaccinated or who have recovered from COVID could still benefit from the drug, says Dr. Priya Nori , an infectious disease physician at Montefiore Health System in Bronx, New York, and a professor at Albert Einstein College of Medicine. Taking Paxlovid could "help you get back on your feet faster, feel better faster, and be potentially less infectious faster," Nori says.
The Biden administration has been talking up the treatment. "We want all individuals to know about this effective medication, and to have a conversation with their health care provider about if they are eligible, and whether they should be making a plan" for accessing the drug if they do test positive, says Dr. Meg Sullivan, chief medical officer for the Office of the Assistant Secretary for Preparedness and Response in the Department of Health and Human Services.
But some people have had trouble getting the medicine quickly, when it can make a difference in the progression of the disease, despite the administration's effort to make the medicines easy to obtain after a positive test for COVID-19.
Quick access to Paxlovid can be a challenge
Dan Weissmann , 54, tried three different routes to access Paxlovid in the Chicago area when he got COVID in April. First, the nearby CVS Minute Clinic didn't have any appointments readily available. Then, the nurse practitioner at the urgent care clinic he went to initially misunderstood his medical conditions and refused to prescribe the pills. Eventually, his wife tracked down his recently retired primary care doctor, who wrote him a prescription. Weissmann is glad he got the pills; his condition improved after taking them. But he says it took "an unusual amount of knowledge, connections and assertiveness," to obtain them. Luckily, Weissmann, host of the health podcast An Arm and A Leg , had all three.
In upstate New York, after a few days of COVID symptoms, Pamela Coukos' college-age son tested positive on a recent Friday. "He has one of the risk factors; he seemed pretty sick. And also, it would be better if he didn't spend, like, 10 days feeling terrible because he was in the middle of final exams," Coukos says. But the university health service was closing, and the nearest " test to treat " location wasn't open on weekends. He managed to book a Saturday telehealth appointment with his primary care doctor in his home state of Maryland, who sent a prescription to a pharmacy in upstate New York. A friend drove 26 miles each way to pick it up. The sick student took the pills, recovered by Wednesday and went on to complete his exams. "It was ultimately successful, but more complicated than it needed to be," Coukos says.
COVID pills are authorized by FDA for people at high risk of disease progression – and in practice, the risk criteria have broadened as supply has increased, says Dr. Phyllis Tien , an infectious disease physician at University of California, San Francisco, who serves on the National Institutes of Health's COVID-19 Treatment Guidelines Panel.
During the winter omicron surge, pill supplies were limited, and many health care providers prescribed Paxlovid only to those who were most vulnerable, due to older age or serious underlying illnesses. Now, health conditions such as high cholesterol, depression, smoking-related lung disease, obesity, not being fully vaccinated or boosted – all factors that increase a person's risk for severe COVID outcomes – might qualify a recently infected COVID patient for a course of Paxlovid. "If someone wants it, and is eligible for it, they should be able to access it," Tien says.
A prescription is the key
The antiviral pills require a prescription , and need to be started within five days of symptoms appearing. To get a prescription you'll have to show positive COVID-19 test results, and review your risk factors and any medications you take with a health care provider.
Paxlovid – a combination of two antiviral drugs called nirmatrelvir and ritonavir – can't be taken at the same time as some common supplements and medications, including statins and some birth control pills . "There's a long list of drug-drug interactions," says Jacinda Abdul-Mutakabbir , an assistant professor at Loma Linda University School of Pharmacy, "You want to make sure you bring forth any other medications you may be taking" for medical evaluation before you receive the medication.
The drug could also be dangerous for those with reduced kidney or liver function .
For those who can't take Paxlovid, there are several other early COVID-19 treatments that a health care provider might prescribe, such as remdesivir or monoclonal antibody infusions. Molnupiravir, a five-day pill treatment from Merck, is another option, although it is prescribed far less often than Paxlovid. In a clinical trial the Merck drug reduced hospitalization by only 30%.
Common side effects of taking Paxlovid include a metallic taste, diarrhea, increased blood pressure and muscle aches – all temporary. "While these side effects are not ideal, they're definitely better than what we would see if these individuals were to go forth and develop severe COVID," says Abdul-Mutakabbir.
Here are three ways to access COVID pills, if you're eligible to get them.
Contact your primary care doctor
For those with health insurance and access to their primary care providers or health care team, you can make an in-person or telehealth appointment to get tested (or share your positive test results), assessed for risks and medications and, if eligible, obtain a prescription for the pills.
You'd then get the prescription filled at a nearby pharmacy .
Having a provider that knows your medical history, as well as the details of your current situation, can be very helpful, says Dr. Ulrika Wigert , a family medicine physician at CentraCare in Sauk Center, Minnesota. "Did you test the first day [of symptoms]? Did you test the second day? How sick were you when you tested?" And, if you're starting to feel better by the time you get the medication, do the benefits of taking the medication outweigh any risks? "Having a provider help navigate that on the individual patient basis" can help guide you through an appropriate course of care, she says.
Visit a test-to-treat site
Another route to getting Paxlovid is visiting one of the 2,300 health centers, urgent care clinics and pharmacies that are designated by the government as "test to treat" sites. These are locations that have on-site prescribing capabilities and pills on-hand.
"For individuals who do not have a health care provider, or are unable to access their health care provider within a short time frame...test-to-treat locations offer testing and evaluation with a health care provider to determine if that medication is appropriate, and can dispense the medication on-site," says Sullivan of HHS.
Test-to-treat locations can be found on this map .
Before you go, "check with your health plan to make sure that they are in-network with your plan," says Sabrina Corlette , co-director of the Center on Health Insurance Reforms at Georgetown University, "So theservices you receive there will be fully covered, or at least you'll be subject only to a nominal copayment."
For those without insurance, some of the test-to-treat sites are federally qualified health centers that can provide low cost COVID testing and treatment services to the uninsured.
Try online urgent care
For those who prefer telehealth visits – and may not be able to get an appointment quickly through their primary health care provider – virtual healthcare platforms such as Plushcare , eMed and Truepill offer online visits to test, assess and prescribe COVID medications. The appointments are available at all hours, and may come with some out-of-pocket costs. A prescription can be sent to a nearby pharmacy, or filled and shipped to you , depending on what the service offers.
"The pro of this approach is that it's designed just for this purpose – of testing and treating for COVID," says Montefiore's Nori. "You'll efficiently get the service you need." The cons, she says, are that they don't know your full situation, such as your home context and your medical history. "They're relying on you to convey all your medications, herbal remedies," she says, but it can provide you with timely access to treatment.
What you can do ahead of time
If you're concerned about getting COVID and want to make a plan of action ahead of time, experts recommend four steps:
- Be prepared to test quickly , if you suspect you might have COVID. "Have tests available at home, or know where you can access a testing site," says Sullivan at HHS.
- Know whether you're a person with risk factors. Check to see if you have a condition that puts you at high risk for COVID, and talk with your medical provider about COVID treatments to know if you're eligible and to get questions answered ahead of time.
- Check what your insurance covers , and figure out where you can get a timely consultation. Services in-network are more likely to be covered by your insurance
- Find pharmacies near you that stock Paxlovid , so you know where you can fill a prescription.
And, stay up to date on your COVID-19 vaccinations, to help protect yourself and those around you from getting infected.
Now, a caveat on costs: although the pills themselves are free, there may be some out-of-pocket fees. Testing, getting a health care consultation and prescription, and follow up can cost you, depending on whether you're insured and what your insurance covers, says Georgetown's Corlette . To lower costs, those with health insurance should seek care in-network when possible; for those without insurance, a federally qualified health center may provide services that are free or very low cost, she says.
- molnupiravir
- COVID treatments
Thanks for visiting! GoodRx is not available outside of the United States. If you are trying to access this site from the United States and believe you have received this message in error, please reach out to [email protected] and let us know.

Paxlovid (nirmatrelvir co-packaged with ritonavir)
Important paxlovid updates.
- March 11, 2024: COVID-19 Treatments Locator Tool: Request to Health Care Providers
- February 20, 2024: CMS Revised Letter: Introduction of Prescription Oral Antivirals for COVID-19 to the Commercial Market
- January 29, 2024: FDA revises letter of authorization for the emergency use authorization for Paxlovid
- December 20, 2023: Updates to the Sunsetting the US Government COVID-19 Therapeutics Distribution Program
- December 20, 2023: Frequently Asked Questions About COVID-19 Therapeutics Transition to Commercial Distribution
- November 28, 2023: Transition of Paxlovid to Commercial Distribution
View All Updates
Important Information About Paxlovid
Paxlovid (nirmatrelvir co-packaged with ritonavir) is an oral antiviral drug that should be initiated as soon as possible within 5 days of symptom onset. Paxlovid is available for patients by prescription only. Prescriptions can be obtained from your health care provider or through the Test to Treat program .
On May 25, 2023, the U.S. Food and Drug Administration (FDA) approved Pfizer’s application for Paxlovid to treat adults (18 years of age and older) with a current diagnosis of mild to moderate COVID-19 and who are at high risk for progression to severe COVID-19. At the same time, FDA retained Emergency Use Authorization (EUA) for the treatment of adults and pediatric patients (12 years of age and older weighing at least 40 kg) with a current diagnosis of mild to moderate COVID-19 and who are at high risk for progression to severe COVID-19, including hospitalization or death.
Please see the Eligibility Screening Checklist for additional details.
Paxlovid is available in two package presentations:
- Paxlovid Standard Dose that includes 300 mg nirmatrelvir (two 150 mg tablets) with 100 mg ritonavir (one 100 mg tablet) taken together twice daily for 5 days.
- Paxlovid Reduced Dose is for people with moderate renal impairment (eGFR ≥ 30 mL/min to < 60 mL/min) that includes 150 mg nirmatrelvir (one 150 mg tablet) with 100 mg ritonavir (one 100 mg tablet) taken together twice daily for 5 days. Paxlovid is not recommended for people with severe renal impairment (eGFR < 30 mL/min).
Paxlovid is not a substitute for vaccination in individuals for whom COVID-19 vaccination and a booster dose are recommended. There are known drug interactions with Paxlovid. See the Drug Interaction Checker for more information.
For information on returning undispensed, USG distributed, EUA-labeled supply, please see Sunsetting the U.S. Government COVID-19 Therapeutics Distribution Program .
Emergency Use Authorization and Approval of Paxlovid
On May 25, 2023, Paxlovid received approval to treat adults with mild to moderate COVID-19 in eligible patients and retained the EUA for these patients as well as for children ages 12 and older weighing at least 40 kg (88 pounds).
On January 29, 2024, the Food and Drug Administration (FDA) announced an important revision to the Paxlovid emergency use authorization (EUA), stating that Paxlovid manufactured and labeled in accordance with the EUA (EUA-labeled Paxlovid) currently in U.S. distribution will remain authorized for use through the labeled or extended expiration date, as applicable, or through March 8, 2024, whichever is earlier.
As of March 9, 2024, EUA-labeled Paxlovid (nirmatrelvir co-packaged with ritonavir) no longer authorized for use, regardless of expiry data.
Paxlovid received initial EUA on December 22, 2021, for the treatment of mild to moderate COVID-19 in eligible patients.
The FDA website hosts the latest information on the EUA.
Distribution of Paxlovid
From December 2021 to December 15, 2023, the Administration for Strategic Preparedness and Response (ASPR) within the U.S. Department of Health and Human Services (HHS) worked with jurisdiction health departments, pharmacy partners, Test to Treat sites, long-term care providers, and health centers funded by the Health Resources and Services Administration (HRSA) to support the fair and equitable distribution of Paxlovid. The medication with the EUA label was provided for free by the U.S. government (USG) through December 15, 2023. It is now available through commercial distribution.
Jurisdiction health departments, pharmacy partners, and other central partners assisted with distribution of the product to dispensing sites across the nation, and the distributor shipped product directly to sites, which include pharmacies, doctors’ offices, clinics, hospitals, urgent care centers, and local health departments.
Continued Access to the U.S. Government Paxlovid Supply
With the transition to traditional commercial ordering and distribution of Paxlovid HHS continues to leverage USG-procured supply of Paxlovid to ensure affordable access for all patients, including no cost access for beneficiaries of public programs such as Medicare and Medicaid and for those who are uninsured. HHS took steps to ensure procured product is not lost to expiry and established a separate stockpile for future emergencies.
U.S. Government Patient Assistance Program Operated by Pfizer
Through December 31, 2024, individuals covered under federal programs, such as Medicare or Medicaid, and uninsured patients are eligible for the USG Patient Assistance Program (PAP) operated by Pfizer and can receive Paxlovid at no cost. Patients or health care providers and pharmacists on behalf of patients can enroll to participate in the program at https://paxlovid.iassist.com . This program is supported by USG-procured Paxlovid.
Retail pharmacies can learn more about becoming a Paxlovid PAP participating location by contacting the program vendor at [email protected] .
Federal Entities Authorized to Distribute Paxlovid
Certain federal entities (HRSA-supported health centers, Indian Health Service health centers, Veterans Health Administration facilities, Department of Defense, and others) continue to have access to remaining USG-procured Paxlovid supply for patient care. USG-procured Paxlovid also may be used to support state, local, tribal, or territorial special programs targeting vulnerable populations on a case-by-case basis.
Paxlovid Co-Pay Savings Program
Pfizer is separately operating a Paxlovid Co-Pay Savings Program for eligible privately (commercially) insured patients to ensure affordable access. Health care providers and pharmacists can enroll their patients at Paxlovid.pfizerpro.com .
Paxlovid FDA Resources
- Letter of Authorization
- Fact Sheet for Healthcare Providers
- Fact Sheet for Patients and Caregivers
- Frequently Asked Questions: FDA EUA for Paxlovid (FDA)
- Healthcare Provider Letter about Dispensing Information
- Paxlovid Patient Eligibility Screening Checklist Tool for Prescriber
Hello, I’m Dr. Colin Shepard. I serve as the CDC Liaison to the HHS Assistant Secretary for Preparedness and Response, also called ASPR . I want to talk about one of the therapeutics that can help people with COVID-19—Paxlovid.
What is Paxlovid?
Paxlovid is an oral antiviral used to help fight the coronavirus infection by stopping the coronavirus from replicating in the body. This lowers the viral load, reducing the chances of the illness progressing to more serious symptoms and hospitalization.
Who is eligible for Paxlovid?
Paxlovid is for adults and children 12 and older who are at high risk for developing serious symptoms of COVID-19 that may lead to hospitalization and/or death. For more information about who is at high risk, please see the resources provided in the description.
Paxlovid is for people who have a positive COVID-19 rapid or PCR test with mild to moderate symptoms and who are not in the hospital. It should be administered as early as possible but needs to be given within 5 days of symptom onset.
How is Paxlovid administered?
Paxlovid requires a prescription and is available through a pharmacy or health care clinic including Test to Treat locations where these antivirals are being distributed for use at home. Patients will take a combination of pills twice a day for 5 days.
What are the limitations of use of Paxlovid?
Paxlovid is not for everyone. There are serious drug interactions with some of the ingredients that need to be considered. Paxlovid is not recommended for patients with severe renal or hepatic impairment. It is not appropriate to start Paxlovid if you are already hospitalized for COVID-19.
Paxlovid is one of several COVID-19 therapeutic options . Watch the other Outpatient COVID-19 Therapeutics Videos in this series for more information. Visit us online at https://aspr.hhs.gov/COVID-19 and please see the resources linked in the description to learn more. You can connect with ASPR on social media platforms to stay up to date on our latest posts and information that we share. Please email any questions to [email protected] .
Thank you for your time.
In this video, Colin Shepard, M.D., Centers for Disease Control and Prevention liaison to ASPR, provides more information about the at home use of Paxlovid for treatment of COVID-19.
Paxlovid Frequently Asked Questions
Approval and authorization.
Paxlovid (nirmatrelvir co-packaged with ritonavir) is a preferred oral antiviral for the treatment of mild to moderate COVID-19 illness.
Patients take a combination of pills twice a day for 5 days. Paxlovid should be administered as early as possible following the appearance of any symptoms and needs to be initiated within 5 days of symptom onset.
Paxlovid is approved by the FDA for adults (May 2023). Is the Emergency Use Authorization (EUA) still active for Paxlovid? What are the limitations of authorized use for Paxlovid?
- Yes. The EUA for Paxlovid is still active and authorizes the use of Paxlovid for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kg) who are at high risk for progression to severe COVID-19, including hospitalization or death.
- Paxlovid is not authorized for initiation of treatment in patients requiring hospitalization due to severe or critical COVID-19.
- Paxlovid is not authorized for use as pre-exposure or post-exposure prophylaxis for prevention of COVID-19.
Are there any warnings or precautions that should be taken when prescribing Paxlovid?
Yes, health care providers and patients must be aware of the following warnings and precautions:
- The concomitant use of Paxlovid and certain other drugs may result in potentially significant drug interactions.
- Hypersensitivity reactions: Anaphylaxis and other hypersensitivity reactions have been reported with Paxlovid. If signs and symptoms of a clinically significant hypersensitivity reaction or anaphylaxis occur, immediately discontinue Paxlovid and initiate appropriate medications and/or supportive care.
- Hepatotoxicity: Hepatic transaminase elevations, clinical hepatitis, and jaundice have occurred in patients receiving ritonavir.
- HIV-1 drug resistance: Paxlovid use may lead to a risk of HIV-1 developing resistance to HIV protease inhibitors in individuals with uncontrolled or undiagnosed HIV-1 infection.
Please refer to the Paxlovid fact sheet for health care providers for additional information on warnings and precautions.
How can I order Paxlovid?
As of November 1, 2023, Paxlovid became available for purchase through the commercial market. Ordering of USG-procured Paxlovid was available through December 15, 2023, and is now closed except for federal entity partners. Federal entities can continue to order from the USG supply through the Health Partner Ordering Portal .
Through December 31, 2024, individuals covered under federal programs, such as Medicare or Medicaid, and uninsured patients are eligible for the USG Patient Assistance Program (PAP) operated by Pfizer and can receive Paxlovid at no cost. Health care providers and dispensing locations can provide information to patients regarding eligibility and how eligible patients can enroll in PAP to obtain free product. In addition, Pfizer has established a Co-Pay Savings Program for eligible commercially insured patients. Additional information for patients and health care providers is available.
What are the current recommendations about “rebound” presentation after SARS-CoV-2 infection?
Rebound, which is defined as experiencing recurrence of symptoms and/or SARS CoV-2 antigen positivity after initial resolution, has been observed not only among patients treated with Paxlovid but also occurs in patients receiving no treatment and in patients treated with other COVID-19 therapeutics .
Recent studies suggest patients experiencing rebound have an extremely low probability of developing severe COVID-19, including a study published in CDC's Morbidity and Mortality Weekly Report in December 2023, which found no consistent association between treatment with COVID-19 antivirals and rebound. Further studies on this phenomenon are ongoing.
Additional guidance on the management of patients experiencing rebound is available.
Where can health care professionals get more information?
- NIH Therapeutic Management of Nonhospitalized Adults With COVID-19
- FDA Fact Sheet for Health Care Providers for Paxlovid (nirmatrelvir and ritonavir)
- FDA Frequently Asked Questions for Paxlovid
- Side-by-Side Outpatient Therapeutics Overview
- Therapeutics Clinical Implementation Guide
- COVID-19 Therapeutics Decision Aid
- Conditions associated with higher risk for severe COVID-19
- Email any questions to [email protected] .
Eligibility
Paxlovid is for adults (ages 18 years of age and older) and children (12 years of age and older weighing at least 40 kg) for treatment of mild to moderate COVID‑19 and who are at high risk for progression to severe COVID‑19 disease, including hospitalization and death. Criteria include:
- Have mild to moderate COVID-19 and are within 5 days of symptom onset
- Have one or more risk factors for severe COVID-19
The U.S. Food and Drug Administration’s (FDA) Paxlovid Patient Eligibility Screening Checklist Tool for Prescribers is a useful tool for assessing eligibility.
See FDA’s Fact Sheet for Healthcare Providers for detailed information about Paxlovid.
Who is considered to have a risk factor for severe COVID-19?
Per the current CDC’s Interim Clinical Considerations for COVID-19 Treatment in Outpatients guidelines , risk factors include:
- Age 50 years or older, with risk increasing substantially at age ≥ 65 years
- Being unvaccinated or not being up to date on COVID-19 vaccinations
- Specific medical conditions and behaviors
For additional information, please see the National Institutes of Health’s COVID-19 Treatment Guidelines: Clinical Spectrum of SARS-CoV-2 Infection .
Health care providers should also proactively counsel high-risk patients about the availability of effective therapeutics and discuss a COVID-19 Action Plan with their patients.
What is the benefit of Paxlovid in treating mild to moderate COVID-19?
The benefit of a 5-day treatment course of Paxlovid was demonstrated in the clinical trial that supported the EUA. This study that among non-hospitalized, unvaccinated patients at high risk of progression to severe disease, treatment with Paxlovid reduced the risk of hospitalization or death by 88%. 1
Observational data, including vaccinated patients, from Israel , 2 Hong Kong , 3 and the United States is consistent with benefit in high-risk patients:
- 46% reduction in hospitalizations and deaths compared to the untreated 2
- 65% reduction in death compared to non-users 3
- 51% lower hospitalization rate within 30 days after diagnosis than those who were not prescribed Paxlovid 4
References:
- Jennifer Hammond, et al. New Engl J Medicine . 2022;386(15):NEJMoa2118542. doi: 10.1056/nejmoa2118542
- Ronza Najjar-Debbiny, et al. Clinical Infectious Diseases , 2022; ciac443, https://doi.org/10.1093/cid/ciac443
- Carlos K.H. Wong, et al. Lancet Infectious Disease, 2022; doi: https://doi.org/10.1016/ S1473-3099(22)00507-2
- Melisa M. Shah, et al. MMWR. https://www.cdc.gov/mmwr/volumes/71/wr/mm7148e2.htm?s_cid=mm7148e2_w
Can patients take Paxlovid if they are taking other medications?
Drug-drug interactions are important when considering whether to prescribe Paxlovid. Paxlovid may impact the concentration of concomitantly administered medications.
Despite its potential for drug-drug interactions, many commonly-used medications can be safely co-administered with Paxlovid . The prescriber should perform a thorough medication reconciliation, including over-the-counter medications and supplements, prior to prescribing Paxlovid.
FDA’s Paxlovid Patient Eligibility Screening Checklist Tool for Prescribers includes a helpful table with medications that interact with Paxlovid, and the recommended action for the prescriber.
What are the alternatives to Paxlovid for the patient with mild to moderate COVID-19 illness who cannot take it?
- Veklury (remdesivir) is a preferred antiviral medication for mild to moderate COVID-19. Veklury is given intravenously, once daily for 3 consecutive days.
- Lagevrio (molnupiravir) (oral antiviral) is an alternative medication when preferred therapies authorized or approved by FDA are not clinically appropriate or available.
Testing and Prescriptions
Do patients need a prescription to receive paxlovid.
Yes; a prescription from a authorized health care provider is required.
How can patients get a prescription for Paxlovid and where can the prescription be filled?
Patients can receive a prescription for Paxlovid by three main ways: a health care provider, a Test to Treat site, or an authorized pharmacist.
- An individual’s health care provider remains the best first option for assessment and prescribing for patients who have symptoms consistent with mild to moderate COVID-19.
- For individuals who do not have timely access to their own health care provider, patients can get tested, assessed for COVID-19 therapeutic eligibility, and have their prescription filled at Test to Treat sites nationwide.
- The FDA authorized pharmacists with access to a patient’s health care records to prescribe Paxlovid under certain conditions .
Find COVID-19 medications , including Paxlovid.
Does the EUA require a positive result from a direct SARS-CoV-2 viral test prior to prescribing Paxlovid to a patient who is at high risk for severe COVID-19?
No. Although FDA continues to recommend that authorized prescribers use direct SARS-CoV-2 viral testing to help diagnose COVID-19, FDA removed the requirement for positive test results effective on February 1, 2023. FDA recognizes that, in rare instances, individuals with a recent known exposure (e.g., a household contact with a positive direct SARS-CoV-2 viral test) who develop signs and symptoms consistent with COVID-19 may be diagnosed by an authorized prescriber as having COVID-19 even if they have a negative direct SARS-CoV-2 viral test result. In such instances, the authorized prescriber may determine that treatment with Paxlovid for COVID-19 is appropriate if the patient reports mild to moderate symptoms of COVID-19 and is at high-risk for progression to severe COVID-19, including hospitalization or death, and the terms and conditions of the authorization are met, as detailed in the Fact Sheet for Healthcare Providers .
Are lab results required before a patient can be prescribed Paxlovid?
Assessment of renal and hepatic function is important when considering prescribing Paxlovid.
Licensed physicians and advanced practice providers are not required to perform laboratory testing when prescribing Paxlovid. Providers should use clinical judgement to determine if labs are necessary.
State-licensed pharmacists must have sufficient information, such as access to a patient’s health care records within the past 12 months or consultation with a health care provider in an established provider-patient relationship with the individual patient, to assess for renal and hepatic function in order to prescribe Paxlovid.
Specific information on clinical evaluation considerations to prescribe are in the FDA Fact Sheet for Healthcare Providers .
Patient Information
What is an oral antiviral.
Oral antivirals are pills that stop the virus that causes COVID-19 from making copies of itself in your body. One oral antiviral is called Paxlovid (also known as nirmatrelvir co-packaged with ritonavir), and the other is called Lagevrio (also known as molnupiravir).
How can patients find Paxlovid in their area?
Find available COVID-19 medication . A call center is also available at 1-800-232-0233 (TTY 1-888-720-7489 ) to get help in English, Spanish, and more than 150 other languages.
DIAL is also available to specifically help people with disabilities access services. To get help, call 1-888-677-1199 , Monday through Friday from 9 AM to 8 PM ET or email [email protected] .
How can patients prepare before exposure to COVID-19? Where can patients find more information?
- Patients are encouraged to discuss a COVID-19 Action Plan with their health care providers.
- Patients should have available electronic or printed health records from the past year, including the most recent blood work relating to kidney and liver problems. In addition, they will need a list of all medications they are taking, including over-the-counter medications.
- Learn more about COVID-19 Treatment Information for Patients .
- Fact Sheet for Patients, Parents, and Caregivers
Thresholds, Orders, and Replenishment by Jurisdiction
Shelf Life Extension
Find COVID-19 Medications
- Product Resources
COVID-19 Therapeutics
Locating COVID-19 Therapeutics
- Test to Treat
- Find COVID-19 Medication
- COVID-19 Therapeutics Thresholds by Jurisdiction
- Sunsetting the US Government COVID-19 Therapeutics Distribution Program
- COVID-19 Therapeutic Product Expiration
- Information for Long‑Term Care Facilities
Outpatient COVID-19 Therapeutics
- Lagevrio (molnupiravir)
- Veklury (remdesivir)
- Previously Available Products
Announcements
- Important Updates
- Social Media Digital Toolkit
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- COVID-19 Vaccines
- Occupational Therapy
- Healthy Aging
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Where Can You Get Paxlovid Now? Are You Eligible for the Medication?
Joe Raedle / Getty Images
- Who's Eligible for Paxlovid?
- Can You Get Paxlovid If You Don't Qualify?
Should You Take Paxlovid If Your Symptoms Are Mild?
- Should You Finish the Whole Course If You're Feeling Better?
Does Paxlovid Help Prevent Long COVID?
Should you worry about paxlovid rebound, can you get paxlovid without insurance, key takeaways.
- Paxlovid is an oral antiviral medication that can be taken at home to reduce the risk of COVID-19 hospitalization and death in high-risk individuals.
- Paxlovid requires a prescription and not everyone is eligible for it.
- For now, Paxlovid is still free for U.S. residents.
For people at a higher risk of severe illness from COVID-19, Paxlovid is an effective antiviral treatment for an early coronavirus infection.
A recent study showed that Paxlovid reduces the risk of hospitalization or death from Omicron variants in people aged 50 and older by 44%. For people who were unvaccinated, Paxlovid lowered the risk of hospitalization or death by 81%.
Paxlovid is a combination of two antiviral medications: nirmatrelvir and ritonavir.
"Nirmatrelvir stops the virus from replicating and reduces the amount of virus in the body. Ritonavir helps to boost the levels of nirmatrelvir so it is not broken down too quickly," said Shirin Mazumder, MD , an infectious diseases physician at Methodist Le Bonheur Healthcare in Memphis.
Who's Eligible for Paxlovid?
"Paxlovid is not recommended for everyone," Mazumder said. The antiviral medication is only recommended for people over the age of 12 and are at high risk for developing severe COVID.
This includes older adults or people who have underlying medical conditions such as asthma, diabetes, chronic lung disease, and chronic kidney disease. Here's a list of high-risk underlying conditions that could help determine if you're eligible for the treatment.
Paxlovid is also only meant for use within five days of symptom onset. Your healthcare provider will ultimately decide if you meet the criteria for a prescription. Licensed pharmacists may be able to prescribe the drug as well, but they must consult with your physician or review your medical records to make sure you're not taking any drug that would interact with Paxlovid.
Can You Get Paxlovid If You Don't Qualify?
You'll need a prescription from a doctor in order to obtain Paxlovid. If your healthcare provider determines that you don't meet the criteria, it's unlikely you'll receive a prescription.
"Paxlovid has several different medication interactions so a careful review of someone’s medication history is very important before it is prescribed," Mazumder said. "Even certain over-the-counter medications and supplements can interact with Paxlovid so be sure to discuss any medications that you taking with your healthcare provider."
For Paxlovid to be effective, it should be initiated within five days of the appearance of COVID-19 symptoms, according to Chandra Shekhar Bakshi, DVM, PhD , a professor of pathology, microbiology, and immunology at New York Medical College.
"In other words, the effectiveness of Paxlovid is best if it is taken early during the infection, at which stage the symptoms are still mild," Bakshi said.
More severe symptoms typically develop after this five-day window because of organ damage caused by the virus, he said. If a patient starts this treatment when the symptoms are severe, Paxlovid can not reverse this damage.
Should You Finish the Whole Course If You're Feeling Better?
Paxlovid consists of a five-day course of 30 pills which are to be taken three times per day for five days. Bakshi said it's important to complete the entire course to get the full benefits of the treatment.
Clinical trials show that Paxlovid is effective at preventing the development of severe COVID, hospitalization, or death in 86-89% of cases if the treatment is initiated within three days of the onset of symptoms and a full course of the treatment is completed.
Although research is still limited, a few large studies have shown that high-risk people treated with Paxlovid within five days of testing positive have a lower chance of developing long COVID symptoms after 90 days.
"Long COVID may be a consequence of the initial moderate to severe COVID," Bakshi said. "Therefore, if the initial infection is controlled, the development of long COVID symptoms could be minimized or completely prevented."
Paxlovid rebound is when COVID symptoms return within two to eight days after completing a course of Paxlovid, Mazumder explained. In some cases, people have tested positive again with no symptoms.
According to the CDC, a rebound doesn't mean that someone was reinfected or that they had resistance to Paxlovid. A return of symptoms might be a part of the natural history of the virus, and no additional measures are needed when it comes to treatment for rebound symptoms.
"The good news is that, in general, rebound symptoms tend to be milder," Mazumder said.
Paxlovid is free for U.S. residents as long as the federal supply lasts. When the national stockpile runs out, people might have to pay out-of-pocket costs.
What This Means For You
If you're at a high risk of developing a severe case of COVID-19, speak to your healthcare provider about taking Paxlovid within the first five days of your infection. The drug can be obtained at no cost with a prescription.
The information in this article is current as of the date listed, which means newer information may be available when you read this. For the most recent updates on COVID-19, visit our coronavirus news page .
Dryden-Peterson S, Kim A, Kim AY, et al. Nirmatrelvir Plus Ritonavir for Early COVID-19 in a Large U.S. Health System : A Population-Based Cohort Study . Ann Intern Med . 2023;176(1):77-84. doi:10.7326/M22-2141
U.S. Food and Drug Administration. Fact sheet for healthcare providers: Emergency use authorization for Paxlovid .
U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes pharmacists to prescribe Paxlovid with certain limitations .
Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19 . N Engl J Med . 2022;386(15):1397-1408. doi:10.1056/NEJMoa2118542
Xie Y, Choi T, Al-Aly Z. Association of treatment with nirmatrelvir and the risk of post–covid-19 condition . JAMA Intern Med . 2023;183(6):554-564. doi:10.1001/jamainternmed.2023.0743
Centers for Disease Control and Prevention. Health Advisory: Covid-19 rebound after Paxlovid Treatment .
Kaiser Family Foundation. The End of the COVID-19 Public Health Emergency: Details on Health Coverage and Access .
By Mira Miller Miller is a journalist specializing in mental health, women's health, and culture. Her work is published in outlets ranging from Vice to Healthnews.
Get a Telehealth Visit and Treatment for COVID-19
Have a positive covid test and symptoms get a telehealth visit and a prescription now..

Telehealth Visit with a Provider Online

- Your online visit will be via video or secure messaging based upon your condition and requirements in your state.
- Provide your medical history and a copy of your positive lab report. Our providers can prescribe Paxlovid or other standard medications and natural remedies.
Pharmacist Oversight of Your Medications

- Complete your online registration. A pharmacist will review your medications as needed to help your telehealth provider to best prescribe for you.
- If appropriate, you’ll receive a prescription delivered to your door or ready for pick up.
Realtime Follow Up With our Care Team

- Our virtual medical assistants make sure your prescription is taken care of. We’ll coordinate with your primary care where needed.
- We’ll even send you follow up surveys to check on you. Please let us know how you are doing and how we can help you.
What Medications Are Available in the Test-to-Treat Program?
Paxlovid and Molnupiravir are both Emergency Use Authorized as an investigational drug used to treat mild-to-moderate COVID-19 in adults and children. We will also consider other options for treatment including scientifically backed natural vitamins and supplements as is appropriate for you.
Learn more PAXLOVID MOLNUPIRAVIR OTHER OPTIONS

Just 3 steps to get started

Select an in person or online provider and complete your appointment. We’ll even help you schedule your in person appointment when you complete your registration.

If appropriate, get medications dispensed in person or we’ll arrange for delivery when you get a telemedicine visit.

Telemedicine providers are available 9AM to 10PM EST, but our patient care team is here to assist you 24 hours a day.
AZOVA brings care to you when and where you need it.
- Nearly 1 million visits completed
- Over 10 million COVID tests shipped
- Designed to bring the latest healthcare innovations to you with the most convenience
- US-licensed healthcare providers care for you and your family
Frequently asked questions
What states can i get a telehealth visit in.
This service is available in all 50 states and Washington, D.C. AZOVA provides telehealth services through a network of licensed independent healthcare providers who have each received training in the appropriate use and prescribing of Emergency Use Authorized medications such as Paxlovid, standard prescription medications and vitamins and supplements that may assist in the recovery from COVID-19.
Which Medications are Available through the Test-to-Treat Program?
Your telemedicine provider can prescribe Emergency Use Authorized medications, standard prescription medications, and can also make recommendations for scientifically backed vitamins and supplements where appropriate.
Both Paxlovid (nirmatrelvir tablets; ritonavir tablets) and Molnupiravir are included in the Test-to-Treat program and are available through emergency use authorization (EUA) from the Food and Drug Administration (FDA). Here is the information about the Paxlovid EUA and the Fact Sheet for Patients. Here is the information about the Molnupiravir EUA and the Patient Fact Sheet. We will add new COVID-19 medications to our formulary as they become available.
Additional information on Paxlovid:
Paxlovid has not been approved, but has been authorized for emergency use by FDA under an EUA, for the treatment of mild-to moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kg) with positive results of direct SARSCoV-2 viral testing, and who are at high-risk for progression to severe COVID-19, including hospitalization or death. The emergency use of Paxlovid is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biological products during the COVID19 pandemic under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb3(b)(1), unless the declaration is terminated or authorization revoked sooner.
Additional information on Molnupiravir:
Molnupiravir has not been approved, but has been authorized for emergency use by FDA under an EUA, for the treatment of mild-to-moderate COVID-19 in adults who are at high-risk for progression to severe COVID-19, including hospitalization or death, and for whom alternative COVID-19 treatment options authorized by FDA are not accessible or clinically appropriate. The emergency use of Molnupiravir is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biological products during the COVID-19 pandemic under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization revoked sooner.
Is There a Cost for the Test-to-Treat Medications?
While the US government is covering the cost, Molnupiravir are free of charge. If your telehealth provider prescribes other medications, the cost can be calculated by the pharmacy before your purchase. Please note: The US government could choose to discontinue coverage of the Test-to-Treat medications at any time.
Who is Eligible for Treatment with These Medications?
Telehealth services are only available for individuals 18 years of age or older and who have a symptomatic COVID-19 infection and who have a validated positive COVID-19 viral test report. If you would like your provider to prescribe Paxlovid, please note that you must also weigh at least 40 kg (88.18 lbs) with symptoms that began within the last 5 days and must have at least 1 high risk condition. If Paxlovid is not appropriate for you, your provider will make an alternate recommendation. All prescribing decisions are at the discretion of your telehealth provider.
What Are the Potential Side Effects of these Medications?
Please refer to the Patient Fact Sheet for Paxlovid here.
Please refer to the Patient Fact Sheet for Molnupiravir here.
Do You Offer Refunds?
We do not offer refunds for the cost of telehealth services, medications or COVID test kits.
How Does the Telehealth Visit Work to Get My Medication?
You will complete an online healthcare registration where we will ask you to answer questions about your health, medications and past medical history. We will ask for a photo of your government issued photo ID and any photos of relevant health conditions as appropriate. You will complete the registration and payment and, in most states, your information will be reviewed and you will receive an assessment and plan from your healthcare provider. In a few states, a video visit is required and you will be contacted for a video call with your healthcare provider.
Where appropriate, and as determined by your healthcare provider, you will receive a prescription for one of the Test to Treat medications. We will ship the medication to you or, if you see a healthcare provider in person, you can pick up your prescription locally.
How Long Does it Take to Hear Back From the Healthcare Provider When I Submit a Telemedicine Visit?
You will receive a diagnosis and treatment plan within 24 hours or less from the time you complete your appointment registration.
Can I Get a COVID test through AZOVA?
Yes. You can purchase many different COVID tests here with or without video observation and telehealth proctoring services where you can also receive a validated laboratory report.
Do You Provide Services to Larger Organizations?
Yes. AZOVA is the leading provider of digital health networking technology and services. Our products and services enable governments, employers, health plans and individuals to access convenient healthcare services online and in person.
Can I Use My Insurance?
You can use an HSA or FSA card to pay for your telemedicine visit. You can also submit a claim to your insurance plan for the cost of a COVID test. The United States government is currently covering the cost of Molnupiravir so it is free of charge.
You can chat with AZOVA’s Customer Support team for comprehensive support, including help with your account, testing, and results. We typically respond within 3 minutes or less. Click the messaging icon on the lower right corner of the page to get started.
Talk with Customer Support
You can call the dedicated support line using the number below. Please note that wait times may vary depending on call volume.
Your Safety and Security is our Top Priority

Information presented here is not intended to diagnose or treat any condition and is provided for educational purposes only. Always consult with your healthcare provider before making any healthcare decisions.
If you are having a life threatening emergency, please call 911 immediately.
Other options include:
Monoclonal antibodies.

Vitamins and Supplements

Oral antibiotics

Your telemedicine provider will review your condition, your medications and medical history to determine the best treatment plan for you.
Get Treated Now
Read about our recent product expansion here .

General information about COVID-19 antivirals treatments
Consultations can be requested using the links below based on the state of residence:
- Commonwealth of Massachusetts
- Oregon Health Authority
- Wisconsin Department of Health Services
- Washington State Department of Health
Paxlovid or Molnupiravir are intended for those with mild to moderate COVID-19 who are at high-risk for progression to severe COVID-19, and who are within 5 days of symptom onset. Molnupiravir is only for patients for whom other COVID-19 treatment options approved or authorized by the FDA are not accessible or clinically appropriate. Learn more about Paxlovid on the manufacturer’s website , and review the patient and healthcare provider fact sheets for more information. Learn more about Molnupiravir on the manufacturer’s website , and review the patient and healthcare provider factsheets.
People that have been exposed to someone diagnosed to COVID-19, or if it is unknown if they have been exposed to someone diagnosed with COVID-19, they may be eligible for a consult. Pregnant individuals will also be eligible for a consultation if they reside in Massachusetts, Washington, Oregon or Wisconsin.
A virtual visit for COVID-19 antiviral treatment is available to adults and children 12 years and older.
A parent or guardian is required to register someone aged 12 to 18 years old. A parent/guardian must create an account and add a dependent. A parent/guardian can then request a visit for their dependent. If the visit is for your child or adult dependent, you’ll both need to be present to see the clinician. Eligibility may also be restricted depending on your Color program sponsor’s terms. Eligibility through Color’s partnerships with Massachusetts, Washington, Oregon and Wisconsin is limited to persons who are currently located in those states, and pharmacy products are only shipped to valid addresses in Massachusetts, Washington, Oregon and Wisconsin.
Clinicians will be evaluating whether a patient should be given a prescription for the COVID-19 antiviral treatments Paxlovid or Molnupiravir. If you are interested in other treatment options for COVID-19, please seek care from your healthcare provider.
Paxlovid has not been approved, but has been authorized for emergency use by FDA under an EUA, for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kg) with positive results of direct SARS CoV-2 viral testing, and who are at high-risk for progression to severe COVID-19, including hospitalization or death.
If Paxlovid is appropriate, treatment should be initiated as soon as possible after COVID-19 diagnosis and within 5 days of symptom onset. Paxlovid is not for people that have a history of allergy to nirmatrelvir or ritonavir or other components of these drugs, and it is not for people taking certain other drugs. Paxlovid may cause side effects, some of which may be serious and unexpected. Learn more about Paxlovid on the manufacturer’s website, at https://www.covid19oralrx-patient.com , and review the patient and healthcare provider fact sheets for more information.
The emergency use of Paxlovid is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biological products during the COVID-19 pandemic under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb3(b)(1), unless the declaration is terminated or authorization revoked sooner.
Molnupiravir is authorized for emergency use by FDA under an EUA to treat mild-to-moderate COVID-19 in adults with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progression to severe COVID-19 including hospitalization or death, and for whom other COVID-19 treatment options approved or authorized by the FDA are not accessible or clinically appropriate.
Molnupiravir isn’t authorized for use in people less than 18 years of age, for prevention of COVID-19, for people needing hospitalization for COVID-19, or for use for longer than 5 consecutive days. Molnupiravir isn’t recommended for use in pregnancy as it may cause harm to an unborn baby. Molnupiravir may cause side effects, some of which may be serious and unexpected. Learn more about Molnupiravir on the manufacturer’s website and review the patient and healthcare provider fact sheets for more information.
The emergency use of Molnupiravir is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biological products during the COVID-19 pandemic under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb3(b)(1), unless the declaration is terminated or authorization revoked sooner.
Trademarks referenced herein held by their respective owners.
Your clinician visit is free and will not be charged by insurance. Depending on your insurance, you may be responsible for costs associated with an antivirals prescription you receive. For patients with Medicare, Medicaid, other government insurance programs , or without insurance, enroll in the PAXCESS™ patient support program or call (877) 219-7225 to verify your information for no cost share.
For patients with Commercial insurance , enroll in the PAXCESS™ co-pay savings program or call (877) 219-7225 to receive cost savings on your co-pay.
What to expect from a scheduled virtual visit
Your visit will be performed by a clinician from one of our partner networks. They ensure that their clinicians possess the credentials, training, licensing, experience, and competence to provide high-quality and cost-effective care.
If you scheduled a video visit, you will be placed in a queue and your clinician will send a text message with a link to join the video consultation at the scheduled time. This link can also be found on your Color account dashboard .
The video call will take about 10 minutes. If the clinician cannot complete a video call with you, they will not be able to complete your evaluation.
When you schedule a phone visit, you will be placed in a queue and your clinician will call you after they review your information. Prior to the phone consultation, you will receive a notification that you will receive the call soon. For more details visit your Color account dashboard .
How do I cancel or reschedule my visit?
To cancel or reschedule your visit, contact Support by email at [email protected] or call at (844) 352-6567 from 8 AM to 8 PM EST, 7 days a week. You may also cancel your appointment through your Color account dashboard
For video visits , a clinician will wait up to 10 minutes for you to join the video call. If the clinician cannot complete a video call with you, they will not be able to complete your evaluation. If you miss the visit, you can call Support at (844) 352-6567 and request a new visit.
For phone visits , If you don’t answer the first call, the clinician will attempt to call you one more time within 10 minutes. If you miss both calls, you can call Support at (844) 352-6567 and request a new visit.
Antivirals visits are available in:
- Haitian Creole
- Mandarin Chinese (simplified)
- Mandarin Chinese (traditional)
- Tagalog/Filipino
Pharmacy options and prescriptions
Local Pharmacy Pickup
It is recommended to confirm with your pharmacy that Paxlovid is in stock as well as their operating hours prior to submitting the health questionnaire. This will ensure your consultation will be completed in a timely manner.
Do you need home delivery?
If you need home delivery, please follow up with your selected pharmacy to discuss options. If you are on a Medicaid or Medicare insurance plan or do not have health insurance, you may be eligible for free overnight delivery. Call AssistRx at (877) 450-4412 to learn more.
Check with your pharmacy if they can transfer the prescription to the new pharmacy. If your pharmacy can not be reached, it is recommended to contact the new pharmacy you plan to transfer the Rx to and attempt the transfer from there.
If a transfer is needed, please provide the pharmacy details (address and phone number) and confirm with the pharmacy that Paxlovid is in stock. If you encounter any issue, contact [email protected] and we will assist with the transfer.
What to expect from the COVID-19 Antiviral Treatment Hotline?
No, you do not need a Color account to use the antiviral hotline. If at a later date you would like to see your after-visit summary, you may create an account and link them together or request a copy from Color. Providing an email address is optional, however it is recommended to receive the after-visit summary. If you need assistance with your after-visit-summary, please contact us at [email protected]
Your virtual visit will be with a licensed clinician from one of our partner networks.
When you schedule a phone visit, you’ll be placed in a queue and your clinician will attempt to call you after they review your information. You should receive a notification notifying you that a clinician will call soon, more details can be found on your Color account dashboard . If you don’t answer the first call, the clinician will attempt to call you one more time within 10 minutes. If you miss both calls, you can call Support at (844) 352-6567 and request a new visit. Note: depending on your phone carrier the phone number might appear to be from an unknown caller.
To cancel your visit or schedule another visit, you may do so from your Color account dashboard by clicking “Cancel Consultation”, from there you may contact Support by emailing [email protected] or calling Support at (844) 352-6567 , 7 days a week, for assistance.
If you don’t answer the first call, the clinician will call you one more time in the next 10 minutes. If the clinician can’t reach you after two calls, they won’t be able to complete your visit and you will have to request a new consultation. The caller ID might read as “unknown caller”. If enabled, p lease disable any spam blockers on your phone to ensure the clinician can reach you . If you miss the calls, please call (833) 273-6330 and request a new visit.
No. At this time, visit requests can only be made for dependents through the online flow.
- Marshallese
You can review versions of the documentation collected during intake here:
- https://www.color.com/consent-for-healthcare-services
- https://www.color.com/policies/tos
- https://www.color.com/policies/privacy
- https://www.color.com/policies/notice-of-privacy-practices
After your visit
Your visit details can be accessed from your Visits tab at home.color.com. You can take a screenshot of your visit details page or print from your browser. If you are unable to view your aftercare plan, please contact us at [email protected] and we can email you the after care plan.
Are you able to write a sick slip, work excuse, or return-to-work note?
Although patients will receive a treatment plan, we are unable to write a sick slip, work excuse, or return-to-work note.
If you have questions, you can email [email protected] or call (844) 352-6567 7 days a week.
A medication fact sheet link is available as part of your treatment after care summary. If you experience adverse reactions, please contact your local emergency services or call 911. Color Support is unable to answer any clinical questions or concerns.
Any clinical questions you may have should be directed to your personal care physician or a medical care professional. Color Support is unable to provide clinical advice under any circumstances.
Email : [email protected]
Call : (844) 352-6567
Hours : 5 AM to 8 PM PT (8 AM to 11 PM ET), 7 days a week.

Loading, just a moment...
Press Releases
- Virtual ExpressCare Leads Covid Treatment Citywide with Over 20,000 Prescriptions for Paxlovid and 6,800 Monoclonal Antibody Infusions
- Press Release
- In the Media
- Social Media
- The Remedy Podcast
Virtual Expresscare Leads COVID Treatment Citywide With Over 20,000 Prescriptions for Paxlovid and 6,800 Monoclonal Antibody Infusions
These treatments are projected to have prevented over 1,550 hospital visits and over 300 deaths Virtual ExpressCare is the largest prescriber of Paxlovid in New York City, prescribing 8 times as much as the next highest provider The service provides urgent care 24/7 in over 200 languages, and patients are connected to a provider in minutes
Sep 14, 2022
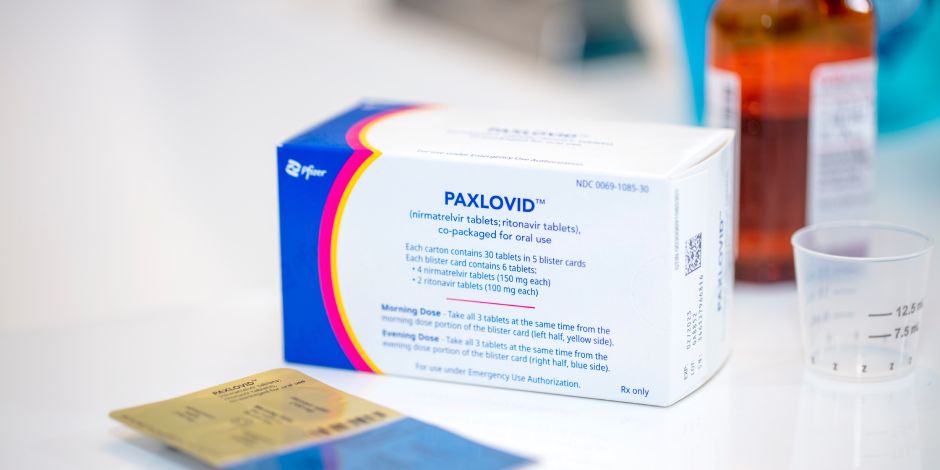
NYC Health + Hospitals today announced its Virtual ExpressCare service has issued over 20,000 prescriptions for Paxlovid and 6,800 monoclonal antibody infusions to treat Covid, potentially preventing over 1,550 hospital visits and over 300 deaths. Virtual ExpressCare is the largest prescriber of Paxlovid in New York City, prescribing 8 times as much as the next highest provider. NYC Health + Hospitals and its Virtual ExpressCare program was one of the first in the nation to offer telehealth visits to prescribe Paxlovid, making it easier for New Yorkers to access the medication and protecting the community by keeping COVID-positive individuals at home. In addition to Covid, Virtual ExpressCare provides urgent care for an array of concerns, including minor injuries, fever and flu, upper respiratory infections, anxiety, depression, and substance use issues. The service is available 24/7 in over 200 languages, and patients are connected to a healthcare provider in minutes. Patients can access Virtual ExpressCare by going to expresscare.nyc or calling 631-EXP-Care (631-397-2273).
“Keeping New Yorkers safe and healthy are our top priorities, which is why we are investing in new weapons to fight the war against COVID-19 and make it easier to get tested and treated,” said New York City Mayor Eric Adams . “Thanks to the 20,000 doses of Paxlovid we’ve distributed through Virtual ExpressCare, thousands of New Yorkers have been able to speak to a provider in minutes and get connected to lifesaving medication. If you feel sick, get tested, get treated, and get back to your life.”
“NYC Health + Hospitals has been critical to the City’s response to Covid, and that continues today as our system leads in prescribing Paxlovid in New York City,” said NYC Health + Hospitals Senior Vice President and Chief Quality Officer Eric Wei, MD . “We have since expanded beyond the five boroughs, and now all New Yorkers should know if they test positive for Covid, they can rely on NYC Health + Hospitals for treatment from the comfort of their own home through our Virtual ExpressCare service.”
“Virtual ExpressCare gives patients a quick and easy way to talk to a provider and see if they qualify for Covid treatment,” said NYC Health + Hospitals Executive Director of ExpressCare Erfan Karim . “We are here all day, every day to help all New Yorkers with their urgent care needs in their preferred language and we bring equitable care into their homes virtually so people can live their healthiest lives.”
“Having access to the latest COVID-19 therapeutics regardless of whether someone has a regular health care provider is critical in our effort to keep New Yorkers from becoming seriously ill or hospitalized,” New York State Department of Health Commissioner Dr. Mary T. Bassett said. “Thanks to the State’s agreement to use NYC Health + Hospitals’ Virtual ExpressCare Platform, thousands of people, particularly in underserved communities throughout New York State, have been evaluated for treatment and received care by calling the State’s new Hotline at 888-TREAT-NY or by calling 212-COVID-19 for those who live in NYC.”
“Treatment, and specifically Paxlovid, have been literal lifesavers for many New Yorkers,” said NYC Health Commissioner Dr. Ashwin Vasan . “They have enabled us to navigate recent waves while keeping hospitalizations comparatively low, and represent a critical ongoing tool to save lives and help us navigate the moment we’re in with COVID-19. NYC Health + Hospitals has been a valued partner is making treatments accessible and we’re grateful for their speed in building this program up.”
Since its launch, Virtual ExpressCare has offered nearly 90,000 virtual visits, more than 40,000 of which have been for Covid. In July, Virtual ExpressCare began offering Covid prescriptions statewide through the State’s new Covid hotline , 888-TREAT-NY. In its first two months, the service has issued nearly 1,000 Covid prescriptions to residents across the state. Virtual ExpressCare also prescribes Paxlovid via the city’s unique Covid hotline, 212-COVID-19, and offers home delivery to patients in New York City coordinated by the NYC Health Department.
Patients who are prescribed Paxlovid can receive same-day or next-day courier delivery of the medication in all five boroughs of New York City.
In June, the New York City Test & Treat Corps began prescribing Paxlovid through the nation’s first mobile Test to Treat program . New Yorkers who test positive for COVID-19 at one of the over 30 mobile Test to Treat units can be evaluated by an onsite clinician and, if eligible, walk away with Paxlovid provided onboard the mobile unit. Since the program’s launch, clinicians have issued approximately 1,500 prescriptions onsite.
Patients who are prescribed a monoclonal antibody infusion can schedule same-day or next-day in-person infusion at 12 locations across the city. The health system can provide transportation for patients to and from their infusion appointments. Follow-up care is also available, including calls from the infusion team 24 to 48 hours after dose and access to Virtual ExpressCare providers to address any post-visit clinical concerns.
There is no out-of-pocket cost to patients for Covid treatment. If a patient has insurance, NYC Health + Hospitals will bill the patient’s insurance for the visit. If a patient is uninsured, the service will help patients obtain coverage through health insurance, NYC Care , or a sliding scale program. NYC Health + Hospitals offers monoclonal antibody treatment at its 11 hospitals with no out-of-pocket costs to patients.
Contact : [email protected]; 212.788.3339
About NYC Health + Hospitals
NYC Health + Hospitals is the largest municipal health care system in the nation. We are a network of 11 hospitals, trauma centers, neighborhood health centers, nursing homes, and post-acute care centers. We are a home care agency and a health plan, MetroPlus. Our health system provides essential services to more than one million New Yorkers every year in more than 70 locations across the city’s five boroughs. Our diverse workforce of more than 43,000 employees is uniquely focused on empowering New Yorkers, without exception, to live the healthiest life possible. Visit us at www.nychealthandhospitals.org and stay connected on Facebook at https://www.facebook.com/NYCHealthSystem or Twitter at @NYCHealthSystem .
COVID-19 Resources
- COVID-19 Data Tracker
- COVID-19 Testing Locations
- COVID-19 Vaccine
- Where & How to Get the COVID-19 Vaccine
- COVID-19 FAQ
- COVID-19 Vaccine Myths
- After Your COVID-19 Vaccine
- How You Can Help
- Pandemic Response Status
- Visitation Restrictions
- For Providers
- COVID-19 ReCOVery Program
Related news
Cardiologist addresses long covid support group.
Apr. 29, 2022
Munson Healthcare Expands Access to COVID-19 Testing
Oct. 26, 2021
Related Blogs
A parents' guide to covid-19: keeping kids safe.
Jul. 16, 2022
7 Steps for a Successful Virtual Doctor Visit
Mar. 30, 2022
Spring Break During COVID-19
Mar. 18, 2022
Stronger Than Ever: A COVID-19 Survivor Story
Jan. 17, 2022
COVID-19 Survivor Stories
Jan. 02, 2022
Hunting Season During COVID-19
Nov. 10, 2021
Should Kids Wear Masks?
Sep. 30, 2021
The Long-Term Health Effects of COVID-19
Jul. 13, 2021
“Why I Got Vaccinated.” Three Teens Share Why They Chose the COVID-19 Vaccine
May. 12, 2021
Therapies for Treating COVID-19
Paxlovid Therapy for Treating COVID-19
Paxlovid is a first line therapy medicine used to treat mild-to-moderate COVID-19. Paxlovid can be used in adults and children who meet the following criteria:
- Are 12 years of age and older
- Weigh at least 88 pounds
- Have a positive test for the COVID-19 virus
- And, are at high risk for progression to severe COVID-19
When started within 5 days of symptom onset, Paxlovid keeps the virus that causes COVID-19 from reproducing, preventing symptoms from progressing. It reduces the risk of hospitalization and death for patients at high risk of disease progression.
To learn more about Paxlovid, visit the MDHHS Paxlovid Information for the Public webpage.
The FDA has authorized the emergency use of Paxlovid for the treatment of mild-to-moderate COVID-19 in adults and children who meet the above criteria.
A healthcare provider will determine if you are a candidate for Paxlovid, which requires a prescription. To further discuss treatment for COVID-19, connect with your primary care provider, or consider Munson Healthcare’s Virtual Urgent Care service.
To determine if Paxlovid is right for you, discuss with your medical provider:
- Any known allergies
- Diagnosis of liver or kidney disease
- If you are pregnant, plan to become pregnant or are breastfeeding a child
- Have any serious illnesses
- Any/all current medications and supplements you take
Currently, Paxlovid is provided by the federal government at no charge. Some dispensing pharmacies may bill insurance providers for the cost of dispensing the medication. Patients should not experience out-of-pocket costs.
Contact your primary care provider, or consider the Munson Healthcare Virtual Urgent Care to discuss treatment for COVID-19 with a healthcare provider.
- Extra 15% off $20+ Pickup orders with code PICKUP15
- Clip your mystery deal!
- Up to 60% off clearance
- Your Account
- Walgreens Cash Rewards
- Prescription Refills & Status
- Vaccination Records
- Order Status & History
- Buy It Again
Select a store
Walgreens virtual healthcare: online doctor & prescriptions.
Online doctor visits, on demand
See a doctor or nurse practitioner online, get a diagnosis and fill prescriptions, if needed, from the comfort of home.

Walk-ins welcome
How it works
Quickly connect with a clinician using our efficient intake process.

What condition can we help with?
Get high-quality care from U.S.-licensed doctors and nurse practitioners for nonemergency conditions.
Illness & infection

Women’s health

Allergy & asthma

Heart health
Sexual health.

Skin & hair

Urgent care
Type of visit: Video visit
Required for treatment: N/A
Live urgent care visits offer personalized discussions with a healthcare provider for a variety of conditions.
We recommend you see an in-person provider for your care if:
- You are experiencing chest pain or palpitations, difficulty speaking or slurred speech, facial droop or numbness, difficulty breathing, difficulty moving or feeling arms or legs, problems with your eyes (other than pink eye), or recently have fainted.
- You are seeking care for ear pain or dental pain.
- You are seeking care for a UTI and your sex assigned at birth is male
- You are pregnant or breastfeeding
- You are seeking controlled substances or durable medical equipment
- You are seeking a physical exam, lab work, or imaging orders
- You are seeking medications for pain, sleep, ADHD or anxiety
- If you are seeking care for strep throat, please note that based on recent clinical guidelines, in-person strep testing is required in many, but not all cases. We do not offer in-person strep testing; however, we can provide guidance on whether it would be appropriate based on your symptoms.
Commonly prescribed medications:
Varies based on patient symptoms. Recommended treatments may include online prescriptions, over-the-counter treatment recommendations, and/or self-care strategies.
Cough, Cold, Sinus Infection
You may be a good candidate for virtual care if:
- You are currently experiencing symptoms consistent with sinusitis, such as runny nose, stuffy nose or congestion
- You have had 3 or more sinus infections within the past year
- Your symptoms have been ongoing for more than 1 month or your symptoms have returned after having recently been treated for sinusitis with antibiotics
- You are having difficulty breathing that is unrelated to nasal congestion, you have pain or stiffness in your neck, changes in vision or a fever greater than 101°F
Depending on your symptoms, our providers may recommend prescriptions, over-the-counter medications, home remedies or seeking in-person care. Prescriptions may include antibiotics (such as Amoxicillin or Augmentin) for sinus infection, Benzonatate (Tessalon Perles) for cough or other symptomatic treatments for colds.
Type of visit: Chat
Required for treatment: Photo of left and/or right eye
- You are experiencing symptoms consistent with pink eye, such as redness, itchiness and crusting of the eye(s)
- You wear contact lenses
- You have a recent history of trauma to the eye or concern about something in your eye
- You have had eye surgery within the past 2 months
- You are experiencing blurred, impaired, or changes in vision, sensitivity to light, flashing lights or floaters, headache with nausea, inability to open eye or keep it open, redness beyond the eye, pain or tenderness in or around the eye
Commonly prescribed medications: Antibiotic eye drops, such as Erythromycin, trimethoprim-polymyxin B and ofloxacin
Required for treatment: Photo of lips
If you are also experiencing an outbreak of genital herpes, please start a genital herpes visit where we can address both oral and genital herpes.
- You have a current oral cold sore outbreak
- You have had 3 or more cold sore outbreaks within the last year
- Your last outbreak was within the past 2 weeks
- You are seeking cold sore suppression/prevention treatment or refills
Commonly prescribed medications: Valacyclovir, Acyclovir, Famiclovir
Type of visit: Chat (ages 18–64); Video Visit (ages 18+)
Required for treatment: Photo of a positive COVID test result from within 5 days of first symptom (such as an at-home test, a test result from Walgreens or another healthcare provider)
- You are interested in a COVID treatment plan that may include Paxlovid
- You are able to upload a photo of your positive COVID test result and you are within 5 days of your first symptoms
- You are experiencing a fever greater than 102°F, dizziness or lightheadedness, chest tightness, chest pain or wheezing, difficulty urinating, lower leg, ankle, or foot swelling, problems with memory and/or confusion, or problems eating/keeping down food or liquids
- You have hepatitis, cirrhosis, elevated liver enzymes or severe renal impairment
Commonly prescribed medications: Paxlovid (nirmatrelvir/ritonavir)
Typical number of refills if prescribed: 1 pack, 0 refills
Required for treatment: Photo of a positive flu test result. If you don’t have a positive flu test, you can schedule a Flu & COVID test for $19.99 at Walgreens.
If you were exposed to someone who tested positive for flu, such as a household member, and you have not been tested for flu, please select Video Visit and you can discuss treatment options with the provider.
- You are able to upload a photo of your positive flu test result with your name, date of birth and date. Or, you were in contact with someone who was diagnosed with flu and you are at high risk for complications from flu (please select Video Visit if you do not have a positive flu test result)
- You have had flu-like symptoms for less than 48 hours, you are at increased risk for complications from flu or you are in contact with someone who is immunocompromised
Commonly prescribed medications: Tamiflu (Oseltamivir)
Birth Control
Required for treatment: Documented blood pressure reading within the last 12 months, such as a photo of a medical record from a patient portal or an at-home blood pressure monitor reading. Photo should include your name, date of birth and date of reading. Don’t have a recent blood pressure reading? Get a blood pressure monitor from Walgreens with 30-minute Pickup or FREE 1-hour Delivery on orders $35+.
- You are interested in the following forms of birth control: oral pill, patch, vaginal ring, diaphragm and internal condoms, starting a new type of birth control or refilling your current form of birth control
- You are a biological female of reproductive age
- You can provide documentation of a blood pressure reading from within the past year
- You use tobacco and are 35 years or older
- You have a history of breast cancer, bariatric surgery, stroke, heart disease, cirrhosis or lupus
- You are seeking Depo-Provera, Phexxi, temporary menses delay, IUD or Nexplanon.
Commonly prescribed medications: Sprintec, Junel, Norethindrone (such as Ortho Micronor), Nuvaring
Typical number of refills if prescribed: One-year supply
Urinary tract infection
Type of visit: Video Visit
- You have had symptoms for less than 2 weeks (pain with peeing, urgent need to pee and/or frequent peeing)
- This is your first UTI in the past 3 months
- Your sex assigned at birth was female
- You are experiencing fever, chills, nausea, vomiting, flank, back, or abdominal pain or unusual vaginal discharge
Commonly prescribed medications: Macrobid, Bactrim, Cephalexin
Emergency contraception
Type of visit: Chat or Video Visit, varies by state. Before you pay for your visit, we will let you know if we need to conduct a Video Visit in your state.
Required for treatment: Height and weight
- You are a biological female
- You had unprotected sex within the past 5 days
- You are vomiting
- You have a history of malabsorptive bariatric procedures
Commonly prescribed medications: Ella (ulipristal), Plan B (levonorgestrel)
Plan B and Ella are both safe and effective forms of emergency contraception to reduce the risk of unintended pregnancy. Plan B is available over the counter while Ella is only available via prescription. Ella can be taken up to 5 days after unprotected sex and has a higher effectiveness rate compared to Plan B. Cost of over-the-counter versus prescription medications may vary based on health insurance coverage.
Yeast Infection
- You are currently experiencing symptoms consistent with yeast infection, such as itchiness, burning and irritation of the vagina
- You have had a yeast infection in the past
- Your sex assigned at birth is female
- You are experiencing abdominal, pelvic or back pain
- You are experiencing nausea, vomiting, fever or chills
- You have a concern for STIs or bacterial infection
- You have visible vaginal lesions
- You experience recurrent yeast infections (4 or more per year)
- You have a recurrent yeast infection within 7 days of recent treatment
- You are seeking preventative treatment
Commonly prescribed medications: Fluconazole
Asthma medication refill
Required for treatment: Photo of your most recent asthma prescription (pill bottle, inhaler or medical record)
- You need a refill (30 day supply) of your most recent asthma medication
- You do not have a prior history of asthma
- You have poorly controlled asthma
- You are currently experiencing an asthma exacerbation
- You have concern for an underlying infection
- It has been more than 1 year since you have last had an asthma management visit with your PCP or specialist
Please note:
- We do not offer treatment adjustments
- We do not offer steroids for exacerbations
- Daily medications will only be refilled if you have been less than 2 weeks without medication
- Episodic medications (such as inhalers) will only be refilled if you have been less than 6 months without medication
Commonly prescribed medications: Albuterol inhaler refills, refills of your current daily medication for asthma
Typical number of refills if prescribed: 30 day supply with 0 refills per year
Patients may not receive more than one prescription every 12 months through this service
Seasonal Allergies
Required for treatment: Photos of eyes, nose and back of throat
- You have recurring seasonal allergy symptoms, which may impact the eyes, nose, sinuses and skin (such as clear nasal discharge, past history of seasonal or environmental allergies and seeking symtomatic relief) (If you are not sure what is causing your symptoms, we recommend seeking care through the Urgent Care visit or Cold, Cough, Sinus Infection visit.)
- You have eye or facial pain, fever or chills, are coughing up yellow or green phlegm, or have yellow or green drainage from the nose
- You are having a severe allergic reaction (such as, shortness of breath, wheezing, swollen lips or tongue)
- You may have a viral infection, eye injury, abrasion or ulceration
- You have a history of asthma
Commonly prescribed medications: Montelukast (Singulair), Fluticasone nasal spray (Flonase), Cetirizine (Zyrtec)
Providers may prescribe medications that are also available over-the-counter. OTC medications may not be covered by insurance and it may be cheaper to get medication through a prescription. Insurance coverage and pricing varies by plan. Typical number of refills if prescribed: Montelukast: 90 tablets, 1 refill
Blood Pressure Medication Refill
Required for treatment: Photo of current prescriptions for high blood pressure (picture of pill bottle(s) or medical record)
- You have been previously diagnosed with hypertension and are seeking a refill of your current medication
- You are on 3 or more high blood pressure medications
- You are experiencing headache, blurry vision, dizziness, nausea or vomiting, severe back pain, confusion, lightheadedness, fainting, dry mouth or thirst, shortness of breath, chest pain or tightness, palpitations or racing heart
- You are seeking a refill of a blood thinner medication
- You have been off of your current medication for more than a week
Commonly prescribed medications: Diuretics, ACE inhibitors, ARBs, Beta Blockers, & Calcium channel blockers, including combination pills
Typical number of refills if prescribed: We provide one 90-day refill within a 12 month period if your provider determines it is clinically appropriate for you. If you need another refill within 12 months, please follow up with your primary care provider.
Erectile dysfunction
Required for treatment:
- Proof of most recent ED prescription (photo of pill bottle or medical record)
- Proof of documented blood pressure reading within the last 12 months (such as a photo of a medical record from a patient portal or an at-home blood pressure monitor reading) Don’t have a recent blood pressure reading? Get a blood pressure monitor from Walgreens with 30-minute Pickup or FREE 1-hour Delivery on orders $35+.
- You have been previously diagnosed with erectile dysfunction and are overdue for a provider visit or need a refill of your medication
- You are a biological male
- You have a documented blood pressure reading within the last 12 months
- You can provide a photo of your most recent ED prescription (pill bottle or medical record)
- You have had elevated blood pressure in the past 12 months
- You have a family history of heart attack or heart disease in persons less than 55 years of age
- You have recently experienced chest pain, dizziness, fainting or seizures or have heart disease
Commonly prescribed medications: Cialis (tadalafil), Viagra (sildenafil), Levitra (vardenafil)
Typical number of refills if prescribed: 90 day supply with 3 refills. Early refills will not be provided.
Genital herpes
Required for treatment: Photo of your most recent prescription medication used for genital herpes
- You have a current outbreak of genital herpes
- You have previously been diagnosed and treated for genital herpes
- You are seeking genital herpes suppression/prevention treatment or refills
- This is your first genital outbreak
- There is concern for a concurrent bacterial infection
- You have atypical lesions on areas of the body that are not the genital or oral region
- You have lesions near the eye area
- You have neck pain, fever, severe headache, nausea or vomiting
- You have had 3 or more outbreaks within the last 12 months
Commonly prescribed medications: Valacyclovir tablet, Acyclovir tablet, Famciclovir tablet
Acne, anti-wrinkle & dark spots
Required for treatment: Photo of front of face, left side of face and right side of face
- You are interested in topical treatment for mild-moderate facial acne, wrinkles, or dark spots
- You have severe scarring, acne on multiple parts of your body or cystic acne
- You need the following prescriptions: isotretinoin (also known as Accutane, Zenatane, Absorica, Myorisan, Claravis, or Amnesteem), oral antibiotics or spironolactone
Commonly prescribed medications: Tretinoin (for acne & anti-wrinkle), Benzoyl Peroxide (for acne), Topical Clindamycin (for acne), Birth control (for hormonal acne), Hydroquinone (for dark spots)
Typical number of refills if prescribed: 2 refills
Men’s hair loss
- Photo of hairline and scalp
- You have male pattern balding (hair loss around your hairline and on the top of your head)
- You are interested in regrowing your hair or preventing future hair loss
- You have significant hair loss on other parts of your body
- You have pain, itching or burning of the scalp
- You have HIV, a weakened immune system, a rheumatological disorder or autoimmune diseases
Commonly prescribed medications: Propecia (finasteride); we do not prescribe Oral Minoxidil
Typical number of refills if prescribed: 90 day supply, 3 refills
When you need care now, not later

No appointment needed
Most visits start within 15 minutes of your request being submitted.
Extended hours, 7 days a week: 8 am-midnight EST (5 am-9 pm PST)
Currently available in: CA, FL, GA, IL, MI, NV, NC, OH and TX

Low & upfront fees
Transparent pricing before your visit with low-cost services from $33 to $75.
Pay with credit card, debit card, HSA or FSA card. Insurance currently not accepted.

Prepare for your visit
To begin your visit, you’ll need to:
- Upload a government-issued photo ID and your photo to verify your identity
- Answer a few medical questions to determine if this service is a good fit for your needs
- Enable your video camera and microphone access for treatments that require a live video visit
- Use the current version of Chrome, Firefox or Safari for best results when accessing the service
Prescriptions made easy

Frequently Asked Questions
- Start a visit request anytime. Select your treatment area and answer clinical intake interview questions regarding your condition. Some treatment areas require you to provide photos, for example, of the affected area or of a previously filled medication.
- The next available provider licensed in your state will send you a message through your patient portal when they are ready to connect. Doctors and nurse practitioners are available between the hours of 8 am to midnight EST (5 am to 9 pm PST), seven days a week. If you request a visit overnight, a provider will reach out to you as soon as they are available, which may be the following morning.
- To get started, click on a treatment area above to learn more about our visit types, what we treat and typical wait times.
All treatment areas require you to upload a government-issued photo ID, as well as a selfie, to verify your identity. All visits require patients to be age 18 or older. Each treatment area has unique requirements to receive care. Please click on a treatment area above to learn more about its requirements.
To access this service, you must be physically located in one of the following states at the time of the visit: CA, FL, GA, MI, IL, NV, NC, OH or TX. Urgent care is not yet available in MI. Please check back soon to learn where we are growing next—we plan to offer virtual care to patients in additional states in the future.
Visits can be conducted on a mobile device, tablet or desktop computer. We recommend using the most recent version of Chrome, Firefox or Safari to access the service. Microsoft Edge is known to cause issues in video-based visits. Internet Explorer is not supported. For video visits, you will need to enable video camera and microphone access to interact with a clinician. Video visits are conducted on a HIPAA-compliant platform. Click on a treatment area above to learn more.
Patient support can be reached at 866-740-7721 between the hours of 8 am to midnight EST (5 am to 9 pm PST), seven days a week. You may also send a message through the patient portal in your Walgreens Virtual Healthcare account.
Payment & insurance
Walgreens Virtual Healthcare visits are $33 - $75. Click on a treatment area above to see the price of each visit type. Insurance is currently not accepted for virtual care visits; however, you may pay for your visit with your HSA/FSA card. Insurance may be used to purchase your prescription, if applicable. We plan to accept insurance for virtual care visits in the future.
Yes, you can use insurance to pay for most medication. Insurance coverage for prescription drugs varies. Please talk with your insurer or pharmacy directly about any questions regarding your prescription drug coverage. The cost of medication is not included in the cost of your visit. Looking to pay for your medication out of pocket? Find lower prescription prices at Walgreens with our free search tool, Walgreens Rx Savings Finder , powered by RxSense ® .
We accept debit, credit and HSA/FSA cards.
The fee you pay is for a clinical assessment, which may be conducted via a live visit or an offline (asynchronous) review of your clinical records by a Walgreens Virtual Healthcare provider. They will review your clinical situation and use their independent clinical judgment to recommend the most appropriate treatment option for you. This may include prescribing medication, recommending over-the-counter medications or home care strategies and/or recommending that you visit an in-person provider for further evaluation (such as an urgent care center, emergency department, primary care provider or specialist). We cannot offer refunds solely because the provider determines a prescription medication is not the best option for you, cannot be safely prescribed based on the information available, does not prescribe your preferred medication or due to issues related to pharmacy medication pickup.
If you are unable to complete your visit, please let us know right away by logging in to your Walgreens Virtual Healthcare account and sending us a chat message, or by calling us at 866-740-7721, so we can cancel your consultation before a provider reviews your intake. Your credit card will be charged after the provider reviews your intake and shares their recommendations for next steps.
If you have questions about your care, please log in to your Walgreens Virtual Healthcare account and send us a chat message or call us at 866-740-7721.
Our provider team is made up of experienced doctors and nurse practitioners who offer high-quality treatment and care. You will be matched with a doctor or nurse practitioner licensed in the state where you are located at the time of your visit. Service availability varies by state.
Providers & prescriptions
After your visit, if you would like your records sent to your primary care physician, you can use the Walgreens Virtual Care patient portal to send us a message and request that a copy of your records be sent to your primary care physician.
Please click on a treatment area above to learn more about what medications are commonly prescribed, as well as common exclusions. We do not prescribe any medications that are listed as controlled substances by the U.S. Drug Enforcement Agency (DEA) or state law. This includes narcotics, amphetamine stimulants and benzodiazepines.
Disclaimers
Walgreens-affiliated medical practices are independently owned and operated by licensed physicians who provide services using the Walgreens virtual care program telehealth platform. For more information about the relationship between Walgreens and the medical practices click here .
Walgreens Health Medical Group California P.C. is a California professional medical corporation utilizing the fictitious name “Walgreens Health Medical Group California P.C.” pursuant to Cal. Bus. & Prof. Code § 2415. To view the Fictitious Name Permit click here .

- Epidemiology and Global Health
- Open access
- Copyright information
Optimal timing maximises Paxlovid benefits for treating COVID-19
- Annotations Open annotations. The current annotation count on this page is being calculated .
Researchers have described the optimal timing for COVID-19 patients to take the antiviral, Paxlovid, to get the most benefit from the treatment, according to a study published April 16 in eLife.
The findings suggest that taking Paxlovid three to five days after COVID-19 symptoms emerge may maximise the drug’s ability to reduce viral loads, minimise viral spread and reduce viral rebound. They also indicate that broader use of Paxlovid during this window might be a powerful tool to help curb the spread of the SARS-CoV-2 virus that causes COVID-19 without the need for potential population-wide restrictions in the future, although additional studies will first be needed in this area.
Paxlovid is used in some parts of the world to protect people most at risk of hospitalisation and death from the virus. However, its uptake has been slow, and some patients have experienced rebounding viral growth after they stopped taking the medication.
“Drugs like Paxlovid that hinder the replication, or spread, of a virus within patients can improve those patients’ outcomes and reduce their infectiousness to others. They may also be administered more widely to help curb pandemic waves,” says Zhanwei Du, Assistant Professor (Research) at the WHO Collaborating Center for Infectious Disease Epidemiology and Control, School of Public Health, University of Hong Kong, and a lead author on the study. “However, to get the most out of these drugs, we first need to understand the optimal timing for taking them and to encourage their wider distribution and uptake.”
To determine the best timing for Paxlovid treatment, Du and colleagues analysed health records from 208 patients hospitalised with mild to moderate COVID-19 in Hong Kong between January 6 and May 1, 2022. Half of the patients included in the analysis (104) were treated with Paxlovid, while the other half received no antiviral treatment.
The research team used data on the patients’ viral loads to create mathematical models of the virus’ behaviour both with and without Paxlovid treatment. Their modelling suggested that the drug reduced viral replication in patients by 90% overall, but its effectiveness varied depending on when it was administered to them.
Those who received Paxlovid treatment three days after their symptoms first appeared had only a 17% chance of experiencing rebounding viral growth after they stopped taking the drug. Treatment in this window also lowered their ability to infect others by 12%, while treatment after five days of symptoms emerging had a less positive effect on their ability to infect others. Patients treated fewer than three days after symptoms began were more likely to have rebounding viral growth after stopping treatment, and the treatment did not reduce their infectiousness to others.
“Our analyses suggest that the optimal treatment window to maximise Paxlovid’s ability to reduce infectiousness and minimise rebound viral growth is between three and five days after symptoms start,” says co-senior author Lauren Ancel Meyers, the Cooley Centennial Professor at the Departments of Integrative Biology, and Statistics and Data Science, at the University of Texas at Austin, US. “The data also suggest timely use of Paxlovid could help lower the risk of SARS-CoV-2 transmission to others.”
However, the authors note that the data do not currently account for the potential emergence of resistance to Paxlovid, which they say would need to be evaluated further before the drug could be used more widely.
“Fast-acting antiviral drugs like Paxlovid have the potential to reduce SARS-CoV-2 transmission while improving patient outcomes,” concludes co-senior author Benjamin Cowling, Professor, Chair of Epidemiology and co-Director of the WHO Collaborating Center for Infectious Disease Epidemiology and Control, School of Public Health, University of Hong Kong. “The development of global distribution programs that provide rapid and equitable access to antivirals could enhance our ability to combat COVID-19 as the virus and the landscape of immunity continues to evolve.”
Media contacts
Emily Packer eLife [email protected] +441223855373
George Litchfield eLife [email protected]
eLife transforms research communication to create a future where a diverse, global community of scientists and researchers produces open and trusted results for the benefit of all. Independent, not-for-profit and supported by funders, we improve the way science is practised and shared. In support of our goal, we’ve launched a new publishing model that ends the accept/reject decision after peer review. Instead, papers invited for review will be published as a Reviewed Preprint that contains public peer reviews and an eLife assessment. We also continue to publish research that was accepted after peer review as part of our traditional process. eLife receives financial support and strategic guidance from the Howard Hughes Medical Institute , Knut and Alice Wallenberg Foundation , the Max Planck Society and Wellcome . Learn more at https://elifesciences.org/about .
To read the latest Epidemiology and Global Health research published in eLife, visit https://elifesciences.org/subjects/epidemiology-global-health .
A retrospective cohort study of Paxlovid efficacy depending on treatment time in hospitalized COVID-19 patients
Be the first to read new articles from elife.


[149] How useful is Paxlovid in 2024?
18 Apr 2024 [149] How useful is Paxlovid in 2024?
Jump to: Abstract | Full Text | Plain Language Summary | Infographic | Conclusions | References

click on the image to open high resolution version of the infographic
Plain Language Summary
Should i take paxlovid tm for covid-19.
BOTTOM LINE:
For most people with COVID-19, Paxlovid TM will:
- NOT make you feel better faster
- NOT lower your chance of dying or going to the hospital
What is Paxlovid TM ?
Paxlovid TM (nirmatrelvir-ritonavir) is a medication for some people who have COVID-19. It is meant to lower the chance of dying or going to the hospital because of COVID-19. Paxlovid TM is for people who:
- have tested positive for COVID-19 within the last 5 days, and
- are at high risk of dying or going to the hospital because of COVID-19.
What does “high risk” mean?
Only people at high risk should be offered Paxlovid TM . This includes those who are:
- severely immunocompromised (for example, those who have received an organ transplant, or are being treated for blood cancer), or
- moderately immunocompromised (for example, those being treated for cancer, taking immunosuppressants, some people with HIV)
For such people, Paxlovid TM may lower the chance of dying or going to the hospital. Ask your doctor or nurse practitioner if you think you might be at high risk.
What if I am not “high risk”?
If you are not in the high-risk categories above, Paxlovid TM is unlikely to make you feel better faster, or lower your chance of dying or going to the hospital because of COVID-19. You could also experience side effects from taking Paxlovid TM . The chance of hospitalization or death from COVID-19 in BC is now very low.
Has the definition of “high risk” changed?
Yes. Early in the COVID-19 pandemic, certain things were thought to make people much more likely to get very sick from COVID-19. This included older age and conditions like stroke, diabetes, or heart disease. Now, most people are vaccinated or have already had COVID-19. The current circulating strains of the virus are less likely to make someone very sick compared to early in the pandemic.
Does Paxlovid TM have side effects?
People taking Paxlovid TM are more likely to notice side effects compared with those who don’t. Side effects can include altered taste, diarrhea, muscle aches, or nausea. Your prescriber and pharmacist will need to check if your other medications are safe to take with Paxlovid TM . You may have to stop some of your usual medications when taking Paxlovid TM .
Background: In 2024 the risk of hospitalization or death due to COVID-19 is low for most people under age 80 in British Columbia (BC). However, for those at high risk of complications, the antiviral drug combination nirmatrelvir-ritonavir (NMV-r, marketed as Paxlovid TM ) has shown promise in reducing the composite risk of hospitalization and death from COVID-19.
Methods: We evaluated the effectiveness of NMV-r based on data from 3 randomized controlled trials conducted by Pfizer and an observational study conducted by the Therapeutics Initiative in British Columbia. In addition to the impact of NMV-r on COVID-19-related hospitalization or death, we considered the impact of vaccination status on the drug’s effectiveness.
Results: The EPIC-HR trial showed a significant reduction in hospitalization and death in high-risk, unvaccinated patients during 2021. In contrast, the 2021-2022 EPIC-SR trial found no benefit for lower-risk, vaccinated patients. The BC observational study found statistically significant risk reductions in high-risk groups, but not in an expanded eligibility group. NMV-r does not affect symptom duration or prevent transmission and is not known to prevent long-COVID.
Conclusions: NMV-r may benefit high-risk COVID-19 patients, but it offers little advantage for lower-risk, vaccinated individuals. As Paxlovid TM will cost Canadians about $1,400 per treatment, cost-effectiveness is a significant consideration. The ongoing PANORAMIC and CanTreatCOVID clinical trials may provide further insights into NMV-r’s long-term effects on COVID-19 and on healthcare utilization.

How useful is Paxlovid ™ in 2024?
Vignette: During a possible future resurgence of the COVID-19 pandemic, two patients test positive and ask you to prescribe antiviral therapy. One is 47 years old, up to date on his COVID vaccinations, but overweight and worried about his lungs because he smokes. Having paid taxes for years, he feels that he deserves access to treatment. Your second patient is 78 and also fully immunized; but she has diabetes, heart failure and is undergoing treatment for breast cancer. Should you prescribe Paxlovid TM for both, or only for one?
Summary and Conclusions
- The risk of hospitalization or death resulting from COVID-19 in 2024 is low in British Columbia for most people under 80 years of age.
- For people at high risk of complications from COVID-19, results of the EPIC-HR trial and BC observational data are congruent. The antiviral drug combination nirmatrelvir-ritonavir (NMV-r) also known by its brand name Paxlovid TM reduces the composite risk of hospitalization with COVID-19, and death from any cause.
- In contrast, people who are vaccinated are at lower risk and are unlikely to benefit.
- NMV-r does not shorten symptoms or prevent transmission, and is not known to prevent long-COVID.
- A large ongoing trial in the United Kingdom and the similar CanTreatCOVID trial in Canada may provide further information within 1-2 years.
- Paxlovid TM is expected to cost Canadians about $1,400 for a 5-day course of therapy. Before prescribing it, consider the cost of treating what may be hundreds of patients to prevent one event.
Evidence has emerged gradually as to who is most likely to benefit from NMV-r for COVID-19
In January 2022 Health Canada approved the antiviral drug combination nirmatrelvir-ritonavir (NMV-r) also known by its brand name Paxlovid TM . A year later, Therapeutics Letter 141 provided an interim analysis of the effects of NMV-r on COVID-19–related hospitalization or death from any cause in British Columbians eligible for treatment. 1
By then, Pfizer Inc. had completed 3 double-blind randomized clinical trials (RCTs) of NMV-r as part of a program entitled Evaluation of Protease Inhibition for COVID-19 (EPIC). Reported numbers of participants differ between a March 16, 2023 US FDA Briefing Document 2 and the sponsor’s published reports. Numbers shown below are from the references cited:
- EPIC-HR: 5 days of NMV-r vs placebo for 2,246 unvaccinated people with confirmed infection deemed at “high risk” from COVID-19 who were enrolled between July 16 and December 9, 2021 and predominantly infected with the Delta variant. Results published online on February 16, 2022 demonstrated a clinically and statistically significant reduced risk of hospitalization and death in this early pandemic high-risk population. 3 Therapeutics Letter 141 summarized these results. 1
- EPIC-SR: 5 days of NMV-r vs placebo for 1,296 people with confirmed infection deemed at “standard risk” who were fully vaccinated with risk factors for progression or unvaccinated, enrolled between August 25, 2021 and July 25, 2022 and infected with either Delta or Omicron variants. Pfizer announced by media release on June 14, 2022 that EPIC-SR did not achieve its primary endpoint of shortening symptom duration. 4 On August 14, 2023 it posted results at clinicaltrials.gov 5 and published formal results on April 4, 2024. 6 Below, we discuss these results further.
- EPIC-PEP: 5 or 10 days of NMV-r vs placebo for post-exposure prophylaxis in 2,954 people with infected household contacts , enrolled between September 9, 2021 and April 12, 2022. Pfizer announced by media release on April 29, 2022 that numerical reductions of infections were not statistically significant. 7 It posted results to clinicaltrials.gov on May 6, 2023 8 but has yet to publish EPIC-PEP.
This Therapeutics Letter highlights the final results of our BC observational study, and contextualizes our findings with results from the newly published EPIC-SR trial.
What can we learn from the EPIC trials?
The 3 EPIC trials were double-blinded, randomized, placebo-controlled trials (DBRCT) designed, sponsored, and conducted by Pfizer.
EPIC-HR (“High-Risk”) included unvaccinated patients who were expected to be at high risk for complications from COVID-19, including from immunosuppression, smoking, cardiovascular disease, and cancer. 3 The median age was 46 and 13% of participants were 65 or older. Its primary endpoint was the composite of hospitalization with COVID-19 or death from any cause by day 28 post-enrolment. NMV-r reduced this endpoint by an absolute risk reduction of 5.6%, 95% confidence interval 4.0% to 7.2%, number needed to treat (NNT) = 18 to prevent 1 hospitalization or death. ( Table )
In contrast, EPIC-SR (“Standard-Risk”) included patients without the high-risk factors of participants in EPIC-HR. In this trial, 57% were fully vaccinated against COVID-19, the median age was 42, and only 5% were 65 or older. 6 The primary endpoint was time to sustained alleviation of symptoms. COVID-19–related hospitalization or death from any cause was a secondary endpoint. No difference was observed in any of these endpoints. ( Table )
It is important to appreciate that EPIC-HR was conducted in unvaccinated people during the Delta wave of the COVID-19 pandemic. EPIC-SR also included 43% unvaccinated participants and overlapped the Delta and Omicron waves. Thus the generalizability of the EPIC findings is unknown for treatment of vaccinated patients in 2024 or subsequently, and for people infected with an Omicron variant.
The EPIC-PEP trial also spanned the period of Delta and then Omicron variant prevalence. 8 While these results remain unpublished, the US FDA concluded that the difference between a 2.4% to 2.6% short-term infection rate amongst NMV-r treated household contacts of an infected patient vs. 3.9% amongst placebo recipients was neither clinically meaningful nor statistically significant. 2
One challenge to understanding the EPIC trial results is whether advanced age, of itself, increases serious outcomes from COVID-19 that can be mitigated by NMV-r treatment. EPIC-HR defined as “high risk” anyone over age 60, but participants were unvaccinated (during the second year of the pandemic). 9 The trial report refers to only 268 people age ≥65. 3 In EPIC-SR, anyone age ≥18 without high-risk comorbidities was eligible and considered as having “standard risk” for COVID-19 complications. 10 This included 65 people age ≥65 who were fully vaccinated, the oldest was 87. Thus, age alone is at best an arbitrary marker for risks from COVID-19, and needs to be contextualized among other risk factors such as vaccination status and comorbidities.
Observational study of NMV-r in BC: final results
Therapeutics Initiative researchers used BC Ministry of Health datasets to analyze the 28-day risk of COVID-19-related hospitalization or death from any cause in the groups eligible for treatment. 11 This mimics the outcomes studied in EPIC-HR. To minimize confounding bias, we limited our analysis to the subset of British Columbia residents to whom NMV-r was dispensed (“index cases”), and for whom we could match a person (control) of the same age (±2 years), sex, and who was infected with laboratory-confirmed COVID-19 infection within a month of the paired index case. We also matched for propensity scores, a common pharmacoepidemiologic method to control for imbalances in comorbidities between groups.
We studied 4 groups of patients, of which 3 included people considered “clinically extremely vulnerable” (CEV) by virtue of medical conditions previously designated in early 2021 to prioritize COVID-19 vaccinations. The first 2 groups included people age ≥18 who were severely (CEV1) or moderately (CEV2) immunocompromised. A third (CEV3) comprised people who were not immunocompromised, but with medical conditions that engender a high risk for complications from COVID-19. A fourth “Expanded Eligibility” group allowed wider access to NMV-r.
The BC eligibility criteria for NMV-r treatment, updated in November 2023 are available online. 12 However, they may change due to efficacy and effectiveness evidence accumulated since 2021, as suggested by the Canadian Drug Expert Committee in January 2024. 13
The Table juxtaposes results from the EPIC-HR and EPIC-SR DBRCTs with observational findings from BC patients with a positive polymerase chain reaction (PCR) test who were eligible for and received NMV-r, and from matched controls between February 1, 2022, and February 3, 2023, when most British Columbians were fully vaccinated. The median age of these BC patients and controls was 70.
Compared with controls who did not receive NMV-r, treatment was associated with statistically significant absolute risk reductions in the composite outcome of 2.5% in the CEV1 group, and 1.7% in the CEV2 group. There was a non-statistically significant absolute risk reduction of 1.3% in the CEV3 group, but a non-statistically significant absolute risk increase in the Expanded Eligibility group. In this group, the BC cohort most similar to people studied in the EPIC-SR trial, our results also did not identify a benefit from NMV-r (Paxlovid TM ).
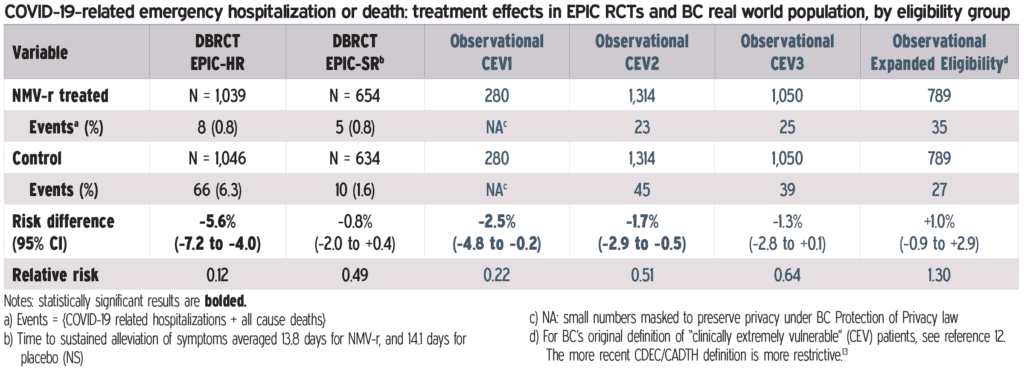
What can we tell patients who contract COVID-19?
For most people in British Columbia, the risk of hospitalization with COVID-19 or death from COVID-19 is now very low. In October 2023, scientists at the BC Centre for Disease Control published their analysis of severe outcome risks due to first infections among residents of BC’s Lower Mainland. 14 The most recent interval studied was July 31 to December 3, 2022.
For the latter part of 2022 they estimated the following risks for people newly infected with COVID-19:
Ages 60 to 69:
- death: about 3 per 10,000 infected (18 events among 69,830 people);
- hospitalization for COVID-19: about 16 per 10,000 (112 events).
Ages 70 to 79:
- death: about 11 per 10,000 (35 events among 31,440 people);
- hospitalization for COVID-19: about 73 per 10,000 (228 events).
Given these estimates, even if NMV-r could hypothetically have reduced the risk of death or hospitalization by 50% in people aged 70 to 79 (for which no evidence exists), the number needed to treat (NNT) to prevent one death during the second half of 2022 would have been about 1,800. To prevent one hospitalization, the NNT would have been about 275. The BCCDC authors emphasize that their estimates refer to people infected for the first time. 14 However, severe outcome risks may be even lower – and the corresponding NNTs even higher – among people who are fully vaccinated and have also acquired natural immunity from a COVID-19 infection.
The EPIC-SR and EPIC-PEP randomized clinical trials did not show that NMV-r can reduce symptom duration, or prevent COVID-19 after exposure. Currently, there is also no evidence that early treatment with NMV-r for acute COVID-19 infection can prevent long-COVID. 13 We also do not know whether NMV-r can prevent hospitalization of typical residents in long-term care.
NMV-r relies on ritonavir to prolong the half-life of nirmatrelvir by potent inhibition of CYP3A, an important drug-metabolizing enzyme in the liver and the small intestine. 15 Because CYP3A metabolizes about half of commonly used drugs, using NMV-r requires special attention to potential toxicity from other medications. The most common adverse effects are taste alteration and diarrhea, but patients need to understand that NMV-r is also potentially dangerous to some.
Will more information from RCTs be forthcoming?
PANORAMIC is a large publicly funded open label trial in the United Kingdom (UK), designed and run from Oxford University. 16 It began by evaluating treatment of early COVID-19 with molnupiravir, a different antiviral drug licensed in the UK, from December 2021 to late April 2022. In 25,708 people (mean age 57 years, 94% having received at least 3 vaccine doses) molnupiravir did not prevent hospitalization or death, compared with usual care alone. 17 It was also not cost-effective for overall health utilization. 18 Molnupiravir is not approved in Canada except for use in clinical trials. 19
Between April 2022 and the end of recruitment on March 28, 2024, the PANORAMIC trialists also randomized 3,516 participants to open-label NMV-r versus usual care. 20 This part of the PANORAMIC trial will eventually provide additional information about NMV-r, including whether it can reduce long-term persistence of symptoms and healthcare utilization at 3 and 6 months after treatment. 16 , 21
The Canadian Institutes of Health Research (CIHR), Health Canada, and the Public Health Agency of Canada sponsored a similar trial, CanTreatCOVID , which began to randomize Canadian patients to NMV-r or usual care in January 2023. 22 The trial protocol mirrors PANORAMIC’s practical approach to self-enrolment through a website or referral by clinicians. Anyone age 50 years or older who tests positive for COVID-19 is eligible to participate, as well as younger adults with chronic conditions. Patients can self-refer at www.cantreatcovid.org , and preliminary results are expected in 2025. The study website shows 454 Canadians had enrolled as of April 17, 2024.
Is NMV-r therapy worth the cost?
This is an important question for an expensive drug. In Canada, a 5-day course of Paxlovid TM has a retail cost of about $1,400 (including dispensing). 13 Roughly 300 people currently take NMV-r each week in BC. Similar utilization could total nearly $22 million/year. Healthcare resources are always in high demand, and money devoted to drugs is not available for other parts of the healthcare system. For perspective, $1,400 approximates the hospital cost of a surgical procedure such as cholecystectomy or hernia repair, can vaccinate about 75 people against COVID-19, or cover about 40 routine patient visits with a family doctor.
Vignette resolution: Your 47 year-old patient is unlikely to benefit from NMV-r, so you review his COVID-19 vaccination history and advise him about current booster options and recommendations. But your 78 year-old may have a substantially higher risk for complications from COVID-19 due to her pre-existing chronic conditions, and meets criteria recently proposed by the Canadian Drug Expert Committee. 13 Again, you review vaccination status, but as her symptoms began yesterday and she tested positive, you prescribe NMV-r after checking for interactions with her usual drug therapy.
BC primary care physicians and nurse practitioners can sign up on a secure platform to access their own personalized prescribing Portraits (8 topics currently available and more are being produced). If you qualify and have already signed up, log in to view your Portraits.
- Therapeutics Initiative. Paxlovid in British Columbia: Interim real-world analysis. Therapeutics Letter 141, January-February 2023. https://ti.ubc.ca/letter141
- Food and Drug Administration. FDA briefing document: Antimicrobial Drugs Advisory Committee meeting, March 16, 2023 . https://www.fda.gov/media/166197/download Errata: https://www.fda.gov/media/166198/download
- Hammond J, Leister-Tebbe H, Gardner A, et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. New England Journal of Medicine. 2022; 386(15):1397-1408. DOI: 10.1056/NEJMoa2118542
- Pfizer. Pfizer Reports Additional Data on PAXLOVID™ Supporting Upcoming New Drug Application Submission to US FDA. June 14, 2022. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-reports-additional-data-paxlovidtm-supporting
- US National Library of Medicine. Evaluation of Protease Inhibition for COVID-19 in Standard-Risk Patients (EPIC-SR). https://classic.clinicaltrials.gov/ct2/show/results/NCT05011513
- Hammond J, Fountaine RJ, Yunis C, et al. Nirmatrelvir for Vaccinated or Unvaccinated Adult Outpatients with Covid-19. New England Journal of Medicine. 2024; 390(13):1186-1195. DOI: 10.1056/NEJMoa2309003
- Pfizer. Pfizer Shares Top-Line Results from Phase 2/3 EPIC-PEP Study of PAXLOVIDTM for Post-Exposure Prophylactic Use. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-shares-top-line-results-phase-23-epic-pep-study
- US National Library of Medicine. A Study of a Potential Oral Treatment to Prevent COVID-19 in Adults Who Are Exposed to Household Member(s) With a Confirmed Symptomatic COVID-19 Infection. https://classic.clinicaltrials.gov/ct2/show/results/NCT05047601
- Pfizer. EPIC-HR Protocol C4671005: Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. New England Journal of Medicine. 2022; 386(15) https://nejm.org/doi/suppl/10.1056/NEJMoa2118542/suppl_file/nejmoa2118542_protocol.pdf
- Pfizer. EPIC-SR Protocol C4671002: Nirmatrelvir for Vaccinated or Unvaccinated Adult Outpatients with Covid-19. New England Journal of Medicine 2024; 390(13) https://www.nejm.org/doi/suppl/10.1056/NEJMoa2309003/suppl_file/nejmoa2309003_protocol.pdf
- Dormuth CR, Kim JD, Fisher A, et al. Nirmatrelvir-Ritonavir and COVID-19 Mortality and Hospitalization Among Patients With Vulnerability to COVID-19 Complications. JAMA Network Open 2023; 6(10):e2336678. DOI: 10.1001/jamanetworkopen.2023.36678
- BC COVID Therapeutics Committee (CTC). Clinical Practice Guide for the Use of Therapeutics in Mild-Moderate COVID-19. November 2023 Update. http://www.bccdc.ca/Health-Professionals-Site/Documents/COVID-treatment/ClinicalPracticeGuide_Therapeutics_MildModerateCOVID.pdf
- Canadian Agency for Drugs, Technology and Health Assessment. CADTH Reimbursement Recommendation Nirmatrelvir-Ritonavir (PaxlovidTM). January 2024. https://www.cadth.ca/sites/default/files/DRR/2024/SR0808%20Paxlovid%20-%20Draft%20CADTH%20Recommendation.pdf
- Skowronski DM, Kaweski SE, Irvine MA, et al. Risk of hospital admission and death from first-ever SARS-CoV-2 infection by age group during the Delta and Omicron periods in British Columbia, Canada. CMAJ Canadian Medical Association Journal 2023; 195(42):E1427-1439. DOI: 10.1503/cmaj.230721
- Pfizer Canada. PaxlovidTM Product Monograph. Revised January 2, 2024. https://covid-vaccine.canada.ca/info/pdf/paxlovid-pm-en.pdf
- University of Oxford. PANORAMIC: Platform Adaptive trial of NOvel antiviRals for eArly treatMent of COVID-19 in the Community. https://www.panoramictrial.org
- Butler CC, Hobbs FDR, Gbinigie OA, et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomized controlled trial. Lancet 2023; 401(10373):281-293. DOI: 10.1016/S0140-6736(22)02597-1
- Png ME, Harris V, Grabey J, et al. Cost-utility analysis of molnupiravir plus usual care versus usual care alone as early treatment for community-based adults with COVID-19 and increased risk of adverse outcomes in the UK PANORAMIC trial. British Journal of General Practice 2024. DOI: 10.3399/BJGP.2023.0444
- Health Canada Drug and Health Product Portal. Summary of Cancellation for Molnupiravir (*Lagevrio). April 26, 2023. https://dhpp.hpfb-dgpsa.ca/review-documents/resource/RDS1683828447298
- Butler C. A clinical trial investigating novel treatments for COVID-19 in the community. ISRCTN registry. 2024. DOI: 10.1186/ISRCTN30448031
- University of Oxford. PANORAMIC trial documents. Updated: October 12, 2023. https://panoramictrial.org/for-healthcare-professionals/documents Protocol: https://panoramictrial.org/files/protocols/panoramic_protocol_v8-0_04oct2023.pdf
- Pinto A, et al. Canadian Adaptive Platform Trial of Treatments for COVID in Community Settings (CanTreatCOVID). 2024. https://cantreatcovid.org
Lauren Pelley
CBC News Health – Second Opinion: Who needs Paxlovid now? New guidelines suggest only highest-risk groups should get COVID drug https://www.cbc.ca/news/health/paxlovid-cadth-guidelines-1.7177570
Save my name, email, and website in this browser for the next time I comment.
Notify me when new comments are added.

IMAGES
VIDEO
COMMENTS
Telehealth is a quick and easy way to see if Paxlovid, a COVID-19 treatment pill, is ... Virtual consultations are available in English, Spanish, Haitian ... (833) 450-3461 (compatible with Video Relay Services) Accessibility accommodations for telehealth visits, including American Sign Language (ASL) interpretation and captioning (CART or ...
Visit a pharmacy clinic. Some pharmacies have store-based clinics with authorized staff who can test, evaluate, prescribe, and dispense Paxlovid. However, if the clinic is not affiliated with the Test to Treat program, you could be charged for the evaluation. If your insurance does not cover it, the fees can range from $50 to $150.
* To learn more about Paxlovid, the manufacturer Pfizer has developed this fact sheet. Our doctors cannot prescribe other oral or IV treatments, including Lagevrio (molnupiravir), the antiviral drug Veklury (remdesivir) and monoclonal antibodies (for COVID-19 treatment). The fastest way to receive care is to request a visit on the mobile app or ...
Telehealth is a free and easy way to see if COVID-19 oral antivirals such as Paxlovid, are right for you. Oral antivirals, which are COVID-19 treatment pills taken by mouth, are available by prescription only. ... If you have tested positive for COVID-19 and want to schedule a virtual telehealth appointment with a health care provider to ...
An online visit costs between $0-$89. Doctor On Demand is a covered benefit for over 98 million Americans. If you're covered by your employer or insurance, then you'll pay $0. No insurance, no problem. You can use Doctor On Demand starting at $89 per visit. See a doctor now for your COVID-19 symptoms. Get the app to get started.
Today, a patient who searches for "Paxlovid" may find an ad from telehealth provider PlushCare to "get the FDA authorized Paxlovid prescribed" in a 15-minute online visit, if eligible ...
Visit a test-to-treat site. Another route to getting Paxlovid is visiting one of the 2,300 health centers, urgent care clinics and pharmacies that are designated by the government as "test to ...
This is why it's important to provide recent kidney blood test results to your pharmacist if you're receiving pharmacist-prescribed Paxlovid. The standard dose of Paxlovid is 300 mg (2 tablets) of nirmatrelvir and 100 mg (1 tablet) of ritonavir by mouth every 12 hours for 5 days. The Paxlovid dose for people with mild to moderate kidney ...
Paxlovid (nirmatrelvir co-packaged with ritonavir) is an oral antiviral drug that should be initiated as soon as possible within 5 days of symptom onset. Paxlovid is available for patients by prescription only. ... Visit us online at https: //aspr.hhs.gov/COVID-19 and please see the resources linked in the description to learn more. You can ...
Paxlovid is an oral antiviral medication that can be taken at home to reduce the risk of COVID-19 hospitalization and death in high-risk individuals. Paxlovid requires a prescription and not everyone is eligible for it. For now, Paxlovid is still free for U.S. residents. For people at a higher risk of severe illness from COVID-19, Paxlovid is ...
Paxlovid has not been approved, but has been authorized for emergency use by FDA under an EUA, for the treatment of mild-to moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kg) with positive results of direct SARSCoV-2 viral testing, and who are at high-risk for progression to severe COVID-19 ...
Work with your healthcare professional to get prescribed PAXLOVID, if appropriate. Once you fill your prescription, start taking PAXLOVID that morning or evening, or at the time your healthcare professional recommends. Remember, PAXLOVID must be started within the first 5 days of COVID‑19 symptoms.
A virtual visit for COVID-19 antiviral treatment is available to adults and children 12 years and older. ... Paxlovid has not been approved, but has been authorized for emergency use by FDA under an EUA, for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kg) with ...
Get paxlovid medication . Here's what our doctors recommend. Also see child or teen concerns. Learn more about COVID-19 treatments. Complete an E-visit questionnaire for quick advice, prescriptions, and/or tests. Start E-visit. Call 866-454-8855 for appointments and advice, 24/7. Call now
NYC Health + Hospitals today announced its Virtual ExpressCare service has issued over 20,000 prescriptions for Paxlovid and 6,800 monoclonal antibody infusions to treat Covid, potentially preventing over 1,550 hospital visits and over 300 deaths. Virtual ExpressCare is the largest prescriber of Paxlovid in New York City, prescribing 8 times as ...
If you test positive for COVID-19, schedule a virtual visit with a primary care provider to determine if you are eligible for this treatment. Please note: ... be age 18 and older for molnupiravir or age 12 and older and weigh at least 88 pounds for Paxlovid. If you are pregnant or breastfeeding, please talk with your health care provider about ...
The authors randomly assigned 1,296 patients to receive either Paxlovid or placebo every 12 hours for 5 days. The median time to sustained relief of symptoms was 12 days in the Paxlovid group and 13 days in the placebo group. Five participants (0.8%) in the Paxlovid group and 10 (1.6%) in the placebo group were hospitalized for COVID-19.
INDICATION PAXLOVID is indicated for the treatment of mild-to-moderate coronavirus disease 2019 (COVID-19) in adults who are at high risk for progression to severe COVID-19, including hospitalization or death. PAXLOVID is not approved for use as pre-exposure or post-exposure prophylaxis for prevention of COVID-19.
Munson Healthcare is now offering Paxlovid therapy for treating patients with mild or moderate COVID-19 symptoms. Virtual Urgent Care | Ask-a-Nurse: 231-935-0951 | Visitor Policy ... 7 Steps for a Successful Virtual Doctor Visit . Mar. 30, 2022 Spring Break During COVID-19. Mar. 18, 2022 Stronger Than Ever: A COVID-19 Survivor Story ...
Prepare for your visit. To begin your visit, you'll need to: Upload a government-issued photo ID and your photo to verify your identity. Answer a few medical questions to determine if this service is a good fit for your needs. Enable your video camera and microphone access for treatments that require a live video visit.
An antiviral medication taken by mouth. Paxlovid is the recommended choice for many people, but it might interfere with other medications you may be taking. Paxlovid is the first oral antiviral pill to be approved by the FDA to treat COVID-19 in adults. Paxlovid is available for 12-17 year-olds under the FDA's emergency use authorization.
Simulation/modelling study. Researchers have described the optimal timing for COVID-19 patients to take the antiviral, Paxlovid, to get the most benefit from the treatment, according to a study published April 16 in eLife. The findings suggest that taking Paxlovid three to five days after COVID-19 symptoms emerge may maximise the drug's ...
E-visits. Answer a quick self-service questionnaire and get a response with advice/treatment from a physician within 2 hours. Average wait time. Varies by region of care. Hours.
Please note, as of March 1, 2024 this is no longer a free service provided by the state of California. However, you can use the code "COVIDCA" for 20% off your Virtual COVID-19 visit. Call +1 (888) 897-1244 if you have questions, need help booking an appointment or don't have an email address to use. Sort: Lowest price.
Abstract. Background: In 2024 the risk of hospitalization or death due to COVID-19 is low for most people under age 80 in British Columbia (BC). However, for those at high risk of complications, the antiviral drug combination nirmatrelvir-ritonavir (NMV-r, marketed as Paxlovid TM) has shown promise in reducing the composite risk of hospitalization and death from COVID-19.