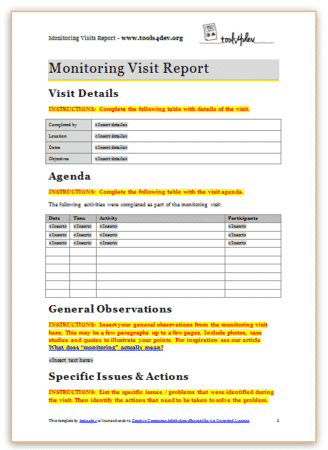
tools4dev Practical tools for international development


Monitoring visit report template
The purpose of a monitoring visit (sometimes called a supervision visit or a field visit) is to make sure that project activities are implemented the way they are described in the plan. It normally involves meeting with the people running the project, meeting with the participants, and observing the activities.
At the end of a monitoring visit, it is important to prepare a report that describes what you found. These reports will document any discrepancies between the plan and actual implementation, as well as improvements made by the project team.
This monitoring visit report template is appropriate when:
- You need to report the results of a monitoring visit, supervision visit, or field visit.
This monitoring visit report template is NOT appropriate when:
- Your organisation or donor already has a standard template for monitoring visit reports (in which case use their template).
Photo by U.S. Mission Uganda
Tags Monitoring & Evaluation
About Piroska Bisits Bullen
Related Articles

What can international development learn from tech start-ups?
13 May 2021

Social Enterprise Business Plan Template
12 May 2021

How to write an M&E framework – Free video tutorial & templates
10 September 2017

- Core Curriculum
- Web Seminars
- On-Boarding Programs
- On-Site Training
- On-Demand eLearning Courses
- Web Seminar Archives
- CCAD Programs
- GCP Training Assessments
- Mock Audits Findings Based Training
- Virtual Meetings Support Services
- Customized eLearning Solutions
- Licensing of Barnett's Content
- SOP Development Training
- Acquisition Integration
- Training Subscription
- Publications
- Course Catalog

- Training Courses
- GCP Training & Assessments
- Audits and Mock Inspections
- Licensing of Barnett's Content
- SOP Development & Training

Barnett International's Core Curriculum courses are comprehensive role-based clinical research training programs designed to provide industry professionals with hands-on training in a dynamic virtual setting. Held four times a year during our “Clinical Research Training Weeks,” courses offer clinical research-focused, performance-based training and core competency development.
How to Write Effective Monitoring Reports and Communications
Upcoming Courses
Modal body text goes here.

Clinical Monitors (CRAs) must document many details of the happenings at investigational sites, including Confirmation Letters to sites, Monitoring Visit Reports, Follow-Up Letters to sites, Telephone Contact Reports, Email/Faxes to sites, and Queries and Notes to File (NTF). All of these become essential documents as they demonstrate the compliance of the monitor and, thus, the sponsor in the conduct of the clinical trial. These are all eligible for inspection by the regulatory authorities at any time both during and after the study is completed and submitted for product approval. This is the same regulation for drugs, biologics, and devices. Effective writing skills are, therefore, extremely important so that we show the diligence and detail involved in effective monitoring. Increasingly, we notice that the Confirmation Letters, Monitoring Visit Reports, and Follow-Up Letters have discrepancies. This may be simple date inconsistencies, or critical data credibility issues. It is important that the monitor be aware of the importance of these issues in the review of study documentation. This module will provide some practical solutions to addressing document deficiencies as well as provide a practical understanding of how these documents provide evidence for the regulated activities of the investigator and the sponsor.
The monitor visit starts with a well-written Confirmation Letter informing the investigator and investigator’s staff of the expectations of the upcoming visit. An accurate and complete Monitoring Visit Report details all of the activities of the monitor in meeting the sponsor’s obligation during the actual monitor visit, including action items and demonstrable management of the site by the monitor. Queries must be well-written if they are to be understood by the study coordinator or Principal Investigator at the site. The Follow-Up Letter, which must detail the progress made on this visit and highlight any deficiencies for which the monitor expects resolution must agree with the action items listed in the Monitoring Visit Report. Written documentation of Telephone Contacts must be direct, accurate, and timely; other communications between monitor visits need to be associated with the proper events as well. This module will provide an understanding of the information required, importance of timely and well-documented discussions, and proper methods of filing this key documentation. This workshop is invaluable for the CRA as well as the individual who critiques the various reports.

- Describe the requirements of documenting monitoring activities
- Implement strategies for effective writing outside of the monitor visit
- Effectively manage site and sponsor activities and document them appropriately
- Recognize the importance of a well written Monitoring Visit Report
- Evaluate well-written and poorly-written material from actual studies
- Identify the appropriate use of Notes to File in both patient-related and study-related situations
- Write effective documents for various types of monitor visits

- Clinical Research Associates/Monitors
- Lead Clinical Research Associates
- Contract Clinical Research Associates
- Clinical Research Associate Managers
- Project and/or Study Managers
- Project and/or Clinical Trial Assistants
- Quality Assurance Personnel

Gary B. Freeman, M.S.
Click here for complete trainer biographies

Day 1: 9:00 a.m. – 4:00 p.m.
- Confirmation Letters, Follow-Up Letters
- Queries, Monitoring Visit Report
- Communication Outside the Monitor Visit (telephone, email faxes, Notes to File)

- Review a Monitoring Visit Report and evaluate examples of well-written and poorly written documentation of issues, deviations, and action items for follow-up
- Write sections of a Monitoring Visit Report based on a scenario provided
- Draft a Follow-up letter given some issues to review in the Monitoring Visit Report
- Critique Confirmation and Follow-up letters
- Discuss the importance of providing consistent information
- Critique a Telephone Contact Report
- Discuss the value of proper filing of documentation related to the visit but conducted outside of the actual visit Review several scenarios and associated NTFs and evaluate if the NTF was the most appropriate manner for managing and documenting the issue
- Learners are encouraged to bring specific work-related document samples for evaluation in light of best practices and GCP standards

Registration fees include assorted breakfast items that will be available each day ½ hour prior to the start of the seminar. Also included is a Networking Lunch that will be served each training day.
Special rates are available for multiple attendees from the same organization. Contact Melissa Dolen at 781-972-5418 to discuss your options and take advantage of the savings.
Click here for our seminar cancellation policy

Hold this course at your company!
For more information, contact Naila Ganatra at (215) 413-2471.
The Art and Science of Site Monitoring Visit Reports
An experienced CRA may follow all the rules and requirements for writing a site monitoring visit report (SMVR), but there are nuances that can make the difference between a report that simply follows that formula and one that really paints a picture its audience can understand.
Roslyn Hennessey, senior project manager at Westat describes the ideal SMVR as “a snapshot in time of what’s happening at the site.” To develop that snapshot, start by asking who will read the report, she advises. There are several types of audiences for SMVRs, all of which have slightly different interests:
- The sponsor wants to know about the performance of the site — is it in compliance with the protocol, are improvements needed, are there any major concerns;
- The PI and site staff want to know if additional training or procedural changes are needed;
- Regulatory authorities and auditors want to know if the site is in compliance and, if not, what corrective action was taken;
- Future monitors need enough information to ensure a smooth hand-off from one CRA to another.
It’s important to give all of the key stakeholders the “heart or the core of the information that they are most interested in,” she says.
And although an SMVR should be comprehensive, it also needs to be concise to avoid burying important information or tiring the reader. Writing an SMVR is an exercise in deciding what information needs to go in and what needs to stay out.
“We really need to get more efficient in terms of going just from point A to B,” she says, “but in that A to B, we need to include some important things.”
Hennessey recommends starting at the end. A monitor usually will have an overall message to convey in the report and should choose the information that supports that message. “Know what you want to say ahead of time and write to your conclusion,” she says. “What do I need to say to get me to that conclusion?”
Be analytical in what you write, she advises. A good SMVR is more than an “information dump.” When writing the report, provide the reader with some context instead of just transcribing notes. Explain what those notes indicate, Hennessey urges. Look for common denominators in the findings and identify root causes of problems. Pay attention to trends, from a single visit and across several visits.
In addition to being analytical, an SMVR should be objective. Elizabeth Weeks-Rowe, a clinical research training consultant, advises SMVR authors to keep the human factor in mind. A monitor may encounter many different personalities in a trial, but they should not be reflected in the site report.
“Taking the emotion out of verbiage is critical,” says Weeks-Rowe. “Be factual, not emotional.” If, for example, a principal investigator is less than professional, this impression should not be included in the report. Instead, address any outcome of that behavior. Making sure that all findings are actionable helps avoid an emotional narrative, she says.
Hennessey notes that an attentive monitor can enhance communication between the site and the sponsor. “Sometimes the investigators feel like the sponsor is too far removed,” she says, and wonder “where is my voice heard?” The investigator may feel more comfortable communicating with the CRA, who then passes the information on to the sponsor via the SMVR.
The last step in writing an effective SMVR is recommending corrective actions, Hennessey says. All issues/findings noted in the report should have an associated action and/or resolution. Have a “no finding left behind” policy, she advises. But, she cautions, don’t be overly prescriptive. It’s important that the PI and site staff take responsibility for problems and solutions.
Both Hennessey and Weeks-Rowe stress taking care of the basics, carefully reviewing the SMVR for misspellings and grammatical errors. Flaws in the presentation, Hennessey says, distract from the content and message and ultimately hurt the credibility of the monitor and the company.
Ultimately, the SMVR must be able to stand the test of time, Hennessey says. Trials can last for years and go through several CRAs along the way. “It’s not often that a CRA gets to work with a site from initiation to closeout,” she notes. And a regulatory request for further information or an audit can come at any time. In one case, she says, the FDA came back to question the SMVR 10 years after the fact.
Upcoming Events
Effective root cause analysis and capa investigations for drugs, devices and clinical trials, 2024 avoca quality consortium summit, featured products.

Surviving an FDA GCP Inspection: Resources for Investigators, Sponsors, CROs and IRBs

Best Practices for Clinical Trial Site Management
Featured stories.

Thought Leadership: Remote Patient Monitoring Gives New View of Safety in Cardiac Clinical Trials

Ask the Experts: Applying Quality by Design to Protocols

Clinical Trials Need Greater Representation of Obese Patients, Experts Say

FDA IT Modernization Plan Prioritizes Data-Sharing, AI, Collaboration and More
Standard operating procedures for risk-based monitoring of clinical trials, the information you need to adapt your monitoring plan to changing times..
The Best Tools and Resources for Productive Clinical Trial Monitoring Visits
If you work in Clinical Research, you know that there are different requirements for each type of Monitoring visit. Whether you are conducting a Pre-Study Visit (PSV), Site Initiation Visit (SIV), Interim Monitoring Visit (IMV)/Routine Monitoring Visit (RMV), or a Close-Out Visit (COV), each visit comes with its own set of nuances that the Clinical Research Associate (CRA) needs to be prepared for prior to traveling on-site or conducting the visit remotely. When a CRA’s visit goes well, the study can continue to move forward and stay on track for success.
When the goals of a Monitoring visit are not met, there is an increased risk that a Trial will be delayed and/or over budget or Patient Safety may be put at risk. Thankfully, there are ways to avoid unproductive Monitoring visits!
Monitoring Clinical Trials is one of the most important responsibilities for a CRA. With purposeful planning and organization, along with helpful Clinical Trial Monitoring tools and resources, CRAs can flourish in this role!
In this article, we focus on how a CRA can prepare for an Interim Monitoring Visit (IMV) but these tools and resources can be applied to all visits conducted either on-site or remotely by CRAs.
Typical CRA Responsibilities During an Interim Monitoring Visit
CRA responsibilities are based on the type of Visit being conducted. IMVs are the most common visit to occur during a Study’s lifecycle and usually happen every 4-6 weeks, depending on Site Enrollment. Here are the general tasks that CRAs address at an IMV or RMV:
- Ensure Study eligibility by reviewing the Source documentation, medical records, and Case Report Form entries for all patients, especially those recently randomized. This includes the Informed Consent Form (ICF) collected at Screening, Randomization data, and Follow-up data, as well as Labs, ECGs, and vitals.
- Perform a review of all reported Safety issues such as Adverse Events, Serious Adverse Events, and Adverse Events of Special Interest.
- Examine the Investigational Product (IP) and Study supplies.
- Evaluate Study personnel to ensure all are appropriately delegated and trained to perform Study procedures.
- Assess the Site facilities for any changes since the Pre-Study Visit.
- Meet with the Principal Investigator (PI) and other Site staff to review the overall progress of the Study and the CRA’s findings. Provide re-training if needed and answer any questions the site may have.
The Association of Clinical Research Professionals (ACRP) created this extensive checklist of tasks for a monitoring visit.
During each visit, CRAs are responsible for documenting everything they observe and discuss. Following the visit, this information is compiled into a Clinical Monitoring Report. Here are 5 guidelines for writing an useful clinical monitoring report from MasterControl.
The Best Tools to Bring to a Monitoring Visit
The Clinical Research Monitoring tools that you bring have a meaningful impact on the success of a Monitoring visit.
Here are the three best Clinical Trial Monitoring tools to have at the Study site – or in your briefcase!
- The Visit To Do List Pad makes planning your visit easier. Keep it with you while you are on site to help you stay focused and on task. If you’d prefer to skip the paper, the Visit To Do List is also available as a Digital Download!
- Throughout your Monitoring visit, record Action Items on the Action Item Carbonless Pad . At the end of your Visit, leave one copy with the Site staff so that everyone is on the same page for the Action Items that need to be completed. Use the other copy to write your Monitoring Visit Report and Follow-up Letter. The Action Item Carbonless Pad is available for FREE for a limited time: https://clinessentials.com/clinical-trial-monitoring-tool/ . Download your copy today!
- Last, take your Monitoring a step further with CRA Audit Notes that keep you organized – and give the site staff a visual indication of the tasks that must be accomplished. This simple upgrade from the yellow sticky notes typically used during monitoring help ensure your Action Items are addressed in record time. Along with being one of the best Site Monitoring tools, they also improve communication and effectiveness for Clinical Research teams!
If you are interested in a variety of the Monitoring tools listed above, check out the Bundles that allow you to purchase items at a discounted rate.
These Clinical Trial Monitoring tools alleviate high-stress, nonstop, jam-packed Site visits– making visits more productive, organized, and successful!
Steps for a Successful Monitoring Visit
Being prepared for a Monitoring visit is instrumental to the success of the Visit. When both the Clinical Research Associate and the Research Site are prepared, Monitoring visits run smoothly and efficiently.
With proper communication and a simple 5 step process , preparing for a Monitoring visit does not have to be overly time-consuming. Plus, spending time in advance will be time saved ensuring a productive, organized visit!
- Plan the visit. Make sure that your plans are based on the Protocol or directions from the Clinical Research team (Clinical Trial Manager, Lead CRA and/or Sponsor).
- Pack so that you are prepared . This free downloadable checklist gives our Top 5 Workbag Essentials and is a great way to simplify your packing and keep you organized.
- Review the Study Protocol and have a thorough understanding of the entire document.
- Highlight the Action Items. If you have questions or actions needing to be addressed during the visit, try to send them to the site in advance so they can be ready to address during your visit. It is always more effective to discuss and resolve questions or actions items while on site. Once you leave the site it may be challenging to get the site to make it a priority or you will have to review remotely.
- Bring Clinical Trial Monitoring tools! (See The Best tools listed above!)
For more tips, check out this YouTube video about how CRAs should prepare for a routine monitoring visit.
Conclusion As a leader and essential member of the Research team, the Site staff will appreciate and respect you for your thoroughness and organization – and this makes for open communication and a better working relationship!
Introduce your Research teams to the time-saving tools that lead to successful monitoring visits. These same tools can also be used at the site level to keep the trial moving and on the right track in between Monitoring visits.
For more Clinical Trial Monitoring tools and resources that help Clinical Research Professionals become more efficient, visit www.clinessentials.com.
By: Tiffany Ashton MAS, CCRA, Director of Clinical Operations at ClinEssentials

Share this article
Other posts.

Design of Virtual Focus Group Checklist

Learn eCORE Annual Review

Considerations for Design of Virtual Focus Groups

Unique Challenges of Conducting Population-based Surveys in Low- and Middle-Income Countries

Learn eCORE Presents a Free Research Compliance Webinar Hosted by VA BIO

Tips for Achieving Clinical Trials Success through Lean Six Sigma and Stakeholder Collaboration

The Key Information Section in Consent – Why, What, and How

The Clinical Project Manager’s Role in Ensuring Success in Clinical Trials

An Interview with Dr. Stephen Rosenfeld

Is Deception in Research Ethical?

What is a Certificate of Confidentiality and Does My Research Need One?

Successful IRB Submissions Part 2: Common Mistakes Noted During IRB Review

Successful IRB Submissions Part 1: Three Simple Tips

Understanding Investigator Conflict of Interest (ICOI)

New Value-Added Feature

Resources for Ethical Issues in the Use of Artificial Intelligence (AI) in Human Subjects Research

Reid Blackman on AI ethics

Human Research Protections in Research with Real World Data

Learn eCORE Year In Review

Learn eCORE’s Ann Hardy to present at the 2022 PRIM&R Conference

Diversity and Inclusion in the All of Us Research Program Part II: How All of Us is Creating Diverse Data Sets and Encouraging Their Broad Use

Diversity and Inclusion in the All of Us Research Program Part I: Enrolling Diverse Participants

Defining Research for IRB Review of SBER Studies

What is Human Subjects Research?

Designing Health Literate Clinical Research

Diverse Clinical Trials and Health Equity

What Is Social, Behavioral, and Educational Research (SBER) and How Does It Differ From Biomedical Research?

Long COVID: The Pandemic Within the Pandemic?

Learn eCORE Partners with NIH Research Initiative

Clinical Trials: A Participant’s Perspective

Ethical Issues for Research Involving Participants with Diminished Capacity to Consent

Decentralized Clinical Trials: Here to Stay?

The Pandemic’s Silver Lining: Increasing Participant-Centered Research Approaches

Are The Belmont Report Principles Still Relevant for Today’s Research?

Is Learn eCORE Training Right for You?
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar
- Skip to footer
The Medical Device Business Journal — Medical Device News & Articles | MassDevice
5 guidelines for writing a useful clinical monitoring report
January 31, 2019 By Sponsored Content

Taking Your Report from Good to Great
By Brandy Chittester, Chief of Clinical Operations
A well-written monitoring report is an essential part of documenting clinical trial oversight. In addition to being required by ISO and ICH guidelines, it also tells the story of the clinical trial to the FDA, demonstrating site performance and sponsor oversight during an FDA inspection.
Unfortunately, this important task often doesn’t get the attention it deserves. Between scheduling visits, traveling and conducting the visits, it can fall down on a monitor’s priority list.
Whether you’re a monitor out in the trenches or a sponsor overseeing a study at a high level, here are a few important guidelines you and your staff should follow to ensure your clinical monitoring reports are accurate and complete.
1. Do Your Homework Before the Site Visit
To ensure all the information will be available to write the report, you should be thoroughly prepared for the site visit. Before you arrive on site, you should be able to answer the following questions:
- What data should be source-verified?
- How many queries are outstanding?
- Will the regulatory documents should be reviewed and what updates are needed?
- What data and action items still are outstanding?
A monitor’s time on site is often limited. Taking the time to look into these issues ahead of time will help you prioritize tasks and make the most of the time you have.
In addition to site-specific preparation, you must also be sure to understand the visit report template and the purpose of each question, since these templates can vary from one sponsor to the next. Likewise, you should always consult the monitoring plan for the study to be sure to complete activities during the visit as required by the sponsor.
2. Take Good Notes During the Visit
To make sure you don’t miss an important step, you should keep the monitoring report template open throughout the visit, making note of activities as they are completed.
We can’t overstate the importance of taking good notes. So many things that happen during a monitoring visit seem obvious at the time (like a follow-up item to correct a source worksheet or to re-review select data points), but in many cases, those items are forgotten soon after you leave a site.
Some suggested methods for keeping notes might be for you to mark off sections of the report as tasks are completed or use highlighting or another font color to show what requires assistance from the site. Although monitors always want to cross off every item on the list during a site visit, there almost always will be items that require follow-up later. No matter how you choose to do this, you need make note of what items require your attention after the visit to ensure nothing slips through the cracks.
Each monitor will find his or her own way of taking notes, but filling in the report during the visit is a good way to keep important issues top of mind.
3. Write the Report as Soon as Possible
The report should be written as soon as possible after the visit. The best-case scenario is to write the report before preparing for and going on the next visit, but this is not always possible or practical. The next best practice would be to write one report before writing the next. When a report sits incomplete for a few weeks and other visits have taken place in the meantime, the likelihood that the report will be accurate and complete is low, even with the most thorough notes.
4. Check Reports Carefully
A great monitoring report should be clear, concise and grammatically correct. Sloppy oversights, such as grammar mistakes and carrying over information from a previous report, can diminish confidence in your work. Copying and pasting information from a previous report may seem like a shortcut, but it leaves too much room for error. You should start fresh and check your work carefully when you’re finished.
5. Be Sure the Report Only Includes Essential Information
A good clinical monitoring report should be a summary of items you reviewed during the visit—no more, no less.
Too much narrative or detailed descriptions of what was in compliance can make finding what was out of compliance more difficult. You also should avoid documenting details that are already on record elsewhere. For example, if an adverse event has been submitted to the sponsor, there is no reason to include detailed information about the event again in the monitoring report.
Bonus: Take the Report from Good to GREAT
In general, monitors do an acceptable job documenting in their reports the issues noted during site visits. However, a monitor’s job doesn’t end with simply documenting issues; he or she also needs to document the efforts made to bring the site into compliance. Additionally, a monitor should document any discussions or actions taking place to prevent issues from reoccurring. Adding such details to monitoring reports illustrates the ongoing efforts by the sponsor and site to work together to address issues in real time and ensure the study stays compliant.
Writing effective monitoring reports requires an in-depth knowledge of the job, the study protocol, the site, their practices, the sponsor’s procedures, the monitoring plan, the report template and, of course, the regulations.
As a leading medical device CRO, IMARC Research has extensive experience in writing monitoring reports. Before you write your next monitoring report, download this resource for more tips and a checklist you can follow to ensure your clinical monitoring reports are as complete, accurate and useful as possible.
More recent news
- Philips gets judge’s OK to settle Respironics recall economic losses
- How Philips’ significant respiratory devices recall unfolded
- Roivios wins FDA breakthrough nod for renal assist device

MASSDEVICE MEDICAL NETWORK
Devicetalks webinars, podcasts, & discussions.
- PRO Courses Guides New Tech Help Pro Expert Videos About wikiHow Pro Upgrade Sign In
- EDIT Edit this Article
- EXPLORE Tech Help Pro About Us Random Article Quizzes Request a New Article Community Dashboard This Or That Game Popular Categories Arts and Entertainment Artwork Books Movies Computers and Electronics Computers Phone Skills Technology Hacks Health Men's Health Mental Health Women's Health Relationships Dating Love Relationship Issues Hobbies and Crafts Crafts Drawing Games Education & Communication Communication Skills Personal Development Studying Personal Care and Style Fashion Hair Care Personal Hygiene Youth Personal Care School Stuff Dating All Categories Arts and Entertainment Finance and Business Home and Garden Relationship Quizzes Cars & Other Vehicles Food and Entertaining Personal Care and Style Sports and Fitness Computers and Electronics Health Pets and Animals Travel Education & Communication Hobbies and Crafts Philosophy and Religion Work World Family Life Holidays and Traditions Relationships Youth
- Browse Articles
- Learn Something New
- Quizzes Hot
- This Or That Game New
- Train Your Brain
- Explore More
- Support wikiHow
- About wikiHow
- Log in / Sign up
- Education and Communications
- Official Writing
- Report Writing
How to Write a Visit Report
Last Updated: March 30, 2024 References
This article was co-authored by Madison Boehm . Madison Boehm is a Business Advisor and the Co-Founder of Jaxson Maximus, a men’s salon and custom clothiers based in southern Florida. She specializes in business development, operations, and finance. Additionally, she has experience in the salon, clothing, and retail sectors. Madison holds a BBA in Entrepreneurship and Marketing from The University of Houston. This article has been viewed 653,518 times.
Whether you’re a student or a professional, a visit report helps you document the procedures and processes at an industrial or corporate location. These reports are fairly straightforward. Describe the site first and explain what you did while you were there. If required, reflect on what you learned during your visit. No additional research or information is needed.
Writing a Visit Report
Explain the site's purpose, operations, and what happened during the visit. Identify the site's strengths and weaknesses, along with your recommendations for improvement. Include relevant photos or diagrams to supplement your report.
Describing the Site

- Reports are usually only 2-3 pages long, but in some cases, these reports may be much longer.
- In some cases, you may be asked to give recommendations or opinions about the site. In other cases, you will be asked only to describe the site.
- Ask your boss or instructor for models of other visit reports. If you can't get a model, look up samples online.

- If you visited a factory, explain what it is producing and what equipment it uses.
- If you visited a construction site, describe what is being constructed and how far along the construction is. You should also describe the terrain of the site and the layout.
- If you’re visiting a business, describe what the business does. State which department or part of the business you visited.
- If you’re visiting a school, identify which grades they teach. Note how many students attend the school. Name the teachers whose classes you observed.

- Who did you talk to? What did they tell you?
- What did you see at the site?
- What events took place? Did you attend a seminar, Q&A session, or interview?
- Did you see any demonstrations of equipment or techniques?

- For example, at a car factory, describe whether the cars are made by robots or humans. Describe each step of the assembly line.
- If you're visiting a business, talk about different departments within the business. Describe their corporate structure and identify what programs they use to conduct their business.
Reflecting on Your Visit

- Is there something you didn’t realize before that you learned while at the site?
- Who at the site provided helpful information?
- What was your favorite part of the visit and why?

- For example, you might state that the factory uses the latest technology but point out that employees need more training to work with the new equipment.
- If there was anything important left out of the visit, state what it was. For example, maybe you were hoping to see the main factory floor or to talk to the manager.

- Tailor your recommendations to the organization or institution that owns the site. What is practical and reasonable for them to do to improve their site?
- Be specific. Don’t just say they need to improve infrastructure. State what type of equipment they need or give advice on how to improve employee morale.
Formatting Your Report

- If you are following a certain style guideline, like APA or Chicago style, make sure to format the title page according to the rules of the handbook.

- Don’t just say “the visit was interesting” or “I was bored.” Be specific when describing what you learned or saw.

Sample Visit Report

Community Q&A
You Might Also Like

- ↑ http://services.unimelb.edu.au/__data/assets/pdf_file/0010/471286/Site_Reports_for_Engineers_Update_051112.pdf
- ↑ https://www.examples.com/business/visit-report.html
- ↑ https://www.thepensters.com/blog/industrial-visit-report-writing/
- ↑ https://eclass.aueb.gr/modules/document/file.php/ME342/Report%20Drafting.pdf
About This Article

To write a visit report, start by including a general introduction that tells your audience where and when you visited, who your contact was, and how you got there. Once you have the introduction written out, take 1 to 2 paragraphs to describe the purpose of the site you visited, including details like the size and layout. If you visited a business, talk about what the business does and describe any specific departments you went to. Then, summarize what happened during your visit in chronological order. Make sure to include people you met and what they told you. Toward the end of your report, reflect on your visit by identifying any strengths and weaknesses in how the site operates and provide any recommendations for improvement. For more help, including how to format your report, read on! Did this summary help you? Yes No
- Send fan mail to authors
Reader Success Stories
Betty Tarutia
Jul 9, 2020
Did this article help you?
Jayani Rathnayake
Aug 6, 2019
Jun 13, 2019
Atremedaki Phawa
Aug 19, 2019

Featured Articles

Trending Articles

Watch Articles

- Terms of Use
- Privacy Policy
- Do Not Sell or Share My Info
- Not Selling Info
wikiHow Tech Help Pro:
Level up your tech skills and stay ahead of the curve

Providing courses, products & services so Clinical Research Professionals can thrive & achieve fulfilling careers.
How to Plan a Productive Site Monitoring Visit
by ClinEssentials Team | Feb 28, 2022 | Tools for Research Professionals , Clinical Research Associates , Clinical Research Coordinators , Tips for Research Professionals | 0 comments

Many Clinical Research Associates are unprepared for their clinical trial monitoring visits, which is an important part of their role. It is understandable – CRAs are busy and it can be hard to find time for planning where there are so many other tasks to complete right away.
Monitoring visits are when CRAs ensure their studies are staying in compliance and on track. Creating a plan for the allotted time is the best way to ensure a productive, efficient monitoring visit.
What Happens at a Clinical Trial Monitoring Visit
Monitoring visits are typically very full days – jam-packed with all of the action items the CRA and the site need and want to accomplish.
A CRA may visit a site for site initiation, an Interim Monitoring Visit (IMV) – also called a Routine Monitoring Visit (RMV), or a study close-out. IMVs are the most common and usually happen every 4-6 weeks. However, monitoring plans differ and are determined by the Sponsor or CRO before a trial begins.
3 objectives of a monitoring visit
While there are many things that a CRA wants to successfully complete at a monitoring visit, there are three main objectives for each visit:
- Discover any data errors and/or discrepancies
- Evaluate the site staff’s understanding of the protocol and procedures
- Determine compliance
Standard tasks, meetings and goals of a monitoring check-in visit
Tasks are based on the reason for the visit. Since an Interim Monitoring Visit is the most common, here are the general business items that a CRA will address at a monitoring check-in visit:
- Review the regulatory binder, study documentation, and CRF entries
- Audit screening, enrollment, visit, and follow up data
- Conduct Source Data Verification
- Perform a safety assessment
- Examine the investigational product and study supplies
- Evaluate study personnel and site facilities
- Meet with the Principal Investigator and site staff
The Association of Clinical Research Professionals (ACRP) created this extensive checklist of tasks for a monitoring visit .
CRAs are responsible for documenting everything they observe, notice, and discuss during a visit. Following the visit, this information is compiled into a clinical monitoring report. Here are 5 guidelines for writing a useful clinical monitoring report from MasterControl.
Consequences of being Unprepared for a Monitoring Visit
Being prepared for a monitoring visit is instrumental in the success of the visit. When both the Clinical Research Associate and the research site are prepared, monitoring visits run smoothly and efficiently. The patient volunteers, clinical research team, and CRO or Sponsor are all negatively affected when the CRA or the site staff has not taken the time to prepare for a monitoring visit.
Scheduling conflicts and unavailability of key team members can lead to important meetings that have to wait until the next visit.
The site may not have the necessary documents or the regulatory binder ready for the CRA to review, which is one of the primary activities for most monitoring visits.
When monitoring visits are not successful and all essential tasks and meetings do not occur, the trial can be delayed – and potentially over budget. Sponsors and CROs may lose trust in the site and the staff.
3 Steps for a Successful Monitoring Visit
With proper communication and a simple 3 step process, preparing for a monitoring visit does not have to be overly time-consuming. Plus, spending time in advance will be time saved through an efficient, organized visit!
- Plan the visit. Download this free checklist that will lead to a well-planned visit. It even includes a list of documents to have on your computer and in your work bag. (The checklist can be modified based on the protocol or directions from the research team.)
- Review the study protocol and have a thorough understanding of the entire document.
- Bring clinical trial monitoring tools! Use the Visit To Do List to stay focused and on task. Provide the site staff with a copy of the Action Item List – and use the other copy to write the Monitoring Visit Report and Follow-Up Letter.
For more tips, check out Dan Sfera’s YouTube video about how CRAs should prepare for a site monitoring visit .
Stop showing up unprepared for a monitoring visit! As a leader and essential member of the research team, the site staff will appreciate and respect you for your thoroughness and organization – and this will be evident in their work!
For more great ideas and monitoring tools that help Clinical Research Professionals become more efficient, visit www.clinessentials.com .
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Submit Comment
More Resources


How to Get into Clinical Research
by ClinEssentials Team | Feb 7, 2024 | Clinical Research Careers , Clinical Research Coordinators , Clinical Trial Assistants , Tips for Research Professionals

How Clinical Trial Managers Are Involved with Study Recruitment
by ClinEssentials Team | Jan 10, 2024 | Clinical Research Careers , Clinical Trial Managers , Tips for Research Professionals

CRA Responsibilities for Each Stage of a Clinical Trial
by ClinEssentials Team | Dec 27, 2023 | Clinical Research Careers , Clinical Research Associates , Tips for Research Professionals

How to Review a Clinical Trial Protocol (Free Checklist Included)
by ClinEssentials Team | Nov 8, 2023 | Clinical Research Careers , Clinical Trial Managers , Tips for Research Professionals , Tools for Research Professionals
Gaining Efficiencies in the Monitoring Visit Report Process
In a previous post , we explored how the use of monitoring reviewer comments in Vault CTMS improves monitoring efficiency. In this post, Ora discusses how they are using the feature to optimize trial processes.
Ora, Inc . is a full-service research organization specializing in pre-clinical and clinical ophthalmic drug and device development. Monitoring is one of our largest groups with the greatest number of users, so streamlining processes and reducing costs is critical.
We continue to optimize our business processes and innovate with Vault CTMS by adopting new features as soon as Veeva releases them. We implemented monitoring reviewer comments in our Vault two days after the feature was released in May 2019. Our goal was to streamline the 60-80 monitoring events we have each month. Since going live with the feature, CRAs have added over 3,000 reviewer comments, which prompted us to do a deeper analysis of the comments.
Deep Dive: Ora’s Experience & Lessons Learned with Monitoring Reviewer Comments
Monitoring reviewer comments are captured as reportable data in Vault CTMS and configurable dashboards give us the ability to visualize trends and drill deeper into the data. We created dashboards that show at-a-glance KPIs, including:
- The number of reviewer comments over time
- The top five questions with the most comments
- IMV (interim monitoring visit) reviewer comments by monitoring event section (e.g, protocol, IRB and regulatory, facilities and equipment, eCRF and SDV, etc.)
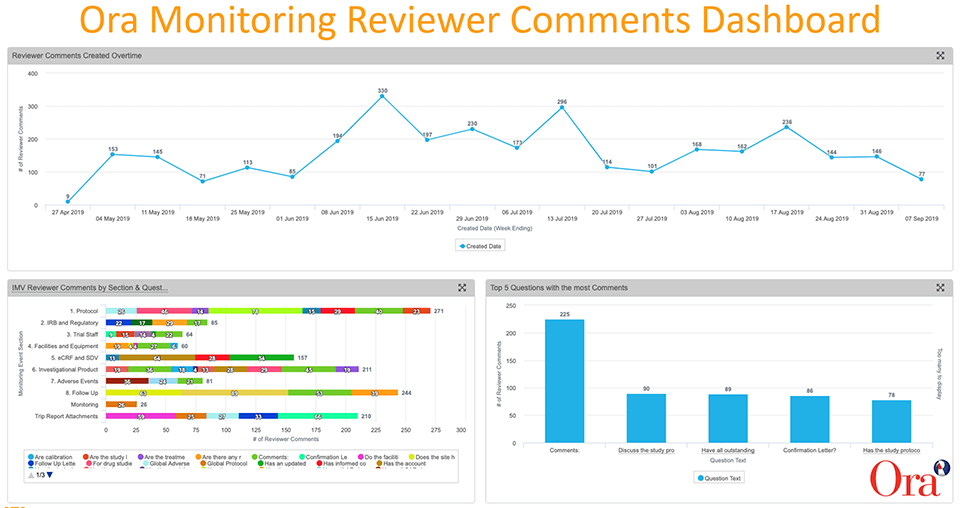
The analysis of the data allowed us to identify trends, spot issues, and take action.
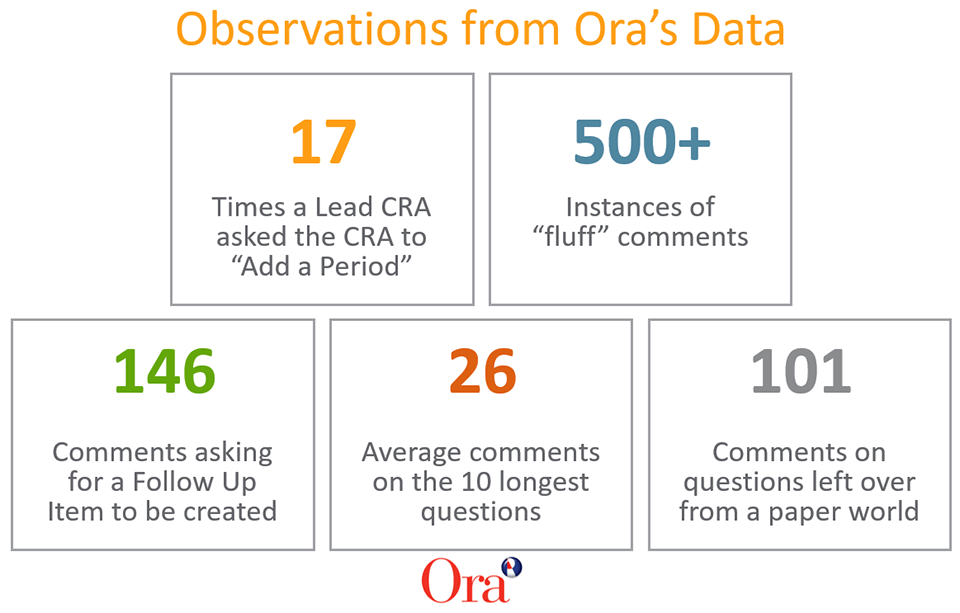
1. FINDING: Reviewer comments were significantly higher for site evaluation visits (SEVs). SEVs have an average of 32 comments per monitoring event, compared to IMVs with an average of 8.8 comments.
ACTION: Investigate comments logged on SEVs to determine why there are 4x as many comments on those reports compared to IMVs. We are currently analyzing the data to determine if the high number of comments are a result of CRAs receiving new information about sites, if they need refresher training, or if the SEV questions need to be simplified. Once the root cause is identified, we can take data-driven action.
2. FINDING: Open-ended questions (where users can type in their response) and longer questions have the highest number of comments. We have a question called “Comments?” on our Vault monitoring events, where CRAs can type any response. This question had the highest number of review comments (180+ responses). We also found questions that exceed 100 characters tend to be more confusing for CRAs, as we suspect they are unsure what should be populated in the field.
ACTION: Restructure the questions. We will reduce the number of open-ended questions, and also cut down the character count, as the data shows that shorter, succinct questions have fewer comments.
3. FINDING: 17% of comments were responses such as “ok” or “resolved.” We have 500+ instances of “fluff” comments. For example, a study manager may ask a CRA to update a protocol deviation, and the CRA responds to the comment with “ok” or “resolved,” then resolves both reviewer comments.
ACTION: Retrain CRAs on the correct process for resolving monitoring reviewer comments. CRAs will have a refresher course so they know they can resolve the comment, rather than reply.
4. FINDING: Some reviewer comments are approved after the required 10 days. Ora’s compliance procedures require that monitoring events are approved within 10 days. Most comments are resolved within the first three days. However, we found instances where comments were resolved after 10 days (and thus monitoring events were approved after our 10-day SLA).
ACTION: View weekly dashboard to ensure we are compliant with company policies. We are closely monitoring when reviewer comments aren’t resolved within the required timeline to determine if there are deeper issues that require corrective action.
5. FINDING: Latent questions from previous paper-based trip reports are still active. These are questions left over from our days of paper trip reports. However, Vault CTMS automatically generates confirmation and follow-up letters and relates those documents to the monitoring event. This duplicates data, which we found is confusing to CRAs who must answer a question that is automatically populated by the field on the monitoring event.
ACTION: Remove redundant questions from monitoring events. Our aim is to alleviate confusion and speed the process of completing the form.
We look forward to taking action on these patterns and continuing to streamline and optimize trial management with Vault CTMS. Watch this 13-minute video to learn more about our experience with monitoring reviewer comments.
Interested in learning more about how Veeva can help?
Dashpivot article – Site Visit Report example

Site Visit Report example
What is a site visit report.
A site visit report is a formal document that provides a detailed account of a visit to a particular location or project site.
It records the observations, activities, conditions, discussions, and any deviations or issues identified during the visit.
The report often includes recommendations or action items based on these findings.
It serves as an official record, aids in tracking progress or compliance, and can guide future decision-making.
What does the site visit report example cover?
Here's what's covered in the site visit report example:
- Report Title: Clearly indicating it's a "Site Visit Report."
- Project Name/Title: Name of the project or site.
- Location: Address or description of the site visited.
- Date of Visit: The exact date the visit took place.
- Prepared By: Name of the person or team who prepared the report.
- Introduction/Objective: A brief section detailing the purpose and objectives of the site visit.
- Attendees/Participants: A list of individuals present during the visit, including their roles or affiliations.
- Summary of Activities/Observations: A concise overview of what was done and seen during the visit.
- Project Progress: Status of ongoing work.
- Safety Measures: Observations related to safety precautions, PPE usage, and potential hazards.
- Quality of Work: Comments on the quality of work done so far.
- Equipment & Resources: Status and condition of machinery, tools, and other resources.
- Personnel: Feedback on staff performance, skill levels, or interactions.
- Issues or Concerns Identified: Any problems, discrepancies, or potential risks noticed during the visit.
- Recommendations: Based on observations and identified issues, suggest corrective actions, improvements, or next steps.
- Photos and Diagrams: Visual documentation can be invaluable in a site report. Include relevant photos with clear captions to illustrate points made in the report.
- Conclusion: Sum up the main findings and the overall impression from the site visit.
- Next Steps/Follow-Up Actions: Any scheduled follow-up visits, tasks to be done, or decisions to be made after the site visit.
- Attachments/Appendices: Additional materials, notes, or detailed data supporting the report's content.
- Signatures: Depending on the report's formality, it might be necessary for the person preparing the report and perhaps a superior or project stakeholder to sign off on its contents.
A well-prepared site visit report should be clear, concise, and structured. It provides a factual and objective account of the visit and serves as a vital tool for communication, decision-making, and record-keeping.
Site Visit Report example and sample
Below is an example of a site visit report in action. You can use this example in its entirety or sample it as needed.

Use a free Site Visit Report template based on this Site Visit Report example
Digitise this site visit report example.
Make it easy for your team to fill out site visit reports by using a standardised site visit report template .
The free digital site visit report comes pre-built with all the fields, section and information from the site visit report example above for your team to carry out detailed reports.
Customise the report with any extra information you need captured from your site visit reports with the drag and drop form builder.
Distribute your digital site visit report for your team on mobile or tablet so they can fill it out on site while the information is still fresh and at hand.
Create digital workflows for your site visit reports
Make it easy for your team to request, record and sign off on site visit reports by utilising a dedicated a site visit report app .
Automated workflows move a site visit request from planning to recording to signoff a smooth and simple process.
Quickly and easily share completed site visit reports as perfectly formatted PDF or CSV so your team is always across what's been recorded.
Take photos of site progress on site via your mobile or tablet, attach directly to your site visit reports with automatic timestamps, geotagging, photo markup and more.

Site diary template
Complete and organise your daily diaries more efficiently.

Meeting Minutes template
Capture, record and organise those meeting minutes.

Progress Claim template
Streamline and automate the progress claim process to get paid faster and look more professional.
Sitemate builds best in class tools for built world companies.
About Nick Chernih
Nick is the Senior Marketing Manager at Sitemate. He wants more people in the Built World to see the potential of doing things a different way - just because things are done one way doesn't mean it's the best way for you.
Leave a Comment Cancel Reply
Save my name, email, and website in this browser for the next time I comment.
How to write a good monitoring and evaluation report - guidelines and best practices
July 27, 2021.
Reporting is an integral part of any monitoring and evaluation plan or framework. Good reporting enables organisations to communicate the value of their work and their impact while allowing them to demonstrate aid effectiveness and enhance performance, collaboration, learning and adaptation within their organisation and throughout the entire project cycle.
In this article, we will explain what monitoring and evaluation reporting is, how it’s done and how your organisation can benefit from periodic reporting. Plus, stay with us as we walk you through some of the best practices of M&E reporting – M&E report formats and frequency, what to include in your M&E report and some top guidelines from experts to help you streamline your reporting process and write reports that are credible and constructive.
What is monitoring and evaluation (M&E) reporting and how is it done?
Reporting is the documentation and communication of M&E results to appropriate audiences at specified times. The key purpose of reporting may be to account for funds expended, to provide rich data for the decision-making process or to improve targeting and coordination of investments and on-ground actions. Reporting can be done at a project or program level. Most M&E reports include financial summary of a project as well as updates on its progress and achievements, activities undertaken, inputs supplied, money disbursed, key findings, results, impacts, plus, conclusions and recommendations from the interventions that have been compiled from various monitoring and evaluation activities and data sources.
The goal of reporting is to present these collected and analyzed data as information or evidence to key stakeholders and investors to utilize and to increase their confidence in the project and the implementing team.
Benefits of periodic monitoring and evaluation (M&E) reporting
Periodic reporting on M&E data helps internal staff and management teams to assess and communicate their transparency and accountability to their stakeholders, partners, funders, beneficiaries and others. It enables them to identify and interpret the progress their interventions have made against their set targets and indicators and its impact in the community of interest and its people.
M&E reports also help the team to test the effectiveness of their underlying assumptions, project activities, design, strategy and suggest ways for future adaptation and improvements. Moreover, M&E reports allow the team to identify and share challenges they have encountered, unexpected changes that have emerged in the process, along with underlying reasons for under-performance or shortcomings of existing management and monitoring systems and their proposed recommendations and action plans for improvements of subsequent work plans and sustainability of their results.
M&E reports help the stakeholders, partners, donors and others involved in the project to grasp a clear picture of the performance of the project and its real impact on the ground, helping them make evidence-based decisions to improve the current intervention and design better projects in the future. These reports also help the higher-up management teams to make adjustments to their internal operations and make recommendations for the state or country level policy amendments. Moreover, the evidence from such reports also help the donors to direct aid and funding to where it’s needed most – to address the most critical issues and help the most vulnerable communities in need.
Monitoring and Evaluation (M&E) reports - frequency and formats
Each organisation is different and so are its projects, hence every organisation has its own unique reporting system. M&E reports can be produced and distributed on a weekly, bi-weekly, monthly, quarterly, bi-annually or on an annual basis. Weekly and bi-weekly reports are usually concise and shared with the internal team and some external stakeholders to keep them up to date on the project progress against their targets, budget, any changes made to the project or the implementing team etc. However, monthly, quarterly bi-annual or annual reports are much more comprehensive and include more details and evidence on the progress of the intervention, project inputs, activities, outputs, outcomes, lessons learned, recommendations etc. These are shared with a wider audience, including partners, donors and other stakeholders.
Many organisations report on their M&E data in a traditional narrative format in the form of paper reports. However, with the emergence of digital technology and approaches, many others are adopting new and innovative mediums to report, such as videos, recorded audio, interactive media, mapping, data visualization, interactive dashboards, online presentations and more. How often and in which format reports are produced and distributed depends on the organisation and its reporting system. The frequency and format also depend on the nature of the project, its M&E plan and log frames, the resources available, the requirements of the donors and the audience of the report – how and by whom reporting data will be utilized etc.
Note that many organisations report on their monitoring and evaluation activities together, however, there are exceptions. In some organisations, monitoring and evaluation reporting is done separately. Monitoring is done by implementing staff members and it’s undertaken more frequently than evaluation. Evaluation on the other hand could be undertaken by internal staff or external consultants.
See how TolaData’s configurable dashboards can add value to your organisation’s reporting processes.
Some key points to keep in mind before writing your M&E report
- Have you identified indicators for each project activity? Indicators must be relevant and easy to track, measure and report. Here’s how to create indicators that make sense.
- Have you identified key monitoring and evaluation questions and determined what data will need to be collected and which tools and methods will be used to collect them?
- How will you collect, consolidate and analyse your data and distil insights from it? Here’s how you can integrate data from multiple sources and tools.
- How will you compile and consolidate the findings and results and include them in your report?
- How frequently will you send out your report and in what format?
- Be mindful of the audience and timing of your report. M&E reports are effective only when they are submitted to the right people at the right time and facilitates corrective decision making.
What goes into an M&E report?
Please note that this list includes some common elements included in an M&E report. As mentioned above, every organisation and every project is different and so is their reporting system. Therefore, you will have to adjust this list according to the nature of your project, the requirements of your donors and stakeholders and the audience of your report.

This list has been adapted from the Evaluation Report Checklist by USAID .
Guidelines for writing credible and constructive monitoring and evaluation (M&E) reports.
The following guidelines have been adapted from the document – Monitoring and Evaluation Guidelines from the UN World Food Programme.
- To help ensure efficiency, the purpose of reporting should be clearly defined. Be sure to include a section in the introduction describing the need to produce this report and its anticipated use.
- Make sure the i nformation you are providing is accurate, complete, reliable, timely, relevant and easy to understand.
- Be clear who your audience is and ensure that the information is meaningful and useful to them. If needed, tailor the content, format and timing of the report to suit the audiences’ needs. Information is of little value if it is too late or infrequent for its intended purpose.
- Consistency is key. Reporting should adopt units and formats that allow comparison over time, enabling progress to be tracked against indicators, targets and other agreed-upon milestones.
- Make sure your report is concise and the layout clean and consistent.
- Focus on results and accomplishments and link the use of resources allocated to their delivery and use.
- Be sure to include a section describing the data sources and data collection methods used so that your findings are objectively verifiable.
- Write in plain language that can be understood by the target audience. Avoid complex jargons and details if possible and be consistent in your use of terminology, definitions and descriptions of partners, activities and places. Be sure to define any technical terms or acronyms in the annex section.
- Make use of graphs and charts to communicate your findings. Present complex data with the help of figures, summary tables, maps, photographs, and graphs that are easier to understand.
- Include references for sources and authorities.
- Include a table of contents for reports over 5 pages in length.
- Make sure your reporting system is cost effective. Avoid excessive, unnecessary reporting. Information overload is costly and can burden information flow and the potential of using other more relevant information.
- Be open to feedback. Make sure to include an email address, a physical address or a telephone number for the recipients to send their feedback on the report.
As we can see, there are many benefits of good and timely reporting and it should be a part of every development project and its monitoring and evaluation system. However, many organisations continue to report on their progress only as a part of the donor requirement, without giving much heed to the huge prospect of collaboration, learning, adaptation and improvement. Therefore, a good reporting system should have a balance of all these elements, plus quality project management and good coordination and communication flow within the team.
We hope you found our article helpful. If you have any comments or suggestions on how we can improve it, please leave a comment below.
Key References
- Monitoring and evaluation guidelines, UN World Food Programme
- Reporting, INTRAC
- Evaluation report checklist, USAID
By Chandani Lopez Peralta, Content Marketing Manager at TolaData.
22 thoughts on “How to write a good M&E report – guidelines & best practices”
Thank you very much for the useful information
Our pleasure, Hassani. So glad to hear that you found our article helpful. Do check our Blog section for more resources on M&E — https://www.toladata.com/blog/
thank you so much just checked it out, you guys are doing a great job and now you have made my job much easier. Jackpot here!
I like the simplicity of explanations and clarity on topics. Best wishes
Thank you for your lovely comment, Benedicta!
This document is quite enriching and had greatly enhanced my reporting skills. From now henceforth my reports will be much more improved and quite concise.
Glad to be of help, Mercy! In case you are looking for more insightful resources on M&E related topics, do check out our blog section – https://www.toladata.com/blog/
Thank you very much. I have found your articles very enlightening. Do you have any on how to budget for M&E? Also is M&E reported separately from the report produced by project officers or both are merged
Thank you for your feedback, Amina! To answer your question, it depends on your donor’s reporting requirements. Some choose to report on M&E activities separately while others merge them with their project reports. We do not have a separate article on budgeting for M&E yet but we will definitely add that to our list.
Thanks a lot. I realized how M&E is important and how useful for us.
Glad to hear that, Thein Zaw!
This was helpful and enlightening Thanks
I really love this write-ups. Thank you so much.
I realized that M&E report is the best Thanks alot
When evaluating project management software, it is important to consider the compatibility of the software with your current project management process.
very helpful
Thank you so much, content is so informative
thanks its very intersting
I have an inquiry, for the report conclusion, do we need to support this section with numbers or percentages or no need!!
Thank so much for the resources it actually help a lot
thank you for your assistance it has helped me alot
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
- Data Collection
- Data Management
- Indicator Tracking
- Indicator Aggregation
- Custom Solutions
- IATI Reporting
- TolaData Partners
- Client Testimonials
- Help Center
- Quick Start Guide
- Knowledge Base
- Release Notes
- Case Studies
- Feature Focus
© TolaData 2024

The TolaBrief Newsletter
A monthly round-up of news and useful links on the digitisation of the sustainable development sector, from the team at TolaData

GxP Lifeline
How to write a useful clinical monitoring report.

Documentation is among the least glamorous and most burdensome aspects of compliance . For regulators, nothing happened if it wasn’t documented. Some companies err on the side of caution by requiring too much documentation without focusing on the documentation’s purpose. However, it’s also important to not neglect documentation when it is needed. One place where this balance is particularly important is in site clinical monitoring .
Site monitoring reports serve a regulatory purpose by proving the site monitoring happened. Their usefulness extends beyond simply checking a box on the compliance checklist. A well-written report shouldn’t be an afterthought but should be an integral part of the site visit. The report and visit are essential parts of proper clinical trial management .
#1: Clinical Monitoring Report Preparation
Site monitoring has a purpose. Keep that purpose in mind as you prepare. There are requirements in the study protocol that must be met, some of which pertain to what information needs to be included in the report. Depending on how or if the clinical trial is using centralized or remote monitoring, some of the information for the report can be collected before the monitor is even on-site. If those methods are used, reviewing documentation and data can be done beforehand. Remote access to that information is also useful when it comes to writing the report after the visit.
An important component of preparing is to look at past clinical monitoring reports and audit reports, if any have been conducted. If a previous site interim visit reported a problem, that requires follow-up during the next visit. Time on-site is limited, so these issues should be prioritized. A risk-based approach to monitoring will also indicate certain areas that require precedent.
#2: Note Taking
Taking notes takes time, but it’s worth it in situations where you couldn’t possibly remember everything. The monitoring report template should be open throughout the visit, both to keep you on track and to give you a place to write notes. Everyone has their own system of taking notes, but your focus should be on the areas most important to regulators — patient safety and data quality. This is the whole reason for doing the site monitoring, so take notes that demonstrate adherence to protocol, good clinical practices (GCP) , and regulations. Or notes on how the site is not adhering to those requirements.
#3: Monitoring Follow-Up
Your clinical monitoring visit and the ensuing report aren’t isolated incidents. They will be shaped by previous visits and affect future ones. Hopefully, issues from the past site monitoring reports that you reviewed while preparing for the visit have been resolved. If not, include it in your notes and the final report. Any corrective action the site has implemented should prevent recurrence. The site and monitor should work together to find solutions that will bring the site into compliance. Your report should also include any items that will need follow-up during the next visit.
Besides follow-up for a single site, monitors should also be aware of how any issues might reflect a trend across the whole study. If multiple sites are having the same issue, it might have more to do with the study protocol than the sites themselves.
#4: Write Your Clinical Monitoring Report ASAP
Taking great notes is critical, but the best time to write the report is when it’s fresh on your mind. There might be details that didn’t make it into your notes that would soon be forgotten. Or you might look back at your notes later and realize something that made sense in the moment no longer does. It’s important to write the report quickly because human memory is fallible. Going along with that, if multiple site visits are scheduled for the same day, there’s danger in remembering something from one site as pertaining to another. At the very least, the report for one site should be written before visiting the next.
#5: Be Clear and Concise in Your Report
A site monitoring report shouldn’t read like a doctoral thesis. But it also can’t be a collection of bullet points. There’s a balance that monitors have to achieve between having too much information and not enough. The report absolutely should include:
- The date of the visit.
- Name of people involved (from the site and sponsor).
- Summary of what was reviewed.
- Description of any issues or potential issues (e.g., nonconformance, problems with data, etc.)
- Description of any corrective action, who is responsible for that action, and when that action will be completed.
While there’s no wordcount requirement for a site monitoring report, the summary and descriptions do need to be detailed enough to verify the monitoring plan was followed.
Documenting what happens during a clinical monitoring visit is just as important as the visit itself. It’s essential for showing the site is compliant with regulations and the study protocol. It also demonstrates the improvements sites make when issues are discovered and who is responsible for them. Reports from previous visits serve as a starting point for the next visit and ensure follow-up items aren’t forgotten. Keeping all this in mind, the site monitoring report needs to be given forethought and attention to make it a useful document.

Sarah Beale is a content marketing specialist at MasterControl in Salt Lake City, where she writes white papers, web pages, and is a frequent contributor to the company’s blog, GxP Lifeline. Beale has been writing about the life sciences and health care for over five years. Prior to joining MasterControl she worked for a nutraceutical company in Salt Lake City and before that she worked for a third-party health care administrator in Chicago. She has a bachelor’s degree in English from Brigham Young University and a master’s degree in business administration from DeVry University.
View {title}
- to the content
- to the main navigation
- to the service navigation
Monitoring Site Initiation Visit (SIV) Report Template
Carefully assessing whether a site is ready to start a trial is a crucial step towards mitigating risks when conducting a trial. This template includes a step-by-step checklist for monitors and covers all the important aspects of a site initiation visit (SIV). It complements our series of monitoring templates covering all aspects of monitoring from the starting line to the finish line. These templates are freely accessible and are suitable for all sites doing clinical research.
Is your site ready to start a trial?
The up-to-date and user-friendly Monitoring Site Initiation Visit (SIV) Report Template can be used to report a site initiation visit.
This template is a step-by-step checklist with convenient drop-down lists. It guides monitors through all the important aspects of a site initiation visit (SIV), so they can assess whether a site is ready to start a clinical trial. It is part of a series of monitoring templates that includes:
- Monitoring Close-Out Visit (COV) Template
- Monitoring Visit Report Template
- Monitoring Plan Template
- Routine Monitoring Visit and Close-Out Visit (RMV-COV) Report Template
This template is suitable for any monitor engaged in clinical research in Switzerland and abroad. We particularly recommend using this template within the SCTO’s CTU Network for investigator-initiated, multicentre studies. It can also be used as a self-assessment form for study sites in anticipation of a site initiation visit.
This template was developed by the SCTO’s Monitoring Platform and first published in August 2021 and last revised in March 2023 (V3).
Monitoring Site Initiation Visit Report, cover page
Monitoring Site INitiation Visit Report, page 1
Monitoring Site Initiation Visit Report, page 2
Monitoring Site Initiation Visit Report, page 3
Monitoring Site Initiation Visit Report, page 4
Monitoring Site Initiation Visit Report, page 5
Monitoring Site Initiation Visit Report, page 6
This template is licensed under CC BY-NC 4.0. Its content can be shared and adapted as long as you follow the terms of the license. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc/4.0/ .
- Monitoring Site Initiation Visit Report V3 (last revised in March 2023) (docx, 1.28 MB)
Our templates and tools are free. As a publicly funded organisation, we strive to inform the public about the impact of our work and to continually improve our services. We therefore kindly ask you to leave us your email address so we can contact you with a short one-time survey. You may download the document without providing your personal data.
Questions or suggestions?
Feel free to contact us with any questions or feedback related to this resource!
Before continuing on our website: We use cookies on our website to ensure that it functions smoothly and to analyse website use. Please read our data privacy statement and accept the use of cookies.

Clinical Trial Monitoring Reports and How to Write Them
Among the most important aspects of study is observation. Overseeing the advancement of any stage, measure, process, and procedure in real time is essential to the accurate conclusion of any clinical trial undertaking. Normal monitoring actions are needed to guarantee caliber, efficiency, compliance within predefined and regulations fundamentals. In addition, it guarantees comprehensiveness, and precision within clinical investigation. Such actions also ensure that the trial isn't just conducted in compliance with Standard Operating Procedures (SOPs). They must also function to validate it is correctly reported and recorded. Consider enrolling in the Clinical Research Coordinator course or the CRA training provided by CCRPS.
There demands something vital as a part in the execution of trials. And this thing is known as trial development reports.
Such a report ought to be carefully prepared and it must summarize the means that a study is done. It also ought to point out recruiting progress and procedures; should emphasize and clarify adjustments to the analysis, and ought to point security issues. If you're involved in such reporting or need an in-depth understanding of the procedures, the Advanced Clinical Research Project Manager Certification might be of interest.
Aside from the ethics committee, researchers could also need to present yearly improvement reports of an investigation (such as any applicable alterations or dangers) to spouses, encouraging associations, and/or organizations, along with other interested parties if needed. To understand more about these requirements and get certified, the ICH-GCP course is an excellent resource.
If you're thinking about getting important skills on GCP or you also would like to upgrade your own know-how, subscribe to our comprehensive Good Clinical Practice class here .
In summary, tracking and reporting processes is an incredibly significant function in clinical trials. The right conduct of these procedures ensure compliance with legislation, regulations, and predetermined conditions. In addition, they make certain that the study doesn't pose any dangers to wellbeing. Progress reports, subsequently, empower practitioners, specialists, researchers, ethics committees, as well as others involved to keep a tab on the trial and its own advancement. Clinical professionals need to signal any alterations or risks to react to them timely, correctly, and efficiently. For further training, consider the Pharmacovigilance Certification to deepen your knowledge in monitoring drug safety.
The objective of progress reports would be to accumulate and outline upgrades, key facets, along with summaries of a continuing trial. It's very crucial to be aware that progress reports must be filed to institutional evaluation board/independent integrity questionnaire (IRB/IEC), following a trial that has obtained positive opinion.
One other important issue to mention is there are many different forms and when it comes to submitting progress reports that researchers must take into consideration before proceeding.
Precisely, these kinds are:
Printing name and date of entry ought to be composed also. A digital copy is also needed to be delivered to the interested websites and committees inside a 30-day interval following the reporting procedure was completed.
The period of time whereby an advance report ought to be filed is at least one time in a year. Nevertheless, based upon the situation as well as the RECs' needs, these reports might be passed in more often, although the analysis is still going and till its official conclusion date.
Take courses from CCRPS and learn more on how to become a clinical research professional. For detailed guidance on submission requirements and processes, the Medical Monitor Certification and Advanced Principal Investigator Physician Certification can provide extensive knowledge.
Discover more from Clinical Trials Assistant Training | Clinical Research Training | Certified Clinical Research Professionals Course to further your career in clinical research management.

CRA Exam Questions
2018 clinical research associate (cra) salaries estimated from 1,850 american employees.
33+ SAMPLE Visit Report Templates in Google Docs | Pages | PDF | MS Word
Visit report templates in google docs | pages | pdf | ms word, 33+ sample visit report templates, what is a visit report, the basic format of a visit report, how to write a proper visit report, what are some examples of a visit report, how many pages does a visit report have, what is a trip report memo.

Visit Report Template

Sample School Visit Report
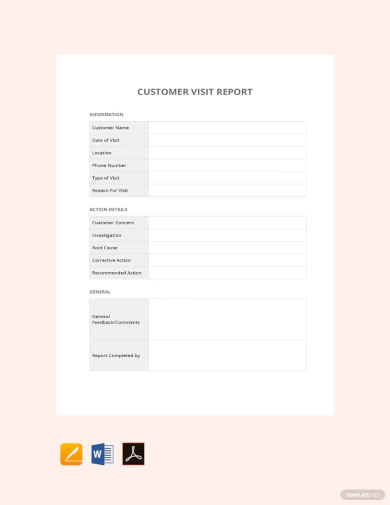
Customer Visit Report Template

Field Visit Report

Sample Site Visit Report
Customer Visit Report Outline

Sample Industry Visit Report

New Customer Visit Report Template

Construction Site Visit Report

Sample Customer Visit Report

Free School Visit Report Template

Sample Official Overseas Visit Report

Weekly Site Visit Report

Project Field Visit Report

Recommendation Study Visit Report

Observation Site Visit Reports for Engineers

Simple Industrial Visit Report

School Lab Visit Analysis Report
Building Construction Property Visit Report

Medical College Visit Report
Sample Location Visit Report

Monitoring Visit Report Summary

Marketing Team Site Visit Report
School Academic Visit Report Template

Chemical Exposure Visit Report
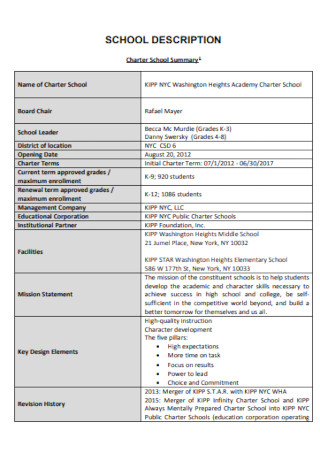
Business Renewal Site Visit Report

Management Conference Visit Report

Pre-Event Site Visit Report Example in PDF
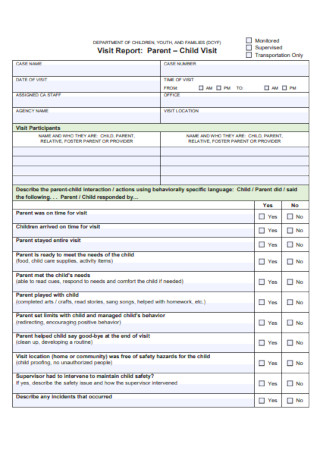
Sample Parent Visit Report Format

Home Tour Visit Report Template

Report of Research Visit

Daily Food Sponsor Visit Report Example

Sample Civil Site Monitoring Visit Report

Why Are Visit Reports Important?
Step 1: determine your purpose, step 2: be observant and write what happened, step 3: reflect on your visit, step 4: download a template and insert the details, step 5: organize details according to the format.
- Site visit report
- Business visit report
- Field trip visit report
- Industrial visit report
- Monitoring visit report
Share This Post on Your Network
File formats, word templates, google docs templates, excel templates, powerpoint templates, google sheets templates, google slides templates, pdf templates, publisher templates, psd templates, indesign templates, illustrator templates, pages templates, keynote templates, numbers templates, outlook templates, you may also like these articles, 12+ sample construction daily report in ms word | pdf.
Introducing our comprehensive sample Construction Daily Report the cornerstone of effective project management in the construction industry. With this easy-to-use report, you'll gain valuable insights into daily activities report,…
25+ SAMPLE Food Safety Reports in PDF | MS Word

Proper food handling ensures that the food we intake is clean and safe. If not, then we expose ourselves to illnesses and food poisoning. Which is why a thorough…
browse by categories
- Questionnaire
- Description
- Reconciliation
- Certificate
- Spreadsheet
Information
- privacy policy
- Terms & Conditions
Europe is the fastest-warming continent, at nearly twice the average rate, report says

Europe is the fastest-warming continent and its temperatures are rising at roughly twice the global average, two top climate monitoring organizations reported Monday, warning of the consequences for human health, glacier melt and economic activity.
The U.N.’s World Meteorological Organization and the European Union’s climate agency, Copernicus, said in a joint report that the continent has the opportunity to develop targeted strategies to speed up the transition to renewable resources like wind, solar and hydroelectric power in response to the effects of climate change.
The continent generated 43% of its electricity from renewable resources last year, up from 36% the year before, the agencies say in their European State of the Climate report for last year. More energy in Europe was generated from renewables than from fossil fuels for the second year running.
The latest five-year averages show that temperatures in Europe are now running 2.3 degrees Celsius (4.1 degrees Fahrenheit) above pre-industrial levels, compared to 1.3 degrees Celsius higher globally, the report says — just shy of the targets under the 2015 Paris climate accord to limit global warming to 1.5 degrees Celsius.

“Europe saw yet another year of increasing temperatures and intensifying climate extremes — including heat stress with record temperatures, wildfires, heat waves, glacier ice loss and lack of snowfall,” said Elisabeth Hamdouch, the deputy head of unit for Copernicus at the EU’s executive commission.
The report serves up a continental complement for WMO’s flagship state of the global climate report, which has been published annually for three decades, and this year came with a “red alert” warning that the world isn’t doing enough to fight the consequences of global warming.
Copernicus has reported that March marked the 10th straight month of record monthly temperatures. The average sea-surface temperature for the ocean across Europe hit its highest annual level in 2023, the Europe report said.
The European report focuses this year on the impact of high temperatures on human health, noting that deaths related to heat have risen across the continent. It said more than 150 lives were lost directly last year in connection with storms, floods and wildfires.
The cost of weather- and climate-related economic losses in 2023 were estimated at more than 13.4 billion euros (about $14.3 billion).
“Hundreds of thousands of people were affected by extreme climate events in 2023, which have been responsible for large losses at continental level, estimated to be at least in the tens of billions of euros,” said Copernicus director Carlo Buontempo.
Extreme weather fanned heat waves, wildfires, droughts and flooding, the report said. High temperatures have contributed to a loss of glacier ice on the continent, including in the Alps — which have lost about 10% of their remaining glacier ice over the last two years .
Still, the report’s authors pointed to some exceptions, such as how temperatures were below average in Scandinavia and Iceland even if the mercury was higher than average across much of the continent as a whole.

IMAGES
VIDEO
COMMENTS
The purpose of a monitoring visit (sometimes called a supervision visit or a field visit) is to make sure that project activities are implemented the way they are described in the plan. It normally involves meeting with the people running the project, meeting with the participants, and observing the activities. At the end of a monitoring visit, it is important to prepare a report that ...
There will be many good tips to help you with monitoring visit report completion. So, for those of you that don't know, let's start with the guidelines. Per ICH/GCPs, the monitor should submit a written report to the sponsor after each trial site visit or trial related communication. The guidelines also state what each report should include ...
The up-to-date and user-friendly Monitoring Visit Report Template can be used to report monitoring visits. This template facilitates step-by-step reporting of all matters relevant to the reporting of monitoring visits. The template includes an action list and practical drop-down menus. It is part of a series of monitoring templates that includes:
In conclusion, the different types of monitoring visits, PSVs, SIVs, IMVs, and COVs, are designed to ensure that the study is being conducted in a way that the subjects are safe and the data is valid. The CRA must ensure that sites are performing the study in compliance with the protocol, ICH/GCPs, and regulatory requirements as well as ...
Write sections of a Monitoring Visit Report based on a scenario provided. Draft a Follow-up letter given some issues to review in the Monitoring Visit Report. Critique Confirmation and Follow-up letters. Discuss the importance of providing consistent information. Critique a Telephone Contact Report.
An experienced CRA may follow all the rules and requirements for writing a site monitoring visit report (SMVR), but there are nuances that can make the difference between a report that simply follows that formula and one that really paints a picture its audience can understand. Roslyn Hennessey, senior project manager at Westat describes the ...
The first step toward writing a great monitoring report has little to do with the actual act of writing the report. In order to ensure all the information will be available to write the report, the monitor must be thoroughly prepared for the site visit. This ensures everything will be reviewed and discussed during the visit to allow for the ...
Following the visit, this information is compiled into a Clinical Monitoring Report. Here are 5 guidelines for writing an useful clinical monitoring report from MasterControl. The Best Tools to Bring to a Monitoring Visit. The Clinical Research Monitoring tools that you bring have a meaningful impact on the success of a Monitoring visit.
safeguardingsupporthub.org | Tip Sheet MEL tips | January 2021 1. Planning a visit. ü Be clear on the purpose of your monitoring visit. Be sure to communicate the purpose to the. project staff you will be visiting in advance of your arrival. ü Identify any risks that might arise as a result of the project visit. Anticipate and mitigate risks.
3. Write the Report as Soon as Possible. The report should be written as soon as possible after the visit. The best-case scenario is to write the report before preparing for and going on the next ...
1. Add a title page to the beginning of your report. The title should be the name of the visit and site, such as "Visit to Airplane Factory" or "Corporate Headquarters Visit Report." Under the title, include your name, your institution, and the date of the visit. Do not put any other information on this page.
Bring clinical trial monitoring tools! Use the Visit To Do List to stay focused and on task. Provide the site staff with a copy of the Action Item List - and use the other copy to write the Monitoring Visit Report and Follow-Up Letter. For more tips, check out Dan Sfera's YouTube video about how CRAs should prepare for a site monitoring visit.
Initial (first)monitoring visit. If you were recently hired for a CRA position in a new pharmaceutical company, you would need to do the next steps prior to scheduling the first monitoring visit: - Familiarize with the company's general SOPs and Sponsor's study-specific SOPs (if applicable) relating to the clinical study initiation ...
This visit report template can be used as a starting point to assist in making sure that specific items are checked during a clinical trial. This template can be modified to add/delete items as identified in the trial-specific Clinical Monitoring Plan. Join a network of Victorian cancer researchers, clinicians and consumers to keep your finger ...
On Site Monitoring Visit Report Template TOOL 1.5.3 INSTITUTIONAL LOGO Monitoring Visit Report (On-Site) Protocol title (short) Protocol number Site Investigator Date of visit (day/month/year) Monitor Attendees The following personnel attended this initiation Name (last, first) Title Survey role
The analysis of the data allowed us to identify trends, spot issues, and take action. 1. FINDING: Reviewer comments were significantly higher for site evaluation visits (SEVs). SEVs have an average of 32 comments per monitoring event, compared to IMVs with an average of 8.8 comments. ACTION: Investigate comments logged on SEVs to determine why ...
A site visit report is a formal document that provides a detailed account of a visit to a particular location or project site. It records the observations, activities, conditions, discussions, and any deviations or issues identified during the visit. The report often includes recommendations or action items based on these findings.
Make sure your report is concise and the layout clean and consistent. Focus on results and accomplishments and link the use of resources allocated to their delivery and use. Be sure to include a section describing the data sources and data collection methods used so that your findings are objectively verifiable.
Site monitoring reports serve a regulatory purpose by proving the site monitoring happened. Their usefulness extends beyond simply checking a box on the compliance checklist. A well-written report shouldn't be an afterthought but should be an integral part of the site visit. The report and visit are essential parts of proper clinical trial ...
The up-to-date and user-friendly Monitoring Site Initiation Visit (SIV) Report Template can be used to report a site initiation visit. This template is a step-by-step checklist with convenient drop-down lists. It guides monitors through all the important aspects of a site initiation visit (SIV), so they can assess whether a site is ready to ...
Clinical Trial Monitoring Reports and How to Write Them. Oct 31. Among the most important aspects of study is observation. Overseeing the advancement of any stage, measure, process, and procedure in real time is essential to the accurate conclusion of any clinical trial undertaking. Normal monitoring actions are needed to guarantee caliber ...
Step 2: Be Observant and Write What Happened. As natural beings, we observe various details. And it would be best if you were observant during the whole visit about the locations, progress of the visit, and even time. You will then write what happened or what was observed in a handy notebook or through your gadget.
Europe is the fastest-warming continent and its temperatures are rising at roughly twice the global average, two top climate monitoring organizations reported Monday, warning of the consequences ...