Why Do Our Minds Wander?
A scientist says mind-wandering or daydreaming help prepare us for the future
Tim Vernimmen, Knowable Magazine
:focal(800x602:801x603)/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer_public/a5/8b/a58b6ad5-aabb-49b6-9565-941c9ce047f0/mind-wandering-1200px_web.jpg)
When psychologist Jonathan Smallwood set out to study mind-wandering about 25 years ago, few of his peers thought that was a very good idea. How could one hope to investigate these spontaneous and unpredictable thoughts that crop up when people stop paying attention to their surroundings and the task at hand? Thoughts that couldn’t be linked to any measurable outward behavior?
But Smallwood, now at Queen’s University in Ontario, Canada, forged ahead. He used as his tool a downright tedious computer task that was intended to reproduce the kinds of lapses of attention that cause us to pour milk into someone’s cup when they asked for black coffee. And he started out by asking study participants a few basic questions to gain insight into when and why minds tend to wander, and what subjects they tend to wander toward. After a while, he began to scan participants’ brains as well, to catch a glimpse of what was going on in there during mind-wandering.
Smallwood learned that unhappy minds tend to wander in the past, while happy minds often ponder the future . He also became convinced that wandering among our memories is crucial to help prepare us for what is yet to come. Though some kinds of mind-wandering — such as dwelling on problems that can’t be fixed — may be associated with depression , Smallwood now believes mind-wandering is rarely a waste of time. It is merely our brain trying to get a bit of work done when it is under the impression that there isn’t much else going on.
Smallwood, who coauthored an influential 2015 overview of mind-wandering research in the Annual Review of Psychology, is the first to admit that many questions remain to be answered.
This conversation has been edited for length and clarity.
Is mind-wandering the same thing as daydreaming, or would you say those are different?
I think it’s a similar process used in a different context. When you’re on holiday, and you’ve got lots of free time, you might say you’re daydreaming about what you’d like to do next. But when you’re under pressure to perform, you’d experience the same thoughts as mind-wandering.
I think it is more helpful to talk about the underlying processes: spontaneous thought, or the decoupling of attention from perception, which is what happens when our thoughts separate from our perception of the environment. Both these processes take place during mind-wandering and daydreaming.
It often takes us a while to catch ourselves mind-wandering. How can you catch it to study it in other people?
In the beginning, we gave people experimental tasks that were really boring, so that mind-wandering would happen a lot. We would just ask from time to time, “Are you mind-wandering?” while recording the brain’s activity in an fMRI scanner.
But what I’ve realized, after doing studies like that for a long time, is that if we want to know how thinking works in the real world, where people are doing things like watching TV or going for a run, most of the data we have are never going to tell us very much.
So we are now trying to study these situations . And instead of doing experiments where we just ask, “Are you mind-wandering?” we are now asking people a lot of different questions, like: “Are your thoughts detailed? Are they positive? Are they distracting you?”
How and why did you decide to study mind-wandering?
I started studying mind-wandering at the start of my career, when I was young and naive.
I didn’t really understand at the time why nobody was studying it. Psychology was focused on measurable, outward behavior then. I thought to myself: That’s not what I want to understand about my thoughts. What I want to know is: Why do they come, where do they come from, and why do they persist even if they interfere with attention to the here and now?
Around the same time, brain imaging techniques were developing, and they were telling neuroscientists that something happens in the brain even when it isn’t occupied with a behavioral task. Large regions of the brain, now called the default mode network , did the opposite: If you gave people a task, the activity in these areas went down.
When scientists made this link between brain activity and mind-wandering, it became fashionable. I’ve been very lucky, because I hadn’t anticipated any of that when I started my PhD, at the University of Strathclyde in Glasgow. But I’ve seen it all pan out.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer_public/4c/58/4c5891e3-0206-47df-9255-a8d5e196de9f/g-default-mode-network-alt_web.jpg)
Would you say, then, that mind-wandering is the default mode for our brains?
It turns out to be more complicated than that. Initially, researchers were very sure that the default mode network rarely increased its activity during tasks. But these tasks were all externally focused — they involved doing something in the outside world. When researchers later asked people to do a task that doesn’t require them to interact with their environment — like think about the future — that activated the default mode network as well.
More recently, we have identified much simpler tasks that also activate the default mode network. If you let people watch a series of shapes like triangles or squares on a screen, and every so often you surprise them and ask something — like, “In the last trial, which side was the triangle on?”— regions within the default mode network increase activity when they’re making that decision . That’s a challenging observation if you think the default mode network is just a mind-wandering system.
But what both situations have in common is the person is using information from memory. I now think the default mode network is necessary for any thinking based on information from memory — and that includes mind-wandering.
Would it be possible to demonstrate that this is indeed the case?
In a recent study, instead of asking people whether they were paying attention, we went one step further . People were in a scanner reading short factual sentences on a screen. Occasionally, we’d show them a prompt that said, “Remember,” followed by an item from a list of things from their past that they’d provided earlier. So then, instead of reading, they’d remember the thing we showed them. We could cause them to remember.
What we find is that the brain scans in this experiment look remarkably similar to mind-wandering. That is important: It gives us more control over the pattern of thinking than when it occurs spontaneously, like in naturally occurring mind-wandering. Of course, that is a weakness as well, because it’s not spontaneous. But we’ve already done lots of spontaneous studies.
When we make people remember things from the list, we recapitulate quite a lot of what we saw in spontaneous mind-wandering. This suggests that at least some of the activity we see when minds wander is indeed associated with the retrieval of memories. We now think the decoupling between attention and perception happens because people are remembering.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer_public/f4/73/f473de94-c653-4155-be90-aa06c5d9a827/g-brain-regions-mind-wandering-alt_web.jpg)
Have you asked people what their minds are wandering toward?
The past and future seem to really dominate people’s thinking . I think things like mind-wandering are attempts by the brain to make sense of what has happened, so that we can behave better in the future. I think this type of thinking is a really ingrained part of how our species has conquered the world. Almost nothing we’re doing at any moment in time can be pinpointed as only mattering then.
That’s a defining difference. By that, I don’t mean that other animals can’t imagine the future, but that our world is built upon our ability to do so, and to learn from the past to build a better future. I think animals that focused only on the present were outcompeted by others that remembered things from the past and could focus on future goals, for millions of years — until you got humans, a species that’s obsessed with taking things that happened and using them to gain added value for future behavior.
People are also, very often, mind-wandering about social situations . This makes sense, because we have to work with other people to achieve almost all of our goals, and other people are much more unpredictable than the Sun rising in the morning.
Though it is clearly useful, isn’t it also very depressing to keep returning to issues from the past?
It certainly can be. We have found that mind-wandering about the past tends to be associated with negative mood.
Let me give you an example of what I think may be happening. For a scientist like me, coming up with creative solutions to scientific problems through mind-wandering is very rewarding. But you can imagine that if my situation changes and I end up with a set of problems I can’t fix, the habit of going over the past may become difficult to break. My brain will keep activating the problem-solving system, even if it can’t do anything to fix the problem, because now my problems are things like getting divorced and my partner doesn’t want any more to do with me. If such a thing happens and all I’ve got is an imaginative problem-solving system, it’s not going to help me, it’s just going to be upsetting. I just have to let it go.
That’s where I think mindfulness could be useful, because the idea of mindfulness is to bring your attention to the moment. So if I’d be more mindful, I’d be going into problem-solving mode less often.
If you spend long enough practicing being in the moment, maybe that becomes a habit. It’s about being able to control your mind-wandering. Cognitive behavioral therapy for depression, which aims to help people change how they think and behave, is another way to reduce harmful mind-wandering.
Nowadays, it seems that many of the idle moments in which our minds would previously have wandered are now spent scrolling our phones. How do you think that might change how our brain functions?
The interesting thing about social media and mind-wandering, I think, is that they may have similar motivations. Mind-wandering is very social. In our studies , we’re locking people in small booths and making them do these tasks and they keep coming out and saying, “I’m thinking about my friends.” That’s telling us that keeping up with others is very important to people.
Social groups are so important to us as a species that we spend most of our time trying to anticipate what others are going to do, and I think social media is filling part of the gap that mind-wandering is trying to fill. It’s like mainlining social information: You can try to imagine what your friend is doing, or you can just find out online. Though, of course, there is an important difference: When you’re mind-wandering, you’re ordering your own thoughts. Scrolling social media is more passive.
Could there be a way for us to suppress mind-wandering in situations where it might be dangerous?
Mind-wandering can be a benefit and a curse, but I wouldn’t be confident that we know yet when it would be a good idea to stop it. In our studies at the moment, we are trying to map how people think across a range of different types of tasks. We hope this approach will help us identify when mind-wandering is likely to be useful or not — and when we should try to control it and when we shouldn’t.
For example, in our studies, people who are more intelligent don’t mind wander so often when the task is hard but can do it more when tasks are easy . It is possible that they are using the idle time when the external world is not demanding their attention to think about other important matters. This highlights the uncertainty about whether mind wandering is always a bad thing, because this sort of result implies it is likely to be useful under some circumstances.
This map — of how people think in different situations — has become very important in our research. This is the work I’m going to focus on now, probably for the rest of my career.

Get the latest Science stories in your inbox.
- Search Menu
- Volume 2024, Issue 1, 2024 (In Progress)
- Volume 2023, Issue 1, 2023
- Special Issues
- Author Guidelines
- Journal Policies
- Submission Site
- Open Access
- Why publish with this journal?
- Call For Papers
- About Neuroscience of Consciousness
- About the Association for the Scientific Study of Consciousness
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic


Article Contents
Introduction, mind wandering, cognitive control, why the mind wanders, explanations, predictions, philosophical implications, acknowledgments.
- < Previous
Why does the mind wander?
- Article contents
- Figures & tables
- Supplementary Data
Joshua Shepherd, Why does the mind wander?, Neuroscience of Consciousness , Volume 2019, Issue 1, 2019, niz014, https://doi.org/10.1093/nc/niz014
- Permissions Icon Permissions
I seek an explanation for the etiology and the function of mind wandering episodes. My proposal—which I call the cognitive control proposal—is that mind wandering is a form of non-conscious guidance due to cognitive control. When the agent’s current goal is deemed insufficiently rewarding, the cognitive control system initiates a search for a new, more rewarding goal. This search is the process of unintentional mind wandering. After developing the proposal, and relating it to the literature on mind wandering and on cognitive control, I discuss explanations the proposal affords, testable predictions the proposal makes, and philosophical implications the proposal has.
Makes a novel and empirically tractable proposal regarding why the mind wanders
Offers novel explanations of data on mind wandering
Offers predictions for future work on mind wandering
Integrates literature on cognitive control with the literature on mind wandering
Discusses implications for a philosophical account of the nature of mind wandering
Minds wander
Some wander more than others, but human ones wander a lot. A much-cited estimate, due to Killingsworth and Gilbert (2010) , has it that the awake human mind spends from a third to half its time wandering. That’s a big range, a rough estimate, and there are good reasons to be suspicious of it (see Seli et al. 2018 ). The actual number will likely depend a bit upon the nature of mind wandering, a bit upon whether we have the right measure to produce such an estimate, and of course a bit on individual variability. Estimates aside, though, introspection reports that the mind wanders surprisingly often. My question here is this.
Why does it happen?
Sub-questions include the following. What drives the mind to wander? Does anything drive it to wander? Is the transition from focused thought to meandering thought random? Is it a failure of control, or is there some dark purpose behind these mental movements?
In the next section, I set the table by discussing a few interesting features of mind wandering, as well as a few recent proposals about its etiology, and its function. It is easy to conflate these two, since if mind wandering has a function its etiology may very well help illuminate it, but the questions are distinct. Here, I am more interested in why mind wandering happens—about its etiology. It turns out, though, that on my proposal mind wandering happens for good functional reasons. I develop this proposal, which I call the cognitive control proposal, in Cognitive control and Why the mind wanders sections. In Explanations section, I discuss some explanations this proposal makes possible. In Predictions section, I discuss some predictions that could confirm or disconfirm the proposal. In Philosophical implications section, I discuss implications for a philosophical account of the nature of mind wandering.
By referring to this phenomenon as mind wandering, a term familiar to the lay person, we hope to elevate the status of this research into mainstream psychological thinking (946).
As Murray et al. (2019) report, since that review, usage of “mind wandering” has risen dramatically. Only the Smallwood and Schooler paper used the term in a title or abstract in 2006. In 2018, the term appeared in 132 titles or abstracts.
Increased attention to the range of phenomena grouped together by “mind wandering” is salutary. But theorists recognize that the range of processes the term groups may contain multiple etiologies and processing signatures. Accordingly, theorists have proposed many sub-types of mind wandering, categorizing episodes of mind wandering in at least three distinct ways.
The first two involve a conception of mind wandering as defined in part by the contents of a mind-wandering episode, where the contents are unrelated to a task an agent was performing, or was supposed to perform. But there are various ways for an agent to engage in task-unrelated thought. Some categorize mind-wandering episodes in terms of a relationship to an agent’s intention: mind wandering might occur intentionally or unintentionally ( Giambra 1995 ; Seli et al. 2016 ). A second way to categorize mind-wandering episodes is in terms of a relationship to external stimuli. One might here distinguish between distraction, when the mind is prompted to wander by external stimuli, and mind wandering, when the mind is prompted to wander by internal processes, independently of any particular stimuli (see Stawarczyk et al. 2013 ). Or one could argue that distraction, especially sustained distraction, is a legitimate mind wandering as well.
A third way to characterize mind wandering is not in terms of its contents, but rather its dynamics. So, e.g., Christoff et al. (2016) characterize mind wandering as a species of spontaneous thought, with distinct dynamics. Mind wandering is distinguished from creative thought, and rumination, and other types of mental episodes, by relation to the presence or absence of various constraints on the episode (e.g., what they call “deliberate” and “automatic” constraints).
From a certain height, it appears that these different characterizations may not be in competition. Perhaps there are many routes to mind wandering. Perhaps some of them overlap. Perhaps different questions can be answered by focusing on certain routes in certain contexts. Reasonably, Seli et al. (2018) have recently argued in favor of mind wandering as a natural kind, with different sub-types grouped together by relations akin to family resemblance: “We propose that the field acknowledge mind-wandering to be a multidimensional and fuzzy construct encompassing a family of experiences with common and unique features” (2018, 482).
Methodological and conceptual clarity will simply require, in empirical manuscripts, something like the following sentence: “Here, we conceptualized mind-wandering as ________, and operationally defined it for our participants as ________.” Critically, this approach allows researchers the freedom to study whatever features of mind-wandering they wish, while providing the required specificity about aspects of the experience being explored. (488)
In the same spirit, I note here the sub-type of mind wandering that concerns me. I am interested in unintentional mind wandering—episodes of mind wandering that are neither initiated nor governed by any reportable intention of the agent. This category may cross-cut any relationship to external stimuli, in the sense that unintentional mind wandering could be externally or internally initiated. And it may demonstrate dynamics that are distinct from other sub-types of mind wandering.
Unintentional mind wandering could in principle happen non-consciously. But the literature on human mind wandering has it pegged as a feature of the conscious mind. That is to say, when the mind wanders, what wanders is the stream of consciousness—processes of conscious mentation. So, one key way to study mind wandering is to ask people whether or how often their mind has wandered. People offer reports about it. They recognize that they have been mind wandering. This is not because of mind wandering’s phenomenological signature. It is rather because people have a sense that they were once up to something, and then, more or less unbeknownst to them, they began to be up to something else. Thomas Metzinger (2013) speaks of this as the self-representational blink: an unnoticed shift from pursuing one task to doing whatever it is we do when the mind wanders. Recognizing that your mind has been wandering is always slightly surprising, because you did not plan for things to go in that way. From your perspective, it seems that they just did .
This is puzzling. But calling a mental episode unintentional need not imply that mind wandering is maladaptive, or that it has no function. Indeed, the very frequency with which it occurs had led many to suggest that it must have some functional role (e.g., Baird et al. 2011 ). It may not, of course. Perhaps, we survive in spite of how mentally addled we all are. But it is at least plausible that there is a function.
Some accounts of mind wandering might be taken to deny this. McVay and Kane (2010a ) and Kane and McVay (2012) , e.g., have argued that mind wandering reflects a failure of executive control. They note that a negative correlation exists between working memory capacity and a tendency to experience task-unrelated thoughts (see also Randall et al. 2014 ). Some such correlation is plausible. When one experiences task-unrelated mentation, something has clearly gone wrong. One has failed to stay on task.
But this also fails to imply that mind wandering has no function. Kane and McVay note that the correlation between working memory capacity and task-unrelated thought is not terribly strong: “WMC accounts for only about 5% of the variability in [task-unrelated thought] TUT rates (and vice versa)” (2012, 352). It is possible that mind wandering is both a failure in one sense and adaptive in another.
[W]e found evidence for the hypothesis that cognitive control abilities are specifically involved in the flexible adjustment of mind-wandering to task demands. As was hypothesized, high-WMC participants showed higher levels of TUT adjustment than did low-WMC participants. Thus, a more flexible coordination of the stream of thought appears to be characteristic of high-WMC individuals: They engage in TUTs when situational demands are low but reduce TUTs in attention-demanding situations. (1313)
This hypothesis is consistent with work that has demonstrated that as cognitive control resources diminish with age, the propensity to mind wander diminishes as well ( Maillet and Schacter 2016 ).
If we are to believe that mind wandering is associated with deployments of cognitive control, we need evidence that when agents mind wander, they engage in thought processes that may be beneficial. Some evidence for this is that when agents mind wander, their thoughts very frequently go to non-occurrent goals and needs, and to mentation about how to satisfy these goals in the future ( Klinger 1999 ; Baird et al. 2011 ).
Indeed, as Irving and Thompson (2019) note, it seems that it is possible to manipulate the content of mind wandering episodes by giving agents specific goals. Morsella et al. (2010) told some participants they would, in the near future, have to answer questions about the states in America. Then they gave the participants a different task. About 70 percent of these participants’ task-unrelated thoughts were about U.S. geography. Similarly, Mac Giolla et al. (2017) gave some participants a real future task, and told different participants to only pretend to have (or to lie about having) the same future task. Those participants with genuine intentions reported much more spontaneous thought about the future task than participants without genuine intentions.
It is also possible to manipulate mind wandering by reminding agents of their goals. Kopp et al. (2015) had participants either construct a list of their plans for the week or list features of a car. Participants then performed a reading task. Participants who had just reviewed a set of their own plans and goals reported much more mind wandering during the reading.
There is thus an apparent tension within mind wandering. When the mind wanders (at least unintentionally), agents are distracted from the current task, and performance suffers. But when the mind wanders, it tends to find non-occurrent goals the agent possesses, generating planning that could be beneficial. What’s more, greater cognitive control is associated with increases in mind wandering, especially when task demands are low.
Recall my original question: why does the mind wander? Two related questions that could help: What causes it to start, and what explains what happens as it wanders?
My proposed answer runs through recent work on cognitive control, and on what kinds of mechanisms drive allocations of cognitive control resources. I discuss this work in the next section.
A remarkable feature of the human cognitive system is its ability to configure itself for the performance of specific tasks through appropriate adjustments in perceptual selection, response biasing and the on-line maintenance of contextual information. The processes behind such adaptability, referred to collectively as cognitive control … ( Botvinick et al. 2001 , 624)
Rouault and Koechlin likewise emphasize processes of regulation towards certain ends: “Cognitive control refers to mental processes that evolve as regulating adaptive behavior beyond basic reinforcement and associative learning processes” (2018, 106).
There is a danger here, analogous to the one just discussed regarding definitions of mind wandering, in including far too many process-types under the same heading. “Cognitive control” includes processes like the construction and maintenance of a task set, the switching from one task set to another, the deployment of attention in various ways, the deployment of inhibition, and the monitoring of an agent’s progress towards goal achievement. To get better at understanding how these processes work together (or don’t), it helps to have a label. But the nature of the system is only loosely delineated.
Given this, there is room for differing emphases. So, e.g., Adele Diamond characterizes cognitive control processes as “a family of top-down mental processes needed when you have to concentrate and pay attention, when going on automatic or relying on instinct or intuition would be ill-advised, insufficient, or impossible” (136). This characterization is useful, but not definitive. For the kind of cognitive control processes, I have in mind here might be considered top-down, but do not activate only when agents need to deploy attention. These processes operate outside of the agent’s awareness, influencing the agent’s thought and action in subtle and difficult to detect ways.
So, e.g., Kurzban et al. (2013) have argued that one subtle way cognitive control mechanisms influence thought and action is by generating an experience of effort related to the performance of some task. They hypothesize that the experience of effort is the result of sub-personal computations that determine the current task’s value, as well as the value of nearby available tasks, and output a determination of the opportunity cost of persisting on the current task. The experience of effort is hypothesized to be a signal to the agent to switch tasks.
Kurzban et al. ’s proposal has received a lot of attention. Few agree with all of the specifics, but most agree with the general perspective that sub-personal monitoring mechanisms are concerned to determine the value of succeeding in the current task, as well as the cost of continuing engagement in the current task, and are concerned to, in some sense, direct the agent or her cognitive control resources in a more fruitful way.
Perhaps the most mature theory characterizing the mechanisms that constitute the allocation of cognitive control is the Expected Value of Control theory (see Shenhav et al. 2013 , 2017 ). The general idea is that the cognitive control system “specifies how much control to exert according to a rational cost-benefit analysis, weighing these effort costs against attendant rewards for achieving one’s goals” ( Lieder et al. 2018 , 2). Lieder et al. add to this idea a sophisticated model of how the cognitive control system might come to learn the value of the various control signals it can deploy, and might rely upon what it learns to guide cognition in adaptive ways.
Lieder et al. characterize the position the cognitive control system is typically in as a Markov decision process, specified over certain parameters, driven by reinforcement learning. Those parameters are the initial state of the system, the set of states the system could be in, the set of possible actions (or moves, or operations) the system could take, the conditional probabilities of transitioning between states, and a reward function. Lieder et al. further characterize the actions the system could take as “control signals that specify which computations the controlled systems should perform” (4).
Given this setup, the main aim is to maximize reward via the specification of control signals. The way the system does this is by way of learning algorithms. The system builds and updates a model that specifies transition probabilities between states given different control signals, and that maps these probabilities onto a reward function. The reward function balances the reward associated with an outcome (a new state), together with the computational costs of specifying the computation required to drive the system towards the outcome. So, what the system is designed to do is to take the action (specify the control signal or the package of control signals) that has the highest expected value, given the probabilities of where the action takes the system, and the costs of taking the action.
The hypothesis here is that “the cognitive control system learns to predict the context-dependent value of alternative control signals” (5), and that these predictions determine which actions the system takes.
In cases in which the context is relatively well-known, Lieder et al. posit that the system will depend upon relationships between features of the internal state of the agent and features of the context, and will perform weighted sum calculations to determine the value of various possible actions. Cases in which the context is not well-known are more difficult. But Lieder et al. propose that in such cases the system may utilize exploration strategies to teach itself the value of various actions in the novel situation. These exploration strategies involve drawing samples of the value of control signals in previously encountered contexts, averaging over them, and again selecting the control signal that provides the highest expected value.
Lieder et al. note that “This model is very general and can be applied to model cognitive control of many different processes” (6). And they offer a proof of concept for it, by demonstrating that their model outperforms alternative models across a range of processing types.
These processing types involve learning what features of a task are predictive of reward. Some of them are quite simple. One task on which their model performed well-involved learning where to allocate attention, based upon variable reward offered for attending to different locations. A second task involved learning the difference between colors that indicate reward, and colors that do not. That the model predicts basic learning of this sort is good, but not too surprising.
The expected value of computation depends not only on the rewards for correct performance but also on the difficulty of the task. In easy situations, such as the congruent trials of the Stroop task, the automatic response can be as accurate, faster, and less costly than the controlled response. In cases like this, the expected value of exerting control is less than the EVOC of exerting no control. By contrast, in more challenging situations, such as incongruent Stroop trials, the controlled process is more accurate and therefore has a positive EVOC as long as accurate performance is sufficiently important. Therefore, on incongruent trials the expected value of control is larger than the EVOC of exerting no control. Our model thus learns to exert control on incongruent trials but not on congruent trials. Our model achieves this by learning to predict the EVOC from features of the stimuli. This predicts that people should learn to exert more control when they encounter a stimulus feature (such as a color or word) that is predictive of incongruence than when they encounter a feature that is predictive of congruence. (19)
Of course, agents are rarely aware that a system (or coordinated collection of mechanisms) within them is busy learning the value of different modes of responding, and guiding the way that they deploy cognitive control resources. We are not here explaining explicit deliberation or planning. But we are getting insight into the processes—sub-personal, if you like—that create the cognitive ocean in which more explicit processes swim. What’s more, we are getting insight into the kinds of learning that drive cognitive control operations that agents have to simply live with. Shifts of attention, pulls to engage in various computational operations, a sense of what operations are valuable in what contexts—these are rarely things we explicitly consider. Rather, we depend upon this background to engage in explicit cognition and intentional action.
With this as background, I can suggest an interesting possibility, leading to a proposal regarding the etiology and function of mind wandering.
The possibility is this. Depending on the cognitive control system’s model of the value of various control signals, in cases containing relatively little expected value the system may select a package of control signals leading to exploration. These would be cases in which the goal is to find a new and better goal. And the method, which remains here unclear—although one could imagine it involving shifts of attention, construction of task sets involving imagination, inhibition of current goals, etc.—might be generally described as disengagement from the present task in order to set out upon a search for a more valuable task.
The cognitive control proposal, then, is this. Mind wandering is caused by the cognitive control system precisely when, and because, the expected value of whatever the agent is doing—usually, exercising control towards the achievement of some occurrent goal—is deemed too low, and this “too low” judgment generates a search for a better goal, or task. Perhaps, e.g., the estimation of expected value dips below a value threshold attached to the package of control signals that generate exploration for another goal, or task. Or perhaps the value is always computed in comparison with available options, such that mind wandering is sometimes initiated even in the face of a rewarding current task.
This is a straightforwardly empirical proposal, and should be assessed in terms of the explanations it affords, and by whether the predictions it makes are confirmed or disconfirmed. Before I discuss explanation and prediction, however, I wish to note two things.
First, it would certainly be useful if the cognitive control system contained such an operation. Humans are sophisticated agents, with multiple needs and goals potentially in play in most waking life situations. Fixation on one goal alone, or working towards the satisfaction of one goal at a time, is not a great strategy for flourishing. For, first, if one gets stuck on a difficult goal, or if it becomes apparent (i.e. apparent at least to some system tasked with calculating such a thing) that the present goal is not as rewarding as once calculated, it is much wiser to disengage and seek a better goal. And, second, in many situations progress towards multiple goals at once is possible. All one needs is the capacity to divide attention somewhat, or the capacity to hold multiple goals in mind—or at least within some accessible place—and one might waste much less time. Notice, further, that the above points may hold even if dividing the mind amongst multiple goals leads to performance decrements. Perfect performance is not always required. So long as mediocre performance allows one to satisfy goals and needs, accepting mediocre performance will be a good strategy.
Second, explicit cognitive control already does contain such an operation. Sometimes a task becomes too effortful, too uncomfortable, or too boring. Sometimes—after one has just awakened from a long nap, e.g.—there’s no obvious task at hand. In such cases performing a search for a high-value goal is a familiar operation that we perform explicitly. In other cases, we do not leave behind the current task, but we rather utilize deliberation, prospection, imagination, and other processes in order to find sub-goals, or means to achieve the goal that is currently structuring behavior. These modes of exploration towards discovery of a high-value goal are explicit. Our question here is whether the cognitive control system implicitly—i.e., in the absence of an explicit or conscious formation of intention to do so—initiates mind wandering as a similar mode of exploration, and for similar reasons. The proposal is that it does.
Here are explanations this proposal affords.
First, this proposal offers an explanation for the initiation of mind wandering episodes. These episodes are initiated without the agent’s explicit consent. But they do frequently occur. One possible explanation is that the agent necessarily loses control in these instances. That characterizes the initiation of a mind wandering episode as random. A better explanation, I submit, is that while the initiation of a mind wandering episode is, in one sense, a failure—a failure of the current goal and task set to persist—it is, in another sense, a smart move. It is smart because it results from a cognitive control system that is more or less constantly attempting to determine the value of selecting packages of control signals, and that will act when discrepancies in value are calculated. Note, incidentally, that this could be extended to cases in which the agent is pursuing no particular goal, or has no current task. The system need not always compare value between goals. It might be useful, e.g., to tag expected levels of reward to particular environments, perhaps by averaging over the kinds of rewards an environment-type provides. If agents associate one type of environment—a party, e.g.,—to a plethora of rewarding experiences, then a signal that this environment is near—one can hear party music, e.g.,—might lead the mind to wander in the direction of the kinds of experiences the rewarding environment provides.
The fact that the initiation of mind wandering episodes is smart helps to additionally explain a second fact, namely, that agents with higher levels of cognitive control mind wander more frequently when the current task is easy or non-rewarding.
This is not to deny that mind wandering episodes may sometimes be initiated by affectively salient stimuli, or other distractors. Nor is it to deny the existence of completely unguided, or otherwise guided, episodes of mind wandering. I am not in a position to deny that, e.g., a case of spreading activation in a semantic network could qualify as unintentional mind wandering. It may very well be—indeed it seems plausible—that only some cases of unintentional mind wandering are controlled in the way I here propose. Note, however, that even if this is right, the cognitive control system may be able to interact with uncontrolled mind wandering processes. In some cases, uncontrolled mind wandering could be commandeered if a valuable goal suggests itself.
Third, this proposal offers an explanation for the fact that mind wandering episodes tend to go to other goals the agent possesses. This is a natural place for a process to go if that process is structured by an aim to find a more rewarding goal than the one from which the agent has just disengaged. For it will be much more cost-effective to find existent goals, perhaps by querying memory, than to explore the environment and to construct entirely new goals (although of course this may happen, especially when the environment easily affords novel and rewarding goals).
Fourth, this proposal might be integrated with extant explanations of aspects of mind wandering. Consider, e.g., the decoupling hypothesis ( Antrobus et al. 1970 ; Smallwood et al. 2003 ; Smallwood and Schooler 2006 )—the idea that once mind wandering is underway, domain-general cognitive processes are engaged to maintain the mind wandering episode, by keeping attention decoupled from perceptual input, and by aiding the “continuity and integrity” of the agent’s train of thought ( Smallwood 2013 , 524). As Smallwood (2013) notes, the decoupling hypothesis does not seek to explain the initiation of mind wandering. The cognitive control proposal is consistent with it. That is, the proposal is consistent with domain-general resources being deployed to assist mind wandering episodes. The main comment I wish to make here is that the decoupling hypothesis becomes more plausible, and data on the deployment of domain-general resources in mind wandering more transparent, if the entire process of mind wandering can be seen as goal-directed, where the goal is set by the cognitive control system.
This proposal is also consistent with work on the recruitment of neural areas during mind wandering. Christoff et al. , e.g. ( Christoff et al. 2009 ; Fox et al. 2015 ), have found that episodes of mind wandering recruited not only core areas of the default mode network—medial PFC, posterior cingulate/precuneus, and posterior temporoparietal cortex—but also dorsal anterior cingulate cortex and dorsolateral prefrontal cortex, “the 2 main regions of the executive network” ( Christoff et al. 2009 , 8722). Christoff et al. plausibly link the involvement of the executive network with task performance decrements. The cognitive control proposal adds the possibility that executive network recruitment is associated with the goal-directed nature of (at least some) unintentional mind wandering.
Consider, further, recent work on the dynamics of mind wandering. In a recent review, Christoff et al. (2016) rightly notice that much research on mind wandering has been content-based, “assessing the contents of thoughts in terms of their relationship to an ongoing task or activity” (722). They seek, instead, to offer a taxonomy of thought-types in terms of their dynamics—of how they operate over time. They propose two dimensions along which the dynamics of thought may be influenced. The first dimension is characterized in terms of the degree to which thought is constrained by mechanisms that are “flexible, deliberate, and implemented through cognitive control” (719). The paradigm here is the intentional generation of a deliberative process, or the intentional maintenance of attention on a task. The second dimension is characterized in terms of the degree to which thought is constrained by mechanisms that are automatic, in that they “operate outside of cognitive control to hold attention on a restricted set of information” (719). There are many ways thought may be automatically distracted—Christoff et al. mention affectively salient stimuli as one example.
Within our framework, mind-wandering can be defined as a special case of spontaneous thought that tends to be more-deliberately constrained than dreaming, but less-deliberately constrained than creative thinking and goal-directed thought. In addition, mind-wandering can be clearly distinguished from rumination and other types of thought that are marked by a high degree of automatic constraints, such as obsessive thought. (719)
Now, this is not an explanation of why the mind wanders. It is, instead, a mapping of mind wandering onto a broader taxonomy of cognitive kinds, with special attention given to other modes of spontaneous thought. This taxonomy is useful for a number of reasons. For example, Christoff et al. map their taxonomy onto areas of the brain. So they say, e.g., that the part of the default network that centers on the medial temporal lobe is likely to be involved in the generation of mind wandering, as well as, via “its involvement in contextual associative processing” (724), the conceptual variability of some episodes of mind wandering. They also link the hippocampus to mind wandering, suggesting that it may contribute to the “imaginative construction” of hypothetical scenarios. Such mapping work from aspects of spontaneous thought onto activity patterns in large-scale brain networks affords fruitful suggestions for future study of the kinds of psychological patterns and activities that characterize mind wandering over time.
But there are possibilities and explanations that this approach does not (yet) address, and that potentially have consequences for the taxonomy of cognitive kinds that they offer.
Creative thinking may be unique among other spontaneous-thought processes because it may involve dynamic shifts between the two ends of the spectrum of constraints. The creative process tends to alternate between the generation of new ideas, which would be highly spontaneous, and the critical evaluation of these ideas, which could be as constrained as goal-directed thought in terms of deliberate constraints and is likely to be associated with a higher degree of automatic constraints than goal-directed thought because creative individuals frequently use their emotional and visceral reactions (colloquially often referred to as “gut” reactions) while evaluating their own creative ideas. (Box 1, 720)
I suggest that mind wandering is similarly complex. If the cognitive control proposal is correct, then in at least some cases mind wandering is initiated by processes of cognitive control, even though the goal driving mind wandering is not set explicitly by the agent. This could be captured by adding layers onto Christoff et al. ’s taxonomy, deepening explanations of the etiology and function of each kind of spontaneous thought. And these deeper explanations at each place could be expected to bear fruit for understanding the dynamics of spontaneous thought. In particular, we might hope to find patterns in the neural dynamics that are predictive of the onset as well as the termination of mind wandering episodes, and that differentiate it from dreaming, creative thought, and perhaps from rumination. If the cognitive control proposal is correct, one task would be to map these patterns onto the expected value calculations the cognitive control system is performing. We would expect the dynamics of mind wandering to reflect the initiation of a search for a more rewarding goal, and to reflect attempts to make progress on this search. But now I’m jumping ahead, to predictions the proposal generates.
The cognitive control proposal makes predictions. Confirmation of these would be good news; disconfirmation would be bad news.
First, given the explanation offered for the initiation of mind wandering episodes, the proposal predicts that increases in reward for satisfying an occurrent goal would correlate with decreases in propensity to mind wander. It is well-confirmed that increasing reward leads to boosts in performance level, and to overcoming any purported “ego-depletion,” even for very boring tasks. Paradigms that have established this result could be used to test for the place of mind wandering in the behavioral data.
Second, the proposal predicts that increases in reward for non-occurrent goals the agent possesses would increase mind wandering. We have already seen that reminding agents of goals they possess, or of goals they will soon need to attempt to satisfy, leads to more mind wandering in the direction of these goals. The prediction here is more specific. If one were to, e.g., notify participants that they were soon to perform a task associated with some level of reward, and then to put participants through a low reward task, the prediction is that tendency to mind wander towards this task would be associated with the discrepancy in reward between the current and upcoming task.
Third, this proposal draws upon a view of the cognitive control system on which the learning of values associated with goals, and the learning of values associated with stimuli features predictive of goals, is crucial. So the proposal, plus plausible assumptions about reinforcement learning processes, predicts that it is possible to train participants to associate stimuli with certain goals, and that registration of such stimuli would generate mind wandering to the degree that the associated goal is rewarding. Very costly goals would produce little mind wandering. Cheap but rewarding goals would produce more.
And it may be possible to extend this result. It depends on what the agent associates with rewarding goals. Above I suggested that the system need not always compare value between explicit goals, and that the value computation might include an association between expected levels of reward and particular environments. If so, simply placing an agent in such environments would manipulate levels of unintentional mind wandering.
It may be useful to distinguish predictions this proposal makes from a related proposal: the current concerns hypothesis. The current concerns hypothesis (for which, see Klinger et al. 1973 ; Smallwood and Schooler 2006 ) has it that mind wandering is caused by a shift in salience—when one’s current goals (or concerns: here I use these terms interchangeably), become more salient than the external environment, one’s mind begins to wander. As Smallwood explains the view, “attention will be most likely to shift to self-generated material when such information offers larger incentive value than does the information in the external environment” (2013, 524). This proposal is distinct from mine in the following ways. First, I propose a specific mechanism, connected with recent modeling work in cognitive control, to explain the onset of mind wandering. Thus far, of course, the proposal can be seen as a specification of the current concerns hypothesis. Second, this mechanism initiates mind wandering not by turning attention to one’s current concerns, but by directed thought to search for a more valuable goal than the present one. So the cognitive control proposal makes predictions the current concerns hypothesis does not. For example, the cognitive control proposal predicts that propensity to mind wander could be increased by devaluing the present goal, independently of the salience of any of one’s current goals. That is, no matter how much one’s current goals or concerns lack salience, once could increase mind wandering by devaluing the occurrent goal. And it predicts that mind wandering will not turn directly to one’s other goals—the mind may wander to the environment, rather than to internal concerns, since this is one way the agent may attempt to find a more rewarding task. So we should, e.g., be able to find episodes of more intense environmental scanning as a part of the mind wandering episode. Indeed, if the environment is expected to contain valuable options, one would predict that this is where attention will go, rather than to any internal space of concerns.
This is not to deny that mind wandering represents a failure in some sense. McVay and Kane (2010b ) have argued that mind wandering represents an executive control failure. What fails is a process of goal maintenance: “we suggest that goal maintenance is often hijacked by task-unrelated thought (TUT), resulting in both the subjective experience of mind wandering and habit-based errors” (324). The possibility I am raising is that failures of goal-maintenance could in another sense be successes of a different process. Indeed, perhaps processes of goal-maintenance are closely related to the value-based process of estimating the expected value of continuing on some task, or of searching for a new task, that I propose underlies unintentional mind wandering.
In sum, the proposal is plausible on its face. If correct, it promises to explain a range of data regarding mind wandering, and to explain the—from the agent’s conscious perspective very puzzling—initiation of mind wandering episodes. The proposal may also contribute to explanations of the dynamics of mind wandering. The predictions this proposal makes are testable, and work in this direction might take steps towards further integrating knowledge of how cognitive control works with knowledge of how mind wandering works.
I wish finally to relate this proposal to two leading philosophical accounts of mind wandering. Both of these accounts aim to capture mind wandering quite generally. I have noted in Mind wandering section that this is not my aim. Here, I want only to discuss implications for these more general accounts of mind wandering, if the cognitive control proposal about unintentional mind wandering is on track.
[T]he ability to control the conscious contents of one’s mind in a goal-directed way, by means of attentional or cognitive agency. This ability can be a form of rational self-control, which is based on reasons, beliefs, and conceptual thought, but it does not have to be. What is crucial is the “veto component”: Being mentally autonomous means that all currently ongoing processes can in principle be suspended or terminated. This does not mean that they actually are terminated, it just means that the ability, the functional potential, is given and that the person has knowledge of this fact. M-autonomy is the capacity for causal self-determination on the mental level. (2013, 4)
I think the brush strokes Metzinger uses are too broad. I doubt we have veto control over every conscious process ongoing at a time. But I do think he locates an interesting phenomenon. In unintentional mind wandering, our knowledge (or awareness) that we might suspend, terminate, or re-direct aspects of the stream of consciousness lapses.
My question is this. Should we think of this lapse as the agent’s loss of control? As Metzinger has it, mind wandering essentially involves a lack of ability, and a lack of control—what he calls veto control. I agree that unintentional mind wandering does involve a loss of one kind of control. But I would underline the fact that there are multiple ways for a system to exercise control. Some of these involve consciousness in crucial ways. Some likely do not ( Shepherd 2015 ). Knowledge that one can exercise control in some way at a moment can be useful. But a system may be well-designed, and exercise control in finding or executing goals, even if the system is not explicitly aware of processes that are performing these functions at a time.
Further, there are multiple ways for a system or an agent to possess an ability. The mind wandering agent may lack the ability to suspend, terminate, or re-direct elements of the stream of consciousness in virtue of her knowledge or awareness that she can do so. But she may retain the ability to suspend, terminate, or re-direct elements of the stream of consciousness in virtue of other features—perhaps in virtue of signals that emanate from the cognitive control processes I have emphasized.
This is not a merely verbal distinction. It is about how we understand the constitution of agency, and the kinds of properties that should be ascribed to mind wandering. If the cognitive control proposal is right, mind wandering emerges as an interesting case in which the seams of agency pull apart somewhat—we fail to notice that a non-conscious mechanism has turned the stream of consciousness in a different direction. But there may be good functional reasons for this operation, and it may contribute to an agent’s overall capacities to control the self in various environments and contexts.
An agent A’s attention is unguided if and only if A is not habitually guided to focus her attention on any information. In particular, she does not satisfy the counter-factual condition for attentional guidance: There is no information i such that, if A’s attention isn’t focused on i, she will notice, feel discomfited by, and thereby be disposed to correct this fact. (567)
I am not sure this is right. Mind wandering episodes are sometimes short. Sometimes they stop, it seems to me, precisely because we feel a sense that we were recently up to something, and we feel a pull to return. The cognitive control proposal might be able to explain this—one good move for the cognitive control system, in case of a failure to find a more rewarding task or goal, would be to return to the previous task.
Irving is aware that when it wanders, the mind frequently circles back to the agent’s goals. Does this not suggest guidance of some sort? Irving explains the tension by distinguishing between guidance and motivation. Motivated behavior only requires that an agent’s beliefs, desires, or goals are causal antecedents of the behavior. Guided behavior, by contrast, is explicated in terms of dynamics: it “involves the online monitoring and regulation of behavior” (563). Irving claims that mind wandering may be motivated, but it is not guided.
This aspect of Irving’s account does not compare favorably with the cognitive control proposal—if, of course, future work confirms the proposal. For Irving’s account offers no explanation of how causation by some belief or desire or goal helps explain how or why the wandering mind frequently turns to the agent’s goals. The cognitive control proposal has it that the wandering mind finds goals because that aim is what initiated and governs the mind wandering episode.
Further, if my proposal is right it is not entirely correct to think of mind wandering as unguided. It is, admittedly, not guided by any explicit intention the agent forms. In one sense of “guided,” then, Irving is right. But on the cognitive control proposal, mind wandering is a cognitive control process, and it does have a purpose. It seems purposeless to us in part because it is an interesting case in which some of the seams of agency pull apart somewhat—we do not notice that a non-conscious mechanism has turned the stream of consciousness in a different direction. And it seems purposeless to us in part because the course of the stream of consciousness during mind wandering is, as the cognitive control system plans it, meandering. It is meandering because the goal is to search, to explore, until a more rewarding task is found.
If these considerations are on track, we should say that mind wandering takes the form of a conscious but non-consciously guided process the aim of which is to find a rewarding goal or task. The connection with the cognitive control system explains the guidance aspect—the functionality of mind wandering—and affords the possibility of integration with work on the dynamics of mind wandering. The non-conscious aspect of the guidance explains the air of mystery surrounding mind wandering, why it seems purposeless, and why it seems to come about randomly.
In this article, I have asked why the mind wanders. I focused on a sub-type of mind wandering—mind wandering that occurs independently of any reportable intention. I proposed that unintentional mind wandering is sometimes initiated and sustained by aspects of cognitive control. Unintentional mind wandering is caused by the cognitive control system precisely when, and because, the expected value of whatever the agent is doing—usually, exercising control towards achievement of some occurrent goal—is deemed too low, and this “too low” judgment generates a search for a better goal, or task.
This proposal generates testable predictions, and suggests open possibilities regarding the kinds of computations that may underlie unintentional mind wandering. My hope is that by connecting research on mind wandering with research on cognitive control resource allocation, fruitful strategies for modeling these computations may be taken from cognitive control research and deployed to help explain the initiation and dynamics of mind wandering episodes.
The cognitive control proposal also points us towards a fuller picture of human agency. In this picture, action control and intelligent thought are stitched together by conscious and non-conscious processes operating in concert. Future empirical work is critical to the confirmation of this picture, and to filling in the many unspecified details. This is so not least because, if the proposal I offer is on track, agents are not introspectively aware of the (good) rationale behind many mind-wandering episodes.
The author acknowledges two sources of support. First, funds from European Research Council Starting Grant 757698, awarded under the Horizon 2020 Programme for Research and Innovation. Second, the Canadian Institute for Advanced Research’s Azrieli Global Scholar programme on Mind, Brain, and Consciousness.
Conflict of interest statement . None declared.
Antrobus JS , Singer JL , Goldstein S , et al. Mindwandering and cognitive structure . Trans N Y Acad Sci 1970 ; 32 : 242 – 52 .
Google Scholar
Baird B , Smallwood J , Schooler JW. Back to the future: autobiographical planning and the functionality of mind-wandering . Conscious Cogn 2011 ; 20 : 1604 – 11 .
Boyd R. Realism, anti-foundationalism and the enthusiasm for natural kinds . Philos Stud 1991 ; 61 : 127 – 48 .
Botvinick MM , Braver TS , Barch DM , et al. Conflict monitoring and cognitive control . Psychol Rev 2001 ; 108 : 624.
Braem S , Verguts T , Roggeman C , et al. Reward modulates adaptations to conflict . Cognition 2012 ; 125 : 324 – 32 .
Christoff K , Gordon AM , Smallwood J , et al. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering . Proc. Natl. Acad. Sci 2009 ; 106 : 8719 – 24 .
Christoff K , Irving ZC , Fox KC , et al. Mind-wandering as spontaneous thought: a dynamic framework . Nat Rev Neurosci 2016 ; 17 : 718.
Christoff K , Mills C , Andrews-Hanna JR , et al. Mind-wandering as a scientific concept: cutting through the definitional haze . Trends Cogn Sci 2018 ; 22 : 957 – 9 .
Fox KC , Spreng RN , Ellamil M , et al. The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes . Neuroimage 2015 ; 111 : 611 – 21 .
Giambra LM. A laboratory method for investigating influences on switching attention to task-unrelated imagery and thought . Conscious Cogn 1995 ; 4 : 1 – 21 .
Irving ZC. Mind-wandering is unguided attention: accounting for the “purposeful” wanderer . Philos Stud 2016 ; 173 : 547 – 71 .
Irving ZC , Thompson E ( 2019 ). The philosophy of mind wandering. In: Kieran F , Kalina C (eds), Oxford Handbook of Spontaneous Thought and Creativity . New York, NY : Oxford University Press .
Google Preview
Killingsworth MA , Gilbert DT. A wandering mind is an unhappy mind . Science 2010 ; 330 : 932.
Kane MJ , McVay JC. What mind wandering reveals about executive-control abilities and failures . Curr Direct Psychol Sci 2012 ; 21 : 348 – 354 .
Klinger EC. Thought flow: properties and mechanisms underlying shifts in content. In: Singer JA , Salovey P (eds), At Play in the Fields of Consciousness: Essays in the Honour of Jerome L. Singer . Mahwah, NJ : Erlbaum , 1999 , 29 – 50 .
Klinger E , Gregoire KC , Barta SG. Physiological correlates of mental activity: Eye movements, alpha, and heart rate during imagining, suppression, concentration, search, and choice . Psychophysiology 1973 ; 10 : 471 – 477 .
Kopp K , D’Mello S , Mills C. Influencing the occurrence of mind wandering while reading . Conscious Cogn 2015 ; 34 : 52 – 62 .
Kurzban R , Duckworth A , Kable JW , et al. An opportunity cost model of subjective effort and task performance . Behav Brain Sci 2013 ; 36 : 661 – 679 .
Levinson DB , Smallwood J , Davidson RJ. The persistence of thought: evidence for a role of working memory in the maintenance of task-unrelated thinking . Psychol Sci 2012 ; 23 : 375 – 380 .
Lieder F , Shenhav A , Musslick S , et al. Rational metareasoning and the plasticity of cognitive control . PLoS Comput Biol 2018 ; 14 : e1006043.
Mac Giolla E , Granhag PA , Ask K. Task-related spontaneous thought: a novel direction in the study of true and false intentions . J Appl Res Mem Cogn 2017 ; 6 : 93 – 103 .
Maillet D , Schacter DL. From mind wandering to involuntary retrieval: age-related differences in spontaneous cognitive processes . Neuropsychologia 2016 ; 80 : 142 – 156 .
Meier ME. Is there a positive association between working memory capacity and mind wandering in a low-demand breathing task? A preregistered replication of a study by Levinson, Smallwood, and Davidson (2012) . Psychol Sci 2019 ; 30 : 789 – 797 .
McVay JC , Kane MJ. Does mind wandering reflect executive function or executive failure? Comment on Smallwood and Schooler (2006) and Watkins (2008) . Psychol Bull 2010a ; 136 : 188 – 197 .
McVay JC , Kane MJ. Adrift in the stream of thought: the effects of mind wandering on executive control and working memory capacity. In: Gruszka A, Matthews G, Szymura B (eds), Handbook of Individual Differences in Cognition. New York, NY : Springer , 2010b , 321 – 34 .
Metzinger TK. The myth of cognitive agency: subpersonal thinking as a cyclically recurring loss of mental autonomy . Front Psychol 2013 ; 4 : 931.
Morsella E , Ben-Zeev A , Lanska M , et al. The spontaneous thoughts of the night: how future tasks breed intrusive cognitions . Soc Cogn 2010 ; 28 : 641 – 650 .
Murray S , Krasich K , Schooler JW , et al. Conceptual and methodological considerations for investigations of the task-unrelated-thought (TUT) variety of mind wandering, 2019 ; doi: 10.31234/osf.io/fsg57.
Randall JG , Oswald FL , Beier ME. Mind-wandering, cognition, and performance: a theory-driven meta-analysis of attention regulation . Psychol Bull 2014 ; 140 : 1411.
Rouault M , Koechlin E. Prefrontal function and cognitive control: from action to language . Curr Opin Behav Sci 2018 ; 21 : 106 – 111 .
Rummel J , Boywitt CD. Controlling the stream of thought: Working memory capacity predicts adjustment of mind-wandering to situational demands . Psychon Bull Rev 2014 ; 21 : 1309 – 1315 .
Seli P , Risko EF , Smilek D , et al. Mind-wandering with and without intention . Trends Cogn Sci 2016 ; 20 : 605 – 617 .
Seli P , Beaty RE , Cheyne JA , et al. How pervasive is mind wandering, really? Conscious Cogn 2018 ; 66 : 74 – 78 .
Seli P , Carriere JS , Wammes JD , et al. On the clock: evidence for the rapid and strategic modulation of mind wandering . Psychol Sci 2018 ; 29 : 1247 – 1256 .
Shenhav A , Botvinick MM , Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function . Neuron 2013 ; 79 : 217 – 240 .
Shenhav A , Musslick S , Lieder F , et al. Toward a rational and mechanistic account of mental effort . Annu Rev Neurosci 2017 ; 40 : 99 – 124 .
Shepherd J. Conscious control over action . Mind Lang 2015 ; 30 : 320 – 344 .
Smallwood J. Distinguishing how from why the mind wanders: a process-occurrence framework for self-generated mental activity . Psychol Bull 2013 ; 139 : 519 – 535 .
Smallwood J , Baracaia SF , Lowe M , et al. Task unrelated thought whilst encoding information . Conscious Cogn 2003 ; 12 : 452 – 484 .
Smallwood J , Schooler JW. The restless mind . Psychol Bull 2006 ; 132 : 946 – 958 .
Stawarczyk D , Cassol H , D'Argembeau A. Phenomenology of future-oriented mind-wandering episodes . Front Psychol 2013 ; 4 : 425.
Email alerts
Citing articles via.
- About Association for the Scientific Study of Consciousness
Affiliations
- Online ISSN 2057-2107
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Hum Neurosci
Towards a Neuroscience of Mind-Wandering
Michal gruberger.
1 Functional Brain Center, Wohl Institute for Advanced Imaging, Tel-Aviv Sourasky Medical Center, Tel-Aviv, Israel
2 Department of Psychology, Tel-Aviv University, Tel-Aviv, Israel
Eti Ben-Simon
3 Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel
Yechiel Levkovitz
4 The Emotion-Cognition Research Center, Shalvata Mental Health Center, Hod-Hasharon, Israel
Abraham Zangen
5 Department of Neurobiology, Weizmann Institute of Science, Rehovot, Israel
Talma Hendler
Mind-wandering (MW) is among the most robust and permanent expressions of human conscious awareness, classically regarded by philosophers, clinicians, and scientists as a core element of an intact sense of self. Nevertheless, the scientific exploration of MW poses unique challenges; MW is by nature a spontaneous, off task, internal mental process which is often unaware and usually difficult to control, document or replicate. Consequently, there is a lack of accepted modus operandi for exploring MW in a laboratory setup, leading to a relatively small amount of studies regarding the neural basis of MW. In order to facilitate scientific examination of MW the current review categorizes recent literature into five suggested strategies. Each strategy represents a different methodology of MW research within functional neuroimaging paradigms. Particular attention is paid to resting-state brain activity and to the “default-mode” network. Since the default network is known to exert high activity levels during off-task conditions, it stands out as a compelling candidate for a neuro-biological account of mind-wandering, in itself a rest-based phenomenon. By summarizing the results within and across strategies we suggest further insights into the neural basis and adaptive value of MW, a truly intriguing and unique human experience.
“Thoughts meander like a restless wind inside a letter box they tumble blindly as they make their way across the universe” John Lennon
Introduction
Mind-wandering (MW) refers to ongoing mentation which occurs spontaneously, and largely autonomously, whenever an awake individual is not engaged in a cognitively demanding task. Alternative names to the term “MW” (Smallwood and Schooler, 2006 ; Mason et al., 2007 ) in past and recent literature include “day dreaming” (Giambra, 1979 ), “task-unrelated images and thought” (Giambra and Grodsky, 1989 ), “stimulus independent thought” (Teasdale et al., 1995 ), “task-unrelated thought” (Smallwood et al., 2003 ), “incidental self-processing” (Gilbert et al., 2005 ), “inner speech” (Morin, 2009 ), and “spontaneous thought” (Christoff et al., 2008 ).
Conceptualized as a core element of what William James defined as the “stream of consciousness” (James, 1892 ), MW, in various names and forms, has gained considerable attention in ancient and modern philosophy and in theoretical psychology. The robust, autonomous, and continual nature of this psychological process has led writers to suggest that rather than being an undesired lapse of attention to the external world (William James remarked, when he was accused of being absent-minded, that he was really just present-minded to his own thoughts; Barzun, 1983 ), MW must have an important adaptive value for healthy cognition (Christoff et al., 2008 ; Baars, 2010 ). Yet much like the neural basis of MW, its adaptivity and the nature of its interaction with other cognitive processes remain a scientific blind spot.
In the relatively short history of cognitive neuroscience, which has inherited much of its models, paradigms, and findings from behavioral and cognitive psychology, MW is virtually absent (Smallwood and Schooler, 2006 ) as a subject of research. The reluctance in the scientific arena to study MW can be accounted for by its non-behavioral characteristics when compared to more conventionally studied mental functions: MW occurs in the absence of any external cue; it is often unintended and even unaware; it takes its own course – probably driven by internally generated cues; and it is hard to trace back, replicate or report. However, a recent paradigm shift in functional neuroimaging holds a great promise for the development and establishment of MW research. The discovery of the “default-mode network” (DMN; Raichle et al., 2001 ) and the following realization of the significance of spontaneous resting-state neural activity (Raichle, 2009 ) dramatically launched a prosperous path in the scientific exploration of MW.
Default-mode network relates to a functionally meaningful neural network, which includes the medial prefrontal cortex (MPFC), the precuneus, the posterior cingulate cortex, and the inferior parietal and lateral temporal cortices (Figure (Figure1). 1 ). In comparison to other functional neural networks, DMN has unique patterns of activity (Gusnard et al., 2001 ; Raichle et al., 2001 ): both in terms of energy consumption and in terms of the blood oxygen-level dependent (BOLD) signal, activation levels in this network were shown to descend below baseline during cognitively demanding tasks. Moreover, this network shows high activation levels at rest compared to task. These activation patterns and their possible functional meaning have received considerable attention in recent years, using independent as well as combined neuroimaging techniques (e.g., Ben-Simon et al., 2008 ). Studies with clinical populations shed additional light on the critical functionality of the DMN by demonstrating that malfunctioning of the DMN is associated with several neurological, psychiatric, and psychological pathologies (for a review see Buckner et al., 2008 ).

Results of overviewed studies in relation to DMN regions . DMN-related results of studies overviewed in this review, categorized by strategy, superimposed on a template brain. Light-grey markings denote DMN areas (in accordance with Buckner et al., 2008 ) dorsal and ventral medial prefrontal cortex, lateral temporal cortices, precuneus, and posterior cingulate cortex. Strategies which have not been employed in neuroimaging studies (strategy A2) or strategies which are concerned with degree of connectivity rather than degree of activation in brain areas (strategy B3) are not represented in this figure.
Since the first reports describing it, DMN rest-related activity had been suggested to comprise a neural correlate of MW (Gusnard et al., 2001 ). This proposition was based on two main features of the DMN: First of all, like MW, DMN activity occurs during rest and shows a reverse correlation with cognitive load (Mason et al., 2007 ). Secondly, task-related activations in the medial prefrontal and parietal areas, which comprise substantial elements of the DMN, have been shown to occur during self-related tasks (Northoff and Bermpohl, 2004 ; Spreng et al., 2009 ). This has led writers to suggest that rest-related activations in these areas might subserve MW, in itself a process of self-related mentation (Baars, 2010 ).
With the exceedingly growing body of information on neural activity in the wakeful, resting state, the shortage in accepted modus operandi regarding the scientific examination of MW has become a bottle neck, restraining further examination of the functionality of the DMN on the one hand and of the neural basis of MW on the other. However, several pioneering attempts have been made to study the relation between DMN activity and MW, yielding striking results. Converging the solutions to the challenge of quantifying and scientifically studying MW presented in these studies portrays an array of potential strategies to address the question of a DMN–MW association.
The current review aims to facilitate the scientific exploration of the neural correlates of MW by overviewing existing literature and defining, respectively, five methodological strategies for studying MW within a functional neuroimaging paradigm. Two of these strategies include direct measurements of MW (strategies A1 and A2), whether in real time – during rest or task performance, or retrospectively. Three additional strategies (Strategies B1, B2, and B3) rely on theoretical assumptions regarding MW and self related or cognitive functioning, as well as on the known functionality of networks emerging from connectivity analysis performed on data acquired during the resting state. Through the prism of these five strategies, we review existing literature and findings regarding MW published mainly in the recent decade. Each strategy will be presented in light of its advantages and disadvantages as well as the degree of its fitting to various paradigms and data analysis techniques in experimental neuroimaging.
Strategies for Studying the Neural Correlates of Mind-Wandering
The current section overviews methodologies and results from a representative sample of a decade of literature, mainly functional neuroimaging (PET or fMRI) studies, regarding the relation between DMN activation patterns and MW. The inclusion criterion for studies in this overview was that they bring forward the question of the relation between rest-related DMN activity and rest-related phenomenological experience. Importantly, studies of self-related functions were only included if they state a specific hypothesis regarding rest-related neural and psychological functioning. Table Table1 1 lists the studies presented in this review, categorized by strategy. A visual illustration of the results obtained by these studies is presented in Figure Figure1 1 .
A summary of overviewed studies and their DMN-related results .
This table depicts overviewed studies sorted by strategy and their DMN-related results. Areas of DMN were chosen with accordance to Buckner et al. ( 2008 ). Numbers represent Brodmann areas. PFC, prefrontal cortex; DL, dorso lateral; DM, dorso medial; VM, ventro medial; P/ACC, posterior/anterior cingulate cortex; TPJ, temporoparietal junction .
The below differentiation between indirect and direct strategies could also be discussed in terms of determining the dependent and the independent variables within a functional neuroimaging setup: in the case of the indirect strategies, cognitive load or self-relatedness are being experimentally manipulated (i.e., independent variable) and are expected to cause a change in the measured neural signal of the DMN (i.e., dependent variable); in the case of the direct strategies, the degree of DMN activation is manipulated (i.e., independent variable) by altering rest and task while scanning, and the consequent change in the degree of MW is being assessed following scanning (i.e., dependent variable). Table TableA2 A2 in Appendix summarizes typical dependent and independent variables according to each strategy.
Direct strategies for measuring mind-wandering
Strategies for directly quantifying the degree of MW represent a straightforward attempt to overcome its non-explicit nature, and essentially make conventional experimental methods applicable for studying it. For example, one can use the obtained degree of reported MW to categorize sessions or subjects into groups before analyzing, or to correlate it with the degree of activation in selected brain regions of interest or in the whole brain. The greatest challenge, however, is that in contrast to most behavioral measurements, the actual tracking of MW, or even its mere verbalizing in real time, tampers with its very occurrence (Filler and Giambra, 1973 ): an individual busy with reporting her own MW is less free to engage in spontaneous MW comparing to when left to rest quietly. This can probably account for the relatively few studies which have attempted to directly quantify MW in the history of cognitive neuroscience, and possibly, for the even fewer methods developed to do so. Several quantifications techniques have nonetheless, been employed, some attempting at real-time assessment of the degree of MW while others focusing on post hoc questioning of subjects.
Strategy A1: real-time MW sampling
Mind-wandering can occur with or without awareness of its occurrence (“meta-awareness”; Christoff et al., 2009 ). Nevertheless, one can normally report if a thought was occurring in their mind or not, if interrupted and asked to do so at a given time point. This is the rational underlying the MW sampling (also known as “thought sampling” or “thought probing”) technique (McKiernan et al., 2006 ; Mason et al., 2007 ; Christoff et al., 2009 ; Stawarczyk et al., 2011 ). Several approaches have been introduced for MW sampling in the neuroimaging set up, but a typical one uses a probing tone in even or uneven intervals, during either a rest or a task scan; subjects are instructed to indicate whether they were experiencing a spontaneous thought (i.e., unrelated to task performance) at the time the tone was presented (or, in a similar version, since the previous probe; Giambra, 1995 ). In block-design neuroimaging studies, each scan session is then scored according to the rate of “yes” answers given in it out of the overall number of tones presented in the session. The degree of MW occurrence is found to correlate with degree of neural activity in the DMN, as illustrated in Figure Figure1. 1 . In ERP studies, EEG recordings adjacent to the pressings are analyzed separately for “on-task” vs. “off-task” reports. Using this method, Kam et al. ( 2011 ) demonstrated that the P1 component to a visual or auditory probe was reduced during off task, implying a reduction in sensory level processing during MW.
An interesting body of research based on this strategy examines the link between MW (referred to as “task-unrelated thought”) and errors during task performance (Smallwood et al., 2003 , 2008 ). These studies demonstrate that when MW intrudes during task performance, and attentional lapses occur, task performance is impaired. Based on this line of research it may be suggested that MW competes with task performance on a limited capacity of attentional resources, in effect representing a state of “perceptual decoupling” (Smallwood et al., 2011a ). This corresponds well with the idea discussed later on in this paper on the reverse correlation between MW and executive networks in the brain.
In yet another, less common, version of MW sampling, subjects are requested to press a button each time a thought comes into mind (Giambra, 1989 ). Using this type of report, Braboszcz and Delorme ( 2010 ) asked subjects to press a button as soon as they realized their mind was wandering during a task of counting breaths. These presses were later used as an ERP analysis, showing reduced P200 responses to auditory stimuli, and reduced ability to identify the oddball auditory stimuli (smaller N100 during MW). In addition, frequency analysis showed that MW was associated with higher delta and theta power and lower alpha and beta power compared to task performance. Despite the suitability of this method for ERP studies, as reported by the authors themselves, this version seems to be less favorable and can hardly be found in neuroimaging studies, probably because it imposes greater meta-awareness and concentration from subjects and thus interferes with the natural occurrence of MW.
The strategy of MW sampling presents a clear advantage of being a real time, direct and quantified measurement of MW occurrence. One should bear in mind, though, that to the best of our knowledge it has never been systemically tested for validity and reliability, and thus it is mainly justified by its straight-forwardness and intuitiveness.
Strategy A2: retrospective evaluation of MW
Mind-wandering requires peace of mind; disturbances tend to interrupt its natural flow. In other words, an informative report regarding MW at a given time period, without interfering with its occurrence, may better be collected retrospectively, after a session has ended (notably, even then, the contents of MW is not always straightforwardly accessible to memory). Surprisingly, though, designated structured psychological questionnaires for explicitly assessing MW in healthy individuals are scarce. The very few examples which can be found in the literature (Giambra, 1979 ; Klinger and Cox, 1987 ; Matthews et al., 1999 ) did not seem to survive the transition from psychological behavioral research to neuroscience. Consequently, and unfortunately, there is no accumulated body of literature regarding the neural basis of MW, and virtually no experience in the field obtained by retrospective questioning of MW using validated experimental instruments designated for this matter.
One inspiring study which could be considered an example for this approach is a PET study by D'Argembeau et al. ( 2005 ). In this study subjects had to rate the total amount of thoughts experienced, whatever their content, using an in-house developed questionnaire immediately following scanning (a similar approach is found earlier in Mcguire et al. 1996 ). An alternative to using in-house developed questionnaires is to use established questionnaires of experiences which according to theoretical and clinical literature are related to MW. In such a study (Gruberger et al., 2008 ), questionnaires for measuring dependent self awareness and degree of dissociation were applied to assess the degree of interference in MW during rest. The underlying hypothesis was that artificial interference with the normal process of MW will manifest itself as disruption in self awareness and as a sense of dissociation, which indeed was corroborated by the results. A third noteworthy example is the Resting State Questionnaire (ReSQ) published recently by Delamillieure et al. ( 2010 ) explicitly for usage in a functional neuroimaging setup. The ReSQ consists of 62 items organized by five main types of mental activity: visual mental imagery, inner language, somatosensory awareness, inner musical experience, and mental manipulation of numbers. Using a 0–100% scale, the participant retrospectively and quantitatively rates the proportion of time spent in each mental activity during the resting-state fMRI acquisition. Whether this tool will or will not eventually gain the confidence of the research community, its great importance lies in that it represents a pioneering effort to encompass the richness and individual nature of MW into a standardized questionnaire.
Indirect strategies for measuring mind-wandering
Indirect strategies – strategies in which MW is not directly measured – are typically based on the conceptualization of MW as self-related and as more prevalent during rest than during tasks of high cognitive demand. The hypothesis could be framed as follows: if DMN neural activity during rest is the neural basis of MW, then DMN activations during rest and during a given task should be more similar when the task shares common characteristics to MW, i.e., is characterized by low cognitive load and high self relevance.
The advantage of the indirect strategies is straightforward: they avoid measuring MW directly, thus overcoming its non-quantifiable nature and the lack of validated behavioral MW measures. Instead, they use accepted task-related behavioral measures (mostly validated or previously published) and modulate their self-relatedness or their degree of cognitive load.
Strategy B1: parametric modulation of self-relatedness
James's “spiritual self” (James, 1892 ), Gallagher's “narrative self” (Gallagher, 2000 ), Dennett's “non-minimal self” (Dennett, 1991 ), and Damasio's “autobiographical self” (Damasio, 1998 ), are just a few examples of how MW is often present within theoretical models of the self. It is typically represented as a module of its own, distinct both from “lower,” more basic, senses of consciousness as well as from “higher” self-related executive functions. Contemporary neuro-scientists also tend to agree that the “stream of consciousness” is inseparable from the ongoing, constant, sense of self (Damasio, 1998 ; Gusnard, 2005 ; Beer, 2007 ). According to this notion, MW, whether its content is directly related to the thinker or not, is a self-related, self-generated, self-sustaining function (Baars, 2010 ); it serves as an integral part of self awareness, a pre-requisite for healthy psychological functioning.
The conceptualization of MW as a private case of self-related functioning produces a hypothesis for an overlap between the neural basis of self-related tasks and the neural basis of MW. This hypothesis has been translated in some studies into a rational for comparing neural activations during self-related tasks to neural activations at rest, when MW is assumed to occur most.
Though not the first to suggest a relation between rest-related neural activity and MW, the first paper to specifically associate MW with DMN activity was published by Gusnard et al. ( 2001 ), as part of a series of publications (Raichle et al., 2001 ) introducing the concept of the DMN. In this fMRI study, neural activations during rest were compared both to a subjective, emotional judgment task (“internally cued condition”) and to a neutral judgment task (“externally cued condition”). In accordance with the above prediction, neural activations in DMN-related PFC areas were found to be more similar to the activations at rest during the internally cued condition than during the externally cued condition (see Table Table1 1 for summarized results). Paradigms similar in contrasting a self-related task with a similar non-self-related task can be found in additional fMRI studies (Johnson et al., 2002 ; Goldberg et al., 2006 ; Schneider et al., 2008 ; Andrews-Hanna et al., 2010 ), and in the PET study described earlier (D'Argembeau et al., 2005 ). Results in all of these studies indicate greater activations (or lesser de-activations) in brain areas associated with the DMN, mostly MPFC areas, during self-related tasks than during non-self-related tasks, when compared to rest. These elevated activations were shown to last beyond the duration of the stimuli and into the rest period following stimulation (Schneider et al., 2008 ). The majority of these papers (except for Johnson et al., 2002 ) demonstrate that DMN activations during self-related conditions were more similar to DMN rest-related activity patterns, and suggest that this result might imply a possible functional role of rest-related DMN activations in spontaneous self-related mental activity.
As shown in separate studies as well as in convergence, this is a useful strategy for investigating the functional role of areas within the DMN while staying within the boundaries of accepted neuroimaging paradigms. One drawback of this strategy is the potential of over stretching the concept of self, which may cause confounding the self-relatedness of a task with other characteristics like its emotional valence (e.g., Gusnard et al., 2001 ). Therefore, one should pay special attention that the parameter modulated between study conditions is indeed as specific to self-relatedness as possible.
Strategy B2: parametric modulation of cognitive load
The distinction of ongoing spontaneous mentation from other, task related, mental functions dates back to James ( 1892 ), and has been recognized almost solely by theoretical psychology and philosophy over the years (Gallagher, 2000 ). However, this very classification of MW as the mental function characterizing the un-engagement of attentional resources directly magnifies its potential to be scientifically explored.
The strategy of parametric modulation of cognitive load has been used in the context of studying the functionality of rest-related DMN activity. In this strategy, the contrast of interest when analyzing imaging data is not the commonly used task minus rest contrast, but rather the contrast of rest minus task. Researchers try to demonstrate that the lower the cognitive load in a given task condition, the higher the activations in DMN areas during this task, leading to a smaller difference between DMN activations during the task compared to rest. Indeed, this was found to be the case in fMRI studies such as McKiernan et al. ( 2006 ), Christoff et al. ( 2004 ), and Mason et al. ( 2007 ), and in Wicker et al.’s ( 2003 ) meta-analysis of PET studies. In the case of McKiernan et al. ( 2006 ) and Mason et al. ( 2007 ), behavioral measures (described in strategy A1) were added to the study to further establish a more direct association between high DMN activations during low cognitive demand and MW.
This strategy yields results which correspond well with theoretical accounts of MW as well as with the lay intuition that MW is the “default” mental state when the mind is free to engage in it. In addition to its intuitiveness, and thus its simplicity, the advantage of this strategy is in its robustness: it was found to be replicated across virtually any behavioral task tested (Shulman et al., 1997 ; Mazoyer et al., 2001 ; Wicker et al., 2003 ), which makes it accessible and easy to implement. It should be taken into account, however, that executive functioning and MW are probably not as anti-correlated as these studies may depict. MW may involve executive processes like memory, planning, computing, etc., as is reflected by findings of executive networks co-activated with DMN during MW (Christoff et al., 2009 ). Thus, rather than assuming mutual exclusiveness, the degree and direction of the association between neural activity of the DMN and of executive networks during MW should be studied in greater experimental resolution.
Strategy B3: paradigm-free analysis of neuronal dynamics
Brain activity is combined of activations of neurons which comprise anatomical and functional networks. Recent advances in functional and computational neuroimaging have provided new tools for examining functional interactions between time series of signals obtained from different brain regions, catalyzing the examination of functional connectivity in the resting brain. This type of analysis does not require a behavioral paradigm (“paradigm-free”) and indeed is often implemented on data acquired solely when subjects lie resting in the imaging device (the validity of these signals is discussed in Box 1). In fMRI, analysis methods of the resting-state signal can typically be placed into hypothesis dependent and hypothesis free methods (Van Den Heuvel and Hulshoff Pol, 2010 ), both resulting in connectivity maps – whether correlational or anti-correlational (Uddin et al., 2009 ). These maps demonstrate anatomical networks which, interestingly, greatly overlap with known functional neural networks. The DMN is one of those emerging networks and thus its relation to MW can be further characterized in terms of functional connectivity.
Box 1. Validation of spontaneous BOLD fluctuations acquired during rest.
The neuronal basis of spontaneous resting-state fMRI signals was initially regarded by skeptics as problematic, potentially representing merely unknown parameters of noise as well as known physiological ones. However recent observations increasingly support and validate the neuronal basis of resting-state fMRI signals (Adapted from Van Den Heuvel and Hulshoff Pol, 2010 ):
- The first and probably most compelling evidence for the resting-state signal is that most resting-state patterns tend to occur between brain regions overlapping in known functional and neuroanatomical regions (Salvador et al., 2005 ; Damoiseaux et al., 2006 ; Van Den Heuvel et al., 2008 ).
- The second observation relates to the frequency of rest-related signals revealing that the observed spontaneous BOLD signals are mainly dominated by lower frequencies (<0.1 Hz) with only a minimal contribution of higher frequency cardiac and respiratory oscillations (>0.3 Hz) (Cordes et al., 2000 , 2001 ).
- Lastly, an (indirect) association exists between the frequency profiles of slow spontaneous resting-state fMRI and electrophysiological recordings of neuronal firing (Nir et al., 2008 ) and between spontaneous BOLD fluctuations and simultaneous measured fluctuations in neuronal spiking (Shmuel et al., 2002 ; Shmuel and Leopold, 2008 ).
Altogether these findings advocate toward the validity of the neural signal acquired during the resting state and the legitimacy of its scientific exploration.
Two studies are brought here to exemplify the usage of a paradigm-free strategy in further characterizing the relation between MW and DMN spatio-temporal dynamics. Horovitz et al. ( 2008 ) utilized this strategy to determine whether DMN activity can be de-coupled from conscious awareness. In this study, the level of functional connectivity within the DMN persisted both during the resting state and during light sleep. The authors conclude that DMN connectivity “does not require or reflect the level of consciousness that is typical for wakefulness” (p. 679), which seems to undermine the idea of a functional involvement of DMN activity in MW. Nevertheless, two alternative explanations are offered by the authors: the first is that these results only decouple wakeful awareness from the degree of connectivity within the DMN, but not from the amplitude of its activity ; the other is that light sleep is sometimes characterized by the existence of dream-like reverie activity (a mental activity similar to MW) which like MW may also be a functional product of DMN activity.
Another study by the same group (Horovitz et al., 2009 ) demonstrated altered correlations between DMN network components during different states of consciousness, most notably a reduced involvement of the MPFC during sleep. The authors suggest that among the DMN components, the frontal cortex may play an important role in the sustenance of conscious awareness.
In favor of this strategy, it can be claimed that as some indication exists for the effect of previous task performance on neural activity at subsequent rest (Northoff et al., 2010 ), a paradigm-free study design which consists of rest alone will produce results which are more unbiased. In any case, studies of this strategy call attention to the fact that beyond relative degree of neural activity, more holistic parameters of neural dynamics need to be explored to truly characterize the DMN–MW relation, such as temporal and spatial patterns of DMN activity.
Discussion and Future Directions
In this review we portray the evolvement of the neuroscience of MW, in hope to lay the grounds for additional research to come. Undoubtfully, studies like the ones overviewed here serve to narrow the gap between theoretical understanding of MW and its scientific exploration. Nevertheless, MW is still by large a mystery, and much work remains to complete the puzzle. In Box 2 we put forward several ideas which stem from existing findings in hope of contributing to future research.
Box 2. Mind-wandering: questions for future research.
Understanding MW using brain imaging techniques holds a promise for this field of research. Listed here are a few lines of thought that could constitute an initial framework for future MW studies:
- Temporal patterns of MW: What are the spatio-temporal dynamics which correspond to MW in the human brain? How are they represented in terms of brain connectivity?
- Control of MW: what is MW's locus of control in the brain? Do internal and external abruptions of MW result in similar neural outcome? Interfering with MW occurrence by different type of tasks (e.g., tasks which require external vs. internal attention) could offer preliminary answers.
- MW and consciousness: What is the nature of the relationship between consciousness and MW? Is MW simply an expression of conscious experience much like an actor on a stage or is it a substantial part of consciousness giving rise to the stage itself? If MW is indeed a substantial part of conscious experience one would expect similar neural correlates of both phenomena.
- MW and pathologies: Which functions does MW serve and how are they disrupted when MW does not occur? Both the very mechanism and the contents of MW are of great interest to clinical psychology and psychiatry. Psychiatric and neuronal pathologies associated with MW malfunctioning may shed light on understanding the role of MW in healthy psychological functioning.
- The contents of MW: In this review we put little emphasis on the ever changing contents of MW. This is not to say that they are of no importance, only that the studies described here were interested in the common mechanism underlying this changing flow of contents. Future research might very well attempt to segregate neural patterns during MW which are responsible for the experience of different contents or even different time directions (e.g., future or past) as explored by Smallwood et al. ( 2009 ).
To begin answering such questions, the scientific community must agree upon theoretical definitions as well as normalized, standardized behavioral measures of MW. In the functional neuroimaging field one also needs advanced validated computational methods for studying the temporal dynamics of neural activations in long sequences such as common in rest.
Mind-wandering can be studied under different contexts involving a wide array of experimental questions. Accordingly, as we tried to exemplify in this review, there is no absolute optimal way to study it, but rather it is important to make an informed, educated choice when studying it within a neuroimaging paradigm. For instance, MW sampling provides valuable information about inter-subject and intra-subject differences in the degree of MW, while sacrificing the integrity of its natural, untouched flow; In contrast parametric modulation of cognitive load does not interfere with the natural course of MW and also enables statistical analysis of inter-group variance, with the compromise of MW being only implied, and not directly measured. Figure Figure2 2 depicts a flow chart of relevant considerations in making the most advantageous choice for a given experimental setup.

Flowchart of goodness of fit of different strategies according to study aims . A flowchart which may assist researchers wishing to explore mind-wandering using functional neuroimaging paradigms. According to study aims one should decide on the appropriate strategy taking into consideration advantages and disadvantages of each strategy as discussed in the text.
In addition to portraying modes of operation for the scientific examination of MW, overviewing the neuroscience of MW so far provides a few insights into this neural and mental process.
Functionality of mind-wandering
The robustness of the experience of MW across ages, cultures, and individuals (Singer and McCraven, 1961 ), suggests it holds a vital role in human psychology. As to the specific role of MW, we suggest several ideas based on current literature which may inspire future research.
MW serves “self” functions
As detailed in the context of strategy B1, there are theoretical (Gallagher, 2000 ), neuroanatomical (Gusnard, 2005 ; Northoff et al., 2006 ), and intuitive grounds to claim that MW is a self-related cognitive function, which serves to create and maintain an integrated, meaningful sense of self out of various aspects of self-related information and cognition. Northoff et al. ( 2006 ), for instance, conceptualizes MW as a “psychological baseline,” a form of continuous self-referential processing which is evident during non-task conditions and which ultimately forms our subjective experience of a “continuous stream of subjective experience” or “phenomenal time” where past, present, and future are no longer divided but integrated.
MW enables the projection of a “self” to past and future events
The idea that MW serves processes of future planning and simulation is based on theory and common experience, and is strongly supported by the fact that the DMN includes areas such as the posterior cingulate cortex, the precuneus, and the hippocampus, which are known to take part in such mental processes (Buckner et al., 2008 ). Behaviorally, it has been shown that the contents of MW will tend toward prospecting or retrospecting according to the self relevance of a given context (Smallwood et al., 2009 ), suggesting that MW serves to integrate past and present experiences for the purpose of future planning. Moreover, Smallwood et al. ( 2011b ) suggest that self reflection associated with future-oriented thinking is an integral part of the autobiographical memory system. Interestingly, temporal locus of MW has even been shown to be related to the direction of apparent physical movement through space (forward/backward), implying a functional link between MW temporality and sensory spatio-temporal input (Miles et al., 2010 ).
Altogether, this idea corresponds well with Tulving's idea of “autonoetic consciousness,” which is claimed to be selective to the human kind and which enables mentally traveling into the past and the future (Tulving, 2005 ).
MW serves as a learning and consolidation mechanism by augmenting the associative abilities of the brain
According to this proposition, spontaneous mental processing during wakefulness resembles in its function, in its effects and, to a certain extent, in its neural basis, the off-line processing that occurs during sleep. This relatively recent idea is presented by contemporary writers (Christoff et al., 2008 ; Baars, 2010 ) and already takes into account what is known about DMN activation patterns. According to this notion, it could be suggested that task performance would improve following MW in a similar way when following sleep (Stickgold et al., 2001 ).
Mind-wandering-executive functioning relation: an integrative approach
Converging results from studies like the ones overviewed here provide verification for a strong negative association between MW and executive functioning. This association, mentioned earlier to be part of the rational for strategy B2 (Parametric modulation of cognitive load), is supported by behavioral as well as neuro-scientific evidence (e.g., DMN activity). In light of the infancy of MW research, this in itself is a highly instrumental insight.
Nevertheless, recent lines of evidence suggest that this association is not exclusive. The first is found in the activation of executive prefrontal and parietal brain areas, in addition to DMN areas, as contributing to MW (Christoff et al., 2009 ). The second is found in the gradual increase of DMN activity found in strategies B1 and B2 as cognitive load decreases and self-relatedness increases, which suggests that some DMN activity did occur even in lower self relevance or higher cognitive load conditions. The third line of evidence is brought by studies which show involvement of DMN areas during online task performance (Assaf et al., 2009 ).
Though assuming a dichotomy between MW and “executive” neural networks proved useful for the beginning of MW research, a more mature approach might suggest studying the interplay between MW and executive functions and their underlying neural mechanisms (Smallwood and Schooler, 2006 ). In consistence with this line of thought, Spreng et al. ( 2010 ) suggest that a third anatomically interposed “frontoparietal control network” mediates planning across domains, flexibly coupling with either the default or dorsal attention network in support of internally vs. externally focused cognition, respectively.
Rather than eliminating them, MW probably serves various cognitive functions such as prospective planning, self monitoring, etc. (Baars, 2010 ). A better understanding of the interplay between MW and executive functioning can be achieved by further implementation of the five strategies defined here, in turn contributing altogether to the understanding of the adaptive value of MW with respect to human cognition and affect.
Mind-wandering: the neural basis of its integration and segregation
Portraying the results of the overviewed studies suggests that MW involves activities in distributed brain areas (see Table Table1 1 and Table TableA1 A1 in Appendix). These findings of different activations might underlie specific aspects of the MW process and in turn may serve to deconstruct MW, both theoretically and operationally, into elements according to its content or to the additional mental functions which are involved in it (e.g., emotion, autobiographical memory, mental time traveling, etc.). Examining the different DMN activations according to strategy, as illustrated in Figure Figure1, 1 , implies that some sub-areas within the DMN are common to MW in any context while others are more typically unique to a specific strategy. For example, on an impressionist level only, it could be suggested that across strategies lateral correlates of MW are found more dominantly in the left than in the right hemisphere and can be commonly regarded as part of the network associated with high-level semantic processing. However other correlates of MW do differ between strategies, with the ventral MPFC and precuneus more sensitive to modulation of cognitive load, and dorso-medial MPFC areas more sensitive to self-relatedness.
It is of no doubt that such impressions require a comprehensive quantitative meta-analysis which is beyond the scope of this review. Nevertheless, such a neuro-functional differentiation implies that each strategy might reveal, in addition to the network underlying MW, the neural basis of a specific aspect within the large construct of MW. A functionally based deconstruction of the DMN has already been suggested (Spreng et al., 2009 ; Andrews-Hanna et al., 2010 ; Stawarczyk et al., 2011 ) and could prove fruitful for further scientific examination of MW; Similar MW studies utilizing such refined and specific definitions may shed additional light on differential neural processes which underlie diverse aspects of MW.
Concluding Remarks
Mind-wandering is a universal phenomenon which accompanies much of our daily lives from childhood to adulthood. Its exploration has a vast potential in leading us to a better and more profound understanding of our ongoing mental selves, and in fact, of the basic properties of conscious experience.
The study of MW is at an exciting position of forming into a field of research of its own. Its relevance to a wide array of disciplines, from neuroscience to philosophy to the clinical world ensures that it will draw a growing number of researchers in the near future. We hope that this review serves to set the milestones for a better scientific understanding of this remarkable, unique human quality.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
A summary of overviewed studies and their non-DMN-related results .
This table depicts overviewed studies sorted by strategy and their non-DMN-related results. Areas were chosen only if evident in more than one of the presented studies. Numbers represent Brodmann areas. LTC, lateral temporal cortex; MTC, medial temporal cortex .
Study variables according to strategy .
This table summarizes typical independent and dependent variables, as well as contrast of interest (relevant to neuroimaging analysis) in each of the five strategies .
- Andrews-Hanna J. R., Reidler J. S., Sepulcre J., Poulin R., Buckner R. L. (2010). Functional-anatomic fractionation of the brain's default network . Neuron 65 , 550. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Assaf M., Kahn I., Pearlson G. D., Johnson M. R., Yeshurun Y., Calhoun V. D., Hendler T. (2009). Brain activity dissociates mentalization from motivation during an interpersonal competitive game . Brain Imaging Behav. 3 , 24–37 10.1007/s11682-008-9047-y [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baars B. J. (2010). Spontaneous repetitive thoughts can be adaptive: postscript on “mind wandering” . Psychol. Bull. 136 , 208–210 [ PubMed ] [ Google Scholar ]
- Barzun J. (1983). A Stroll with William James. New York, NY: HarperCollins [ Google Scholar ]
- Beer J. S. (2007). The default self: feeling good or being right? Trends Cogn. Sci. (Regul. Ed.) 11 , 187–189 [ PubMed ] [ Google Scholar ]
- Ben-Simon E., Podlipsky I., Arieli A., Zhdanov A., Hendler T. (2008). Never resting brain: simultaneous representation of two alpha related processes in humans . PLoS ONE 3 , e3984. 10.1371/journal.pone.0003984 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Braboszcz C., Delorme A. (2010). Lost in thoughts: neural markers of low alertness during mind wandering . Neuroimage 54 , 3040–3047 10.1016/j.neuroimage.2010.10.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Buckner R., Andrews-Hanna J., Schacter D. (2008). The brain's default network . Ann. N. Y. Acad. Sci. 1124 , 1–38 [ PubMed ] [ Google Scholar ]
- Christoff K., Gordon A., Smith R., Vancouver B. C. (2008). “The role of spontaneous thought in human cognition,” in Neuroscience of Decision Making , ed. Vartanian O. A. M. (Philadelphia: Psychology Press; ). [ Google Scholar ]
- Christoff K., Gordon A. M., Smallwood J., Smith R., Schooler J. W. (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering . Proc. Natl. Acad. Sci. U.S.A. 106 , 8719–8724 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Christoff K., Ream J. M., Gabrieli J. D. (2004). Neural basis of spontaneous thought processes . Cortex 40 , 623–630 10.1016/S0010-9452(08)70158-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cordes D., Haughton V. M., Arfanakis K., Carew J. D., Turski P. A., Moritz C. H., Quigley M. A., Meyerand M. E. (2001). Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data . AJNR Am. J. Neuroradiol. 22 , 1326. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cordes D., Haughton V. M., Arfanakis K., Wendt G. J., Turski P. A., Moritz C. H., Quigley M. A., Meyerand M. E. (2000). Mapping functionally related regions of brain with functional connectivity MR imaging . AJNR Am. J. Neuroradiol. 21 , 1636. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- D'Argembeau A., Collette F., Van Der Linden M., Laureys S., Del Fiore G., Degueldre C., Luxen A., Salmon E. (2005). Self-referential reflective activity and its relationship with rest: a PET study . Neuroimage 25 , 616–624 10.1016/j.neuroimage.2004.11.048 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Damasio A. R. (1998). Investigating the biology of consciousness . Philos. Trans. R. Soc. Lond. B Biol. Sci. 1879–1882 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Damoiseaux J. S., Rombouts S., Barkhof F., Scheltens P., Stam C. J., Smith S. M., Beckmann C. F. (2006). Consistent resting-state networks across healthy subjects . Proc. Natl. Acad. Sci. U.S.A. 103 , 13848. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Delamillieure P., Doucet G., Mazoyer B., Turbelin M.-R., Delcroix N., Mellet E., Zago L., Crivello F., Petit L., Tzourio-Mazoyer N., Joliot M. (2010). The resting state questionnaire: An introspective questionnaire for evaluation of inner experience during the conscious resting state . Brain Res. Bull. 81 , 565–573 [ PubMed ] [ Google Scholar ]
- Dennett D. C. (1991). Consciousness Explained. Boston: Little Brown & Co [ Google Scholar ]
- Filler M. S., Giambra L. M. (1973). Daydreaming as a function of cueing and task difficulty . Percept. Mot. Skills 37 , 503. [ PubMed ] [ Google Scholar ]
- Gallagher S. (2000). Philosophical conceptions of the self: implications for cognitive science . Trends Cogn. Sci. (Regul. Ed.) 4 , 14–21 [ PubMed ] [ Google Scholar ]
- Giambra L. M. (1979). Sex differences in daydreaming and related mental activity from the late teens to the early nineties . Int. J. Aging Hum. Dev. 10 , 1–34 [ PubMed ] [ Google Scholar ]
- Giambra L. M. (1989). Task-unrelated-thought frequency as a function of age: a laboratory study . Psychol. Aging 4 , 136–143 10.1037/0882-7974.4.2.136 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Giambra L. M. (1995). A laboratory method for investigating influences on switching attention to task-unrelated imagery and thought . Conscious. Cogn. 4 , 1–21 [ PubMed ] [ Google Scholar ]
- Giambra L. M., Grodsky A. (1989). Task-unrelated images and thoughts while reading . Imagery Curr. Perspect. 26–31 [ Google Scholar ]
- Gilbert S. J., Frith C. D., Burgess P. W. (2005). Involvement of rostral prefrontal cortex in selection between stimulus-oriented and stimulus-independent thought . Eur. J. Neurosci. 21 , 1423–1431 [ PubMed ] [ Google Scholar ]
- Goldberg I. I., Harel M., Malach R. (2006). When the brain loses its self: prefrontal inactivation during sensorimotor processing . Neuron 50 , 329–339 10.1016/j.neuron.2006.03.015 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gruberger M., Hendelr T., Harel E. V., Harari H., Levkovitz Y., Zangen A. (2008). I think therefore I am: alterations in the sense of self by stimulation of the prefrontal cortex . NeuroImage , 41 , S41–S180 [ Google Scholar ]
- Gusnard D. A. (2005). Being a self: considerations from functional imaging . Conscious. Cogn. 14 , 679–697 [ PubMed ] [ Google Scholar ]
- Gusnard D. A., Akbudak E., Shulman G. L., Raichle M. E. (2001). Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function . Proc. Natl. Acad. Sci. U.S.A. 98 , 4259–4264 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Horovitz S. G., Braun A. R., Carr W. S., Picchioni D., Balkin T. J., Fukunaga M., Duyn J. H. (2009). Decoupling of the brain's default mode network during deep sleep . Proc. Natl. Acad. Sci. U.S.A. 106 , 11376. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Horovitz S. G., Fukunaga M., De Zwart J. A., Van Gelderen P., Fulton S. C., Balkin T. J., Duyn J. H. (2008). Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG fMRI study . Hum. Brain Mapp. 29 , 671–682 10.1002/hbm.20428 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- James W. (1892). Psychology. Cleveland: World [ Google Scholar ]
- Johnson S. C., Baxter L. C., Wilder L. S., Pipe J. G., Heiserman J. E., Prigatano G. P. (2002). Neural correlates of self reflection . Brain 125 , 1808–1814 10.1093/brain/awf181 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kam J. W. Y., Dao E., Farley J., Fitzpatrick K., Smallwood J., Schooler J. W., Handy T. C. (2011). Slow fluctuations in attentional control of sensory cortex . J. Cogn. Neurosci. 23 , 460–470 [ PubMed ] [ Google Scholar ]
- Klinger E., Cox W. M. (1987). Dimensions of thought flow in everyday life . Imagin. Cogn. Pers. 7 , 125–128 [ Google Scholar ]
- Mason M. F., Norton M. I., Van Horn J. D., Wegner D. M., Grafton S. T., Macrae C. N. (2007). Wandering minds: the default network and stimulus-independent thought . Science 315 , 393–395 10.1126/science.1131295 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Matthews G., Joyner L., Gilliland K., Campbell S. E., Falconer S., Huggins J. (1999). Validation of a comprehensive stress state questionnaire: towards a state “big three” . Personal. Psychol. Eur. 7 , 335–350 [ Google Scholar ]
- Mazoyer B., Zago L., Mellet E., Bricogne S., Etard O., Houde O., Crivello F., Joliot M., Petit L., Tzourio-Mazoyer N. (2001). Cortical networks for working memory and executive functions sustain the conscious resting state in man . Brain Res. Bull. 54 , 287–298 [ PubMed ] [ Google Scholar ]
- Mcguire P. K., Paulsau E., Frackowiak R. S., Frith C. D. (1996). Brain activity during stimulus independent thought . Neuroreport 7 , 2095–2099 [ PubMed ] [ Google Scholar ]
- McKiernan K. A., D'angelo B. R., Kaufman J. N., Binder J. R. (2006). Interrupting the “stream of consciousness”: an fMRI investigation . Neuroimage 29 , 1185–1191 10.1016/j.neuroimage.2005.09.030 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Miles L. K., Karpinska K., Lumsden J., Macrae C. N. (2010). The meandering mind: vection and mental time travel . PLoS ONE 5 , e10825. 10.1371/journal.pone.0010825 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Morin A. (2009). “Inner speech and consciousness,” in The Encyclopedia of Consciousness , ed. Banks W. (NY: Elsevier; ), 389–402 [ Google Scholar ]
- Nir Y., Mukamel R., Dinstein I., Privman E., Harel M., Fisch L., Gelbard-Sagiv H., Kipervasser S., Andelman F., Neufeld M. Y. (2008). Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex . Nat. Neurosci. 11 , 1100–1108 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Northoff G., Bermpohl F. (2004). Cortical midline structures and the self . Trends Cogn. Sci. (Regul. Ed.) 8 , 102. [ PubMed ] [ Google Scholar ]
- Northoff G., Heinzel A., De Greck M., Bermpohl F., Dobrowolny H., Panksepp J. (2006). Self-referential processing in our brain – a meta-analysis of imaging studies on the self . Neuroimage 31 , 440–457 10.1016/j.neuroimage.2005.12.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Northoff G., Qin P., Nakao T. (2010). Rest-stimulus interaction in the brain: a review . Trends Neurosci. 33 , 277–284 10.1016/j.tins.2010.02.006 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Raichle M. E. (2009). A paradigm shift in functional brain imaging . J. Neurosci. 29 , 12729–12734 10.1523/JNEUROSCI.4366-09.2009 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Raichle M. E., Macleod A. M., Snyder A. Z., Powers W. J., Gusnard D. A., Shulman G. L. (2001). A default mode of brain function . Proc. Natl. Acad. Sci. U.S.A. 98 , 676–682 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Salvador R., Suckling J., Schwarzbauer C., Bullmore E. (2005). Undirected graphs of frequency-dependent functional connectivity in whole brain networks . Philos. Trans. R. Soc. Lond. B Biol. Sci. 360 , 937. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Schneider F., Bermpohl F., Heinzel A., Rotte M., Walter M., Tempelmann C., Wiebking C., Dobrowolny H., Heinze H. J., Northoff G. (2008). The resting brain and our self: self-relatedness modulates resting state neural activity in cortical midline structures . Neuroscience 157 , 120–131 10.1016/j.neuroscience.2008.08.014 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shmuel A., Leopold D. A. (2008). Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: implications for functional connectivity at rest . Hum. Brain Mapp. 29 , 751–761 10.1002/hbm.20580 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shmuel A., Yacoub E., Pfeuffer J., Van De Moortele P. F., Adriany G., Hu X., Ugurbil K. (2002). Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain . Neuron 36 , 1195–1210 10.1016/S0896-6273(02)01061-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shulman G. L., Corbetta M., Buckner R. L., Fiez J. A., Miezin F. M., Raichle M. E., Petersen S. E. (1997). Common blood flow changes across visual tasks: I. Increases in subcortical structures and cerebellum but not in nonvisual cortex . J. Cogn. Neurosci. 9 , 624–647 [ PubMed ] [ Google Scholar ]
- Singer J. L., McCraven V. G. (1961). Some characteristics of adult daydreaming . J. Psychol. 51 , 151–164 10.1080/00223980.1961.9916467 [ CrossRef ] [ Google Scholar ]
- Smallwood J., Brown K. S., Tipper C., Giesbrecht B., Franklin M. S., Mrazek M. D., Carlson J. M., Schooler J. W. (2011a). Pupillometric evidence for the decoupling of attention from perceptual input during offline thought . PLoS ONE 6 , e18298. 10.1371/journal.pone.0018298 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Smallwood J., Schooler J. W., Turk D. J., Cunningham S. J., Burns P., Macrae C. N. (2011b). Self-reflection and the temporal focus of the wandering mind . Conscious. Cogn. (in press). 10.1016/j.concog.2010.12.017 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Smallwood J., Mcspadden M., Schooler J. W. (2008). When attention matters: the curious incident of the wandering mind . Mem. Cognit. 36 , 1144. [ PubMed ] [ Google Scholar ]
- Smallwood J., Nind L., O'connor R. C. (2009). When is your head at? An exploration of the factors associated with the temporal focus of the wandering mind . Conscious. Cogn. 18 , 118–125 [ PubMed ] [ Google Scholar ]
- Smallwood J., Schooler J. W. (2006). The restless mind . Psychol. Bull. 132 , 946. [ PubMed ] [ Google Scholar ]
- Smallwood J. M., Baracaia S. F., Lowe M., Obonsawin M. (2003). Task unrelated thought whilst encoding information . Conscious. Cogn. 12 , 452–484 [ PubMed ] [ Google Scholar ]
- Spreng R. N., Mar R. A., Kim A. S. N. (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis . J. Cogn. Neurosci. 21 , 489–510 [ PubMed ] [ Google Scholar ]
- Spreng R. N., Stevens W. D., Chamberlain J. P., Gilmore A. W., Schacter D. L. (2010). Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition . Neuroimage 53 , 303–317 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stawarczyk D., Majerus S., Maquet P., D'Argembeau A. (2011). Neural correlates of ongoing conscious experience: both task-unrelatedness and stimulus-independence are related to default network activity . PLoS ONE 6 , e16997. 10.1371/journal.pone.0016997 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stickgold R., Hobson J. A., Fosse R., Fosse M. (2001). Sleep, learning, and dreams: off-line memory reprocessing . Science 294 , 1052. [ PubMed ] [ Google Scholar ]
- Teasdale J. D., Dritschel B. H., Taylor M. J., Proctor L., Lloyd C. A., Nimmo-Smith I., Baddeley A. D. (1995). Stimulus-independent thought depends on central executive resources . Mem. Cognit. 23 , 551–551 [ PubMed ] [ Google Scholar ]
- Tulving E. (2005). Episodic Memory and Autonoesis: Uniquely Human? New York: Oxford University Press [ Google Scholar ]
- Uddin L. Q., Clare Kelly A., Biswal B. B., Xavier Castellanos F., Milham M. P. (2009). Functional connectivity of default mode network components: correlation, anticorrelation, and causality . Hum. Brain Mapp. 30 , 625. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Van Den Heuvel M., Mandl R., Pol H. H. (2008). Normalized cut group clustering of resting-state FMRI data . PLoS ONE 3 , e2001. 10.1371/journal.pone.0002001 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Van Den Heuvel M. P., Hulshoff Pol H. E. (2010). Exploring the brain network: a review on resting-state fMRI functional connectivity . Eur. Neuropsychopharmacol. 20 , 519–534 10.1016/j.euroneuro.2010.03.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wicker B., Ruby P., Royet J.-P., Fonlupt P. (2003). A relation between rest and the self in the brain? Brain Res. Rev. 43 , 224. [ PubMed ] [ Google Scholar ]

How to Tame Your Wandering Mind
Learn to take steps to deal with distraction..
Posted April 24, 2022 | Reviewed by Jessica Schrader
- Understanding Attention
- Find counselling to help with ADHD
- We can tame our mind-wandering.
- Three tips can help you use mind-wandering to your advantage.
- These include making time to mind-wander and controlling your response to it.

Researchers believe that when a task isn’t sufficiently rewarding, our brains search for something more interesting to think about.
You have a big deadline looming, and it’s time to hunker down. But every time you start working, you find that, for some reason, your mind drifts off before you can get any real work done. What gives? What is this cruel trick our brains play on us, and what do we do about it?
Thankfully, by understanding why our mind wanders and taking steps to deal with distraction, we can stay on track. But first, let’s understand the root of the problem.
Why do our minds wander?
Unintentional mind-wandering occurs when our thoughts are not tied to the task at hand. Researchers believe our minds wander when the thing we’re supposed to be doing is not sufficiently rewarding, so our brains look for something more interesting to think about.
We’ve all experienced it from time to time, but it’s important to note that some people struggle with chronic mind-wandering : Though studies estimate ADHD afflicts less than 3% of the global adult population, it can be a serious problem and may require medical intervention.
For the vast majority of people, mind-wandering is something we can tame on our own—that is, if we know what to do about it. In fact, according to Professor Ethan Kross, director of the Emotion & Self Control Laboratory at the University of Michigan and author of Chatter: The Voice in Our Head, Why It Matters, and How to Harness It , mind-wandering is perfectly normal.
“We spend between a third to a half of our waking hours not focused on the present,” he told me in an email. “Some neuroscience research refers to our tendency to mind-wander as our ‘default state.’”
So why do we do it?
“Mind-wandering serves several valuable functions. It helps us simulate and plan for the future and learn from our past, and it facilitates creative problem-solving,” Kross explained. “Mind-wandering often gets a bad rep, but it’s a psychological process that evolved to provide us with a competitive advantage. Imagine not being able to plan for the future or learn from your past mistakes.”
Is mind-wandering bad for you?
“Like any psychological tool, however, mind-wandering can be harmful if used in the wrong context (i.e., when you’re trying to focus on a task) or inappropriately (i.e., when you worry or ruminate too much),” according to Kross. In other words, mind-wandering is a problem when it becomes a distraction. A distraction is any action that pulls you away from what you planned to do.
If, for instance, you intended to work on a big project, such as writing a blog post or finishing a proposal, but instead find yourself doing something else, you’re distracted.
The good news is that we can use mind-wandering to our advantage if we follow a few simple steps:
1. Make time to mind-wander
Mind-wandering isn’t always a distraction. If we plan for it, we can turn mind-wandering into traction. Unlike a distraction , which by definition is a bad thing, a diversion is simply a refocusing of attention and isn’t always harmful.
There’s nothing wrong with deciding to refocus your attention for a while. In fact, we often enjoy all kinds of diversions and pay for the privilege.
A movie or a good book, for instance, diverts our attention away from real life for a while so we can get into the story and escape reality for a bit.
Similarly, if you make time to allow your mind to drift and explore whatever it likes, that’s a healthy diversion, not a distraction.
The first step to mastering mind-wandering is to plan time for it. Use a schedule maker and block off time in your day to let your thoughts flow freely. You’ll likely find that a few minutes spent in contemplation can help you work through unresolved issues and lead to breakthroughs. Scheduling mind-wandering also lets you relax because you know you have time to think about whatever is on your mind instead of believing you need to act on every passing thought.
It’s helpful to know that time to think is on your calendar so you don’t have to interrupt your mind-wandering process or risk getting distracted later.
2. Catch the action
One of the difficulties surrounding mind-wandering is that by the time you notice you’re doing it, you’ve already done it. It’s an unconscious process so you can’t prevent it from happening.

The good news is that while you can’t stop your mind from wandering, you can control what you do when it happens.
Many people never learn that they are not their thoughts. They believe the voice in their head is somehow a special part of them, like their soul speaking out their inner desires and true self. When random thoughts cross their mind, they think those thoughts must be speaking some important truth.
Not true. That voice in your head is not your soul talking, nor do you have to believe everything you think.
When we assign undue importance to the chatter in our heads, we risk listening to half-baked ideas, feeling shame for intrusive thoughts, or acting impulsively against our best interests.
A much healthier way to view mind-wandering is as brain static. Just as the random radio frequencies you tune through don’t reveal the inner desires of your car’s soul, the thoughts you have while mind-wandering don’t mean much—unless, that is, you act upon them.
Though it can throw us off track, mind-wandering generally only lasts a few seconds, maybe minutes. However, when we let mind-wandering turn into other distractions, such as social-media scrolling, television-channel surfing, or news-headline checking, that’s when we risk wasting hours rather than mere minutes.
If you do find yourself mentally drifting off in the middle of a task, the important thing is to not allow that to become an unintended action, and therefore a distraction.
An intrusive thought is not your fault. It can’t be controlled. What matters is how you respond to it—hence the word respon-sibility.
Do you let the thought go and stay on task? Or do you allow yourself to escape what you’re doing by letting it lead you toward an action you’ll later regret?
3. Note and refocus
Can we keep the helpful aspects of mind-wandering while doing away with the bad? For the most part, yes, we can.
According to Kross, “Mind-wandering can easily shift into dysfunctional worry and rumination. When that happens, the options are to refocus on the present or to implement tools that help people mind-wander more effectively.”
One of the best ways to harness the power of mind-wandering while doing an important task is to quickly note the thought you don’t want to lose on a piece of paper. It’s a simple tactic anyone can use but few bother to do. Note that I didn’t recommend an app or sending yourself an email. Tech tools are full of external triggers that can tempt us to just check “one quick thing,” and before we know it, we’re distracted.
Rather, a pen and Post-it note or a notepad are the ideal tools to get ideas out of your head without the temptations that may lead you away from what you planned to do.
Then, you can collect your thoughts and check back on them later during the time you’ve planned in your day to chew on your ideas. If you give your thoughts a little time, you’ll often find that those super important ideas aren’t so important after all.
If you had acted on them at the moment, they would have wasted your time. But by writing them down and revisiting them when you’ve planned to do so, they have time to marinate and may become less relevant.
However, once in a while, an idea you collected will turn out to be a gem. With the time you planned to chew on the thought, you may discover that mind-wandering spurred you to a great insight you can explore later.
By following the three steps above, you’ll be able to master mind-wandering rather than letting it become your master.

Nir Eyal, who has lectured at Stanford's Graduate School of Business and the Hasso Plattner Institute of Design, is the author of Indistractable: How to Control Your Attention and Choose Your Life.
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- International
- New Zealand
- South Africa
- Switzerland
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Therapy Center NEW
- Diagnosis Dictionary
- Types of Therapy

Understanding what emotional intelligence looks like and the steps needed to improve it could light a path to a more emotionally adept world.
- Coronavirus Disease 2019
- Affective Forecasting
- Neuroscience

It’s normal for your mind to wander. Here’s how to maximise the benefits
Psychology researcher, Bond University
Associate Professor in Psychology, Bond University
Disclosure statement
The authors do not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and have disclosed no relevant affiliations beyond their academic appointment.
Bond University provides funding as a member of The Conversation AU.
View all partners
Have you ever found yourself thinking about loved ones during a boring meeting? Or going over the plot of a movie you recently watched during a drive to the supermarket?
This is the cognitive phenomenon known as “ mind wandering ”. Research suggests it can account for up to 50% of our waking cognition (our mental processes when awake) in both western and non-western societies .
So what can help make this time productive and beneficial?
Mind wandering is not daydreaming
Mind wandering is often used interchangeably with daydreaming. They are both considered types of inattention but are not the same thing.
Mind wandering is related to a primary task, such as reading a book, listening to a lecture, or attending a meeting. The mind withdraws from that task and focuses on internally generated, unrelated thoughts.
On the other hand, daydreaming does not involve a primary, active task. For example, daydreaming would be thinking about an ex-partner while travelling on a bus and gazing out the window. Or lying in bed and thinking about what it might be like to go on a holiday overseas.
If you were driving the bus or making the bed and your thoughts diverted from the primary task, this would be classed as mind wandering.

The benefits of mind wandering
Mind wandering is believed to play an important role in generating new ideas , conclusions or insights (also known as “aha! moments”). This is because it can give your mind a break and free it up to think more creatively.
This type of creativity does not always have to be related to creative pursuits (such as writing a song or making an artwork). It could include a new way to approach a university or school assignment or a project at work. Another benefit of mind wandering is relief from boredom, providing the opportunity to mentally retreat from a monotonous task.
For example, someone who does not enjoy washing dishes could think about their upcoming weekend plans while doing the chore. In this instance, mind wandering assists in “passing the time” during an uninteresting task.
Mind wandering also tends to be future-oriented. This can provide an opportunity to reflect upon and plan future goals, big or small. For example, what steps do I need to take to get a job after graduation? Or, what am I going to make for dinner tomorrow?

Read more: Alpha, beta, theta: what are brain states and brain waves? And can we control them?
What are the risks?
Mind wandering is not always beneficial, however. It can mean you miss out on crucial information. For example, there could be disruptions in learning if a student engages in mind wandering during a lesson that covers exam details. Or an important building block for learning.
Some tasks also require a lot of concentration in order to be safe. If you’re thinking about a recent argument with a partner while driving, you run the risk of having an accident.
That being said, it can be more difficult for some people to control their mind wandering. For example, mind wandering is more prevalent in people with ADHD.
Read more: How your brain decides what to think
What can you do to maximise the benefits?
There are several things you can do to maximise the benefits of mind wandering.
- be aware : awareness of mind wandering allows you to take note of and make use of any productive thoughts. Alternatively, if it is not a good time to mind wander it can help bring your attention back to the task at hand

context matters : try to keep mind wandering to non-demanding tasks rather than demanding tasks. Otherwise, mind wandering could be unproductive or unsafe. For example, try think about that big presentation during a car wash rather than when driving to and from the car wash
content matters : if possible, try to keep the content positive. Research has found , keeping your thoughts more positive, specific and concrete (and less about “you”), is associated with better wellbeing. For example, thinking about tasks to meet upcoming work deadlines could be more productive than ruminating about how you felt stressed or failed to meet past deadlines.
- Consciousness
- Daydreaming
- Concentration
- Mind wandering

Program Manager, Teaching & Learning Initiatives

Lecturer/Senior Lecturer, Earth System Science (School of Science)

Sydney Horizon Educators (Identified)

Deputy Social Media Producer

Associate Professor, Occupational Therapy
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 12 January 2017
Maintenance and Representation of Mind Wandering during Resting-State fMRI
- Ying-hui Chou 1 , 2 , 3 ,
- Mark Sundman 1 ,
- Heather E. Whitson 4 , 5 ,
- Pooja Gaur 6 ,
- Mei-Lan Chu 7 ,
- Carol P. Weingarten 8 ,
- David J. Madden 7 , 8 ,
- Lihong Wang 7 , 9 ,
- Imke Kirste 7 ,
- Marc Joliot 10 ,
- Michele T. Diaz 11 ,
- Yi-Ju Li 12 ,
- Allen W. Song 7 , 13 &
- Nan-kuei Chen 3 , 7 , 13 , 14 , 15
Scientific Reports volume 7 , Article number: 40722 ( 2017 ) Cite this article
4289 Accesses
23 Citations
7 Altmetric
Metrics details
- Neural circuits
- Neuroscience
Major advances in resting-state functional magnetic resonance imaging (fMRI) techniques in the last two decades have provided a tool to better understand the functional organization of the brain both in health and illness. Despite such developments, characterizing regulation and cerebral representation of mind wandering, which occurs unavoidably during resting-state fMRI scans and may induce variability of the acquired data, remains a work in progress. Here, we demonstrate that a decrease or decoupling in functional connectivity involving the caudate nucleus, insula, medial prefrontal cortex and other domain-specific regions was associated with more sustained mind wandering in particular thought domains during resting-state fMRI. Importantly, our findings suggest that temporal and between-subject variations in functional connectivity of above-mentioned regions might be linked with the continuity of mind wandering. Our study not only provides a preliminary framework for characterizing the maintenance and cerebral representation of different types of mind wandering, but also highlights the importance of taking mind wandering into consideration when studying brain organization with resting-state fMRI in the future.
Similar content being viewed by others
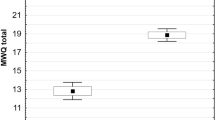
Relationships between resting-state EEG functional networks organization and individual differences in mind wandering
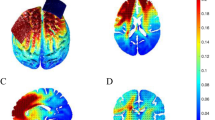
Prefrontal tDCS is unable to modulate mind wandering propensity or underlying functional or effective brain connectivity
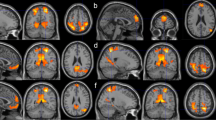
Longitudinal effects of meditation on brain resting-state functional connectivity
Introduction.
Over the past two decades, resting-state functional connectivity measured by functional magnetic resonance imaging (fMRI) has played an essential role in understanding brain functional networks in healthy and patient populations 1 , 2 , 3 , 4 , 5 . Resting-state functional connectivity is measured by the temporal co-activation level of spontaneous fMRI signals between spatially distinct brain regions in the absence of a perceptual or behavioral task 6 . Although the participants are not engaged in any particular task, there is increasing evidence that spontaneous thoughts (known as mind wandering, daydreaming, self-generated mental activity or task-unrelated thought) that are minimally constrained by external perception emerge during fMRI scans and may potentially affect resting-state fMRI data 7 , 8 . Mind wandering during resting-state fMRI has been assessed using different approaches. Questionnaires can be administered, following the resting-state fMRI scan, in which participants are asked to report the presence and frequency of spontaneous thoughts across various domains. Resting-state fMRI studies have employed several types of retrospective measures to assess spontaneous thoughts: Amsterdam Resting-State Questionnaire (ARSQ) 9 , New York Cognition Questionnaire (NYC-Q) 10 , 11 , 12 , and Resting-State Questionnaire (ReSQ) 13 , 14 . Alternatively, mind-wandering has been assessed using experience or thought sampling in conjunction with resting-state fMRI scanning 15 , 16 , 17 , 18 , 19 , 20 . While regions within the default mode network are involved in mind-wandering, a number of other brain regions outside the default mode network also show associations with various contents and forms of spontaneous thoughts 10 , 15 , 16 , 21 , 22 . These findings contribute to an increasingly diverse and complex understanding of the spontaneous thoughts that may occur during resting-state fMRI scans, and thus provoke more questions on the impact of mind-wandering on fMRI data.
For example, previous studies using the ReSQ have indicated that, on average, participants reported spending about 40% and 30% of time on visual and auditory mental imagery, respectively, during resting-state fMRI scans 5 , 13 , 14 . The remaining portion of the scan was filled with a variety of spontaneous thought domains including those pertaining to somatosensory awareness, inner musical experience, and manipulation of numbers 13 , 14 . This gives rise to the questions that form the analytical focus of our study. How is the continuity of spontaneous thoughts supported? Is the mechanism underlying the support of spontaneous thoughts comparable across different domains? Are different thought domains represented by divergent functional connections across the cerebral cortex? Recent studies have observed the non-static nature of resting-state functional connectivity across a single fMRI scan 23 , 24 , 25 , 26 , 27 . Will regulation of mind wandering contribute to the temporal changes in resting-state functional connectivity?
To address these questions, first, we employed multiple regression analyses to identify functional connections that exhibited a significant group difference in connectivity between participants who spent more time in a self-reported spontaneous thought and participants who spent less time in the same thought domain during resting-state fMRI (e.g., those who reported spending a lot of time in auditory mental imagery compared to those who reported spending little or no time on such wandering thought). The functional connections exhibiting a significant group difference in connectivity for a specific spontaneous thought domain would be indicative of the neural correlates associated with sustaining this spontaneous thought. Second, we investigated whether group effects on functional connectivity would vary between earlier and later parts of the resting-state fMRI data time points. Our goal is to provide a framework for studying the maintenance and cerebral representation of mind wandering, and understanding the impact of mind wandering on the acquired resting-state fMRI data.
Behavioral Responses
Each participant completed a post-resting-state-fMRI interview using the Resting-State Questionnaire (ReSQ) 13 to assess spontaneous thoughts during the resting-state fMRI scans. Participants were asked to estimate the proportion of time (on a 0–100% scale) spent during the resting-state fMRI scans in each of the following five spontaneous thought domains: auditory mental imagery/inner language (AUDI/LANG), visual mental imagery (VIMG), somatosensory awareness (SEN), inner musical experience (MUS), and mental manipulation of numbers (NUM). Descriptions of each thought domain are included in the Methods section. On average, the participants reported spending the greatest amount of time in the AUDI/LANG (36.7%) domain, followed by VIMG (26.1%), SEN (22.5%), MUS (8.5%), and NUM (6.2%). For data analyses of each domain of spontaneous thought, participants were split into two groups (higher vs. lower percentage groups). The higher percentage group included participants whose estimated percentage of time spent in a specific thought domain was greater than the 75 th percentile (i.e., upper quartile across all the participants), while the lower percentage group included the remainder of the participants. No significant differences in age were found between the two groups, for any of the individual thought domains. Figure 1 illustrates the estimated proportion of time spent in each thought domain for the higher and the lower percentage groups.
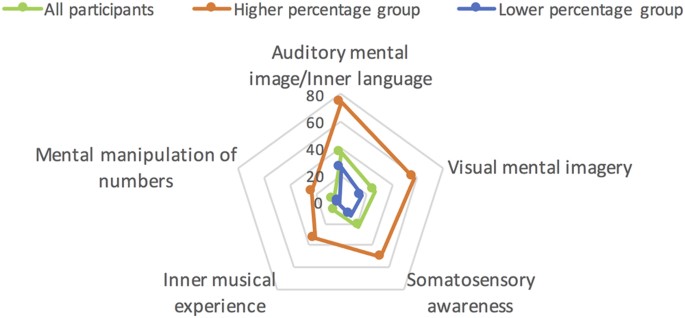
Estimated proportion of time spent in each thought domain of mind wandering.
Matrix-Based Connectivity Analysis Results
In contrast to seed-based analysis that relies on prior knowledge for choosing seed regions, the matrix-based approach employed in this study thoroughly examines functional connectivity between every pair of regions across the whole brain. Our matrix-based functional connectivity analysis procedures are described in detail in the Methods section and illustrated in Fig. 2 .
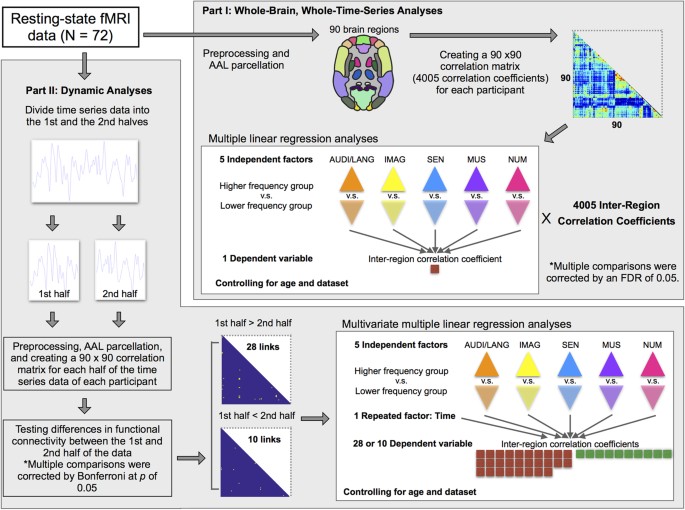
Summary of functional connectivity analysis procedures.
Part I: Whole-brain, whole-time-series analyses
The first goal of our study was to identify functional connections that exhibited significantly different connectivity between groups of higher- and lower-frequency of mind wandering during fMRI scans (see Part I in Fig. 2 ). The resting-state fMRI data were preprocessed and parceled into a set of 90 brain regions using Automated Anatomical Labeling (AAL) template 28 . Inter-regional functional connectivity was estimated using the pairwise Pearson correlation statistics, resulting in 4005 ([90 × 89]/2) correlation coefficients for each participant. We examined group effects on functional connectivity of individual links by performing multiple linear regression analysis 4005 times. Each regression model included 5 independent factors (i.e., group effects of each thought domain), with each controlled for the others, and 1 dependent variable (i.e., the functional connectivity value of an individual link). Our resting-state data were aggregated from three unpublished datasets (see Methods section). Therefore, we added the “dataset” as a covariate in the regression models to control for any variability across datasets.
The analyses yielded 2 significant functional links for the thought domain of AUDI/LANG, corrected for multiple comparisons at a false discovery rate (FDR) of 0.05 29 . The two functional links ( Fig. 3A ) were connected between the left insula and the left caudate nucleus, t (65) = −4.64, p = 0.000017, and between the left insula and the right caudate nucleus, t (65) = −5.01, p = 0.000004. For both functional links, participants in the higher percentage group for AUDI/LANG exhibited a significantly more negative connectivity relative to the participants in the lower percentage group ( Fig. 3B and C ). As described in the Discussion section, bilateral caudate nuclei are brain regions involved in brain state maintenance, and the left insula supports switching between different mental states 30 . No significant associations with functional links were identified for other thought domains. The results suggest that the decrease in functional connectivity of connections between the left insula and bilateral caudate nuclei was associated with the continuity of spontaneous thought related to AUDI/LANG.
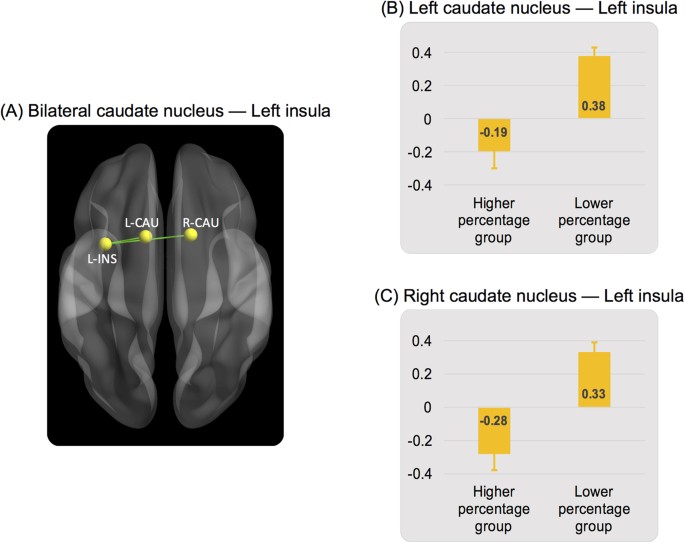
( A ) Functional connections between the left insula (L-INS) and bilateral caudate nuclei (CAU) were associated with the continuity of spontaneous thought for auditory mental imagery/inner language (AUDI/LANG). Data were derived from whole time series data. ( B ) and ( C ) Participants who reported spending more time in mind wandering associated with AUDI/LANG (higher percentage group) exhibited a more negative functional connectivity compared to participants who reported spending less time in AUDI/LANG (lower percentage group). Error bars denote standard errors.
Part II: Dynamic analyses
The second goal of our study was to investigate whether group effects on functional connectivity links would vary between earlier and later portions of the resting-state time series data (see Part II in Fig. 2 ). To this end, we examined whether there was an interaction effect between group and timing of the resting-state fMRI data time course profiles. First, we divided each participant’s time series data into halves (i.e., the 1 st half and the 2 nd half of the time series data, see Fig. 2 ). For each half, the preprocessed fMRI data were parceled using the AAL template 28 , and 4005 ([90 × 89]/2) correlation coefficients were estimated for each half of the time series data of each participant (as stated in the previous section). We then examined differences in functional connectivity between the 1 st half and the 2 nd half of the time series data by performing paired sample t tests on each connectivity value of the 4005 inter-regional functional links.
The analysis yielded 38 functional links for which mean connectivity differed significantly between the first and second halves of the scan, with Bonferroni correction for multiple comparisons (alpha = 0.05/4005 ≈ 0.000012) to minimize false positives. Among the 38 links, 28 links exhibited decreased functional connectivity from the 1 st half to the 2 nd half of the time series data. These links temporally changed their connectivity either from positive to negative, from more positive to less positive, or from less negative to more negative and we called these 28 links “decreasing links”. An additional 10 links exhibited increased temporal functional connectivity in the second half of the scan and they are termed “increasing links”. Among the decreasing links ( Fig. 4A ), most links were connected to the bilateral medial prefrontal cortex (MPFC), primary sensorimotor area, and temporal regions. For the increasing links ( Fig. 4B ), connections were dispersed among visual, temporal, and frontal areas. Additional details of the decreasing and increasing links are presented in Supplementary Table S1 . We then converted the resultant 2 sets of links (i.e., decreasing and increasing links) into 2 binary matrices and used them as inclusive masks in the subsequent analysis.
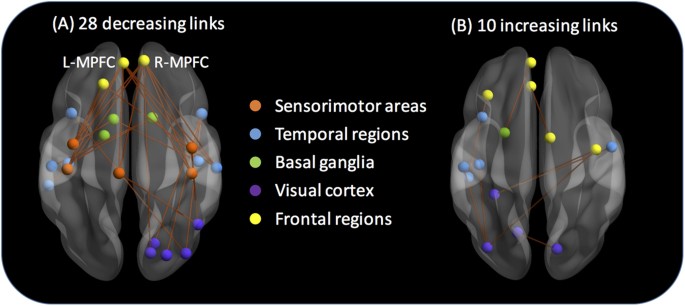
( A ) The majority of the decreasing links were connected to the bilateral medial prefrontal cortex (MPFC), primary sensorimotor cortex, and temporal regions. ( B ) The increasing links were distributed among visual, temporal, and frontal areas.
Within each resultant set of links, we examined Group × Time interaction effects on functional connectivity using a multivariate multiple regression analysis, which estimated a single regression model with more than one dependent variable. The main advantage of the multivariate multiple regression analysis is that all the assessments can be performed in a single step, and thus the risk of false positives associated with repeated assessments (i.e., multiple comparisons) in conventional univariate multiple regression can be inherently eliminated. This analysis was chosen to account for the relationships among several dependent variables and conduct tests of the coefficients across different variables. Our model included 5 independent factors (i.e., group effects of each thought domain), 1 repeated factor (i.e., time: 1 st half vs. 2 nd half of the time series data), 1 covariate (i.e., dataset), and functional connectivity values for a set of links (either all decreasing links or all increasing links) as dependent variables. The multivariate multiple regression analysis yielded two outputs: 1) results of multivariate analysis of variance that tested the overall group effects on functional connectivity across all dependent variables; and 2) results of univariate analysis that examined the group effect on the functional connectivity of each individual dependent variable for each thought domain.
For the decreasing links (i.e., functional connectivity decreasing from the 1 st to the 2 nd half), the multivariate analysis yielded a significant Group × Time interaction effect, F (55, 3575) = 1.43, p = 0.02, for the AUDI/LANG, and an expected, significant time effect, F (55, 3575) = 2.16, p < 0.0001. Post-hoc analysis of the Group × Time interaction for the AUDI/LANG showed that the group effect was significant for the 2 nd half of the time series data ( p = 0.04), but was not significant for the 1 st half of the data ( p = 0.28). While the overall group effect across all dependent variables was not significant for the SEN and VIMG, partially due to a number of potentially less-relevant connections (i.e., not associated with mind wandering) being included in the model, the univariate analysis for the 2 nd half of the data yielded 11 functional links that exhibited a significant group effect for SEN and VIMG in addition to the AUDI/LANG ( Fig. 5A ). Four links associated with the SEN were connected between bilateral MPFC, left paracentral lobule, and right post-central gyrus. Four links associated with the AUDI/LANG were connected between bilateral MPFC, right Heschl gyrus, left superior temporal gyrus, and bilateral caudate nucleus. For the VIMG, 3 links were connected between bilateral MPFC, right superior temporal gyrus, and left dorsolateral superior frontal gyrus. Thus, among the 11 decreasing links that were associated with particular thought domains, 8 were connected to the MPFC, consistent with previous studies indicating its extensive patterns of connectivity between MPFC and other sensory modalities 31 , 32 . These significant group effects on functional connectivity of the decreasing links for the 2 nd half of the time series data indicated that participants in the higher percentage group exhibited more negative functional connectivity in the majority of the connections relative to participants in the lower percentage group ( Fig. 5B and C ). The exception to this pattern involved two links between right Heschl gyrus and bilateral MPFC in which higher functional connectivity was observed among participants who reported higher percentage of AUDI/LANG thought content. Additional statistical results of the univariate analysis are presented in Supplementary Table S2 . For the increasing links (i.e., functional connectivity increasing from the 1 st to the 2 nd half), the expected time effect was significant, F (25, 1625) = 2.31, p = 0.0002. However, neither the group nor the Group × Time interaction effects were significant.
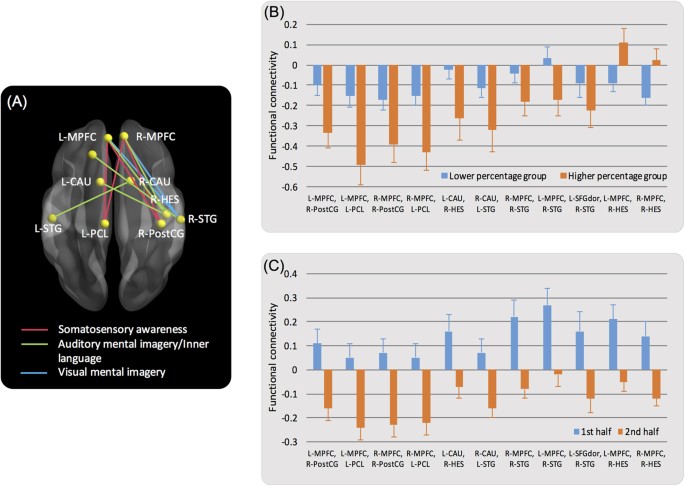
( A ) Eleven functional connections were associated with the continuity of spontaneous thoughts for somatosensory awareness (red), auditory mental imagery/inner language (green), and visual mental imagery (blue). These connections were identified from the 2 nd half time series data of the decreasing links. Spheres represent the centroids of the Automated Anatomical Labeling Template regions as estimated by the BrainNet Viewer 70 . ( B ) Participants in the higher percentage group (orange) exhibited more negative functional connectivity in the majority of links relative to the lower percentage group (blue). Functional connectivity was estimated from the 2 nd half of the time series data. ( C ) Functional connectivity significantly decreased from the 1 st half (blue) to the 2 nd half (orange) of the time series data. Error bars denote standard errors. Abbreviations: L = left; R = right; MPFC = medial prefrontal cortex; PCL = paracentral lobule; PostCG = postcentral gyrus; HES = Heschl gyrus; CAU = caudate nucleus; STG = superior temporal gyrus; SFGdor = dorsolateral part of superior frontal gyrus.
To explore whether we were able to observe comparable findings using seed-based connectivity analysis, we chose the MPFC as the seed region of interest to assess the correspondence between voxel-wise fMRI signals and mind wandering of different domains. Methods and results of the seed-based analysis are shown in the Supplementary Information (in the section of “Seed-based analysis”). Overall, the seed-based connectivity analysis qualitatively reproduces the major findings from the matrix-based analysis.
Altogether, the results obtained from the multivariate multiple regression analysis suggest that 1) the functional connectivity of 11 links (the majority of which connected to the MPFC) was associated with sustaining the spontaneous thoughts in particular domains: AUDI/LANG, SEN, and VIMG, 2) participants who reported spending more time in the AUDI/LANG, SEN, or VIMG exhibited a more negative functional connectivity associated with several links, compared to participants who reported spending less time in each of those same thought domains, and 3) when we examined links for which the functional connectivity tended to decrease over the course of the scan, the relationship between reported thought content and functional connectivity varied between earlier and later portions of the resting-state time series data, with relationships more pronounced in the second half. It is worth noting that, without relying on any a priori hypothesis or pre-selected seeds, we were able to identify the important roles of MPFC for mind wandering, largely in agreement with previous studies 15 , 16 , 22 . We believe that our analytical approach is complementary to the methods used in previous studies, and our proposed discovery-driven matrix-based connectivity analysis is a powerful tool that can potentially add new knowledge to resting-state fMRI and mind wandering research.
Negative functional connectivity and sustaining of spontaneous thoughts
What do these results suggest for the potential underlying mechanism responsible for sustaining spontaneous thoughts during resting-state fMRI? Overall, participants who reported spending more time in spontaneous thought domains tended to exhibit more negative functional connectivity in the majority of links compared to participants who reported spending less time in each of those same thought domains ( Figs 3B,C and 5B ). Furthermore, a number of connections from the decreasing links (i.e., functional connectivity decreasing from the 1 st to the 2 nd half) were related to the continuity of spontaneous thoughts ( Fig. 5 ). The results suggest that this decrease in functional connectivity might be a potential mechanism underlying the maintenance of particular types of spontaneous thoughts during resting-state fMRI, and this mechanism appears to be comparable across different thought domains.
The observed negative functional connectivity associated with the maintenance of mind wandering might be explained by a recently proposed decoupling hypothesis, which postulates that our attention is decoupled or shifted from processing events in the external world to self-generated mental activity to ensure the continuity of mind wandering 33 , 34 , 35 , 36 . Previous support for the decoupling hypothesis primarily comes from electroencephalography (EEG) studies during cognitive tasks 37 , 38 , 39 . In these EEG studies, task-related attention during cognitive activity was characterized by amplitude of event-related potentials (ERPs) 37 , 38 and phase-locking consistency across task trials 39 . The amplitude of ERPs was reduced for participants who engaged in greater amounts of task-unrelated thought 37 , 38 , and task-unrelated thought was associated with a reduction in the trial-to-trial phase-locking consistency to visual events 39 . The findings of these studies illustrate that mind wandering during cognitive tasks is accompanied by a decrease in the processing of task-related information.
Taken together with previous studies 37 , 38 , 39 , 40 , the negative connectivity between brain regions observed in the current study can be interpreted as evidence for increased functional segregation between cortical systems subserving opposite goals or competing representations 41 . The functional segregation might reflect reciprocal modulation or inhibition/suppression through direct or indirect anatomical connections 42 , 43 , 44 . It is to be noted that, while negative correlations have been associated with data preprocessing methods using global signal regression 45 , 46 , a number of studies 42 , 47 , 48 , 49 , 50 , including this current study, observed negative correlations even in the absence of global signal regression. Therefore, the negative connectivity observed in this study could not be an artifact introduced by a global signal regression procedure. Collectively, the evidence presented above has provided initial support for the associations between negative functional connectivity and the underlying neural processes for sustaining mind wandering during resting state fMRI scans.

Neural correlates of sustaining spontaneous thoughts during resting-state fMRI
In this study, we identified two sets of functional connections that were related to the continuity of spontaneous thoughts during resting-state fMRI. First, functional connections between the left insula and bilateral caudate nuclei, associated with AUDI/LANG, were identified from the whole-brain, whole resting-state fMRI time series data ( Fig. 3A ). Second, 11 connections with the majority of links connected to the MPFC, along with a number of domain-specific regions, were identified among the links which exhibited decreased connectivity in the 2 nd half of the time series data ( Fig. 5A ). The connectivity of these links during the second half of the scan was related to sustaining of spontaneous thoughts in the AUDI/LANG, SEN, or VIMG domains.
Previous studies have shown that the insula is highly interconnected with the striatum, including the caudate nucleus 51 , 52 . Both the insula and striatum are complex structures and have been implicated in a wide range of autonomic, affective, sensorimotor, self-referential, and cognitive processes, including language-related function 53 , 54 , 55 . In an extensive review of previous neuroimaging data, Price 56 described a set of brain regions that participate in regulating language-related function. Among the language-related regions, the left insula is specifically involved in articulatory planning, whereas the bilateral caudate nuclei are associated with suppression of unintended responses 56 . In addition to their involvement with language-related function, there is recent evidence indicating that the striatum plays a critical role in brain state maintenance, whereas the insula has a major role in switching between states 30 . In relation to our study, we found negative connectivity between bilateral caudate nuclei and the left insula in participants who reported spending more time in AUDI/LANG and positive connectivity among the same links in participants who reported spending less time in this spontaneous thought ( Fig. 3B and C ). It is possible that, to support the continuity of mind wandering state, the caudate nuclei might exert suppressive effects on the insula to prevent from switching between brain states. Future studies are warranted to elucidate the dynamics between these two brain regions involved maintaining a state of mind wandering.
Among the additional 11 functional links that were related to greater continuity of specific domains of spontaneous thoughts, 8 of them were connected to the MPFC. The MPFC is a hub within the default mode network 3 and has been implicated in self-related processing, such as the retrieval of remote and recent memory associated with autobiography or other self-referential processes, judgments about self and others, and simulations of social interaction 21 , 57 , 58 , 59 , 60 , 61 . Furthermore, the MPFC is characterized by a high-level interconnectivity with multiple sensory modalities such as primary/secondary auditory, somatosensory, and visual cortices 31 , 32 . This multi-modal convergence observed in the MPFC provides an anatomical ground to support the role of the MPFC together with other domain-specific brain regions in the maintenance and representation of spontaneous thoughts across different domains. Therefore, together with previous studies 10 , 15 , 16 , 22 , we propose that the MPFC is a nodal point that serves to support different domains of spontaneous thoughts during resting state.
Mind wandering and variance in resting-state functional connectivity
While we were able to identify functional links related to participant-reported mind wandering behavior from the whole fMRI time series data by assuming temporal uniformity of functional connectivity, we found significant temporal differences in a number of links ( Fig. 4 ). Crucially, some of the decreasing links that were equivalently associated with multiple domains of spontaneous thoughts could only be identified from the 2 nd half of the time series data. This indicates that differences in functional connectivity for the identified links not only manifest between individuals with different levels of self-reported spontaneous thought across specific domains, but also manifest throughout the fMRI time course profile within an individual. In the current study, the fMRI time points were divided into two halves, which revealed an overall trend of decreased functional connectivity for the 11 domain-specific links from the 1 st to the 2 nd half. Future research might include recently developed fMRI protocols of higher temporal sampling and advanced analytic procedures 62 aimed at deconstructing time points into more non-overlapping segments, which may lead to more accurate assessment of dynamic changes in functional connectivity due to mind wandering.
As an exploratory analysis, we divided our dataset 2 (i.e., the one with the longest resting-state fMRI scan time of 520 sec: Supplementary Table S3 ) into four 130-sec segments for segment-specific connectivity measurement. Across the 11 domain-specific functional links of dataset 2, connectivity of the 1 st quarter of time series data was significantly more positive (or less negative) compared to the other 3 quarters ( p < 0.0001), and the connectivity was not significantly different among the 2 nd , 3 rd , and 4 th quarters ( Supplementary Fig. S1 ), suggesting that dynamic changes of functional connectivity in these links likely occurred between the 1 st and 2 nd quarters (i.e., centered around 130 sec after fMRI scans began). This observation could also explain why the maintenance of mind wandering was most pronounced in the 2 nd half of the scan because the 2 nd half of time series data for all 3 datasets began after 130 sec.
Overall, our findings associated with temporal differences in connectivity are consistent with the concept that non-static functional connectivity existed in a number of connections 23 , 24 , 25 , 27 . Further, our findings suggest that the temporal variations in functional connectivity are to some degree of neuronal origin and could be linked with the continuity of mind wandering. In addition, previous studies have found considerable between-subject variability in resting-state connectivity among healthy participants 63 , 64 . Our results suggest this between-subject variance in functional connectivity might be partially attributable to variations in spontaneous thought domain and the time spent in a specific spontaneous thought.
Limitations and Future Directions
Our findings provide a preliminary framework for characterizing the maintenance and cerebral representation of mind wandering during resting-state fMRI. These findings, although insightful and interesting, require replication in larger samples. Several limitations should be acknowledged while interpreting our results. First, the assessment of mind wandering did not account for when the reported mind wandering occurred in the course of the fMRI scan. Future studies that combine real-time resting-state fMRI and probe measures of mind wandering during fMRI scans will be needed to measure the association between transitions of functional connectivity (e.g., from more positive to more negative correlations) and the onset of mind wandering. Second, the mind wandering behavior was determined by self report after the fMRI scan; thus, it could potentially be subject to recall bias. Third, our current study focused on associations between functional connectivity and sustaining of mind wandering. Future investigations using non-invasive brain stimulation techniques (e.g., transcranial magnetic stimulation) to modulate brain activities involved in sustaining of mind wandering are warranted to establish causal relationships between functional connectivity and mind wandering 65 , 66 .
This study demonstrates that sustained mind wandering during resting-state fMRI is associated with decoupled or negative functional connectivity involving the caudate nucleus, insula, MPFC and other domain-specific brain regions. Our findings provide insights into mind wandering at the large-scale network level, and highlight the importance of including the estimated percentage of time and domain of spontaneous thoughts during resting-state fMRI into functional connectivity analyses. Future studies will need to account for these thought processes in analyses of fMRI signals during group comparisons and correlations between functional connectivity and behavioral measures. We expect that the power to detect group differences and to identify imaging-based biomarkers, especially for individual participants and clinical populations, can be enhanced by addressing the variability of mind wandering during resting-state fMRI.
Participants
The study’s participants included 72 right-handed healthy adults (mean age 55 ± 18 years, 37 males) from three unpublished datasets. Participants were excluded if they had a history of neurological or psychiatric disorder; unstable or untreated medical illness (including uncontrolled diabetes or treatment-resistant hypertension); any contraindication to MRI, such as claustrophobia or metallic implants; or raw score less than 27 on the Mini-Mental State Exam 67 . The institutional review board at Duke University Medical Center approved the study, and all data collection and analyses were carried out in accordance with the approved study protocol and the guidelines of the Helsinki Declaration. All of the participants provided written informed consent prior to participation.
Resting-state questionnaire
Each participant completed a post-resting-state-fMRI interview using the adapted ReSQ 13 to assess the content of spontaneous thoughts during the resting-state-fMRI scan. Participants were asked to estimate the proportion of time (on a 0–100% scale) spent during the resting-state-fMRI scan in each of the following five mental activities: AUDI/LANG, VIMG, SEN, MUS, and NUM. AUDI/LANG is defined as remembering/imagining words, sentences or conversations spoken by oneself or others, or talking to oneself with one’s own voice without overt production. VIMG refers to having thoughts in the form of visual images. These visual images could be associated with memory, ongoing learning, or planning of upcoming events. SEN is related to attention attracted by somatosensory sensations such as sensory information from the face or body, or position and movement of our body parts. MUS and NUM is defined as thinking related to music and numbers, respectively. For each participant, the data were log-transformed to normalize population variance. The questionnaires were completed within 30–60 minutes after the resting-state-fMRI scan.
Imaging protocols
Resting-state-fMRI was performed on a 3 Tesla GE scanner. Each participant was scanned on a single occasion, lying still with eyes fixated on a centrally located crosshair. Participants were scanned using three echo planar imaging (EPI) protocols summarized in Supplementary Table S3 . The potential influence of confounders (i.e., dataset and age) was controlled in the following analysis.
fMRI data analysis
The preprocessing of fMRI data was conducted through the Duke Brain Imaging and Analysis Center preprocessing pipelines based on the tools from the Oxford Centre for Functional MRI of the Brain’s Software Library (FSL version 5.0.1, www.fmrib.ox.ac.uk/fsl ) and locally developed Matlab codes (Mathworks, Natick, MA, USA). The first 4 volumes were discarded in order to reach the T1 steady state. The data were corrected for slice-timing differences and motion (six parameters: three translations and three rotations), and were registered to the Montreal Neurological Institute (MNI) 152 template using a 12 degrees of freedom affine transformation implemented in FSL’s Linear Image Registration Tool. All subsequent analyses were conducted in the MNI standard space. We regressed out the 6-parameter rigid body head motion (obtained from motion correction), the averaged time course profiles in the white matter, and the averaged time course profiles in the cerebrospinal fluid regions to reduce non-neuronal contributions to BOLD correlations 68 . We also removed constant offsets and linear drift. Time domain signals with their frequencies less than 0.08 Hz were retained.
A schematic diagram of our matrix-based functional connectivity analysis procedures is illustrated in Fig. 2 . For each participant, the preprocessed low-frequency fMRI data were parceled into a set of 90 brain regions using the AAL template 28 . Each participant’s BOLD time series was averaged within each brain region. We used Pearson correlation as the metric of association between the time series for each pair of the 90 brain regions. This resulted in a 90 × 90 correlation matrix with 4005 ([90 × 89]/2) unique inter-regional correlation coefficients ( r ). These inter-regional r values were transformed to the normal distribution by Fisher’s z transform for further statistical inference 69 . Because age varied widely across participants, we regressed out age-related signals of functional connectivity and then used the residuals for the following statistical analysis.
Statistical analyses
The first goal of the study was to identify functional connections that exhibited a significant group effect (i.e., higher percentage group vs. lower percentage group) on functional connectivity derived from the whole resting-state fMRI time series data (see Part I in Fig. 2 ). For each individual connection, we performed a multiple linear regression analysis to examine the group effects on functional connectivity. Each regression model included 5 independent factors (i.e., group effects of each thought domain), 1 covariate (i.e., dataset), and 1 dependent variable (i.e., functional connectivity value). The independent factor was coded as −1 (lower percentage group) and 1 (higher percentage group). The regression model was performed 4005 times, and multiple comparisons were corrected by an FDR of 0.05 29 .
The second goal of the study was to investigate whether there was an interaction effect between group and timing of the resting-state fMRI time series (see Part II in Fig. 2 ). First, we divided each participant’s time series data into halves (i.e., the 1 st half and the 2 nd half of the time series data). For each half, as for the whole time series data, the preprocessed fMRI data were parceled using the AAL template 28 , and 4005 ([90 × 89]/2) correlation coefficients were estimated for each half of the time series data of each participant. We then examined differences in functional connectivity between the 1 st half and the 2 nd half of the time series data by performing paired sample t tests on each connectivity value of the 4005 inter-regional functional links. The significance criterion was set at a p -value of 0.05 applying Bonferroni correction for multiple comparisons (i.e., alpha = 0.05/4005 ≈ 0.000012), minimizing false positives. The resultant networks were converted into 2 binary matrices (increasing and decreasing links) and used as inclusive masks in the subsequent analysis. Within each resultant set of links, the Group × Time interaction effects on functional connectivity were examined using multivariate multiple regression analysis. The multivariate multiple regression estimated a single regression model with more than one dependent variable (in a single step, where false positives associated with repeated assessments in conventional univariate multiple regression could be inherently eliminated). This approach allowed us to take into account the relationships among several dependent variables and conduct tests of the coefficients across different variables. The regression model included 5 independent factors (i.e., group effects of each thought domain), 1 repeated factor (i.e., time: 1 st half vs. 2 nd half of the time series data), 1 covariate (i.e., dataset), and functional connectivity values of a set of links (decreasing or increasing links) as dependent variables. The multivariate multiple regression analysis yielded two outputs: 1) results of multivariate analysis of variance that tested the overall group effects on functional connectivity across all dependent variables; and 2) results of univariate analysis that examined the group effect on functional connectivity of each individual dependent variable for each thought domain.
Additional Information
How to cite this article: Chou, Y.-h. et al . Maintenance and Representation of Mind Wandering during Resting-State fMRI. Sci. Rep. 7 , 40722; doi: 10.1038/srep40722 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Biswal, B. B. et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA 107 , 4734–4739, doi: 10.1073/pnas.0911855107 (2010).
Article ADS PubMed PubMed Central Google Scholar
Greicius, M. D., Krasnow, B., Reiss, A. L. & Menon, V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100 , 253–258 (2003).
Article ADS CAS PubMed Google Scholar
Raichle, M. E. et al. A default mode of brain function. Proc Natl Acad Sci USA 98 , 676–682, doi: 10.1073/pnas.98.2.676 (2001).
Article ADS CAS PubMed PubMed Central Google Scholar
Raichle, M. E. The restless brain: how intrinsic activity organizes brain function. Philos Trans R Soc Lond B Biol Sci 370 , doi: 10.1098/rstb.2014.0172 (2015).
Mazoyer, B. et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull 54 , 287–298 (2001).
Article CAS PubMed Google Scholar
Biswal, B., Yetkin, F. Z., Haughton, V. M. & Hyde, J. S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34 , 537–541 (1995).
Christoff, K., Gordon, A. M., Smallwood, J., Smith, R. & Schooler, J. W. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America 106 , 8719–8724, doi: 10.1073/pnas.0900234106 (2009).
Mason, M. F. et al. Wandering minds: the default network and stimulus-independent thought. Science 315 , 393–395, doi: 10.1126/science.1131295 (2007).
Stoffers, D. et al. Resting-State fMRI Functional Connectivity Is Associated with Sleepiness, Imagery, and Discontinuity of Mind. PLoS One 10 , e0142014, doi: 10.1371/journal.pone.0142014 (2015).
Article CAS PubMed PubMed Central Google Scholar
Gorgolewski, K. J. et al. A correspondence between individual differences in the brain’s intrinsic functional architecture and the content and form of self-generated thoughts. PLoS One 9 , e97176, doi: 10.1371/journal.pone.0097176 (2014).
Gorgolewski, K. J. et al. A high resolution 7-Tesla resting-state fMRI test-retest dataset with cognitive and physiological measures. Sci Data 2 , 140054, doi: 10.1038/sdata.2014.54 (2015).
Article PubMed PubMed Central Google Scholar
Schaefer, A. et al. Dynamic network participation of functional connectivity hubs assessed by resting-state fMRI. Front Hum Neurosci 8 , 195, doi: 10.3389/fnhum.2014.00195 (2014).
Delamillieure, P. et al. The resting state questionnaire: An introspective questionnaire for evaluation of inner experience during the conscious resting state. Brain Res Bull 81 , 565–573, doi: 10.1016/j.brainresbull.2009.11.014 (2010).
Article PubMed Google Scholar
Doucet, G. et al. Patterns of hemodynamic low-frequency oscillations in the brain are modulated by the nature of free thought during rest. Neuroimage 59 , 3194–3200, doi: 10.1016/j.neuroimage.2011.11.059 (2012).
Tusche, A., Smallwood, J., Bernhardt, B. C. & Singer, T. Classifying the wandering mind: revealing the affective content of thoughts during task-free rest periods. Neuroimage 97 , 107–116, doi: 10.1016/j.neuroimage.2014.03.076 (2014).
Smallwood, J. et al. Representing Representation: Integration between the Temporal Lobe and the Posterior Cingulate Influences the Content and Form of Spontaneous Thought. PLoS One 11 , e0152272, doi: 10.1371/journal.pone.0152272 (2016).
Hurlburt, R. T., Alderson-Day, B., Fernyhough, C. & Kuhn, S. What goes on in the resting-state? A qualitative glimpse into resting-state experience in the scanner. Front Psychol 6 , 1535, doi: 10.3389/fpsyg.2015.01535 (2015).
Kuhn, S., Fernyhough, C., Alderson-Day, B. & Hurlburt, R. T. Inner experience in the scanner: can high fidelity apprehensions of inner experience be integrated with fMRI? Front Psychol 5 , 1393, doi: 10.3389/fpsyg.2014.01393 (2014).
O’Callaghan, C., Shine, J. M., Lewis, S. J., Andrews-Hanna, J. R. & Irish, M. Shaped by our thoughts–a new task to assess spontaneous cognition and its associated neural correlates in the default network. Brain Cogn 93 , 1–10, doi: 10.1016/j.bandc.2014.11.001 (2015).
Van Calster, L., D’Argembeau, A., Salmon, E., Peters, F. & Majerus, S. Fluctuations of Attentional Networks and Default Mode Network during the Resting State Reflect Variations in Cognitive States: Evidence from a Novel Resting-state Experience Sampling Method. J Cogn Neurosci , 1–19, doi: 10.1162/jocn_a_01025 (2016).
Fox, K. C., Spreng, R. N., Ellamil, M., Andrews-Hanna, J. R. & Christoff, K. The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage 111 , 611–621, doi: 10.1016/j.neuroimage.2015.02.039 (2015).
Smallwood, J. et al. The default modes of reading: modulation of posterior cingulate and medial prefrontal cortex connectivity associated with comprehension and task focus while reading. Front Hum Neurosci 7 , 734, doi: 10.3389/fnhum.2013.00734 (2013).
Chang, C. & Glover, G. H. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage 50 , 81–98, doi: 10.1016/j.neuroimage.2009.12.011 (2010).
Allen, E. A. et al. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 24 , 663–676, doi: 10.1093/cercor/bhs352 (2014).
Keilholz, S. D. The neural basis of time-varying resting-state functional connectivity. Brain Connect 4 , 769–779, doi: 10.1089/brain.2014.0250 (2014).
Kucyi, A. & Davis, K. D. Dynamic functional connectivity of the default mode network tracks daydreaming. Neuroimage 100 , 471–480, doi: 10.1016/j.neuroimage.2014.06.044 (2014).
Hutchison, R. M. et al. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage 80 , 360–378, doi: 10.1016/j.neuroimage.2013.05.079 (2013).
Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15 , 273–289 (2002).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Roy Stat Soc B Met 57 , 289–300 (1995).
MathSciNet MATH Google Scholar
Tang, Y. Y., Rothbart, M. K. & Posner, M. I. Neural correlates of establishing, maintaining, and switching brain states. Trends in cognitive sciences 16 , 330–337, doi: 10.1016/j.tics.2012.05.001 (2012).
Hoover, W. B. & Vertes, R. P. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 212 , 149–179, doi: 10.1007/s00429-007-0150-4 (2007).
Barbas, H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull 52 , 319–330 (2000).
Schooler, J. W. et al. Meta-awareness, perceptual decoupling and the wandering mind. Trends in cognitive sciences 15 , 319–326, doi: 10.1016/j.tics.2011.05.006 (2011).
Smallwood, J. et al. Pupillometric evidence for the decoupling of attention from perceptual input during offline thought. PLoS One 6 , e18298, doi: 10.1371/journal.pone.0018298 (2011).
Smallwood, J. Distinguishing how from why the mind wanders: a process-occurrence framework for self-generated mental activity. Psychol Bull 139 , 519–535, doi: 10.1037/a0030010 (2013).
Smallwood, J. & Schooler, J. W. The science of mind wandering: empirically navigating the stream of consciousness. Annu Rev Psychol 66 , 487–518, doi: 10.1146/annurev-psych-010814-015331 (2015).
Barron, E., Riby, L. M., Greer, J. & Smallwood, J. Absorbed in thought: the effect of mind wandering on the processing of relevant and irrelevant events. Psychol Sci 22 , 596–601, doi: 10.1177/0956797611404083 (2011).
Kam, J. W. et al. Slow fluctuations in attentional control of sensory cortex. J Cogn Neurosci 23 , 460–470, doi: 10.1162/jocn.2010.21443 (2011).
Baird, B., Smallwood, J., Lutz, A. & Schooler, J. W. The decoupled mind: mind-wandering disrupts cortical phase-locking to perceptual events. J Cogn Neurosci 26 , 2596–2607, doi: 10.1162/jocn_a_00656 (2014).
Christoff, K. Undirected thought: neural determinants and correlates. Brain Res 1428 , 51–59, doi: 10.1016/j.brainres.2011.09.060 (2012).
Fox, M. D. et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102 , 9673–9678 (2005).
Gopinath, K., Krishnamurthy, V., Cabanban, R. & Crosson, B. A. Hubs of Anticorrelation in High-Resolution Resting-State Functional Connectivity Network Architecture. Brain Connect 5 , 267–275, doi: 10.1089/brain.2014.0323 (2015).
Yatsenko, D. et al. Improved estimation and interpretation of correlations in neural circuits. PLoS Comput Biol 11 , e1004083, doi: 10.1371/journal.pcbi.1004083 (2015).
Yu, C. et al. Functional segregation of the human cingulate cortex is confirmed by functional connectivity based neuroanatomical parcellation. Neuroimage 54 , 2571–2581, doi: 10.1016/j.neuroimage.2010.11.018 (2011).
Murphy, K., Birn, R. M., Handwerker, D. A., Jones, T. B. & Bandettini, P. A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 44 , 893–905, doi: 10.1016/j.neuroimage.2008.09.036 (2009).
Weissenbacher, A. et al. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage 47 , 1408–1416, doi: 10.1016/j.neuroimage.2009.05.005 (2009).
Chen, G., Chen, G., Xie, C. & Li, S. J. Negative functional connectivity and its dependence on the shortest path length of positive network in the resting-state human brain. Brain Connect 1 , 195–206, doi: 10.1089/brain.2011.0025 (2011).
Fransson, P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp 26 , 15–29, doi: 10.1002/hbm.20113 (2005).
Uddin, L. Q., Kelly, A. M., Biswal, B., Castellanos, F. X. & Milham, M. P. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum Brain Mapp 30 , 625–637 (2009).
Chang, C. & Glover, G. H. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage 47 , 1448–1459, doi: 10.1016/j.neuroimage.2009.05.012 (2009).
Fudge, J. L., Breitbart, M. A., Danish, M. & Pannoni, V. Insular and gustatory inputs to the caudal ventral striatum in primates. J Comp Neurol 490 , 101–118, doi: 10.1002/cne.20660 (2005).
Chikama, M., McFarland, N. R., Amaral, D. G. & Haber, S. N. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci 17 , 9686–9705 (1997).
Flynn, F. G. Anatomy of the insula functional and clinical correlates. Aphasiology 13 , 55–78 (1999).
Article Google Scholar
Christopher, L., Koshimori, Y., Lang, A. E., Criaud, M. & Strafella, A. P. Uncovering the role of the insula in non-motor symptoms of Parkinson’s disease. Brain 137 , 2143–2154, doi: 10.1093/brain/awu084 (2014).
Craig, A. D. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci 10 , 59–70, doi: 10.1038/nrn2555 (2009).
Price, C. J. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci 1191 , 62–88, doi: 10.1111/j.1749-6632.2010.05444.x (2010).
Article ADS PubMed Google Scholar
D’Argembeau, A. On the role of the ventromedial prefrontal cortex in self-processing: the valuation hypothesis. Front Hum Neurosci 7 , 372, doi: 10.3389/fnhum.2013.00372 (2013).
Denny, B. T., Kober, H., Wager, T. D. & Ochsner, K. N. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci 24 , 1742–1752, doi: 10.1162/jocn_a_00233 (2012).
Gusnard, D. A., Akbudak, E., Shulman, G. L. & Raichle, M. E. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America 98 , 4259–4264, doi: 10.1073/pnas.071043098 (2001).
Northoff, G. et al. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage 31 , 440–457, doi: 10.1016/j.neuroimage.2005.12.002 (2006).
Turk, D. J., Heatherton, T. F., Macrae, C. N., Kelley, W. M. & Gazzaniga, M. S. Out of contact, out of mind: the distributed nature of the self. Ann N Y Acad Sci 1001 , 65–78 (2003).
Lee, H. L., Zahneisen, B., Hugger, T., LeVan, P. & Hennig, J. Tracking dynamic resting-state networks at higher frequencies using MR-encephalography. Neuroimage 65 , 216–222, doi: 10.1016/j.neuroimage.2012.10.015 (2013).
Chou, Y. H., Panych, L. P., Dickey, C. C., Petrella, J. R. & Chen, N. K. Investigation of long-term reproducibility of intrinsic connectivity network mapping: a resting-state FMRI study. Am J Neuroradiol 33 , 833–838, doi: 10.3174/ajnr.A2894 (2012).
Mueller, S. et al. Individual variability in functional connectivity architecture of the human brain. Neuron 77 , 586–595, doi: S0896-6273(13)00004-4 [pii]10.1016/j.neuron.2012.12.028 (2013).
Axelrod, V., Rees, G., Lavidor, M. & Bar, M. Increasing propensity to mind-wander with transcranial direct current stimulation. Proceedings of the National Academy of Sciences of the United States of America 112 , 3314–3319, doi: 10.1073/pnas.1421435112 (2015).
Kajimura, S., Kochiyama, T., Nakai, R., Abe, N. & Nomura, M. Causal relationship between effective connectivity within the default mode network and mind-wandering regulation and facilitation. Neuroimage 133 , 21–30, doi: 10.1016/j.neuroimage.2016.03.009 (2016).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12 , 189–198 (1975).
Van Dijk, K. R. et al. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 103 , 297–321, doi: 10.1152/jn.00783.2009 (2010).
Fisher, R. A. On the probable error of a coefficient of correlation deduced from a small sample. Metron 1 , 3–32 (1921).
Google Scholar
Xia, M., Wang, J. & He, Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One 8 , e68910, doi: 10.1371/journal.pone.0068910 (2013).
Download references
Acknowledgements
This research was supported by NIH grants R01 NS074045 (NkC), R01 MH098301 (DJM and LW), R01AG043438 (HEW), and R01 AG039684 (DJM).
Author information
Authors and affiliations.
Department of Psychology, University of Arizona, Tucson, AZ, USA
Ying-hui Chou & Mark Sundman
Cognitive Science Program, University of Arizona, Tucson, AZ, USA
Ying-hui Chou
Arizona Center on Aging, University of Arizona, , Tucson, AZ, USA
Ying-hui Chou & Nan-kuei Chen
Department of Medicine and Ophthalmology, Duke University Medical Center, Durham, NC, USA
Heather E. Whitson
Geriatrics Research Education and Clinical Center, Durham Veterans Administration Hospital, Durham, NC, USA
Institute of Imaging Science, Vanderbilt University, Nashville, TN, USA
Brain Imaging and Analysis Center, Duke University Medical Center, Durham, NC, USA
Mei-Lan Chu, David J. Madden, Lihong Wang, Imke Kirste, Allen W. Song & Nan-kuei Chen
Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Durham, NC, USA
Carol P. Weingarten & David J. Madden
Department of Psychiatry, University of Connecticut Health Center, Farmington, CT, USA
Lihong Wang
Neuroimaging Group (GIN), UMR5293, CEA CNRS Université de Bordeaux, Bordeaux, CEDEX, France
Marc Joliot
Department of Psychology, Penn State University, University Park, PA, USA
Michele T. Diaz
Department of Biostatistics and Bioinformatics, Duke University Medical Center, Durham, NC, USA
Department of Radiology, Duke University Medical Center, Durham, NC, USA
Allen W. Song & Nan-kuei Chen
Department of Biomedical Engineering, University of Arizona, Tucson, AZ, USA
Nan-kuei Chen
Department of Medical Imaging, University of Arizona, Tucson, AZ, USA
You can also search for this author in PubMed Google Scholar
Contributions
Study concept and design: Y.C., N.C., and P.G. Acquisition, analysis, or interpretation of data: Y.C., M.S., H.W., P.G., M.C., C.W., D.M., L.W., I.K., M.J., M.D., Y.L., A.S., and N.C. Drafting of the manuscript: Y.C. and N.C. Critical revision of the manuscript for important intellectual content: Y.C., M.S., H.W., P.G., M.C., C.W., D.M., L.W., I.K., M.J., M.D., Y.L., A.S., and N.C. Statistical analysis: Y.C., M.S., Y.L. and H.W. Obtained funding: N.C., D.M., L.W., A.S., and H.W. Administrative, technical, or material support: N.C. and M.S. Study supervision: N.C., D.M., and L.W.
Corresponding author
Correspondence to Nan-kuei Chen .
Ethics declarations
Competing interests.
The authors declare no competing financial interests.
Supplementary information
Supplementary information (pdf 334 kb), rights and permissions.
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Reprints and permissions
About this article
Cite this article.
Chou, Yh., Sundman, M., Whitson, H. et al. Maintenance and Representation of Mind Wandering during Resting-State fMRI. Sci Rep 7 , 40722 (2017). https://doi.org/10.1038/srep40722
Download citation
Received : 13 July 2016
Accepted : 09 December 2016
Published : 12 January 2017
DOI : https://doi.org/10.1038/srep40722
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Mind wandering and task-focused attention: erp correlates.
- Óscar F. Gonçalves
- Gabriel Rêgo
- Paulo S. Boggio
Scientific Reports (2018)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
NBA players turn to meditation to help find their center
NEW YORK — In the minutes before nearly every New York Knicks game this season, as the arena crescendos into a pulsing hive of activity — music blasting, players warming up, fans filing in, vendors hawking overpriced beer — Isaiah Hartenstein creates his own cocoon of calm.
The 7-foot center plops onto the bench, closes his eyes and focuses on his breathing.
“When you’re present, that’s when you play your best,” he said.
That focus, Hartenstein said, has helped him transform from a role player into a starter for a 50-win Knicks team that earned the No. 2 seed in the Eastern Conference playoffs — and it will probably earn him a significant raise on the free agent market this summer. He averaged 7.8 points and 8.3 rebounds during the regular season and anchored the NBA’s ninth-ranked defense.
Hartenstein is part of a growing number of NBA players who embrace meditation, which was first popularized in the league by Hall of Fame coach Phil Jackson. The “Zen Master” led the Chicago Bulls and Los Angeles Lakers to 11 NBA championships between 1991 and 2010 while leading regular team meditation and yoga sessions and emphasizing the importance of mindfulness.
These days, players who regularly practice meditation can be found in every corner of the league, from stars such as Lakers forward LeBron James and Denver Nuggets guard Jamal Murray to role players such as Phoenix Suns forward Royce O’Neale and Sacramento Kings two-way guard Mason Jones.
In a sport that glorifies workaholism, in which success is often measured in pools of sweat, a player taking a moment to step back and focus on his breathing might seem odd. But for some, bringing mindfulness to the court might make the difference between a good game and a bad game.
And as the NBA playoffs ramp up, the spotlight on players’ performance will get even brighter — underscoring the importance of finding peace amid pressure.
“I started to notice that a lot of things in sports is actually a lot more mental than physical,” Hartenstein said. “I noticed that meditation helped me be more present through day-to-day life, but also more present during the game.”
Hartenstein began meditating during the 2020-21 season when he was with the Nuggets after reading a pair of books: “Mind Gym” by Gary Mack and “The Mindful Athlete” by George Mumford, the sports psychologist who worked with Jackson’s Bulls and Lakers teams. These days, in addition to his pregame routine, Hartenstein practices meditation for about 15 minutes in the morning and 30 minutes at night with the app Headspace. Sometimes, if he notices his mind spiraling while on the court, he’ll take a few meditative breaths during a break in the action — without closing his eyes.
“You probably won’t be able to see it unless you really pay attention,” said Hartenstein, who has helped the Knicks take a 2-0 series lead over the Philadelphia 76ers in their first-round playoff matchup. “I’ll do one long breath in, and then at the very end you do another breath, and then you let it out. I’ll do that one to three times. That helps me re-center.”
Jones, who split time this season between the Kings’ main roster and the Stockton Kings, the franchise’s G League affiliate, also turned to mindfulness to stay grounded while on the court — and to find calm amid shuffling between the two teams.
Before every game this season, whether with Sacramento or Stockton, Jones would find a quiet room, sit in a chair and, for 10 minutes, inhale and exhale deeply. He practiced mindfulness during games, too: In the third quarter of Stockton’s G League playoff game against the Santa Cruz Warriors this month, Jones felt his mind wandering. During a timeout, he closed his eyes and took five deep breaths. Then, with 11 seconds left in the fourth quarter, he beat the shot-clock buzzer with a three-pointer that gave the Kings the lead for good.
“Before you get angry, breathe. Before you make any decision, breathe. Close your eyes and breathe,” Jones said of the mind-set that helped him land back in the NBA in February after a two-year stint overseas.
O’Neale, who averaged 25.1 minutes for the Suns after a midseason trade from Brooklyn, also has a routine: He meditates the night before games, and then in the locker room about a half-hour before the opening tip. He lies on the locker room floor, cues up a “spa music” playlist and spends five minutes taking deep breaths. It helps him block out noise from the crowd once play starts.
“It’s like they’re not even there,” O’Neale said.
The conversation around mental health in the NBA has shifted in the past decade, with several teams now employing mental performance coaches. Erwin Valencia, who served with the Knicks in that capacity, helped bridge the gap from Jackson’s days to the modern era.
In 2014, Jackson was hired to be the Knicks’ team president. The following year, Valencia, then the team’s director of training and conditioning, suggested to Jackson that the Knicks integrate meditation, Valencia said. He had grown up idolizing Jackson and described their relationship as similar to “Luke Skywalker and Yoda.”
“We started with him doing these meditations whenever he was in New York,” Valencia said of Jackson. “It ruffled a little bit of the players’ feathers because he would do it at odd times. … He would show up and say, ‘This is the day we do meditations.’ And players were coming out of practice, completely sweaty. They were like, ‘What are we doing?’ Some of the players who were younger were like, ‘What does this old guy want us to do?’ ”
Valencia devised a plan to make meditation more accessible for players from that generation who were glued to their phones. He arranged for the team to have free access to Headspace, which launched in 2012 and had yet to erupt in popularity.
Jackson and the Knicks parted ways in June 2017, but Valencia continued to help players embrace mindfulness. The rest of the league started to catch on, too: Valencia’s relationship with Headspace’s founders paved the way for the app to build a relationship with the NBA, and in 2018, the league reached a partnership with the app: All NBA players and employees were granted access to Headspace, and the league produced guided training videos for the app.
In the years that followed, Valencia led the Knicks in breathing sessions before practice and games. Some players rolled their eyes — including veteran forward Julius Randle, Valencia said. Randle won the league’s most improved player award in 2020-21, but after his play dipped the following season, he approached Valencia about integrating meditation into his daily routine.
Early in the 2022-23 season, MSG Network showed footage of Valencia guiding Randle in a pregame meditation from the Knicks’ bench in the minutes before a game at Madison Square Garden. Their routine, which started with Randle rubbing an essential oil blend on his wrist, continued throughout the season, and Randle excelled: He averaged 25.1 points and 10 rebounds and was named third-team all-NBA.
Valencia also guided Hartenstein and former Knicks forward Obi Toppin in pregame meditations, he said. He left the Knicks after last season, but his influence remains: In addition to Hartenstein, Randle continued his pregame meditations before he suffered a season-ending shoulder injury in January.
“When you’re an athlete, you need to train yourself to find stillness in the middle of madness,” Valencia said. “If you’re so used to practicing a meditative practice, or a mindfulness practice, in the middle of silence all the time, then the moment you get out, you get overwhelmed, and especially in the Garden.
“Having a consistent practice in a space of madness, along with having a practice in a space of peace, allows you to find that peace within the madness.”
Before Game 2 against the 76ers on Tuesday, Hartenstein sat quietly on the bench, his eyes closed as his teammates shot jumpers. About three hours later, with New York trailing in the final minute, he jumped past multiple defenders and grabbed the offensive rebound that led to guard Donte DiVincenzo’s go-ahead three-pointer with 13 seconds remaining. On the next possession, he blocked Philadelphia guard Tyrese Maxey’s layup attempt, sending the Garden into pandemonium and preserving what would become a 104-101 Knicks win.
Hartenstein pumped his fist and roared. His eyes were wide open.
- NBA players turn to meditation to help find their center Earlier today NBA players turn to meditation to help find their center Earlier today
- Clippers star Kawhi Leonard is back from injury. Now comes the hard part. April 24, 2024 Clippers star Kawhi Leonard is back from injury. Now comes the hard part. April 24, 2024
- NBA admits refs missed calls late in Knicks’ wild Game 2 win vs. 76ers April 23, 2024 NBA admits refs missed calls late in Knicks’ wild Game 2 win vs. 76ers April 23, 2024

- Reference Manager
- Simple TEXT file
People also looked at
Review article, towards a neuroscience of mind-wandering.
- 1 Functional Brain Center, Wohl Institute for Advanced Imaging, Tel-Aviv Sourasky Medical Center, Tel-Aviv, Israel
- 2 Department of Psychology, Tel-Aviv University, Tel-Aviv, Israel
- 3 Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel
- 4 The Emotion-Cognition Research Center, Shalvata Mental Health Center, Hod-Hasharon, Israel
- 5 Department of Neurobiology, Weizmann Institute of Science, Rehovot, Israel
Mind-wandering (MW) is among the most robust and permanent expressions of human conscious awareness, classically regarded by philosophers, clinicians, and scientists as a core element of an intact sense of self. Nevertheless, the scientific exploration of MW poses unique challenges; MW is by nature a spontaneous, off task, internal mental process which is often unaware and usually difficult to control, document or replicate. Consequently, there is a lack of accepted modus operandi for exploring MW in a laboratory setup, leading to a relatively small amount of studies regarding the neural basis of MW. In order to facilitate scientific examination of MW the current review categorizes recent literature into five suggested strategies. Each strategy represents a different methodology of MW research within functional neuroimaging paradigms. Particular attention is paid to resting-state brain activity and to the “default-mode” network. Since the default network is known to exert high activity levels during off-task conditions, it stands out as a compelling candidate for a neuro-biological account of mind-wandering, in itself a rest-based phenomenon. By summarizing the results within and across strategies we suggest further insights into the neural basis and adaptive value of MW, a truly intriguing and unique human experience.
“Thoughts meander like a restless wind inside a letter box they tumble blindly as they make their way across the universe” John Lennon
Introduction
Mind-wandering (MW) refers to ongoing mentation which occurs spontaneously, and largely autonomously, whenever an awake individual is not engaged in a cognitively demanding task. Alternative names to the term “MW” ( Smallwood and Schooler, 2006 ; Mason et al., 2007 ) in past and recent literature include “day dreaming” ( Giambra, 1979 ), “task-unrelated images and thought” ( Giambra and Grodsky, 1989 ), “stimulus independent thought” ( Teasdale et al., 1995 ), “task-unrelated thought” ( Smallwood et al., 2003 ), “incidental self-processing” ( Gilbert et al., 2005 ), “inner speech” ( Morin, 2009 ), and “spontaneous thought” ( Christoff et al., 2008 ).
Conceptualized as a core element of what William James defined as the “stream of consciousness” ( James, 1892 ), MW, in various names and forms, has gained considerable attention in ancient and modern philosophy and in theoretical psychology. The robust, autonomous, and continual nature of this psychological process has led writers to suggest that rather than being an undesired lapse of attention to the external world (William James remarked, when he was accused of being absent-minded, that he was really just present-minded to his own thoughts; Barzun, 1983 ), MW must have an important adaptive value for healthy cognition ( Christoff et al., 2008 ; Baars, 2010 ). Yet much like the neural basis of MW, its adaptivity and the nature of its interaction with other cognitive processes remain a scientific blind spot.
In the relatively short history of cognitive neuroscience, which has inherited much of its models, paradigms, and findings from behavioral and cognitive psychology, MW is virtually absent ( Smallwood and Schooler, 2006 ) as a subject of research. The reluctance in the scientific arena to study MW can be accounted for by its non-behavioral characteristics when compared to more conventionally studied mental functions: MW occurs in the absence of any external cue; it is often unintended and even unaware; it takes its own course – probably driven by internally generated cues; and it is hard to trace back, replicate or report. However, a recent paradigm shift in functional neuroimaging holds a great promise for the development and establishment of MW research. The discovery of the “default-mode network” (DMN; Raichle et al., 2001 ) and the following realization of the significance of spontaneous resting-state neural activity ( Raichle, 2009 ) dramatically launched a prosperous path in the scientific exploration of MW.
Default-mode network relates to a functionally meaningful neural network, which includes the medial prefrontal cortex (MPFC), the precuneus, the posterior cingulate cortex, and the inferior parietal and lateral temporal cortices (Figure 1 ). In comparison to other functional neural networks, DMN has unique patterns of activity ( Gusnard et al., 2001 ; Raichle et al., 2001 ): both in terms of energy consumption and in terms of the blood oxygen-level dependent (BOLD) signal, activation levels in this network were shown to descend below baseline during cognitively demanding tasks. Moreover, this network shows high activation levels at rest compared to task. These activation patterns and their possible functional meaning have received considerable attention in recent years, using independent as well as combined neuroimaging techniques (e.g., Ben-Simon et al., 2008 ). Studies with clinical populations shed additional light on the critical functionality of the DMN by demonstrating that malfunctioning of the DMN is associated with several neurological, psychiatric, and psychological pathologies (for a review see Buckner et al., 2008 ).
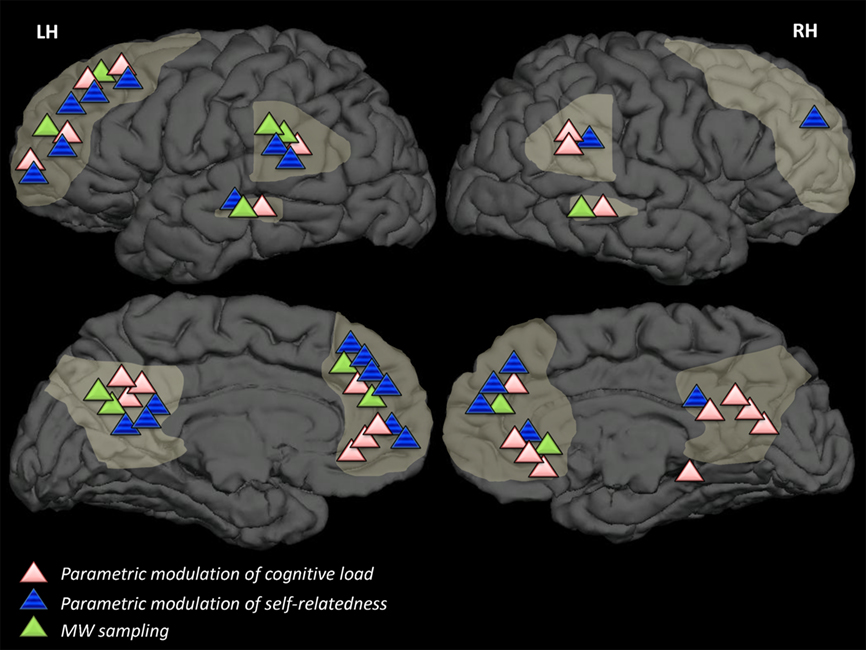
Figure 1. Results of overviewed studies in relation to DMN regions . DMN-related results of studies overviewed in this review, categorized by strategy, superimposed on a template brain. Light-grey markings denote DMN areas (in accordance with Buckner et al., 2008 ) dorsal and ventral medial prefrontal cortex, lateral temporal cortices, precuneus, and posterior cingulate cortex. Strategies which have not been employed in neuroimaging studies (strategy A2) or strategies which are concerned with degree of connectivity rather than degree of activation in brain areas (strategy B3) are not represented in this figure.
Since the first reports describing it, DMN rest-related activity had been suggested to comprise a neural correlate of MW ( Gusnard et al., 2001 ). This proposition was based on two main features of the DMN: First of all, like MW, DMN activity occurs during rest and shows a reverse correlation with cognitive load ( Mason et al., 2007 ). Secondly, task-related activations in the medial prefrontal and parietal areas, which comprise substantial elements of the DMN, have been shown to occur during self-related tasks ( Northoff and Bermpohl, 2004 ; Spreng et al., 2009 ). This has led writers to suggest that rest-related activations in these areas might subserve MW, in itself a process of self-related mentation ( Baars, 2010 ).
With the exceedingly growing body of information on neural activity in the wakeful, resting state, the shortage in accepted modus operandi regarding the scientific examination of MW has become a bottle neck, restraining further examination of the functionality of the DMN on the one hand and of the neural basis of MW on the other. However, several pioneering attempts have been made to study the relation between DMN activity and MW, yielding striking results. Converging the solutions to the challenge of quantifying and scientifically studying MW presented in these studies portrays an array of potential strategies to address the question of a DMN–MW association.
The current review aims to facilitate the scientific exploration of the neural correlates of MW by overviewing existing literature and defining, respectively, five methodological strategies for studying MW within a functional neuroimaging paradigm. Two of these strategies include direct measurements of MW (strategies A1 and A2), whether in real time – during rest or task performance, or retrospectively. Three additional strategies (Strategies B1, B2, and B3) rely on theoretical assumptions regarding MW and self related or cognitive functioning, as well as on the known functionality of networks emerging from connectivity analysis performed on data acquired during the resting state. Through the prism of these five strategies, we review existing literature and findings regarding MW published mainly in the recent decade. Each strategy will be presented in light of its advantages and disadvantages as well as the degree of its fitting to various paradigms and data analysis techniques in experimental neuroimaging.
Strategies for Studying the Neural Correlates of Mind-Wandering
The current section overviews methodologies and results from a representative sample of a decade of literature, mainly functional neuroimaging (PET or fMRI) studies, regarding the relation between DMN activation patterns and MW. The inclusion criterion for studies in this overview was that they bring forward the question of the relation between rest-related DMN activity and rest-related phenomenological experience. Importantly, studies of self-related functions were only included if they state a specific hypothesis regarding rest-related neural and psychological functioning. Table 1 lists the studies presented in this review, categorized by strategy. A visual illustration of the results obtained by these studies is presented in Figure 1 .
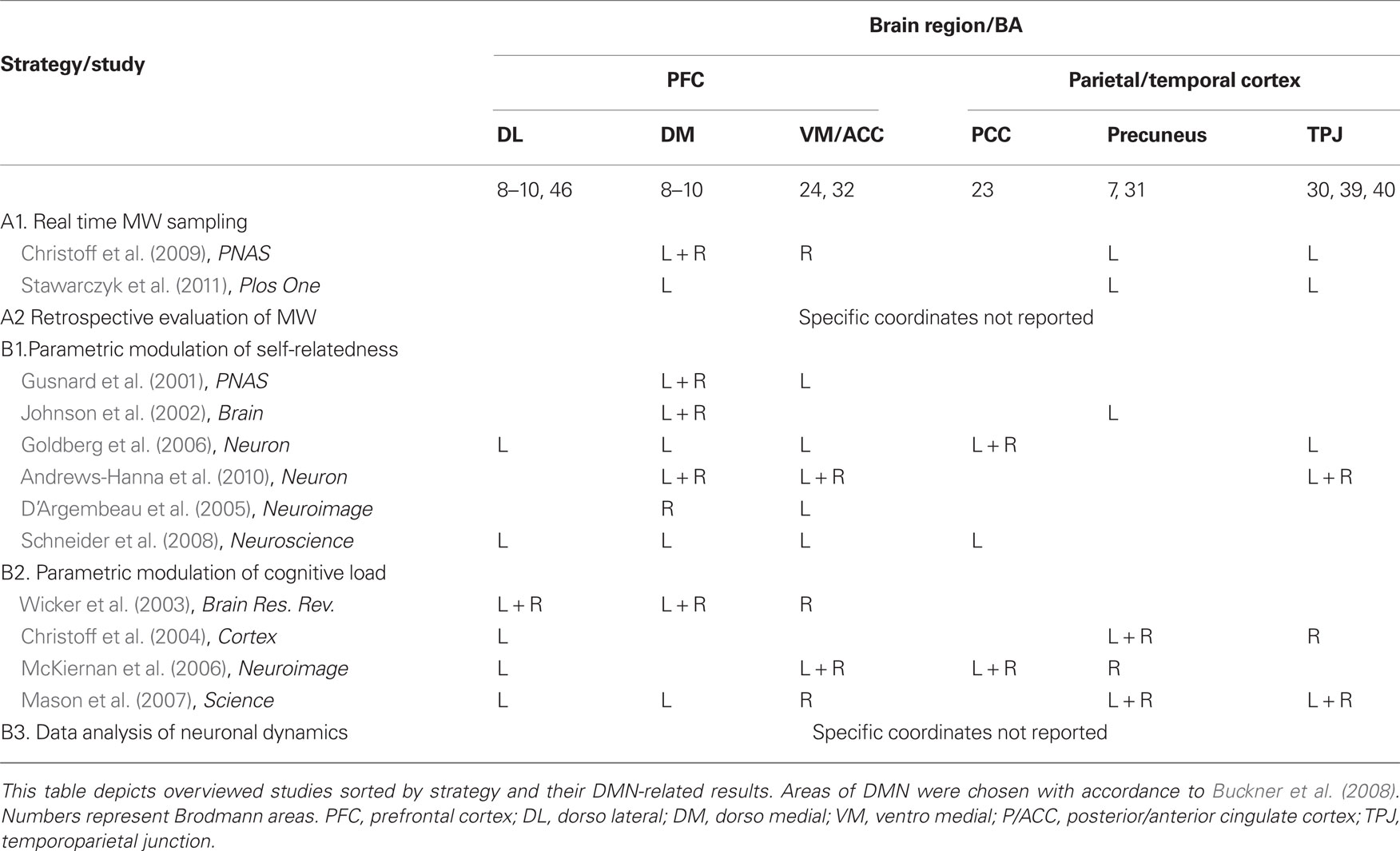
Table 1. A summary of overviewed studies and their DMN-related results.
The below differentiation between indirect and direct strategies could also be discussed in terms of determining the dependent and the independent variables within a functional neuroimaging setup: in the case of the indirect strategies, cognitive load or self-relatedness are being experimentally manipulated (i.e., independent variable) and are expected to cause a change in the measured neural signal of the DMN (i.e., dependent variable); in the case of the direct strategies, the degree of DMN activation is manipulated (i.e., independent variable) by altering rest and task while scanning, and the consequent change in the degree of MW is being assessed following scanning (i.e., dependent variable). Table A2 in Appendix summarizes typical dependent and independent variables according to each strategy.
Direct Strategies for Measuring Mind-Wandering
Strategies for directly quantifying the degree of MW represent a straightforward attempt to overcome its non-explicit nature, and essentially make conventional experimental methods applicable for studying it. For example, one can use the obtained degree of reported MW to categorize sessions or subjects into groups before analyzing, or to correlate it with the degree of activation in selected brain regions of interest or in the whole brain. The greatest challenge, however, is that in contrast to most behavioral measurements, the actual tracking of MW, or even its mere verbalizing in real time, tampers with its very occurrence ( Filler and Giambra, 1973 ): an individual busy with reporting her own MW is less free to engage in spontaneous MW comparing to when left to rest quietly. This can probably account for the relatively few studies which have attempted to directly quantify MW in the history of cognitive neuroscience, and possibly, for the even fewer methods developed to do so. Several quantifications techniques have nonetheless, been employed, some attempting at real-time assessment of the degree of MW while others focusing on post hoc questioning of subjects.
Strategy A1: real-time MW sampling
Mind-wandering can occur with or without awareness of its occurrence (“meta-awareness”; Christoff et al., 2009 ). Nevertheless, one can normally report if a thought was occurring in their mind or not, if interrupted and asked to do so at a given time point. This is the rational underlying the MW sampling (also known as “thought sampling” or “thought probing”) technique ( McKiernan et al., 2006 ; Mason et al., 2007 ; Christoff et al., 2009 ; Stawarczyk et al., 2011 ). Several approaches have been introduced for MW sampling in the neuroimaging set up, but a typical one uses a probing tone in even or uneven intervals, during either a rest or a task scan; subjects are instructed to indicate whether they were experiencing a spontaneous thought (i.e., unrelated to task performance) at the time the tone was presented (or, in a similar version, since the previous probe; Giambra, 1995 ). In block-design neuroimaging studies, each scan session is then scored according to the rate of “yes” answers given in it out of the overall number of tones presented in the session. The degree of MW occurrence is found to correlate with degree of neural activity in the DMN, as illustrated in Figure 1 . In ERP studies, EEG recordings adjacent to the pressings are analyzed separately for “on-task” vs. “off-task” reports. Using this method, Kam et al. (2011) demonstrated that the P1 component to a visual or auditory probe was reduced during off task, implying a reduction in sensory level processing during MW.
An interesting body of research based on this strategy examines the link between MW (referred to as “task-unrelated thought”) and errors during task performance ( Smallwood et al., 2003 , 2008 ). These studies demonstrate that when MW intrudes during task performance, and attentional lapses occur, task performance is impaired. Based on this line of research it may be suggested that MW competes with task performance on a limited capacity of attentional resources, in effect representing a state of “perceptual decoupling” ( Smallwood et al., 2011a ). This corresponds well with the idea discussed later on in this paper on the reverse correlation between MW and executive networks in the brain.
In yet another, less common, version of MW sampling, subjects are requested to press a button each time a thought comes into mind ( Giambra, 1989 ). Using this type of report, Braboszcz and Delorme (2010) asked subjects to press a button as soon as they realized their mind was wandering during a task of counting breaths. These presses were later used as an ERP analysis, showing reduced P200 responses to auditory stimuli, and reduced ability to identify the oddball auditory stimuli (smaller N100 during MW). In addition, frequency analysis showed that MW was associated with higher delta and theta power and lower alpha and beta power compared to task performance. Despite the suitability of this method for ERP studies, as reported by the authors themselves, this version seems to be less favorable and can hardly be found in neuroimaging studies, probably because it imposes greater meta-awareness and concentration from subjects and thus interferes with the natural occurrence of MW.
The strategy of MW sampling presents a clear advantage of being a real time, direct and quantified measurement of MW occurrence. One should bear in mind, though, that to the best of our knowledge it has never been systemically tested for validity and reliability, and thus it is mainly justified by its straight-forwardness and intuitiveness.
Strategy A2: retrospective evaluation of MW
Mind-wandering requires peace of mind; disturbances tend to interrupt its natural flow. In other words, an informative report regarding MW at a given time period, without interfering with its occurrence, may better be collected retrospectively, after a session has ended (notably, even then, the contents of MW is not always straightforwardly accessible to memory). Surprisingly, though, designated structured psychological questionnaires for explicitly assessing MW in healthy individuals are scarce. The very few examples which can be found in the literature ( Giambra, 1979 ; Klinger and Cox, 1987 ; Matthews et al., 1999 ) did not seem to survive the transition from psychological behavioral research to neuroscience. Consequently, and unfortunately, there is no accumulated body of literature regarding the neural basis of MW, and virtually no experience in the field obtained by retrospective questioning of MW using validated experimental instruments designated for this matter.
One inspiring study which could be considered an example for this approach is a PET study by D’Argembeau et al. (2005) . In this study subjects had to rate the total amount of thoughts experienced, whatever their content, using an in-house developed questionnaire immediately following scanning (a similar approach is found earlier in Mcguire et al. 1996 ). An alternative to using in-house developed questionnaires is to use established questionnaires of experiences which according to theoretical and clinical literature are related to MW. In such a study ( Gruberger et al., 2008 ), questionnaires for measuring dependent self awareness and degree of dissociation were applied to assess the degree of interference in MW during rest. The underlying hypothesis was that artificial interference with the normal process of MW will manifest itself as disruption in self awareness and as a sense of dissociation, which indeed was corroborated by the results. A third noteworthy example is the Resting State Questionnaire (ReSQ) published recently by Delamillieure et al. (2010) explicitly for usage in a functional neuroimaging setup. The ReSQ consists of 62 items organized by five main types of mental activity: visual mental imagery, inner language, somatosensory awareness, inner musical experience, and mental manipulation of numbers. Using a 0–100% scale, the participant retrospectively and quantitatively rates the proportion of time spent in each mental activity during the resting-state fMRI acquisition. Whether this tool will or will not eventually gain the confidence of the research community, its great importance lies in that it represents a pioneering effort to encompass the richness and individual nature of MW into a standardized questionnaire.
Indirect Strategies for Measuring Mind-Wandering
Indirect strategies – strategies in which MW is not directly measured – are typically based on the conceptualization of MW as self-related and as more prevalent during rest than during tasks of high cognitive demand. The hypothesis could be framed as follows: if DMN neural activity during rest is the neural basis of MW, then DMN activations during rest and during a given task should be more similar when the task shares common characteristics to MW, i.e., is characterized by low cognitive load and high self relevance.
The advantage of the indirect strategies is straightforward: they avoid measuring MW directly, thus overcoming its non-quantifiable nature and the lack of validated behavioral MW measures. Instead, they use accepted task-related behavioral measures (mostly validated or previously published) and modulate their self-relatedness or their degree of cognitive load.
Strategy B1: parametric modulation of self-relatedness
James’s “spiritual self” ( James, 1892 ), Gallagher’s “narrative self” ( Gallagher, 2000 ), Dennett’s “non-minimal self” ( Dennett, 1991 ), and Damasio’s “autobiographical self” ( Damasio, 1998 ), are just a few examples of how MW is often present within theoretical models of the self. It is typically represented as a module of its own, distinct both from “lower,” more basic, senses of consciousness as well as from “higher” self-related executive functions. Contemporary neuro-scientists also tend to agree that the “stream of consciousness” is inseparable from the ongoing, constant, sense of self ( Damasio, 1998 ; Gusnard, 2005 ; Beer, 2007 ). According to this notion, MW, whether its content is directly related to the thinker or not, is a self-related, self-generated, self-sustaining function ( Baars, 2010 ); it serves as an integral part of self awareness, a pre-requisite for healthy psychological functioning.
The conceptualization of MW as a private case of self-related functioning produces a hypothesis for an overlap between the neural basis of self-related tasks and the neural basis of MW. This hypothesis has been translated in some studies into a rational for comparing neural activations during self-related tasks to neural activations at rest, when MW is assumed to occur most.
Though not the first to suggest a relation between rest-related neural activity and MW, the first paper to specifically associate MW with DMN activity was published by Gusnard et al. (2001) , as part of a series of publications ( Raichle et al., 2001 ) introducing the concept of the DMN. In this fMRI study, neural activations during rest were compared both to a subjective, emotional judgment task (“internally cued condition”) and to a neutral judgment task (“externally cued condition”). In accordance with the above prediction, neural activations in DMN-related PFC areas were found to be more similar to the activations at rest during the internally cued condition than during the externally cued condition (see Table 1 for summarized results). Paradigms similar in contrasting a self-related task with a similar non-self-related task can be found in additional fMRI studies ( Johnson et al., 2002 ; Goldberg et al., 2006 ; Schneider et al., 2008 ; Andrews-Hanna et al., 2010 ), and in the PET study described earlier ( D’Argembeau et al., 2005 ). Results in all of these studies indicate greater activations (or lesser de-activations) in brain areas associated with the DMN, mostly MPFC areas, during self-related tasks than during non-self-related tasks, when compared to rest. These elevated activations were shown to last beyond the duration of the stimuli and into the rest period following stimulation ( Schneider et al., 2008 ). The majority of these papers (except for Johnson et al., 2002 ) demonstrate that DMN activations during self-related conditions were more similar to DMN rest-related activity patterns, and suggest that this result might imply a possible functional role of rest-related DMN activations in spontaneous self-related mental activity.
As shown in separate studies as well as in convergence, this is a useful strategy for investigating the functional role of areas within the DMN while staying within the boundaries of accepted neuroimaging paradigms. One drawback of this strategy is the potential of over stretching the concept of self, which may cause confounding the self-relatedness of a task with other characteristics like its emotional valence (e.g., Gusnard et al., 2001 ). Therefore, one should pay special attention that the parameter modulated between study conditions is indeed as specific to self-relatedness as possible.
Strategy B2: parametric modulation of cognitive load
The distinction of ongoing spontaneous mentation from other, task related, mental functions dates back to James (1892) , and has been recognized almost solely by theoretical psychology and philosophy over the years ( Gallagher, 2000 ). However, this very classification of MW as the mental function characterizing the un-engagement of attentional resources directly magnifies its potential to be scientifically explored.
The strategy of parametric modulation of cognitive load has been used in the context of studying the functionality of rest-related DMN activity. In this strategy, the contrast of interest when analyzing imaging data is not the commonly used task minus rest contrast, but rather the contrast of rest minus task. Researchers try to demonstrate that the lower the cognitive load in a given task condition, the higher the activations in DMN areas during this task, leading to a smaller difference between DMN activations during the task compared to rest. Indeed, this was found to be the case in fMRI studies such as McKiernan et al. (2006) , Christoff et al. (2004) , and Mason et al. (2007) , and in Wicker et al.’s (2003) meta-analysis of PET studies. In the case of McKiernan et al. (2006) and Mason et al. (2007) , behavioral measures (described in strategy A1) were added to the study to further establish a more direct association between high DMN activations during low cognitive demand and MW.
This strategy yields results which correspond well with theoretical accounts of MW as well as with the lay intuition that MW is the “default” mental state when the mind is free to engage in it. In addition to its intuitiveness, and thus its simplicity, the advantage of this strategy is in its robustness: it was found to be replicated across virtually any behavioral task tested ( Shulman et al., 1997 ; Mazoyer et al., 2001 ; Wicker et al., 2003 ), which makes it accessible and easy to implement. It should be taken into account, however, that executive functioning and MW are probably not as anti-correlated as these studies may depict. MW may involve executive processes like memory, planning, computing, etc., as is reflected by findings of executive networks co-activated with DMN during MW ( Christoff et al., 2009 ). Thus, rather than assuming mutual exclusiveness, the degree and direction of the association between neural activity of the DMN and of executive networks during MW should be studied in greater experimental resolution.
Strategy B3: paradigm-free analysis of neuronal dynamics
Brain activity is combined of activations of neurons which comprise anatomical and functional networks. Recent advances in functional and computational neuroimaging have provided new tools for examining functional interactions between time series of signals obtained from different brain regions, catalyzing the examination of functional connectivity in the resting brain. This type of analysis does not require a behavioral paradigm (“paradigm-free”) and indeed is often implemented on data acquired solely when subjects lie resting in the imaging device (the validity of these signals is discussed in Box 1). In fMRI, analysis methods of the resting-state signal can typically be placed into hypothesis dependent and hypothesis free methods ( Van Den Heuvel and Hulshoff Pol, 2010 ), both resulting in connectivity maps – whether correlational or anti-correlational ( Uddin et al., 2009 ). These maps demonstrate anatomical networks which, interestingly, greatly overlap with known functional neural networks. The DMN is one of those emerging networks and thus its relation to MW can be further characterized in terms of functional connectivity.
Box 1. Validation of spontaneous BOLD fluctuations acquired during rest.
The neuronal basis of spontaneous resting-state fMRI signals was initially regarded by skeptics as problematic, potentially representing merely unknown parameters of noise as well as known physiological ones. However recent observations increasingly support and validate the neuronal basis of resting-state fMRI signals (Adapted from Van Den Heuvel and Hulshoff Pol, 2010 ):
• The first and probably most compelling evidence for the resting-state signal is that most resting-state patterns tend to occur between brain regions overlapping in known functional and neuroanatomical regions ( Salvador et al., 2005 ; Damoiseaux et al., 2006 ; Van Den Heuvel et al., 2008 ).
• The second observation relates to the frequency of rest-related signals revealing that the observed spontaneous BOLD signals are mainly dominated by lower frequencies (<0.1 Hz) with only a minimal contribution of higher frequency cardiac and respiratory oscillations (>0.3 Hz) ( Cordes et al., 2000 , 2001 ).
• Lastly, an (indirect) association exists between the frequency profiles of slow spontaneous resting-state fMRI and electrophysiological recordings of neuronal firing ( Nir et al., 2008 ) and between spontaneous BOLD fluctuations and simultaneous measured fluctuations in neuronal spiking ( Shmuel et al., 2002 ; Shmuel and Leopold, 2008 ).
Altogether these findings advocate toward the validity of the neural signal acquired during the resting state and the legitimacy of its scientific exploration.
Two studies are brought here to exemplify the usage of a paradigm-free strategy in further characterizing the relation between MW and DMN spatio-temporal dynamics. Horovitz et al. (2008) utilized this strategy to determine whether DMN activity can be de-coupled from conscious awareness. In this study, the level of functional connectivity within the DMN persisted both during the resting state and during light sleep. The authors conclude that DMN connectivity “does not require or reflect the level of consciousness that is typical for wakefulness” (p. 679), which seems to undermine the idea of a functional involvement of DMN activity in MW. Nevertheless, two alternative explanations are offered by the authors: the first is that these results only decouple wakeful awareness from the degree of connectivity within the DMN, but not from the amplitude of its activity ; the other is that light sleep is sometimes characterized by the existence of dream-like reverie activity (a mental activity similar to MW) which like MW may also be a functional product of DMN activity.
Another study by the same group ( Horovitz et al., 2009 ) demonstrated altered correlations between DMN network components during different states of consciousness, most notably a reduced involvement of the MPFC during sleep. The authors suggest that among the DMN components, the frontal cortex may play an important role in the sustenance of conscious awareness.
In favor of this strategy, it can be claimed that as some indication exists for the effect of previous task performance on neural activity at subsequent rest ( Northoff et al., 2010 ), a paradigm-free study design which consists of rest alone will produce results which are more unbiased. In any case, studies of this strategy call attention to the fact that beyond relative degree of neural activity, more holistic parameters of neural dynamics need to be explored to truly characterize the DMN–MW relation, such as temporal and spatial patterns of DMN activity.
Discussion and Future Directions
In this review we portray the evolvement of the neuroscience of MW, in hope to lay the grounds for additional research to come. Undoubtfully, studies like the ones overviewed here serve to narrow the gap between theoretical understanding of MW and its scientific exploration. Nevertheless, MW is still by large a mystery, and much work remains to complete the puzzle. In Box 2 we put forward several ideas which stem from existing findings in hope of contributing to future research.
Box 2. Mind-wandering: questions for future research.
Understanding MW using brain imaging techniques holds a promise for this field of research. Listed here are a few lines of thought that could constitute an initial framework for future MW studies:
A. Temporal patterns of MW: What are the spatio-temporal dynamics which correspond to MW in the human brain? How are they represented in terms of brain connectivity?
B. Control of MW: what is MW’s locus of control in the brain? Do internal and external abruptions of MW result in similar neural outcome? Interfering with MW occurrence by different type of tasks (e.g., tasks which require external vs. internal attention) could offer preliminary answers.
C. MW and consciousness: What is the nature of the relationship between consciousness and MW? Is MW simply an expression of conscious experience much like an actor on a stage or is it a substantial part of consciousness giving rise to the stage itself? If MW is indeed a substantial part of conscious experience one would expect similar neural correlates of both phenomena.
D. MW and pathologies: Which functions does MW serve and how are they disrupted when MW does not occur? Both the very mechanism and the contents of MW are of great interest to clinical psychology and psychiatry. Psychiatric and neuronal pathologies associated with MW malfunctioning may shed light on understanding the role of MW in healthy psychological functioning.
E. The contents of MW: In this review we put little emphasis on the ever changing contents of MW. This is not to say that they are of no importance, only that the studies described here were interested in the common mechanism underlying this changing flow of contents. Future research might very well attempt to segregate neural patterns during MW which are responsible for the experience of different contents or even different time directions (e.g., future or past) as explored by Smallwood et al. (2009) .
To begin answering such questions, the scientific community must agree upon theoretical definitions as well as normalized, standardized behavioral measures of MW. In the functional neuroimaging field one also needs advanced validated computational methods for studying the temporal dynamics of neural activations in long sequences such as common in rest.
Mind-wandering can be studied under different contexts involving a wide array of experimental questions. Accordingly, as we tried to exemplify in this review, there is no absolute optimal way to study it, but rather it is important to make an informed, educated choice when studying it within a neuroimaging paradigm. For instance, MW sampling provides valuable information about inter-subject and intra-subject differences in the degree of MW, while sacrificing the integrity of its natural, untouched flow; In contrast parametric modulation of cognitive load does not interfere with the natural course of MW and also enables statistical analysis of inter-group variance, with the compromise of MW being only implied, and not directly measured. Figure 2 depicts a flow chart of relevant considerations in making the most advantageous choice for a given experimental setup.
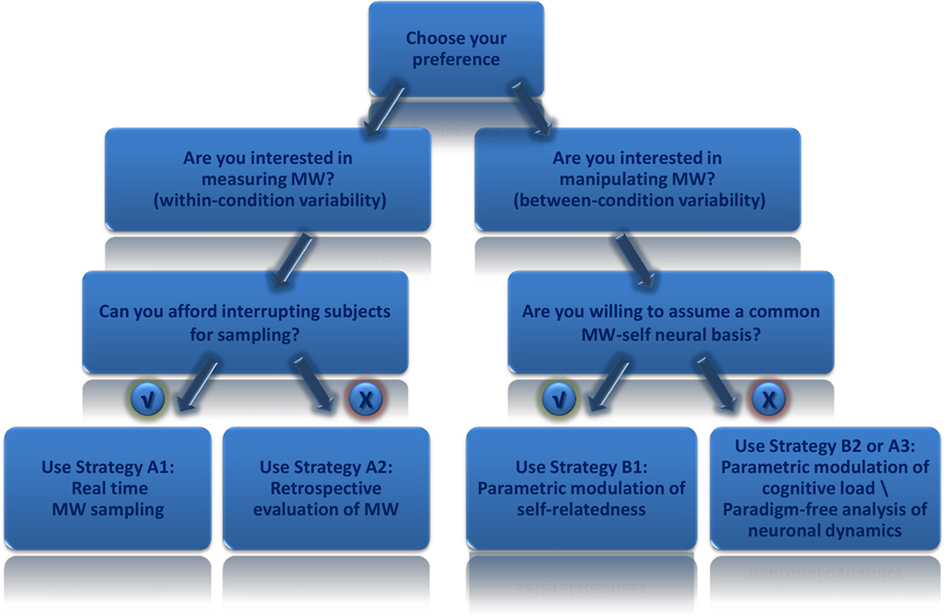
Figure 2. Flowchart of goodness of fit of different strategies according to study aims . A flowchart which may assist researchers wishing to explore mind-wandering using functional neuroimaging paradigms. According to study aims one should decide on the appropriate strategy taking into consideration advantages and disadvantages of each strategy as discussed in the text.
In addition to portraying modes of operation for the scientific examination of MW, overviewing the neuroscience of MW so far provides a few insights into this neural and mental process.
Functionality of Mind-Wandering
The robustness of the experience of MW across ages, cultures, and individuals ( Singer and McCraven, 1961 ), suggests it holds a vital role in human psychology. As to the specific role of MW, we suggest several ideas based on current literature which may inspire future research.
MW serves “self” functions
As detailed in the context of strategy B1, there are theoretical ( Gallagher, 2000 ), neuroanatomical ( Gusnard, 2005 ; Northoff et al., 2006 ), and intuitive grounds to claim that MW is a self-related cognitive function, which serves to create and maintain an integrated, meaningful sense of self out of various aspects of self-related information and cognition. Northoff et al. (2006) , for instance, conceptualizes MW as a “psychological baseline,” a form of continuous self-referential processing which is evident during non-task conditions and which ultimately forms our subjective experience of a “continuous stream of subjective experience” or “phenomenal time” where past, present, and future are no longer divided but integrated.
MW enables the projection of a “self” to past and future events
The idea that MW serves processes of future planning and simulation is based on theory and common experience, and is strongly supported by the fact that the DMN includes areas such as the posterior cingulate cortex, the precuneus, and the hippocampus, which are known to take part in such mental processes ( Buckner et al., 2008 ). Behaviorally, it has been shown that the contents of MW will tend toward prospecting or retrospecting according to the self relevance of a given context ( Smallwood et al., 2009 ), suggesting that MW serves to integrate past and present experiences for the purpose of future planning. Moreover, Smallwood et al. (2011b) suggest that self reflection associated with future-oriented thinking is an integral part of the autobiographical memory system. Interestingly, temporal locus of MW has even been shown to be related to the direction of apparent physical movement through space (forward/backward), implying a functional link between MW temporality and sensory spatio-temporal input ( Miles et al., 2010 ).
Altogether, this idea corresponds well with Tulving’s idea of “autonoetic consciousness,” which is claimed to be selective to the human kind and which enables mentally traveling into the past and the future ( Tulving, 2005 ).
MW serves as a learning and consolidation mechanism by augmenting the associative abilities of the brain
According to this proposition, spontaneous mental processing during wakefulness resembles in its function, in its effects and, to a certain extent, in its neural basis, the off-line processing that occurs during sleep. This relatively recent idea is presented by contemporary writers ( Christoff et al., 2008 ; Baars, 2010 ) and already takes into account what is known about DMN activation patterns. According to this notion, it could be suggested that task performance would improve following MW in a similar way when following sleep ( Stickgold et al., 2001 ).
Mind-Wandering-Executive Functioning Relation: An Integrative Approach
Converging results from studies like the ones overviewed here provide verification for a strong negative association between MW and executive functioning. This association, mentioned earlier to be part of the rational for strategy B2 (Parametric modulation of cognitive load), is supported by behavioral as well as neuro-scientific evidence (e.g., DMN activity). In light of the infancy of MW research, this in itself is a highly instrumental insight.
Nevertheless, recent lines of evidence suggest that this association is not exclusive. The first is found in the activation of executive prefrontal and parietal brain areas, in addition to DMN areas, as contributing to MW ( Christoff et al., 2009 ). The second is found in the gradual increase of DMN activity found in strategies B1 and B2 as cognitive load decreases and self-relatedness increases, which suggests that some DMN activity did occur even in lower self relevance or higher cognitive load conditions. The third line of evidence is brought by studies which show involvement of DMN areas during online task performance ( Assaf et al., 2009 ).
Though assuming a dichotomy between MW and “executive” neural networks proved useful for the beginning of MW research, a more mature approach might suggest studying the interplay between MW and executive functions and their underlying neural mechanisms ( Smallwood and Schooler, 2006 ). In consistence with this line of thought, Spreng et al. (2010) suggest that a third anatomically interposed “frontoparietal control network” mediates planning across domains, flexibly coupling with either the default or dorsal attention network in support of internally vs. externally focused cognition, respectively.
Rather than eliminating them, MW probably serves various cognitive functions such as prospective planning, self monitoring, etc. ( Baars, 2010 ). A better understanding of the interplay between MW and executive functioning can be achieved by further implementation of the five strategies defined here, in turn contributing altogether to the understanding of the adaptive value of MW with respect to human cognition and affect.
Mind-Wandering: The Neural Basis of Its Integration and Segregation
Portraying the results of the overviewed studies suggests that MW involves activities in distributed brain areas (see Table 1 and Table A1 in Appendix). These findings of different activations might underlie specific aspects of the MW process and in turn may serve to deconstruct MW, both theoretically and operationally, into elements according to its content or to the additional mental functions which are involved in it (e.g., emotion, autobiographical memory, mental time traveling, etc.). Examining the different DMN activations according to strategy, as illustrated in Figure 1 , implies that some sub-areas within the DMN are common to MW in any context while others are more typically unique to a specific strategy. For example, on an impressionist level only, it could be suggested that across strategies lateral correlates of MW are found more dominantly in the left than in the right hemisphere and can be commonly regarded as part of the network associated with high-level semantic processing. However other correlates of MW do differ between strategies, with the ventral MPFC and precuneus more sensitive to modulation of cognitive load, and dorso-medial MPFC areas more sensitive to self-relatedness.
It is of no doubt that such impressions require a comprehensive quantitative meta-analysis which is beyond the scope of this review. Nevertheless, such a neuro-functional differentiation implies that each strategy might reveal, in addition to the network underlying MW, the neural basis of a specific aspect within the large construct of MW. A functionally based deconstruction of the DMN has already been suggested ( Spreng et al., 2009 ; Andrews-Hanna et al., 2010 ; Stawarczyk et al., 2011 ) and could prove fruitful for further scientific examination of MW; Similar MW studies utilizing such refined and specific definitions may shed additional light on differential neural processes which underlie diverse aspects of MW.
Concluding Remarks
Mind-wandering is a universal phenomenon which accompanies much of our daily lives from childhood to adulthood. Its exploration has a vast potential in leading us to a better and more profound understanding of our ongoing mental selves, and in fact, of the basic properties of conscious experience.
The study of MW is at an exciting position of forming into a field of research of its own. Its relevance to a wide array of disciplines, from neuroscience to philosophy to the clinical world ensures that it will draw a growing number of researchers in the near future. We hope that this review serves to set the milestones for a better scientific understanding of this remarkable, unique human quality.
Trains Moscow to Elektrostal: Times, Prices and Tickets
- Train Times
- Seasonality
- Accommodations
Moscow to Elektrostal by train
The journey from Moscow to Elektrostal by train is 32.44 mi and takes 2 hr 7 min. There are 71 connections per day, with the first departure at 12:15 AM and the last at 11:46 PM. It is possible to travel from Moscow to Elektrostal by train for as little as or as much as . The best price for this journey is .
Get from Moscow to Elektrostal with Virail
Virail's search tool will provide you with the options you need when you want to go from Moscow to Elektrostal. All you need to do is enter the dates of your planned journey, and let us take care of everything else. Our engine does the hard work, searching through thousands of routes offered by our trusted travel partners to show you options for traveling by train, bus, plane, or carpool. You can filter the results to suit your needs. There are a number of filtering options, including price, one-way or round trip, departure or arrival time, duration of journey, or number of connections. Soon you'll find the best choice for your journey. When you're ready, Virail will transfer you to the provider's website to complete the booking. No matter where you're going, get there with Virail.
How can I find the cheapest train tickets to get from Moscow to Elektrostal?
Prices will vary when you travel from Moscow to Elektrostal. On average, though, you'll pay about for a train ticket. You can find train tickets for prices as low as , but it may require some flexibility with your travel plans. If you're looking for a low price, you may need to prepare to spend more time in transit. You can also often find cheaper train tickets at particular times of day, or on certain days of the week. Of course, ticket prices often change during the year, too; expect to pay more in peak season. For the lowest prices, it's usually best to make your reservation in advance. Be careful, though, as many providers do not offer refunds or exchanges on their cheapest train tickets. Unfortunately, no price was found for your trip from Moscow to Elektrostal. Selecting a new departure or arrival city, without dramatically changing your itinerary could help you find price results. Prices will vary when you travel from Moscow to Elektrostal. On average, though, you'll pay about for a train ticket. If you're looking for a low price, you may need to prepare to spend more time in transit. You can also often find cheaper train tickets at particular times of day, or on certain days of the week. Of course, ticket prices often change during the year, too; expect to pay more in peak season. For the lowest prices, it's usually best to make your reservation in advance. Be careful, though, as many providers do not offer refunds or exchanges on their cheapest train tickets.
How long does it take to get from Moscow to Elektrostal by train?
The journey between Moscow and Elektrostal by train is approximately 32.44 mi. It will take you more or less 2 hr 7 min to complete this journey. This average figure does not take into account any delays that might arise on your route in exceptional circumstances. If you are planning to make a connection or operating on a tight schedule, give yourself plenty of time. The distance between Moscow and Elektrostal is around 32.44 mi. Depending on the exact route and provider you travel with, your journey time can vary. On average, this journey will take approximately 2 hr 7 min. However, the fastest routes between Moscow and Elektrostal take 1 hr 3 min. If a fast journey is a priority for you when traveling, look out for express services that may get you there faster. Some flexibility may be necessary when booking. Often, these services only leave at particular times of day - or even on certain days of the week. You may also find a faster journey by taking an indirect route and connecting in another station along the way.
How many journeys from Moscow to Elektrostal are there every day?
On average, there are 71 daily departures from Moscow to Elektrostal. However, there may be more or less on different days. Providers' timetables can change on certain days of the week or public holidays, and many also vary at particular times of year. Some providers change their schedules during the summer season, for example. At very busy times, there may be up to departures each day. The providers that travel along this route include , and each operates according to their own specific schedules. As a traveler, you may prefer a direct journey, or you may not mind making changes and connections. If you have heavy suitcases, a direct journey could be best; otherwise, you might be able to save money and enjoy more flexibility by making a change along the way. Every day, there are an average of 18 departures from Moscow which travel directly to Elektrostal. There are 53 journeys with one change or more. Unfortunately, no connection was found for your trip from Moscow to Elektrostal. Selecting a new departure or arrival city, without dramatically changing your itinerary could help you find connections.
Book in advance and save
If you're looking for the best deal for your trip from Moscow to Elektrostal, booking train tickets in advance is a great way to save money, but keep in mind that advance tickets are usually not available until 3 months before your travel date.
Stay flexible with your travel time and explore off-peak journeys
Planning your trips around off-peak travel times not only means that you'll be able to avoid the crowds, but can also end up saving you money. Being flexible with your schedule and considering alternative routes or times will significantly impact the amount of money you spend on getting from Moscow to Elektrostal.
Always check special offers
Checking on the latest deals can help save a lot of money, making it worth taking the time to browse and compare prices. So make sure you get the best deal on your ticket and take advantage of special fares for children, youth and seniors as well as discounts for groups.
Unlock the potential of slower trains or connecting trains
If you're planning a trip with some flexible time, why not opt for the scenic route? Taking slower trains or connecting trains that make more stops may save you money on your ticket – definitely worth considering if it fits in your schedule.
Best time to book cheap train tickets from Moscow to Elektrostal
The cheapest Moscow - Elektrostal train tickets can be found for as low as $35.01 if you’re lucky, or $54.00 on average. The most expensive ticket can cost as much as $77.49.
Find the best day to travel to Elektrostal by train
When travelling to Elektrostal by train, if you want to avoid crowds you can check how frequently our customers are travelling in the next 30-days using the graph below. On average, the peak hours to travel are between 6:30am and 9am in the morning, or between 4pm and 7pm in the evening. Please keep this in mind when travelling to your point of departure as you may need some extra time to arrive, particularly in big cities!
Moscow to Elektrostal CO2 Emissions by Train

Anything we can improve?
Frequently Asked Questions
Go local from moscow, trending routes, weekend getaways from moscow, international routes from moscow and nearby areas, other destinations from moscow, other popular routes.
- Skip to main content
- Keyboard shortcuts for audio player
Movie Interviews
'the old oak' follows a small english community amidst the arrival of syrian migrants.

Scott Simon
NPR's Scott Simon speaks to screenwriter Paul Laverty, whose latest collaboration with director Ken Loach is a film titled "The Old Oak."
SCOTT SIMON, HOST:
Ken Loach says "The Old Oak" is his final film after 56 years in the business. The Old Oak's an old pub in a desiccated English mining town in County Durham, where some locals feel uneasy when a group of refugees from war-blighted Syria arrive in 2016. A few people welcome them, offering friendship and support, but more seem to receive them with resentment, suspicion, even worse. They put them here, a character grouses, not Chelsea or Westminster.
(SOUNDBITE OF FILM, "THE OLD OAK")
UNIDENTIFIED ACTOR #1: (As character) Are you going to explain? You didn't even tell us they were coming. When are you going to do that?
DAVE TURNER: (As Dave Turner) We'll be around...
CLAIRE RODGERSON: (As Laura) Listen.
TURNER: (As TJ Ballantyne) ...To explain to everybody.
RODGERSON: (As Laura) But they've got a good point, haven't they?
TURNER: (As TJ Ballantyne) I understand...
RODGERSON: (As Laura) You've got to admit they've got a good point.
TURNER: (As TJ Ballantyne) I understand what they're saying.
RODGERSON: (As Laura) Listen. Like, there's bairns on the bus.
SIMON: TJ, who owns the Old Oak, the last pub in town, is played by Dave Turner. Ebla Mari is Yara, a young refugee with whom he strikes up a friendship. And "The Old Oak" is written by Paul Laverty, who has written every Ken Loach film since 1996. He won the best screenplay award at the 2002 Cannes Film Festival for "Sweet Sixteen." And their film "The Wind That Shakes the Barley" won the 2006 Palme d'Or. Paul Laverty joins us now. Thanks so much for being with us.
PAUL LAVERTY: It's a great pleasure, Scott.
SIMON: Why did you and Ken Loach want to tell this story?
LAVERTY: You know, we've been working together for over 30 years now. And I suppose we knew this was going to be Ken's last film. He was actually turned 86 in the middle of the shoot and is due a rest. He's still as sharp and as bright as ever, but it takes a toll physically, you know, to direct a film, especially the way he does it. He doesn't delegate much. So we really did want to try and just examine the notion of hope and perhaps where we draw nutrition for each other in this mad world, this mad, violent world.
And it was remarkable wandering around these old ex-mining villages, which were once vibrant communities before the miner strike in 1984. It was just seeing how these communities had been decimated after Margaret Thatcher. And then Tony Blair, when he took over, you know, had allowed these communities just to fall into rack and ruin. And so the people who lived there felt that they had lost agency in their lives, lost control of their lives. They were angry and furious. And so when refugees came from Syria and landed there, and many people felt like, you know, why here? Why not in the richer areas? They've got more resources. There was a very mixed reception.
SIMON: It seems important in the film - I imagined you and Ken Loach - that the people in the pub not just be shown as a bunch of drinkers who are stereotypes of bigotry.
LAVERTY: Scott, thanks for saying that. And that was very, very important to us. Anyway, two-dimensional characters or thugs or racists or people who are just angry and furious - it's not very interesting, really, because it becomes just two-dimensional. But what I think is much more interesting is to kind of try and understand why decent people who are sophisticated and understand the world, how their sense of confidence has been worn away, how their sense of well-being, their sense of self-worth, their energy and their empathy for people has been worn down.
SIMON: And how do you get hold of what the people from Syria have been through?
LAVERTY: Well, I think the first obligation is really to listen to them. And then we met some remarkable people there who had just unimaginable lives. I suppose we are dealing with two traumatized communities, but we're not trying to say that the ones of the working class in the U.K. are anywhere near the trauma of these people who have just suffered war. We're not trying to make some specious equation out of that. I mean, what they've gone through in Syria is just unimaginable, you know, just industrial-scale torture and murder of the most brutal kind. And everyone who is in the film was from Syria, apart from Ebla, who played Yara, the main Syrian character.
SIMON: Ebla Mari.
LAVERTY: Ebla Mari, yes. She's a wonderful young woman. She is the only person who is a professional actor. She comes from a theatrical background. All the rest of the Syrians in the film have actually, you know, had to flee Syria because of the war. And Ebla - she actually comes from the Golan Heights, and she actually looks over Syria. She comes from a Druze community. You know, Arabic, obviously, her first language. Her accent was slightly different from her Syrian friends in the film. But she spent a lot of time with them and worked with them on her accent.
SIMON: You and Ken Loach like to work with people who haven't been in films before.
LAVERTY: What Ken has always said from the very beginning - I remember this when we met the very, very first time - was that the casting procedure was really trying to give flesh and blood to the characters as best we can, as imagined in the screenplay. Sometimes, that will be very, very experienced, you know, actors. Cillian Murphy was in our film "The Wind That Shakes the Barley," for example, an actor with tremendous range.
And sometimes, it's people who have never acted before. In this case, we found a wonderful character called Dave Turner. Dave had only done a couple of scenes in our two previous films. But he's a man of great hinterland. He was an ex-trade unionist. He was in the fire brigades union, a man of great sensitivity, and he totally understands the world of TJ Ballantyne, the fictional character. And he even works in a pub. He lives in that area. His accent was perfect. But he understands it in his blood. He hardly needs to think about it, so his instinct was great.
SIMON: Why do you think there seem to be relatively few films about working-class people?
LAVERTY: Well, I think, like in every aspect of our lives, I think you have to examine where power lies. Who has the finance? Who has the money? And let's face it - we live in a world which is dictated by corporations, and then so profit is everything. So perhaps people don't want to make films that celebrate the working class for obvious reasons. Very seldom is the collective celebrated or dug into for the great stories that it has to offer us. But it's a great pity because Ken often says that films should be like a good library where you have a great expanse of different types of books. And unfortunately, I don't think we see that in film and certainly not film that gets properly distributed.
SIMON: I want to ask you about a very quiet and moving scene...
TURNER: (As TJ Ballantyne) Your dolls are very pretty.
SIMON: ...Where a young Syrian girl shows TJ her dolls.
TURNER: (As TJ Ballantyne) Do they have names?
EBLA MARI: (As Yara, speaking Arabic).
UNIDENTIFIED ACTOR #2: (As character) Rahat, Rafif, Sham, Shahad, Amara (ph).
SIMON: What put that scene in your mind?
LAVERTY: And I'm glad you mentioned that scene, Scott. That's just one of these little gifts that come to you, I suppose, when you're trying to dramatize trauma, I suppose. That scene came out of talking to kids who missed their friends because it's like some great big iron fist has smashed into the country, and people have been scattered around the world to the four winds, and they've all ended up in different countries and different spots in the world. But these kids have lost their precious friendships.
What you try to do when you're writing, I suppose, is to write things that seem seamless, that might touch you but also give you insight into the imagination of a child. So, you know, the actual Syrian children in the film just come from local schools close by. The mother - she lives in a little village close by, too. She was really at the heart of the story. So what we found was just remarkable people who shared their lives with us, who gave, you know, flesh and blood to the story.
SIMON: Speaking of flesh and blood, so this is the last film you and Ken Loach will do, you're sure?
LAVERTY: I think so, Scott. I think we've made about 14 feature films together now. So I'd like to see the glass as half full. We've had the most remarkable run together doing films all over the world. And so it's been an absolute privilege for me to work with Ken and also our wonderful producer Rebecca O'Brien, who makes it all possible for us. So rather than mourn it, I'd celebrate just the wonderful journey we've had.
SIMON: Paul Laverty, screenwriter for Ken Loach's new and maybe last film, "The Old Oak." Thank you so much for being with us.
LAVERTY: Great pleasure, Scott, and thank you for having me on your program.
(SOUNDBITE OF BILL FRISELL'S "FARMER")
Copyright © 2024 NPR. All rights reserved. Visit our website terms of use and permissions pages at www.npr.org for further information.
NPR transcripts are created on a rush deadline by an NPR contractor. This text may not be in its final form and may be updated or revised in the future. Accuracy and availability may vary. The authoritative record of NPR’s programming is the audio record.
- Share full article
Advertisement
Supported by
Critic’s Notebook
A Wanderer, Ravel and Suzanne Farrell: Life Is Good at City Ballet
The spring season at New York City Ballet opened with an all-Balanchine program and a vintage miniature from 1975: “Errante,” staged for a new generation.

By Gia Kourlas
With certain dancers, there is an interior drama, an intimate dialogue between movement and music that manages to quiet the air around them, pulling them into greater focus. Mira Nadon, the young New York City Ballet principal , is growing into that place of spellbinding luminosity.
We’ve seen her unflappable elegance, her cool sensuality and her creamy elasticity. But dancing in “Errante,” on the opening program of the company’s spring season that began Tuesday, she displayed a new kind of dancing courage. The ballet, originally known as “Tzigane” after its score by Maurice Ravel, was revived this season with a staging by Suzanne Farrell and a new name, “Errante,” or wandering.
Created for the company’s 1975 Ravel Festival, it was the first ballet George Balanchine choreographed for Farrell upon her return to City Ballet after a rift with Balanchine and time spent in Europe. In Farrell’s restoration, “Errante” is a passionate musical adventure — rich with play, mystery and seduction — that opens with a five-minute solo for its female lead.
As solos go, it’s headstrong and questing, revealing a dancer’s rebellious streak in the choreography’s defiant twists and turns. As for the title change? Tzigane, a word that refers to Romani people, is now considered derogatory. Farrell, who holds the rights to the work, selected “Errante”; the decision to rename was made by Farrell, the George Balanchine Trust and City Ballet, which hasn’t staged the ballet in more than 30 years.
Of the ballet and Farrell, Lincoln Kirstein , a founder of the company, wrote, “Was part of this an echo of her own wandering, of the fact that she had at last returned to her tribe’s encampment, while proclaiming her own increased identity and independence?”
It feels, especially now, like a stand for female autonomy. Starting with Nadon’s casual entrance — a detached, loose walk across the stage as her hands come to rest on the hips — the ballet has a smoldering perfume that heats up over time. Nadon’s sighing shoulders lead her on a path of self-discovery that she fills in with lustrous details. Her elbows rise above her chin like a veil. She flings her arms wildly yet with surgical precision. She arches backward with a rapid shudder of her shoulders.
Ever the wanderer, Nadon seems to be etching her identity onto a role made years before she was born. And like Farrell, she looks great in red, cutting a blazing figure in Joe Eula’s skirt of shredded ribbons, offset by a burgundy bodice with creamy sleeves.
Nadon occasionally snaps her eyes to gaze at the audience. Throughout the violin solo, performed by Kurt Nikkanen, she is a wonder of brazen poise. After stretching her hands forward and slowly wrapping the fingers of one around the pointer finger of another, she whips into tight chaîné turns, pausing to reach and lunge with a daring that seemed to grow from one performance to the next.
When her partner finally appears on the opposite diagonal — Aarón Sanz, dancing with admirable fullness and focus — Nadon has her back to him. Gradually they shift closer until Sanz embraces her around the waist, close but not quite touching.
With whiffs of Hungarian folk dance, they rock on their toes and heels and, eventually, are joined by four couples as a more wild energy overtakes the stage. Nadon spins into a backbend, dangling herself over Sanz’s arm, where she remains as she walks, no, trots — en pointe — across the stage. In moments like these, “Errante” is a rebirth: not a dusty character study from the 1970s, but a vibrant Balanchine miniature imbued with the spirit of the modern world.
On Tuesday, another happy surprise occurred when Farrell, her arm linked in Sanz’s, slipped onto the stage for a bow. Nadon and Sanz, in awe, backed away to applaud along with the crowd. Her appearance was a reminder that preserving Balanchine ballets is a race against time: Former dancers must coach current ones. They knew Balanchine. They knew his counts, and that is everything.
While I could have done without “The Steadfast Tin Soldier” — I don’t need to see a Christmas tree onstage for many more months — the program was a bright start to the season. Despite some rough patches in “Bourrée Fantasque” on opening night, it remained witty and rambunctious, especially the pairings of Emily Kikta and KJ Takahashi, and Emilie Gerrity and Gilbert Bolden III.
Many performances were eye-catching, but Sara Mearns was astounding in the second movement of “Symphony in C” — her dancing now seems to be getting to the essence of a dance — and Alston Macgill and Harrison Coll, making their debuts in the fourth movement, were superbly free. Not every program can end with a ballet as dazzling as “Symphony in C,” but when it does — what a rapturous experience to be listening to Bizet while watching a sea of dancers leaping and spinning in choreographic harmony. It’s not a special effect! This is what human bodies are capable of, and it always blows my mind.
New York City Ballet
Though June 2 at the David H. Koch Theater, nycballet.com
Gia Kourlas is the dance critic for The Times. She writes reviews, essays and feature articles and works on a range of stories. More about Gia Kourlas
Stepping Into the World of Dance
As Harlem Stage’s E-Moves dance series turns 25, Bill T. Jones and other major choreographers discuss its impact on Black dance in New York.
“We the People,” Jamar Roberts’s first dance for the Martha Graham Dance Company, finds the rage and resistance hidden in an upbeat score by Rhiannon Giddens.
In “Nail Biter,” a New York City premiere, the exacting choreographer Beth Gill explores her ballet roots and how to be in her body now.
The choreographer Emma Portner, who has spent her career mixing genres and disciplines , comes to ballet with an eye on its sometimes calcified gender relations.
A childhood encounter with an American soldier in Iraq led Hussein Smko to become a dancer. Now the artist performs on New York stages .
“Deep River” is in many ways an apt title for a dance work by Alonzo King, a choreographer fixated on flow .
- Popular Professionals
- Design & Planning
- Construction & Renovation
- Finishes & Fixtures
- Landscaping & Outdoor
- Systems & Appliances
- Interior Designers & Decorators
- Architects & Building Designers
- Design-Build Firms
- Kitchen & Bathroom Designers
- General Contractors
- Kitchen & Bathroom Remodelers
- Home Builders
- Roofing & Gutters
- Cabinets & Cabinetry
- Tile & Stone
- Hardwood Flooring Dealers
- Landscape Contractors
- Landscape Architects & Landscape Designers
- Home Stagers
- Swimming Pool Builders
- Lighting Designers and Suppliers
- 3D Rendering
- Sustainable Design
- Basement Design
- Architectural Design
- Universal Design
- Energy-Efficient Homes
- Multigenerational Homes
- House Plans
- Home Remodeling
- Home Additions
- Green Building
- Garage Building
- New Home Construction
- Basement Remodeling
- Stair & Railing Contractors
- Cabinetry & Cabinet Makers
- Roofing & Gutter Contractors
- Window Contractors
- Exterior & Siding Contractors
- Carpet Contractors
- Carpet Installation
- Flooring Contractors
- Wood Floor Refinishing
- Tile Installation
- Custom Countertops
- Quartz Countertops
- Cabinet Refinishing
- Custom Bathroom Vanities
- Finish Carpentry
- Cabinet Repair
- Custom Windows
- Window Treatment Services
- Window Repair
- Fireplace Contractors
- Paint & Wall Covering Dealers
- Door Contractors
- Glass & Shower Door Contractors
- Landscape Construction
- Land Clearing
- Garden & Landscape Supplies
- Deck & Patio Builders
- Deck Repair
- Patio Design
- Stone, Pavers, & Concrete
- Paver Installation
- Driveway & Paving Contractors
- Driveway Repair
- Asphalt Paving
- Garage Door Repair
- Fence Contractors
- Fence Installation
- Gate Repair
- Pergola Construction
- Spa & Pool Maintenance
- Swimming Pool Contractors
- Hot Tub Installation
- HVAC Contractors
- Electricians
- Appliance Services
- Solar Energy Contractors
- Outdoor Lighting Installation
- Landscape Lighting Installation
- Outdoor Lighting & Audio/Visual Specialists
- Home Theater & Home Automation Services
- Handyman Services
- Closet Designers
- Professional Organizers
- Furniture & Accessories Retailers
- Furniture Repair & Upholstery Services
- Specialty Contractors
- Color Consulting
- Wine Cellar Designers & Builders
- Home Inspection
- Custom Artists
- Columbus, OH Painters
- New York City, NY Landscapers
- San Diego, CA Bathroom Remodelers
- Minneapolis, MN Architects
- Portland, OR Tile Installers
- Kansas City, MO Flooring Contractors
- Denver, CO Countertop Installers
- San Francisco, CA New Home Builders
- Rugs & Decor
- Home Improvement
- Kitchen & Tabletop
- Bathroom Vanities
- Bathroom Vanity Lighting
- Bathroom Mirrors
- Bathroom Fixtures
- Nightstands & Bedside Tables
- Kitchen & Dining
- Bar Stools & Counter Stools
- Dining Chairs
- Dining Tables
- Buffets and Sideboards
- Kitchen Fixtures
- Wall Mirrors
- Living Room
- Armchairs & Accent Chairs
- Coffee & Accent Tables
- Sofas & Sectionals
- Media Storage
- Patio & Outdoor Furniture
- Outdoor Lighting
- Ceiling Lighting
- Chandeliers
- Pendant Lighting
- Wall Sconces
- Desks & Hutches
- Office Chairs
- View All Products
- Designer Picks
- Side & End Tables
- Console Tables
- Living Room Sets
- Chaise Lounges
- Ottomans & Poufs
- Bedroom Furniture
- Nightstands
- Bedroom Sets
- Dining Room Sets
- Sideboards & Buffets
- File Cabinets
- Room Dividers
- Furniture Sale
- Trending in Furniture
- View All Furniture
- Bath Vanities
- Single Vanities
- Double Vanities
- Small Vanities
- Transitional Vanities
- Modern Vanities
- Houzz Curated Vanities
- Best Selling Vanities
- Bathroom Vanity Mirrors
- Medicine Cabinets
- Bathroom Faucets
- Bathroom Sinks
- Shower Doors
- Showerheads & Body Sprays
- Bathroom Accessories
- Bathroom Storage
- Trending in Bath
- View All Bath
- Houzz x Jennifer Kizzee
- Houzz x Motivo Home
- How to Choose a Bathroom Vanity

- Patio Furniture
- Outdoor Dining Furniture
- Outdoor Lounge Furniture
- Outdoor Chairs
- Adirondack Chairs
- Outdoor Bar Furniture
- Outdoor Benches
- Wall Lights & Sconces
- Outdoor Flush-Mounts
- Landscape Lighting
- Outdoor Flood & Spot Lights
- Outdoor Decor
- Outdoor Rugs
- Outdoor Cushions & Pillows
- Patio Umbrellas
- Lawn & Garden
- Garden Statues & Yard Art
- Planters & Pots
- Outdoor Sale
- Trending in Outdoor
- View All Outdoor
- 8 x 10 Rugs
- 9 x 12 Rugs
- Hall & Stair Runners
- Home Decor & Accents
- Pillows & Throws
- Decorative Storage
- Faux Florals
- Wall Panels
- Window Treatments
- Curtain Rods
- Blackout Curtains
- Blinds & Shades
- Rugs & Decor Sale
- Trending in Rugs & Decor
- View All Rugs & Decor
- Pendant Lights
- Flush-Mounts
- Ceiling Fans
- Track Lighting
- Wall Lighting
- Swing Arm Wall Lights
- Display Lighting
- Table Lamps
- Floor Lamps
- Lamp Shades
- Lighting Sale
- Trending in Lighting
- View All Lighting
- Bathroom Remodel
- Kitchen Remodel
- Kitchen Faucets
- Kitchen Sinks
- Major Kitchen Appliances
- Cabinet Hardware
- Backsplash Tile
- Mosaic Tile
- Wall & Floor Tile
- Accent, Trim & Border Tile
- Whole House Remodel
- Heating & Cooling
- Building Materials
- Front Doors
- Interior Doors
- Home Improvement Sale
- Trending in Home Improvement
- View All Home Improvement
- Cups & Glassware
- Kitchen & Table Linens
- Kitchen Storage and Org
- Kitchen Islands & Carts
- Food Containers & Canisters
- Pantry & Cabinet Organizers
- Kitchen Appliances
- Gas & Electric Ranges
- Range Hoods & Vents
- Beer & Wine Refrigerators
- Small Kitchen Appliances
- Cookware & Bakeware
- Tools & Gadgets
- Kitchen & Tabletop Sale
- Trending in Kitchen & Tabletop
- View All Kitchen & Tabletop
- Storage & Organization
- Baby & Kids
- Housekeeping & Laundry

- View all photos
- Dining Room
- Breakfast Nook
- Family Room
- Bed & Bath
- Powder Room
- Storage & Closet
- Outdoor Kitchen
- Bar & Wine
- Wine Cellar
- Home Office
- Popular Design Ideas
- Kitchen Backsplash
- Deck Railing
- Privacy Fence
- Small Closet
- Stories and Guides
- Popular Stories
- Renovation Cost Guides
- Fence Installation Cost Guide
- Window Installation Cost Guide
- Discussions
- Design Dilemmas
- Before & After
- Houzz Research
- View all pros
- View all services
- View all products
- View all sales
- Living Room Chairs
- Dining Room Furniture
- Coffee Tables
- Home Office Furniture
- Join as a Pro
- Interior Design Software
- Project Management
- Custom Website
- Lead Generation
- Invoicing & Billing
- Landscape Contractor Software
- General Contractor Software
- Remodeler Software
- Builder Software
- Roofer Software
- Architect Software
- Takeoff Software
- Lumber & Framing Takeoffs
- Steel Takeoffs
- Concrete Takeoffs
- Drywall Takeoffs
- Insulation Takeoffs
- Stories & Guides
- LATEST FROM HOUZZ
- HOUZZ DISCUSSIONS
- SHOP KITCHEN & DINING
- Kitchen & Dining Furniture
- Sinks & Faucets
- Kitchen Cabinets & Storage
- Knobs & Pulls
- Kitchen Knives
- KITCHEN PHOTOS
- FIND KITCHEN PROS
- Bath Accessories
- Bath Linens
- BATH PHOTOS
- FIND BATH PROS
- SHOP BEDROOM
- Beds & Headboards
- Bedroom Decor
- Closet Storage
- Bedroom Vanities
- BEDROOM PHOTOS
- Kids' Room
- FIND DESIGN PROS
- SHOP LIVING
- Fireplaces & Accessories
- LIVING PHOTOS
- SHOP OUTDOOR
- Pool & Spa
- Backyard Play
- OUTDOOR PHOTOS
- FIND LANDSCAPING PROS
- SHOP LIGHTING
- Bathroom & Vanity
- Flush Mounts
- Kitchen & Cabinet
- Outdoor Wall Lights
- Outdoor Hanging Lights
- Kids' Lighting
- Decorative Accents
- Artificial Flowers & Plants
- Decorative Objects
- Screens & Room Dividers
- Wall Shelves
- About Houzz
- Houzz Credit Cards
- Privacy & Notice
- Cookie Policy
- Your Privacy Choices
- Mobile Apps
- Copyright & Trademark
- For Professionals
- Houzz vs. Houzz Pro
- Houzz Pro vs. Ivy
- Houzz Pro Advertising Reviews
- Houzz Pro 3D Floor Planner Reviews
- Trade Program
- Buttons & Badges
- Your Orders
- Shipping & Delivery
- Return Policy
- Houzz Canada
- Review Professionals
- Suggested Professionals
- Accessibility
- Houzz Support
- COUNTRY COUNTRY
Custom Fireplace Contractors & Installers in Elektrostal'
Location (1).
- Use My Current Location
Popular Locations
- Albuquerque
- Cedar Rapids
- Grand Rapids
- Indianapolis
- Jacksonville
- Kansas City
- Little Rock
- Los Angeles
- Minneapolis
- New Orleans
- Oklahoma City
- Orange County
- Philadelphia
- Portland Maine
- Salt Lake City
- San Francisco
- San Luis Obispo
- Santa Barbara
- Washington D.C.
- Elektrostal', Moscow Oblast, Russia
Professional Category (1)
- Accessory Dwelling Units (ADU)
Featured Reviews for Custom Fireplace Contractors & Installers in Elektrostal'
- Reach out to the pro(s) you want, then share your vision to get the ball rolling.
- Request and compare quotes, then hire the Fireplace professional that perfectly fits your project and budget limits.
- Electric Fireplace Repair
What should you know about buying a fireplace in Elektrostal'?
Here are some recommendations for when you’re shopping for a fireplace or fire pit:, business services, connect with us.

IMAGES
VIDEO
COMMENTS
Mindfulness and mind wandering are often described as two divergent mental states; 31,32 yet, both are frequently referenced in the context of mental rest. There is a subtle difference in both awareness and engagement with the flow of mental objects that may determine the adaptive or maladaptive nature by which the mental content influences one ...
Physical activity, like a short walk or shaking out your arms and legs in between meetings, can interrupt the cycle of mind wandering and re-energize your focus. 💙 If the mind is wandering, try bringing it back to the present moment through movement. Check out Mindful Movement with Mel Mah. 7. Use grounding exercises.
It is the capacity to retreat and decouple from our immediate environment. Researcher Thomas Metzinger [3] says that mind-wandering is characterized by a loss in our attentiveness and cognitive control. That means, in a mind-wandering state, you have little to no deliberate control over attention on any kind of information and little to no deliberate generation of thoughts or goal-directed ideas.
A scientist says mind-wandering or daydreaming help prepare us for the future. Scientists are beginning to understand when and why minds start to wander. Knowable Magazine. When psychologist ...
Introduction Minds wander. Some wander more than others, but human ones wander a lot. A much-cited estimate, due to Killingsworth and Gilbert (2010), has it that the awake human mind spends from a third to half its time wandering.That's a big range, a rough estimate, and there are good reasons to be suspicious of it (see Seli et al. 2018).The actual number will likely depend a bit upon the ...
Mind-wandering (MW) is among the most robust and permanent expressions of human conscious awareness, classically regarded by philosophers, clinicians, and scientists as a core element of an intact sense of self. ... it stands out as a compelling candidate for a neuro-biological account of mind-wandering, in itself a rest-based phenomenon. By ...
But a review of brain imaging studies led by researchers at UC Berkeley and the University of British Columbia offers a new way of looking at spontaneous versus controlled thinking, challenging the adage that a wandering mind is an unhappy mind. It suggests that increased awareness of how our thoughts move when our brains are at rest could lead ...
New research led by UC Berkeley has come up with a way to track the flow of our internal thought processes and signal whether our minds are focused, fixated or wandering. Using an electroencephalogram (EEG) to measure brain activity while people performed mundane attention tasks, researchers identified brain signals that reveal when the mind is ...
In the past 15 years, mind-wandering and spontaneous thought have become prominent topics in cognitive psychology and neuroscience 2. However, most theories of mind-wandering still overlook the ...
Find counselling to help with ADHD. The first step to mastering mind-wandering is to plan time for it. Use a schedule maker and block off time in your day to let your thoughts flow freely. You ...
Mind wandering is believed to play an important role in generating new ideas, conclusions or insights (also known as "aha! moments"). This is because it can give your mind a break and free it ...
The memory benefit of offline waking rest is comparable to the effect of post-learning sleep, and has been demonstrated for a wide array of types of learning and memory. Periods of offline rest ...
A growing body of literature suggests that we mind wander, we take our mind away from the task at hand, about 50 percent of our waking moments. These might be small little trips that we take away, private thoughts that we have. And when this mind wandering happens it can be problematic.
When your mind is wandering, your brain's "default mode" network is active. Its discovery 20 years ago inspired a raft of research into networks of brain regions and how they interact with each other. ... He found that during rest, when we turn mentally inward, task-negative areas use more energy than the rest of the brain. ...
What I find really interesting about this study is that the Undemanding condition led to more creative problem solving, and more mind-wandering, than the Rest condition. When given the opportunity to sit quietly with no additional task, participants did not think much about the unusual uses task they were given at the beginning of the experiment.
Future studies that combine real-time resting-state fMRI and probe measures of mind wandering during fMRI scans will be needed to measure the association between transitions of functional ...
Jeff Bezos doesn't jam-pack his schedule or set strict time blocks for all his meetings. Instead, the 60-year-old Amazon and Blue Origin founder — currently the second-richest person in the ...
NEW YORK — In the minutes before nearly every New York Knicks game this season, as the arena crescendos into a pulsing hive of activity — music blasting, players warming up, fans filing in ...
Mind-wandering (MW) is among the most robust and permanent expressions of human conscious awareness, classically regarded by philosophers, clinicians, and scientists as a core element of an intact sense of self. Nevertheless, the scientific exploration of MW poses unique challenges; MW is by nature a spontaneous, off task, internal mental process which is often unaware and usually difficult to ...
In 1938, it was granted town status. [citation needed]Administrative and municipal status. Within the framework of administrative divisions, it is incorporated as Elektrostal City Under Oblast Jurisdiction—an administrative unit with the status equal to that of the districts. As a municipal division, Elektrostal City Under Oblast Jurisdiction is incorporated as Elektrostal Urban Okrug.
The journey from Moscow to Elektrostal by train is 32.44 mi and takes 2 hr 7 min. There are 71 connections per day, with the first departure at 12:15 AM and the last at 11:46 PM. It is possible to travel from Moscow to Elektrostal by train for as little as or as much as . The best price for this journey is . Journey Duration.
All the rest of the Syrians in the film have actually, you know, had to flee Syria because of the war. And Ebla - she actually comes from the Golan Heights, and she actually looks over Syria. She ...
DOVER, Del. — While Corey Heim's full-time focus may rest with his No. 11 Tricon Garage Toyota in the NASCAR Craftsman Truck Series, the 21-year-old Georgia native enters the race weekend at ...
Residents of a Moscow region town impacted by power outages have taken to the streets, demanding that local authorities restore heat to their homes as subzero temperatures grip the region, Russian ...
The ballet, originally known as "Tzigane" after its score by Maurice Ravel, was revived this season with a staging by Suzanne Farrell and a new name, "Errante," or wandering.
Fireplaces go beyond physical comforts too, offering psychological and emotional warmth even in modern households. However, new fireplace construction can go wrong, quickly. There is a lot to keep in mind, and there are plenty of safety measures that you need to get right.