
- Introduction
- Palp/Percus
- Auscultation

Palpation/Percussion
Thoracic expansion:.
- Is used to evaluate the symmetry and extent of thoracic movement during inspiration.
- Is usually symmetrical and is at least 2.5 centimeters between full expiration and full inspiration.
- Can be symmetrically diminished in ankylosing spondylitis .
- Can be unilaterally diminished in chronic fibrotic lung disease , extensive lobar pneumonia, large pleural effusions, bronchial obstruction and other disease states.
Percussion:
Percussion is the act of tapping on a surface, thereby setting the underlying structures in motion, creating a sound and palpable vibration. Percussion is used to determine whether underlying structures are fluid-filled, gas-filled, or solid. Percussion:
- Penetrates 5 - 6 centimeters into the chest cavity.
- May be impeded by a very thick chest wall.
- Produces a low-pitched, resonant note of high amplitude over normal gas-filled lungs.
- Produces a dull, short note whenever fluid or solid tissue replaces air filled lung (for example lobar pneumonia or mass) or when there is fluid in the pleural space (for example serous fluid, blood or pus).
- Produces a hyperresonant note over hyperinflated lungs (e.g. COPD ).
- Produces a tympanitic note over no lung tissue (e.g. pneumothorax ).
Diaphragmatic excursion:
- Can be evaluated via percussion.
- Is 4-6 centimeters between full inspiration and full expiration.
- May be abnormal with hyperinflation , atelectasis , the presence of a pleural effusion , diaphragmatic paralysis, or at times with intra-abdominal pathology.
An Initiative of the Program for Bedside Medicine
- See us on youtube
- See us on facebook
- See us on twitter
Pulmonary Exam: Percussion & Inspection
The pulmonary exam is one of the most important and often practiced exam by clinicians. While auscultation is most commonly practiced, both percussion and inspection are equally valuable techniques that can diagnose a number of lung abnormalities such as pleural effusions, emphysema, pneumonia and many others.
Introduction to the Pulmonary Exam
Though taught extensively in early medical training the pulmonary exam is often neglected apart from auscultation.
Percussion During the Pulmonary Exam
The "5-7-9 rule".
- The upper border of liver dullness is defined by:
- 5th intercostal space in the midclavicular line
- 7th intercostal space in the midaxillary line
- 9th intercostal space in the scapular line
- Note: 9th intercostal space is located approximately at the inferior border of the scapula
- Hyperresonance that continues below these boundaries can be suggestive of hyperinflation (e.g. emphysema)
Cardiac dullness
Be able to outline the area of "absolute" cardiac dullness— a fist sized area just to the left of the sternum. If it is not there it suggests emphysema.
Traube's space
- Superiorly: Left 6th rib
- Inferiorly: Left costal margin
- Laterally: Anterior axillary line
- Left pleural effusion (however NOT in left lower lobe pneumonia without effusion as it is the effusion that falls into the costophrenic recess that is above the gastric bubble)
- Splenomegally (less reliable compared to Castell’s Sign)
- Very full colon
- Recently eaten (i.e. stomach is full)
Click here to read an article on the Ludwig Traube.
Tidal Percussion
- Percuss down the back until the normal hyperresonance of the lungs becomes dull over the diaphragm. Then simply have the patient breath in and out deeply while continuing to percuss. The sound should wax and wane.
- Pleural effusion
- Hyperinflation such as emphysema from a maximally contracted diaphragm
Major and Minor Fissures of the Lung
- The major fissure can be located by drawing a line from the T2 spinous process to where the 6th rib meets the sternum. The minor fissure can be approximated by drawing a horizontal line from the 4th rib attachment of the sternum to the major fissure.
- Easier method: Simply ask the patient to put their hands over their head. The scapula will rotate externally and its medial border will outline the major fissure (see figure below).
Historical Perspective of the Pulmonary Exam
Percussion was first described by Dr. Josef Leopold Auenbrugger , an Austrian physician who first observed his father tapping on wine barrels in the cellar of his hotel to determine how much wine was left. The son applied this technique to patients when he became a physician. He is credited with bringing the technique of percussion to the field of medicine. Much of his work occurred around 1760 where he described that by percussing the thorax he could accurately predict the contents of what was inside, as confirmed with post-mortum studies he conducted.
Inspection During the Pulmonary Exam
Signs of copd.
- Inspiratory descent of trachea.
- Use of accessory muscles.
- Pursed lips on exhalation (provides a small amount of PEEP).
- Normal in infancy and increased with aging.
- Prominent angle of Louis (or sternal angle).
- Flaring of the lower costal margins.
- Dahl Sign: Above the knee, patches of hyperpigmentation or bruising caused by constant 'tenting' position of hands or elbows.
- The "subcostal angle" is the angle between the xiphoid process and the right or let costal margin. Normally, during inhalation the chest expands laterally, increasing this angle. When the diaphragms are flattened (as in COPD), inhalation paradoxically causes the angle to decrease.
- Harrison's sulcus: a horizontal grove where the diaphragm attaches to the ribs; associated with chronic asthma, COPD, & Rickets.
REMEMBER : "The side that moves less, is the side of disease!"
Look for signs of volume loss (or gain) on the side that moves less (hollow supraclavicular fossae, intercostal spaces prominent, shoulder droopy, scapula outline more prominent).
Consult the Expert

Dr. Peadar Noone
Dr. Peadar Noone trained in Galway, Dublin, Boston, the UK and Chapel Hill, where he is now Associate Professor of Medicine and Medical Director of the Lung Transplant Program at the University of North Carolina, Chapel Hill.
Clinical Pearl
Insert (in a normal individual) three fingers vertically in the space under the cricoid cartilage, and above the sternal notch. As the person breathes in, the space may reduce to two fingers at most (i.e. the fingers get "squeezed" as the sternum rises with inspiration). In a patient with severe hyperinflation, the crico-sternal distance is much shorter (because the sternum is elevated), maybe 1-2 fingers at most. With inspiration one's fingers get "squeezed" out as the already "high" sternum rises up to the level of the cricoid, thus, in many cases, obliterating the crico-sternal distance altogether. Some clinicians label this sign "tracheal shortening" but strictly speaking, the actual tracheal length does not get shorter. Classically this is seen with severe emphysema / hyperinflation, or severe air trapping. Often accompanied by reduced hepatic and cardiac dullness on percussion, a widened / flared costal angle, and Hoover's sign.
Other Findings in the Chest
- Pectus Excavatum (Funnel Chest) : depression of sternum; in severe cases may compress heart and great vessels.
- Pectus Carinatum (Pigeon chest) : anterior displacement of sternum, usually benign.
- Flail Chest: secondary to multiple rib fractures, depression of diaphragm causes injured area to cave inward producing a "paradoxical thoracic movement" in breathing.
Key Learning Points
- Percussion of the lung exam
- Inspection of the lung exam
Related to Pulmonary Exam: Percussion & Inspection
- Precordial Movements
- Cardiac Second Sounds
- Neck Veins & Wave Forms
- BP & Pulsus Paradoxus
The Stanford Medicine 25
- Aortic Regurgitation Exam
- Ankle Brachial Index
- Ankle and Foot Exam
- Ascites & Venous Patterns
- Bedside Ultrasound
- Breast Exam
- Carpal Tunnel Exam
- Cerebellar Exam
- Deep Tendon Reflexes
- Dermatology Exam: Acne vs. Rosacea
- Dermatology Exam: Learning the Language
- Dermatology Exam: Nevi (Mole) Exam
- Fundoscopic Exam (Ophthalmoscopy)
- Gait Abnormalities
- Hip Region Exam
- Internal Capsule Stroke
- Involuntary Movements and Tremor Diagnosis: Types, Causes, and Examples
- Low Back Exam
- Lymph Node Exam
- Neck Vein Exam
- Pelvic Exam
- Precordial Movements in the Cardiac Exam
- Pulmonary Exam: Percussion & Inspection
- Pupillary Responses
- Pulsus Paradoxus and Blood Pressure Measurement Techniques
- Rectal Exam
- Spleen Exam
- Tarsal Tunnel Exam
- Thyroid Exam
- Tongue Exam
- Liver Disease, Head to Foot
- Visit the 25
- Shoulder Exam Tutorial
- Parkinson's Disease Exam
- Diastolic Murmurs Exam
We have a new app!
Take the Access library with you wherever you go—easy access to books, videos, images, podcasts, personalized features, and more.
Download the Access App here: iOS and Android . Learn more here!
- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs
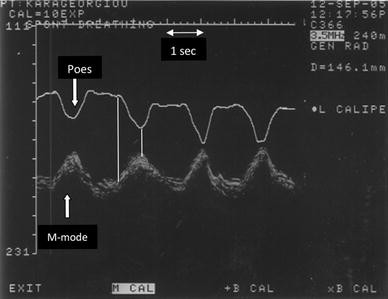
Video 29-08: Demonstration of diaphragm excursion
Demonstration of diaphragm excursion using B-mode. M-mode can then be applied as perpendicular to the diaphragm as possible and activated to obtain a quantitative value. The image was obtained using a 3.5-MHz transducer. The transducer is in longitudinal orientation and placed perpendicular to the chest wall to scan through the 6th intercostal space at the mid axillary line. Video available on AccessMedicine.com .
Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications
- Published: 24 January 2013
- Volume 39 , pages 801–810, ( 2013 )
Cite this article

- Dimitrios Matamis 1 ,
- Eleni Soilemezi 1 ,
- Matthew Tsagourias 1 ,
- Evangelia Akoumianaki 2 ,
- Saoussen Dimassi 2 ,
- Filippo Boroli 2 ,
- Jean-Christophe M. Richard 2 , 3 &
- Laurent Brochard 2 , 3
18k Accesses
365 Citations
5 Altmetric
Explore all metrics
The use of ultrasonography has become increasingly popular in the everyday management of critically ill patients. It has been demonstrated to be a safe and handy bedside tool that allows rapid hemodynamic assessment and visualization of the thoracic, abdominal and major vessels structures. More recently, M-mode ultrasonography has been used in the assessment of diaphragm kinetics. Ultrasounds provide a simple, non-invasive method of quantifying diaphragmatic movement in a variety of normal and pathological conditions. Ultrasonography can assess the characteristics of diaphragmatic movement such as amplitude, force and velocity of contraction, special patterns of motion and changes in diaphragmatic thickness during inspiration. These sonographic diaphragmatic parameters can provide valuable information in the assessment and follow up of patients with diaphragmatic weakness or paralysis, in terms of patient–ventilator interactions during controlled or assisted modalities of mechanical ventilation, and can potentially help to understand post-operative pulmonary dysfunction or weaning failure from mechanical ventilation. This article reviews the technique and the clinical applications of ultrasonography in the evaluation of diaphragmatic function in ICU patients.
Similar content being viewed by others

Diaphragm Ultrasound in the Intensive Care Unit

Diaphragmatic Ultrasound
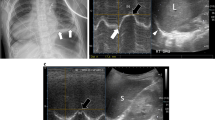
Ultrasound imaging of diaphragmatic motion
Avoid common mistakes on your manuscript.
Introduction
Bedside ultrasonography has become a valuable tool in the management of intensive care unit patients [ 1 , 2 ]. This is especially true in emergency situations where an adequate imaging technique is frequently limited by a variety of factors, including difficulty of patient transportation to the radiology department due to illness severity. Ultrasonography is a noninvasive technique, which has proved to be an accurate, safe, easy to use bedside modality, overcoming many of the standard limitations of imaging techniques.
The diaphragm is the principal respiratory muscle, and its dysfunction predisposes to respiratory complications and can prolong the duration of mechanical ventilation [ 3 – 5 ]. Sonographic evaluation of the diaphragm has recently started to gain popularity in the ICU as specific needs for assessing diaphragmatic function arise in many clinical situations. Abnormal diaphragmatic motion is observed in conditions such as phrenic nerve injury, neuromuscular diseases [ 6 – 11 ], after abdominal [ 12 ] or cardiac surgery [ 4 , 13 ] and in critically ill patients under mechanical ventilation [ 14 – 17 ]. Since diaphragmatic motion plays a prominent role in spontaneous respiration, observation of the diaphragm kinetics seems essential. The use of tools previously available for this purpose is limited due to the associated risks of ionizing radiation (fluoroscopy, computed tomography) or due to their complex and/or highly specialized nature, requiring a skilled operator (transdiaphragmatic pressure measurement, diaphragmatic electromyography, phrenic nerve stimulation, magnetic resonance imaging). Sonography receives increasing recognition as a fast, easy and accurate method of noninvasively evaluating diaphragmatic function at the bedside. In the ICU population, it can quantify normal and abnormal movements in a variety of clinical conditions. In this review, we will show that it can be used for diagnosing diaphragmatic paralysis and recovery [ 3 , 18 , 19 ], serve as a bedside screening test for investigating postoperative diaphragmatic dysfunction [ 4 , 15 , 20 , 21 ] and detect synchronization of spontaneous breathing efforts with the ventilator, potentially allowing an optimized adjustment of the ventilator settings.
Sonographic technique of diaphragmatic evaluation
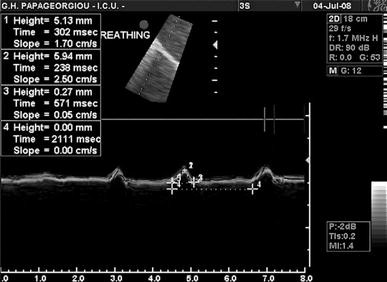
Diaphragmatic sonography is performed using a 3.5–5 MHz phased array probe. The probe is placed immediately below the right or left costal margin in the mid-clavicular line, or in the right or left anterior axillary line and is directed medially, cephalad and dorsally, so that the ultrasound beam reaches perpendicularly the posterior third of the corresponding hemi-diaphragm (Fig. 1 a).The two-dimensional (2D) mode is initially used to obtain the best approach and select the exploration line; the M-mode is then used to display the motion of the anatomical structures along the selected line (Fig. 1 b). This is illustrated in a video placed in the on-line supplement. Patients are scanned along the long axis of the intercostal spaces, with the liver serving as an acoustic window to the right, and the spleen to the left. Normal inspiratory diaphragmatic movement is caudal, since the diaphragm moves toward the probe; normal expiratory trace is cranial, as the diaphragm moves away from the probe (Fig. 1 c). In the M mode, the diaphragmatic excursion (displacement, cm), the speed of diaphragmatic contraction (slope, cm/s), the inspiratory time (Tinsp, s) and the duration of the cycle (Ttot, s) can be measured. In mechanically ventilated patients, evaluation of diaphragmatic motion sometimes necessitates to briefly disconnect the patient from the ventilator to better visualize spontaneous breathing efforts. Of note, many ICU patients may have pleural effusions, consolidation or atelectasis, which, in contrast to what one might expect, allow an easier identification of the hemidiaphragms. The values of diaphragmatic excursion in healthy individuals were reported to be 1.8 ± 0.3, 7.0 ± 0.6 and 2.9 ± 0.6 cm for males, and 1.6 ± 0.3, 5.7 ± 1.0, and 2.6 ± 0.5 cm for females, during quiet, deep breathing and voluntary sniffing, respectively [ 22 ]. Interestingly, the same diaphragmatic excursion values (1.8 cm) were found in ventilated patients who had succeeded in a weaning trial [ 16 ], with no difference between the right and the left hemidiaphragm in both studies. In addition to the measurements of diaphragmatic excursion, the velocity of diaphragmatic contraction (slope, cm/s, Fig. 1 c) can also be measured, like during the assessment of a maximal sniff. The latter is defined as a short, sharp inspiratory effort through the nose and it is thought to be a reproducible and quantitative assessment of diaphragmatic strength [ 23 ] although its role in the respiratory assessment of ICU patients remains to be determined. The slope (speed) of diaphragmatic contraction, during quiet breathing, has been measured at 1.3 ± 0.4 cm/s in forty healthy individuals without any significant differences between males and females [ 24 ].
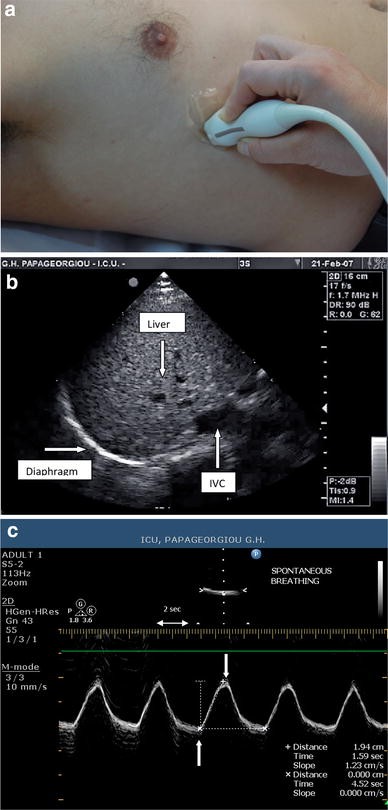
a Probe position for B and M mode diaphragmatic excursion measurements with 3.5–5 MHz probe. b B-mode diaphragm sonography. The bright line reflects the diaphragm. c M-mode diaphragm sonography. Arrows indicate the beginning and the end of the diaphragmatic contraction. The distance between the arrows , indicate an excursion (displacement) of 1.9 cm. The inspiratory time (Tinsp) is measured at 1.6 s, the cycle duration (Ttot) is 4.5 s, and the speed of diaphragmatic contraction (slope), calculated as the diaphragmatic excursion divided by the Tinsp, is 1.2 cm/s
Ultrasound has also been used to evaluate diaphragmatic thickness (tdi, mm) in the zone of apposition of the diaphragm to the rib cage. The zone of apposition is the area of the chest wall where the abdominal contents reach the lower rib cage (Fig. 2 a). In this area, the diaphragm is observed as a structure made of three distinct layers (Fig. 2 b): a non-echogenic central layer bordered by two echogenic layers, the peritoneum and the diaphragmatic pleurae [ 25 ]. To obtain adequate images of diaphragmatic thickness in M mode and 2D mode, a linear high-frequency probe (≥10 MHz) is necessary. The diaphragmatic thickness can be measured during quiet spontaneous breathing (Fig. 3 ) and during a maximal inspiratory and expiratory effort. An index of diaphragmatic thickening, the thickening fraction (TF) can be calculated using the M mode (TF = thickness at end-inspiration − thickness at end-expiration/thickness at end-expiration). Diaphragmatic thickening fraction can be used as an index of diaphragmatic efficiency as a pressure generator [ 26 ].
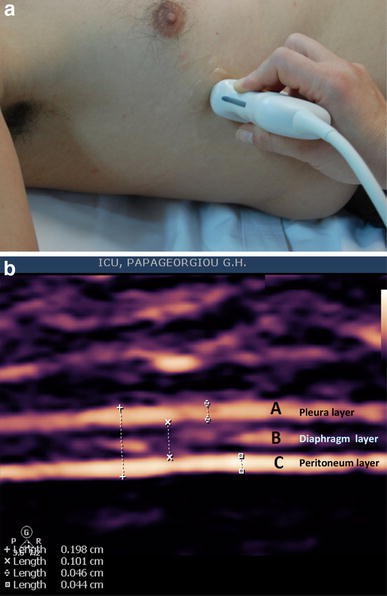
a Probe position for B and M mode diaphragmatic thickness measurements in the zone of apposition with 10–12 MHz probe. b B-mode sonography of the diaphragm in the zone of apposition. A Echogenic diaphragmatic pleura, B non-echogenic central layer, C echogenic peritoneal layer. Notice the thickness measurement of each layer
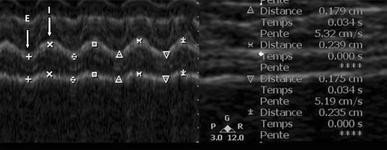
Sonography of the diaphragm in the zone of apposition, in B-mode ( right ) and M-mode ( left ) during quiet breathing. E and I arrows indicates expiration and inspiration, respectively. Notice the diaphragmatic thickening during inspiration and the reproducibility of the thickness measurements during expiration (0.179 and 0.175 cm) and inspiration (0.239 and 0.235 cm)
Normal values of diaphragmatic thickening: In normal individuals, there is a wide range of tdi at functional residual capacity (FRC), ranging between 1.8 to 3 mm. As lung volume increases from the residual volume (RV) to total lung capacity (TLC) there is a mean tdi increase of 54 % (range 42–78 %). Furthermore, the diaphragm also thickens during a maximal inspiratory pressure (Pimax) maneuver at FRC. A thickening ratio of 2.6 can be measured, dividing the diaphragmatic thickness during Pimax at FRC by the diaphragmatic thickness while relaxing at FRC [ 27 , 28 ].
Accuracy and reproducibility
Several studies have addressed the subject of accuracy and reproducibility of ultrasounds to measure the diaphragmatic displacement and thickness in healthy volunteers and in ICU patients.
In a large study measuring diaphragmatic excursion in healthy volunteers, Boussuges [ 22 ] reported that the intraobserver reproducibility was 96 and 94 %, and the interobserver reproducibility 95 and 91 %, during quiet breathing for the right and left diaphragm, respectively. The intraobserver and interobserver reproducibility (intra-class correlation coefficients) of the diaphragmatic excursion measurements reported in ICU patients were found in the same range, between 88 and 99 % [ 4 , 16 ].
Concerning the reproducibility of diaphragmatic thickness measurements during the same session, Vivier et al. [ 26 ] assessed analyser reproducibility, intra - analyser reproducibility (same settings analyzed repeatedly) and inter - analyser reproducibility (same recordings obtained separately by two different ultrasonographers). The values reported for repeatability (intra-class correlation coefficients) were all above 0.97. Coefficients of repeatability ranged around 7–8 % for intra- or inter - analyser repeatability and around 15–18 % for intra- or inter – observer repeatability.
To enhance reproducibility, there are some technical tips for the diaphragmatic echographer. First, one must know that there is little difference in the diaphragmatic excursion between the middle and the posterior part of the diaphragm [ 20 ]. Therefore, there is little reason to bother about the exact location, and the best diaphragmatic delineation in B-mode must be chosen before applying the M-mode. Second, the cursor for diaphragmatic excursion measurements in M-mode should always be as strictly perpendicular as possible with regards to the middle or posterior part of the diaphragm. This can be obtained by rotating the probe or by correcting the M-mode angle with a specific knob on the echo machine. Finally, for diaphragmatic thickness, use of the higher resolution linear probe (≥10 MHz) is necessary.
Limitations of the technique
There are limitations to diaphragmatic sonography, as well as some rules to be respected in order to avoid mistakes and errors in data collection and interpretation. One obvious limitation of diaphragmatic sonography, especially in ICU patients, is a poor acoustic window (poor quality images), and this has been reported to occur between 2 and 10 % [ 15 , 16 , 26 ].
When measuring diaphragmatic excursion, the sonographer should be as perpendicular as possible to the diaphragmatic excursion line, otherwise the accuracy and the repeatability of the diaphragmatic excursion measurements can be seriously affected. If the end point is the diaphragmatic excursion measurement, and the patient is under assisted modes of mechanical ventilation, the measured excursion (cm) will represent the sum of two forces working in the same direction; first, the force of the diaphragmatic contraction by itself, and second, the passive displacement of the diaphragm by the pressure applied by the ventilator. In this case, there is no means to distinguish which part of diaphragmatic displacement is passive, due to the external applied force, or active by the diaphragmatic contraction acting as a negative pressure generator. If the goal is to evaluate diaphragmatic excursion as a force generator without the ventilator assistance, a brief recording (5–10 min) during spontaneous breathing is necessary. On the contrary, if one wants to detect diaphragmatic contractions and better understand patient–ventilator interactions (Figs. 4 , 5 ) including the triggering delay of the ventilator (Fig. 6 ), the above-mentioned technical precautions are not mandatory.
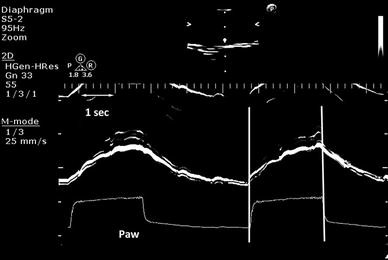
Simultaneous recordings of diaphragmatic contraction in M-mode sonography and airway pressure waveform (Paw), in a patient under pressure support ventilation. Patient–ventilator synchrony is confirmed by the perfect synchronization of the beginning ( first vertical line ) and the end of the diaphragmatic contraction ( second vertical line ) and the triggering and the cycling off of the ventilator
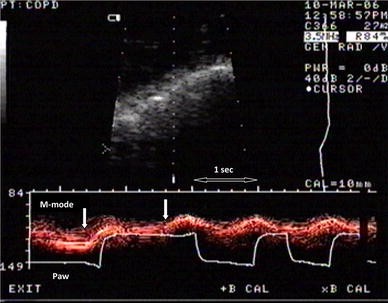
Diaphragmatic contraction in M-mode sonography and Paw in a COPD patient under pressure support ventilation, indicating patient–ventilator asynchrony. In the first assisted breath, ventilator inspiratory time is much longer compared to the second breath. In the first assisted breath, we notice two diaphragmatic contractions ( arrows ); the second diaphragmatic contraction prolongs the inspiratory time of the assisted breath
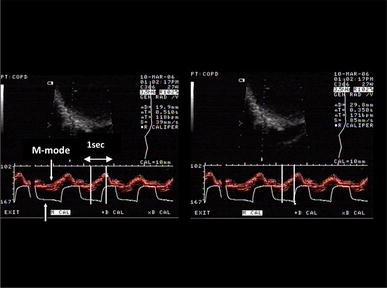
Diaphragmatic contraction in M-mode sonography and airway pressure waveform in a COPD patient under pressure support ventilation illustrating triggering delay. The same picture is on the right and on the left . On the left, the duration of the diaphragmatic contraction is measured at 510 ms ( two vertical lines ). On the right, the time between the beginning of the diaphragmatic contraction and the triggering of the same assisted breath is measured at 350 ms ( two vertical lines ). This indicates that 350 out of 510 ms of the total diaphragmatic contraction has been wasted to overcome intrinsic PEEP before triggering the ventilator
Thickening is only influenced by active contraction, but can be affected by several factors evaluated in normal or sick individuals; most of the studies have been performed in spontaneously breathing subjects [ 8 , 9 , 11 , 19 , 27 , 28 ]. Thickness measurements during spontaneous breathing may be influenced by lung volume in a nonlinear relationship [ 27 , 28 ]. The diaphragmatic thickening is more pronounced above 50 % of the vital capacity [ 27 ], and there is a large increase in thickness between relaxation and 10 % of the inspiratory effort [ 28 ]. Furthermore, there are very few data on thickness in mechanically ventilated patients interacting [ 26 ], or not [ 14 ], with the ventilator.

Sonographic evaluation of diaphragmatic weakness and paralysis
Diaphragmatic paralysis.
The diaphragm is the principal respiratory muscle during quiet breathing, and its dysfunction or paralysis can be observed in many clinical circumstances, such as major cardiac, thoracic or abdominal surgery, spinal injury, critical illness polyneuromyopathy, direct injury of the phrenic nerve or polyradiculoneuritis. Traditionally, methods to diagnose diaphragmatic weakness and paralysis examine the thoracic and abdominal pressures generated during spontaneous inspiration [ 13 ]. Measurement of transdiaphragmatic (Pdi) pressure remains the gold standard for diagnosing bilateral diaphragmatic paralysis. However, Pdi is poorly sensitive and thus ineffective to diagnose unilateral diaphragmatic paralysis, since one efficient hemi-diaphragm is sufficient to generate adequate trans-diaphragmatic pressure during quiet breathing, and this is illustrated by the fact that 6 % of asymptomatic subjects have a dyskinetic diaphragm [ 29 , 30 ]. Fluoroscopic examination of hemi-diaphragmatic motion during a sniff test may be useful in patients with unilateral diaphragmatic paralysis but false negative results can frequently occur [ 3 , 18 , 31 ]. Chest radiographs have a sensitivity of 90 % and a specificity of only 44 % in detecting unilateral diaphragmatic paralysis [ 32 ] Pulmonary function tests used for the diagnosis of respiratory muscle weakness are highly dependent on lung volumes and patient effort [ 33 , 34 ]. Magnetic resonance imaging (MRI) quantitative evaluation may allow the assessment of the excursion, synchronicity and velocity of diaphragmatic motion [ 35 , 36 ]. However, all the above-mentioned techniques are not easily applicable in ICU patients, especially when they are intubated and mechanically ventilated.
Sonography is a simple, noninvasive alternative method of diaphragmatic imaging, ideal for repeated or prolonged examinations, such as those required for the diagnosis and follow up of uni- or bilateral diaphragmatic paralysis. Ultrasound has been used to assess the motion of the diaphragmatic dome [ 20 , 25 , 37 – 39 ]. Theoretically it should share some of the diagnostic limitations of fluoroscopy. Nonetheless, Houston et al. [ 40 ] diagnosed diaphragm motion abnormalities in 22 patients, of which only seven were also identified by fluoroscopy. This may be related to the fact that fluoroscopy images the highest portion of the diaphragm, which is the least moving part of the diaphragm [ 38 , 41 ].
In unilateral or bilateral diaphragmatic paralysis, the negative pressure generated by the other respiratory muscles during inspiration, causes the diaphragm to passively move cranially instead of its normal caudal movement. The M-mode trace of the paralyzed side shows the absence of active or a paradoxical (i.e., cranial) movement (Fig. 2 ) particularly with the sniff test [ 18 ]. Moreover, the M-mode tracing of the diaphragmatic movement direction (cranial vs. caudal) allows distinguishing diaphragmatic weakness from paralysis. During inspiration, in patients with diaphragmatic weakness, one observes a reduced diaphragmatic caudal movement, and in patients with diaphragmatic paralysis, a paradoxical motion (Fig. 7 ) [ 22 , 42 ]. Repeated documentation of diaphragmatic excursion for the same individual in follow-up examinations can provide information regarding the evolution of the paralysis. All the above sonographic measurements are applicable to diaphragmatic paralysis of variable etiology, including brachial plexus neuritis, phrenic nerve injury following cardiac or other surgery [ 11 , 16 , 35 ], spinal cord injury [ 10 ] or idiopathic cases. The qualitative discrimination between reduced and paradoxical inspiratory movement may be of critical importance, since the latter is associated with delayed recovery of the phrenic nerve in cardiothoracic surgery patients, and is responsible for prolonged ventilatory support and hospital stay [ 21 ] probably due to more severe nerve injury.
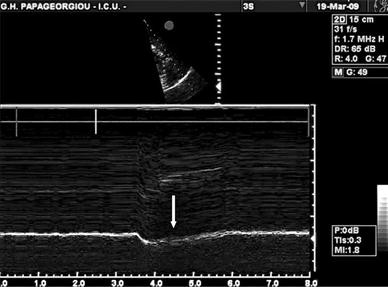
Paradoxical diaphragmatic motion on M-mode sonography in a patient with Guillain-Barré syndrome (the scale at the bottom represents time in seconds). There is a cranial diaphragmatic movement (away from the probe) during spontaneous breathing due to diaphragmatic paralysis. The intercostal muscles recover earlier than the diaphragm and create a negative intrathoracic pressure which displaces the paralytic diaphragm inwards, into the thorax and away from the probe
Diaphragmatic weakness
In neuromuscular diseases, sonography can be used to evaluate the motion of the diaphragmatic dome (diaphragmatic displacement and speed of contraction) and the diaphragmatic thickening in the zone of apposition. In an ultrasonographic study of three patients with amyotrophic lateral sclerosis, Yoshioka et al. [ 11 ] described no change in diaphragmatic excursion and thickness between quiet breathing and maximal inspiratory effort, suggesting a severe impairment of the contractile function of the diaphragm. DeBruin et al. [ 8 ] studied the diaphragmatic thickness in children with Duchenne muscular dystrophy and found that, despite a greater diaphragmatic thickness at FRC, the thickening fraction was less than that of controls during maximum inspiratory effort (1.6 vs. 2.3). Such studies underline the usefulness of diaphragmatic sonography as a noninvasive and handy tool for the diagnosis of diaphragmatic dysfunction in patients with neuromuscular diseases and thus potentially enable the early discrimination of a subpopulation who may eventually need mechanical ventilator support [ 4 , 43 ].
The evaluation of the diaphragm thickness (tdi) in the zone of apposition of the diaphragm to the rib cage can also be an informative approach for weakness or paralysis. Gottesman and McCool [ 3 ] found that tdi of the paralyzed diaphragms was less than 2.0 mm, significantly thinner than that of the normally functioning diaphragms, a finding consistent with their hypothesis that chronic diaphragm paralysis results in atrophy. Measurements of tdi alone, however, may lead to false negative results in the settings of acute paralysis where atrophy has not yet occurred, or to false positive results in small individuals, since tdi varies with weight and height [ 44 ]. Due to the above limitations, to safely diagnose diaphragmatic paralysis, diaphragmatic thickening should be calculated during inspiration according to the formula Δtdi = (tdiTLC − tdiFRC)/tdiFRC (where Δtdi is the change in diaphragm thickness, tdiTLC is diaphragm thickness at TLC and tdiFRC is diaphragm thickness at FRC) [ 3 , 19 ]; all patients with a paralyzed diaphragm exhibited less than 20 % thickening of the diaphragm during inspiration to TLC [ 3 ]. The criterion of Δtdi is useful not only for the initial diagnosis of diaphragmatic weakness or paralysis, but also for monitoring subsequent recovery. The change in Δtdi strongly correlated with changes in vital capacity and the maximal inspiratory pressure reflecting inspiratory muscle strength [ 19 ]. Recently, in ICU patients under mechanical ventilation, it has been shown that diaphragmatic thickening fraction decreased in parallel with the diaphragmatic pressure time product, as soon as the work of breathing was alleviated by incremental levels of pressure support [ 26 ].
Diaphragmatic sonography in ICU patients during partial ventilatory support
A balloon-tipped catheter is traditionally used to measure esophageal and gastric pressure, and to evaluate inspiratory effort. Studies in healthy volunteers and also in patients under assisted modes of ventilation confirm that diaphragmatic M-mode sonography provides a mirror image of the changes in esophageal pressure (Fig. 8 ). Indeed, during inspiration, as the diaphragm contracts and the dome descends, the progressively decreasing esophageal pressure coincides with positive traces on M-mode diaphragmatic sonography; during expiration, esophageal pressure increases while the sonographic trace descends. Therefore, ultrasound can provide a modality that allows demonstration of the patient’s initiation and completion of inspiratory effort in real time, obliterating the need for invasively inserting esophageal balloon catheters for that purpose.
Simultaneous recording of the esophageal pressure and M-mode diaphragmatic sonography. Notice the perfect synchronization of the beginning of diaphragmatic contraction and the drop of the esophageal pressure ( first vertical line ). The second vertical line indicates the end of inspiration and the maximal pressure drop in the esophageal pressure
Diaphragmatic M-mode sonography can provide valuable information in the evaluation of patients during partial ventilatory support. Simultaneous recordings of M-mode diaphragmatic sonography and airway pressures waveforms can allow visualizing that each patient’s inspiratory effort triggers the ventilator appropriately (Fig. 4 ). Therefore, real-time hemi-diaphragmatic sonography could be used in the evaluation of patient–ventilator interactions in clinical practice, in order to detect cases of patient–ventilator asynchrony (Figs. 5 , 6 ). In these cases, diaphragmatic sonography could even allow a proper adjustment of the ventilator settings in order to optimize synchronization of the patient’s inspiratory effort with the assisted mechanical breath. This hypothesis, however, needs to be prospectively tested. Mechanical ventilation in controlled mode and possibly with high levels of partial ventilatory assist, can also result in ventilator-induced diaphragm dysfunction [ 17 , 45 ]. Recent preliminary data suggest that sonographic assessment of the diaphragm can provide a noninvasive measurement of diaphragmatic thickness and allows to observe a progressive diaphragm thinning, as shown in seven patients receiving MV [ 14 ].
Post-operative diaphragmatic dysfunction
Diaphragmatic dysfunction contributes to the etiology of postoperative pulmonary complications after thoracic and abdominal surgery, leading to delayed weaning and prolonged stay in ICU. Kim et al. [ 12 ] demonstrated that the diaphragmatic inspiratory amplitude during deep breathing predicted changes in vital capacity throughout seven postoperative days in patients undergoing liver lobectomy. The best cutoff values of diaphragmatic inspiratory amplitude for detecting 30 and 50 % decreases of vital capacity from preoperative values, as calculated by receiver operating characteristic analysis, were 36 and 24 mm, with sensitivity of 94 and 81 % and specificity of 76 and 91 %, respectively ( p < 0.001). In another study in cardiothoracic surgery patients by Lerolle et al. [ 4 ], a maximal positive diaphragmatic excursion of less than 25 mm was associated with severe diaphragmatic dysfunction as defined by a negative value of the Gilbert index [ 46 ]. The latter is an index which evaluates the diaphragm contribution to respiratory pressure swings during quiet ventilation and is calculated as the ratio of gastric pressure swing to transdiaphragmatic pressure swing; a negative value indicates a paradoxical motion of the diaphragm. This ultrasonographic threshold had an excellent negative likelihood ratio, which was confirmed by assessing patients with uncomplicated postoperative course, none of them having their maximal diaphragmatic excursion <25 mm [ 4 ]. Such studies highlight the advantages of a fully noninvasive technique, which is now increasingly available in the ICU, to focus on patients at high risk for postoperative respiratory complications.
Weaning from mechanical ventilation
Sonography may also be of help during weaning from mechanical ventilation. Jiang et al. [ 15 ] performed a B-mode ultrasonographic evaluation of the diaphragmatic movements by measuring the liver/spleen displacement during spontaneous breathing trials. This examination proved to be a good predictor for extubation outcome. Using a mean cutoff value of 1.1 cm of liver and spleen displacement, the sensitivity and specificity to predict successful extubation was 84.4 and 82.6 % respectively, better than traditional weaning parameters used in the trial, such as rapid shallow breathing index and Pi max [ 15 ]. Patients with adequate spontaneous tidal volume but poor diaphragmatic excursion were more likely to fail a breathing trial compared to patients with adequate spontaneous tidal volume and good diaphragmatic movement; this can be explained by the fact that spontaneous tidal volume represents the result of the combined activation of all respiratory muscles used without specifically measuring the contribution of the diaphragm, whereas diaphragmatic excursion represents the final result of combined diaphragmatic strength, intrathoracic and intra-abdominal pressures [ 15 ]. The authors suggested that diaphragmatic movement was a more sensitive and specific parameter than volume-associated weaning parameters in predicting extubation outcome. Patients who recruit accessory respiratory muscles to maintain adequate tidal volumes may therefore experience more difficulties to sustain spontaneous breathing and fail extubation more often [ 15 ]. Kim et al. [ 16 ] investigated diaphragmatic dysfunction diagnosed by M-mode ultrasonography (vertical excursion <10 mm or paradoxic movements) in 88 medical intensive care unit patients, and they found a prevalence of ultrasonographic diaphragmatic dysfunction of 29 %. Patients with diaphragmatic dysfunction had longer weaning times and total ventilation times than patients without diaphragmatic dysfunction. Their results also suggest that ultrasonography of the diaphragm may be useful in identifying patients at high risk of difficult weaning. However, the role of diaphragmatic excursion as a predictor of extubation outcome in the weaning process remains to be further evaluated.
Future applications and conclusions
Data on diaphragmatic sonography are still scarce compared to cardiac or lung ultrasound applications in ICU patients. For the ICU physician, already familiar with the ECHO machine for cardiac or lung applications, the learning curve of the diaphragmatic sonography is very short. Future research on diaphragmatic sonography in the ICU environment might evaluate the relationship between diaphragmatic thickness and displacement, assess patient-ventilator asynchrony, compare thickness and diaphragmatic displacement with more invasive parameters evaluating the diaphragmatic strength (transdiaphragmatic pressure at rest and during maximal efforts), titrate external PEEP to overcome auto-PEEP in order to improve ventilator trigger delay and synchrony, follow diaphragmatic atrophy or recovery from atrophy in patients suffering from critical illness polyneuromyopathy or assess diaphragmatic function in patients during prolonged or difficult weaning from mechanical ventilation. One additional interesting point could be the assessment of diaphragmatic relaxation. Abnormalities of diaphragmatic relaxation have been reported as a marker of impaired contractile performance [ 47 , 48 ]. Diaphragmatic relaxation rate is so far measured using the trans-diaphragmatic pressure. As illustrated by Figs. 1 c, 4 and 8 , diaphragmatic relaxation could be evaluated noninvasively by diaphragmatic sonography.
Diaphragmatic contraction in M-mode sonography during a spontaneous breathing trial in a patient suffering from critical illness neuromyopathy (the scale at the bottom represent time in seconds). Diaphragmatic weakness is evidenced by the very small diaphragmatic displacement (0.5 cm)
Ultrasonography appears to be a promising tool in the evaluation of diaphragmatic function in ICU patients [ 49 ]. It has the advantage of being fully noninvasive and is becoming widely available in an increasing number of ICUs, bypassing limitations of previously used methods for this purpose. Diaphragmatic ultrasonography provides qualitative and quantitative information regarding diaphragmatic function, as part of an overall respiratory assessment in ICU patients. Apart from clear findings, such as during diaphragmatic paralysis, ultrasonographic evaluation of diaphragmatic function may become helpful in identifying a subpopulation of ICU patients at high risk of further respiratory complications. Further research regarding ultrasonographic diaphragmatic evaluation in pathologies such as sepsis, ventilator-induced diaphragmatic dysfunction and ICU neuromyopathy are anticipated with great interest.
Beaulieu Y, Marik PE (2005) Bedside ultrasonography in the ICU: part 1. Chest 128:881–895
Article PubMed Google Scholar
Beaulieu Y, Marik PE (2005) Bedside ultrasonography in the ICU: part 2. Chest 128:1766–1781
Gottesman E, McCool FD (1997) Ultrasound evaluation of the paralyzed diaphragm. Am J Respir Crit Care Med 155:1570–1574
Article PubMed CAS Google Scholar
Lerolle N, Guerot E, Dimassi S, Zegdi R, Faisy C, Fagon JY, Diehl JL (2009) Ultrasonographic diagnostic criterion for severe diaphragmatic dysfunction after cardiac surgery. Chest 135:401–407
Tobin MJ, Laghi F, Brochard L (2009) Role of the respiratory muscles in acute respiratory failure of COPD: lessons from weaning failure. J Appl Physiol 107:962–970
Ayoub J, Milane J, Targhetta R, Prioux J, Chamari K, Arbeille P, Jonquet O, Bourgeois JM, Prefaut C (2002) Diaphragm kinetics during pneumatic belt respiratory assistance: a sonographic study in Duchenne muscular dystrophy. Neuromuscul Disord 12:569–575
Cohen E, Mier A, Heywood P, Murphy K, Boultbee J, Guz A (1994) Diaphragmatic movement in hemiplegic patients measured by ultrasonography. Thorax 49:890–895
De Bruin PF, Ueki J, Bush A, Khan Y, Watson A, Pride NB (1997) Diaphragm thickness and inspiratory strength in patients with Duchenne muscular dystrophy. Thorax 52:472–475
DePalo VA, McCool FD (2002) Respiratory muscle evaluation of the patient with neuromuscular disease. Semin Respir Crit Care Med 23:201–209
Hardy F, Walker J, Sawyer T (2009) Sonographic measurement of diaphragm movement in patients with tetraplegia. Spinal Cord 47:832–834
Yoshioka Y, Ohwada A, Sekiya M, Takahashi F, Ueki J, Fukuchi Y (2007) Ultrasonographic evaluation of the diaphragm in patients with amyotrophic lateral sclerosis. Respirology 12:304–307
Kim SH, Na S, Choi JS, Na SH, Shin S, Koh SO (2010) An evaluation of diaphragmatic movement by M-mode sonography as a predictor of pulmonary dysfunction after upper abdominal surgery. Anesth Analg 110:1349–1354
Diehl JL, Lofaso F, Deleuze P, Similowski T, Lemaire F, Brochard L (1994) Clinically relevant diaphragmatic dysfunction after cardiac operations. J Thorac Cardiovasc Surg 107:487–498
PubMed CAS Google Scholar
Grosu HB, Lee YI, Lee J, Eden E, Eikermann M, Rose K (2012) Diaphragm muscle thinning in mechanically ventilated patients. Chest: PMID: 22722229
Jiang JR, Tsai TH, Jerng JS, Yu CJ, Wu HD, Yang PC (2004) Ultrasonographic evaluation of liver/spleen movements and extubation outcome. Chest 126:179–185
Kim WY, Suh HJ, Hong SB, Koh Y, Lim CM (2011) Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation. Crit Care Med 39:2627–2630
Petrof BJ, Jaber S, Matecki S (2010) Ventilator-induced diaphragmatic dysfunction. Curr Opin Crit Care 16:19–25
Lloyd T, Tang YM, Benson MD, King S (2006) Diaphragmatic paralysis: the use of M mode ultrasound for diagnosis in adults. Spinal Cord 44:505–508
Summerhill EM, El-Sameed YA, Glidden TJ, McCool FD (2008) Monitoring recovery from diaphragm paralysis with ultrasound. Chest 133:737–743
Harris RS, Giovannetti M, Kim BK (1983) Normal ventilatory movement of the right hemidiaphragm studied by ultrasonography and pneumotachography. Radiology 146:141–144
Kunovsky P, Gibson GA, Pollock JC, Stejskal L, Houston A, Jamieson MP (1993) Management of postoperative paralysis of diaphragm in infants and children. Eur J Cardiothorac Surg 7:342–346
Boussuges A, Gole Y, Blanc P (2009) Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest 135:391–400
Steier J, Kaul S, Seymour J, Jolley C, Rafferty G, Man W, Luo YM, Roughton M, Polkey MI, Moxham J (2007) The value of multiple tests of respiratory muscle strength. Thorax 62:975–980
Soilemezi E, Tsagourias M, Talias MA, Soteriades ES, Makrakis V, Zakynthinos E, Matamis D (2012) Sonographic assessment of changes in diaphragmatic kinetics induced by inspiratory resistive loading. Respirology. doi: 10.1111/resp12011
Ayoub J, Cohendy R, Dauzat M, Targhetta R, De la Coussaye JE, Bourgeois JM, Ramonatxo M, Prefaut C, Pourcelot L (1997) Non-invasive quantification of diaphragm kinetics using m-mode sonography. Can J Anaesth 44:739–744
Vivier E, Mekontso Dessap A, Dimassi S, Vargas F, Lyazidi A, Thille AW, Brochard L (2012) Diaphragm ultrasonography to estimate the work of breathing during non-invasive ventilation. Intensive Care Med 38:796–803
Google Scholar
Cohn D, Benditt JO, Eveloff S, McCool FD (1997) Diaphragm thickening during inspiration. J Appl Physiol 83:291–296
Ueki J, De Bruin PF, Pride NB (1995) In vivo assessment of diaphragm contraction by ultrasound in normal subjects. Thorax 50:1157–1161
Scillia P, Cappello M, De Troyer A (2004) Determinants of diaphragm motion in unilateral diaphragmatic paralysis. J Appl Physiol 96:96–100
Tobin M, Laghi F (1998) Monitoring of respiratory muscle function. In: Tobin M (ed) Principe and practice of respiratory care monitoring. McGraw-Hill, New York, pp 497–544
Alexander C (1966) Diaphragm movements and the diagnosis of diaphragmatic paralysis. Clin Radiol 17:79–83
Chetta A, Rehman AK, Moxham J, Carr DH, Polkey MI (2005) Chest radiography cannot predict diaphragm function. Respir Med 99:39–44
Fiz JA, Montserrat JM, Picado C, Plaza V, Agusti-Vidal A (1989) How many manoeuvres should be done to measure maximal inspiratory mouth pressure in patients with chronic airflow obstruction? Thorax 44:419–421
Wilcox PG, Pardy RL (1989) Diaphragmatic weakness and paralysis. Lung 167:323–341
Kiryu S, Loring SH, Mori Y, Rofsky NM, Hatabu H, Takahashi M (2006) Quantitative analysis of the velocity and synchronicity of diaphragmatic motion: dynamic MRI in different postures. Magn Reson Imaging 24:1325–1332
Kolar P, Sulc J, Kyncl M, Sanda J, Neuwirth J, Bokarius AV, Kriz J, Kobesova A (2010) Stabilizing function of the diaphragm: dynamic MRI and synchronized spirometric assessment. J Appl Physiol 109:1064–1071
Epelman M, Navarro OM, Daneman A, Miller SF (2005) M-mode sonography of diaphragmatic motion: description of technique and experience in 278 pediatric patients. Pediatr Radiol 35:661–667
Houston JG, Morris AD, Howie CA, Reid JL, McMillan N (1992) Technical report: quantitative assessment of diaphragmatic movement–a reproducible method using ultrasound. Clin Radiol 46:405–407
Riccabona M, Sorantin E, Ring E (1998) Application of M-mode sonography to functional evaluation in pediatric patients. Eur Radiol 8:1457–1461
Houston JG, Fleet M, Cowan MD, McMillan NC (1995) Comparison of ultrasound with fluoroscopy in the assessment of suspected hemidiaphragmatic movement abnormality. Clin Radiol 50:95–98
Young DA, Simon G (1972) Certain movements measured on inspiration-expiration chest radiographs correlated with pulmonary function studies. Clin Radiol 23:37–41
Gerscovich EO, Cronan M, McGahan JP, Jain K, Jones CD, McDonald C (2001) Ultrasonographic evaluation of diaphragmatic motion. J Ultrasound Med 20:597–604
Remerand F, Dellamonica J, Mao Z, Ferrari F, Bouhemad B, Jianxin Y, Arbelot C, Lu Q, Ichai C, Rouby JJ (2010) Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med 36:656–664
McCool FD, Benditt JO, Conomos P, Anderson L, Sherman CB, Hoppin FG Jr (1997) Variability of diaphragm structure among healthy individuals. Am J Respir Crit Care Med 155:1323–1328
Hudson MB, Smuder AJ, Nelson WB, Bruells CS, Levine S, Powers SK (2012) Both high level pressure support ventilation and controlled mechanical ventilation induce diaphragm dysfunction and atrophy. Crit Care Med 40:1254–1260
Gilbert R, Auchincloss JH Jr, Peppi D (1981) Relationship of rib cage and abdomen motion to diaphragm function during quiet breathing. Chest 80:607–612
Coirault CCD, Lecarpentier Y (1999) Relaxation of diaphragm muscle. J Appl Physiol 87:1243–1252
Esau SABF, Grassino A, Permutt S, Roussos C, Prady RL (1983) Changes in relaxation rate with diaphragmatic fatigue in humans. J Appl Physiol 54:1353–1360
Lerolle N, Diehl JL (2011) Ultrasonographic evaluation of diaphragmatic function. Crit Care Med 39:2760–2761
Download references
Acknowledgments
The authors are grateful to Aissam Lyazidi for technical assistance in preparation of the manuscript.
Author information
Authors and affiliations.
Intensive Care Unit, Papageorgiou General Hospital, Peripheral Ring Road, N. Efkarpia, Thessaloniki, Greece
Dimitrios Matamis, Eleni Soilemezi & Matthew Tsagourias
Intensive Care Unit, University Hospital of Geneva, Geneva, Switzerland
Evangelia Akoumianaki, Saoussen Dimassi, Filippo Boroli, Jean-Christophe M. Richard & Laurent Brochard
School of Medicine, University of Geneva, Geneva, Switzerland
Jean-Christophe M. Richard & Laurent Brochard
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Dimitrios Matamis .
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MPG 7784 kb)
Rights and permissions
Reprints and permissions
About this article
Matamis, D., Soilemezi, E., Tsagourias, M. et al. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med 39 , 801–810 (2013). https://doi.org/10.1007/s00134-013-2823-1
Download citation
Received : 20 September 2012
Accepted : 18 December 2012
Published : 24 January 2013
Issue Date : May 2013
DOI : https://doi.org/10.1007/s00134-013-2823-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Bedside ultrasonography
- Critically ill
- Diaphragmatic dysfunction
- Find a journal
- Publish with us
- Track your research
Physical Assessment
Assessment of the lungs and thorax continued, additional breath sounds.
If you are unsure of what you are hearing through the stethoscope, or if breath sounds are diminished, ask him/her to breathe deeper and/or open the mouth wider. Perhaps ask him to breath faster; that may enhance the quality of the sounds you are hearing.
Bronchophony
This term represents a test to perform on the patient which may indicate that there is consolidation of the lung. Consolidation refers to increased density of the lung tissue, due to it being filled with fluid and/or blood or mucus. Ask the patient to say the words: “ninety-nine” while you listen through the stethoscope. Normally the sound of “ninety-nine” will sound very faint and muffled. When you listen through normal lung tissue, sounds are normally muffled. If it sounds clear through the stethoscope, there is probably consolidation of the lung and Bronchophony is present. This occurs because sound transmission through consolidated tissues will be greater and clearer because dense tissue transmits sound better than normal “fluffy” lung tissue.
This is a term that indicates that there is consolidation of the lung or possible collapse of the lung. Ask the patient to repeatedly say the sound “ee” while you listen with the stethoscope. Normally, it will sound muffled, but it will remain with the long sound of “ee” when you listen over most of the lung field. If the sound changes to “ay” sound, while the patient is saying “ee” then egophony is present. This indicates consolidation, or that there is fluid in the lungs.
Whispered Pectoriloquy
This is another term to determine the presence of consolidation of the lungs. You will ask the patient to whisper a number or short phrase and repeat it; such as counting, “1, 2, 3” “1, 2, 3”, etc. and listen through the stethoscope. Normally the whispered voice will be distant and very muffled through the stethoscope. If consolidation is present in a section of the lung field, the whispered voice will sound unusually clear and loud, instead of muffled and distant. Consolidation of the lung tissue causes filling of the air spaces of the alveoli and voice transmission through that part of the lung will be unusually clear and louder than normal. Thus if pectoriloquy is present, it indicates consolidation of some portion of the lung field.
COMMON PULMONARY DISORDERS AND PHYSICAL ASSESSMENT FINDINGS USUALLY PRESENT
- Bronchial Asthma:
hyperinflation of lungs, impaired expansion, use of accessory muscles of respiration, prolonged expiration and wheezes present.
- Pneumothorax:
decreased expansion on affected side, hyper resonant or tympanic sounds or even absent sounds in affected areas.
- Pleural Effusion:
decreased expansion of affected side, trachea & heart shifted away from affected side, dullness or flatness or absent breath sounds.
- Atelectasis:
decreased expansion on affected side, dull or flat sound or absent breath sounds, trachea and heart shifted toward affected side.
- Consolidation:
bronchial breath sounds, bronchophony, pectoriloquy, possible splinting on the (pneumonia) affected side.
Summary of Assessment factors:
- When Inspecting
Look for the slope of the ribs, bilateral and symmetrical chest wall expansion, abnormal breathing patterns, thoracic or abdominal breathing.
Look for the shape of the thorax; evaluate anteroposterior diameter relative to lateral diameter of chest wall, pectus carinatum (pigeon breast), pectus excavatum (funnel chest), kyphosis (spine curvature), scoliosis (lateral spine curvature), kyphoscoliosis, and note tracheal position.
Look for breathlessness wheezing, sputum, cough, cyanosis, pallor, eruptions, nodules, scars, neck vein distention, fingers for tobacco stains, finger and toes for clubbing, which can be a sign of chronic respiratory disease.
- When Palpating
Feel for masses, nodules, pain, tenderness, examine the: neck, axillae, supraclavicular fossae for lymph nodes, palpate trachea for midline placement.
Feel for skin temperature and moisture
Feel for other mentioned in the text.
- When Percussing
Listen for symmetry of sounds from each side.
Listen to patient to tell you of pain or tenderness when percussing.
- When Auscultating
Listen for intensity of sounds one each side of the thorax (symmetry)
Listen for normal and abnormal breath sounds.
Following, we will present detailed outlines of the method for assessment. Today, nurses are taking increased responsibility for assessment of lungs, including auscultation. However, there are still many differences in levels of responsibilities among nurses in different hospitals. Some hospitals do not allow any nurses to chart any breath sounds at all. Other facilities want all nurses to listen and record all patients’ breath sounds. There is also every situation in between these two extremes.
We will present guidelines for those nurses who will have this responsibility of listening and charting breath sounds. If you are in a facility that does not allow you to record breath sounds, you may still listen to the lungs and at least chart that you notified someone that the patient sounds “congested.” In most facilities around the country, you may at least chart “congested” lungs if you are not allowed to chart terms like: “rales,” “rhonchi,” etc.
CHARTING THE EXAMINATION FINDINGS
When charting the normal exam, most nurses, for brevity, will chart only that respirations are “normal” and there is no “SOB.” In most cases, that is acceptable for a routine or normal examination. However, it is very possible to be brief and thorough .
- Inspection observe: shape of chest; include deformities width or costal angle, movements of intercostal spaces during respirations use of accessory muscles of respirations local impairment of respiratory movements rate and rhythm of respirations.
Charting of these normal findings might be: resp rate-20/min, regular, no SOB1
- Palpation a. identify areas of tenderness
b. assess any observed areas of abnormality c. assess respiratory excursion (expansive movements of the chest during breathing) d. assess skin condition (temperature, etc.)
- Percussion a. assess any areas of dullness, flatness, tympany
b. assess areas found to be abnormal from previous examinations.
- Auscultation a. assess quality and intensity of breath sounds
b. assess patient for abnormal breath sounds c. assess patient for areas of consolidation
When charting your findings, you may not be sure as to exactly what you are hearing. Most hospitals do not require that palpation and percussion results be charted. If the nurse carefully assesses the breath sounds, those others may not need to be charted, but are still used to confirm the nurse’s assessment of the patient’s problem. If the nurse is unfamiliar with naming the individual breath sounds, you should be very descriptive when charting.
For example: chart the location and sound that you hear….. moist respirations in LLL and RLL……or fine rales in LLL and RLL (either is correct)
Do not feel that you must always tag a name to the type of abnormal respirations that you hear. It is sufficient to accurately describe the abnormal breathing. Another important function is to follow up the results of your exam if there is an abnormality. Your nursing diagnosis will include nursing orders to turn the patient more frequently or to suggest that respiratory therapy be performed on the patient. Therefore, communications is important, but so is the nursing follow-up on your findings.
GUIDE TO ASSESSMENT OF LUNGS AND THORAX
- Assemble Equipment
- History-taking
- Explains Procedure to the patient
- Washes hands
- Gowns or drapes patient to prevent unnecessary exposure
- Provides a quiet place for patient comfort and for auscultation
- Provide adequate lighting
- inspection, palpation, percussion, auscultation
- compares symmetry of thorax (each hemothorax)
- starts at neck, then posterior, right and left lateral, then anterior thorax
- Respiratory rate determination
- Rhythm determination
- Depth determination
- defines boundaries of abnormality is found; describes accurately
- do not allow patient to hyperventilate during the exam.
- avoids bony prominences during the exam (poor sound conduction)
- records findings accurately
POSTERIOR THORAX EXAMINATION
- Patient seated with arms folded across chest
- Inspects symmetry, contour, color, skin condition
- Palpates posterior interspaces for masses, lesions, etc.
- Palpates ribs and scapulae for masses, breaks, etc.
- Evaluates tactile fremitus
- Evaluates respiratory excursion
- Percussion – 5 cm intervals from apex to base contra laterally
- Diaphragmatic excursion
- Ausculate breath sound
- Ausculate voice and whispered sounds
RIGHT AND LEFT LATERAL THORAX
- Patient seated with arms on head
- Begin in the axillae and proceed downward contra laterally using at least 4 or 5 sites for comparison
- Inspects for symmetry, color, condition of skin
- Palpate ribs for masses or bulges
- Palpates tactile fremitus
- Percusses lateral thoraces
- Auscultates breath sounds
- Auscultates voice and whispered sounds
ANTERIOR THORAX
- Patient is supine with arms abducted; child is placed totally flat and head is not allowed to turn.
- Inspect anterior chest for symmetry, contour, color, skin condition
- Palpate ribs and interspaces for bulges and masses
- Palpate for tactile fremitus
- Palpate trachea
- Percuss anterior chest at 5 cm intervals
- Auscultate for breath sounds
- Auscultate for voice and whispered sounds.
CHARTING EXERCISE: This is not part of Posttest for this course: for practice only.
- Chart a brief narrative of a “normal” lung assessment
- Chart on a patient who has COPD with an acute attack.
- General - - - - - -
- Rate, rhythm, depth (difficulty) - - - - -
- Auscultation results - - - - - -
S- (subjective) O- (objective) A- (assessment) P- (plan - -nursing orders)
ADVENTITIOUS SOUNDS:
RALES: (or crackles)
Definition:
- Clusters or showers of sounds
- Produced by bubbling air through the alveoli, bronchioles bronchi
- Non-continuous
- Variable quality:
Fine rales: terminal bronchioles and alveoli, sounds like hair being rubbed between fingers. Medium rales: larger air passages, bubbling sound of opening a carbonated beverage. Coarse rales : louder and lower-pitched from larger passages
- Produced by air travelling through narrowed passages or through mucus in the passages.
- Varying sound quality
- Continuous sound
Types: Fine rhonchi Coarse rhonchi (sonorous) Sibilant rhonchi (wheezes)
FRICTION RUB:
- Coarse grating sound
- Inflamed surfaces of the pleura rub together during respirations
- Usually over anterolateral thorax
Next: PART II MENTAL STATUS ASSESSMENT
- Diaphragmatic Excursion
This test is used for determining the presence of hyperinflated lungs (as in COPD) or phrenic nerve palsy. It uses percussion on the posterior thorax.
Performing the Test
- Percuss along the posterior chest to get a rough idea of where the diaphragm lies under normal breathing. The lungs will be tympanic on percussion whereas the retroperitoneum below the diaphragm will be dull.
- Ask the patient to fully inspire.
- Percuss the new level of dullness and mark this as the inferior level of diaphragmatic excursion.
- Ask patient to fully expire.
- Percuss the new level of dullness and mark this as the superior level of diaphragmatic excusion.
- Repeat on both sides, comparing for symmetry.
Diaphragmatic excursion is usually 5-6 cm.
- Bickley LS. The thorax and lungs. In: Bickley LS, Szilagyi PG. Bates' Guide to Physical Examination and History Taking. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:283-321.
Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
11 Focused Assessment- Respiratory System
Learning objectives.
At the end of this chapter, the learner will:
- Conduct a health history pertaining to the respiratory system.
- Identify anatomic landmarks in identifying underlying structures and the location of physical findings.
- Inspect the thorax for pattern of respiration, skin, symmetry, and use of accessory muscles.
- Auscultate the anterior and posterior thorax for normal breath sounds and adventitious sounds.
- Describe the findings using correct terminology.
- Document the findings of the respiratory exam.
I. Overview of the Respiratory System
The assessment of the respiratory system includes assessing the thorax, lungs, ventilatory function and oxygenation of the body. Focused assessment techniques will be applied intensively in this system: inspect level of consciousness, agitation, skin color, clubbing fingers, shortness of breath, use of accessory muscles, position and alignment of the spine; auscultate breathing sounds; palpate position of the trachea, subcutaneous emphysema; percuss to assess the underlying structure of the chest.
II. Anatomy and Physiology
Identifying thoracic landmarks is essential to the systematic examination of the respiratory system. Click the link below to review anatomy and physiology of the respiratory system. You will need to apply your knowledge in the assessment process.
III. Medical Terminology
Important terms to know and understand:
IV. Step by Step Assessment
Safety considerations:.
- Perform hand hygiene .
- Check room for contact precautions .
- Introduce yourself to patient.
- Confirm patient ID using two patient identifiers (e.g., name and date of birth).
- Explain process to patient.
- Be organized and systematic in your assessment.
- Use appropriate listening and questioning skills.
- Listen and attend to patient cues.
- Ensure patient’s privacy and dignity.
- Apply principles of asepsis and safety .
- Check vital signs .
A more detailed overview of respiratory examination is available at: Lung Exam (text) and Respiratory Sounds (text and video)
Knowledge Check
V. Documentation of Assessment Findings
A sample narrative documentation:
A & O x 4, denies shortness of breath or chest pain, RR18, without use of accessory muscles, symmetrical chest wall movement, clear breath sounds in all lung fields, O2 98% in room air.
VI. Related Laboratory and Diagnostic Procedures/ Findings
A spirometry test (pulmonary function test), have the patient inhale and exhale through a device to check the lung capacity. It can be used to diagnose asthma and chronic obstructive pulmonary disease. A chest X-ray is used to view the structure inside of the chest and is a useful test to diagnose pneumonia. A computerized tomography (CT) scan may also be used to identify respiratory problems that an X-ray cannot detect. Bronchoscopy is an invasive procedure that a fiberscope is inserted into the patient’s airway to examine bronchi. It can be used to retrieve tissues in the airway (biopsy) to diagnose lung cancer or to treat airway blockage or obstruction due to foreign objects.
VII. Learning Exercises

VIII. Attributions and References
- Anderson, R., Doyle, G. R., & McCutcheon, J. A. Clinical procedures for safer patient care. https://pressbooks.bccampus.ca/clinicalproceduresforsaferpatientcaretrubscn/chapter/2-7-head-to-toe-assessment-chest-respiratory-assessment/ Accessed July 4th, 2021.
- Deviant Art: Male torso render image by Illtrytobeapro. Oct. 7, 2013. ODI: https://www.deviantart.com/illtrytobeapro/art/Male-Torso-render-405887318 Accessed July 4th, 2021.
- Doyle, G. R. & McCutcheon, J. A. Step by Step Checklist adapted from https://opentextbc.ca/clinicalskills/chapter/2-5-focussed-respiratory-assessment/
- Khan Academy: Meet the lungs by Rishi Desai. https://www.khanacademy.org/science/high-school-biology/hs-human-body-systems/hs-the-circulatory-and-respiratory-systems/v/meet-the-lungs
- Reyes, F.M. Modi, P., & Le, J.K. (Updated July 10, 2020). Lung Exam. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan. DOI: https://www.ncbi.nlm.nih.gov/books/NBK459253/ Accessed July 5th, 2021.
- Wikipedia contributors. (2019, July 30). Respiratory sounds. In Wikipedia, The Free Encyclopedia . Retrieved 23:46, August 29, 2019, from https://en.wikipedia.org/w/index.php?title=Respiratory_sounds&oldid=908541418
- Wikimedia Commons. Barrel chest adapted from DOI: https://commons.wikimedia.org/wiki/File:%CE%92%CF%85%CF%84%CE%B9%CE%BF%CE%B5%CE%B9%CE%B4%CE%AE%CF%82_%CE%B8%CF%8E%CF%81%CE%B1%CE%BA%CE%B1%CF%82_(barrel_chest).png
Health Assessment Guide for Nurses Copyright © by Ching-Chuen Feng; Michelle Agostini; and Raquel Bertiz is licensed under a Creative Commons Attribution 4.0 International License , except where otherwise noted.
Share This Book
Comparison of Diaphragmatic Stretch Technique and Manual Diaphragm Release Technique on Diaphragmatic Excursion in Chronic Obstructive Pulmonary Disease: A Randomized Crossover Trial
Affiliations.
- 1 Department of Physiotherapy, Kasturba Medical College, Manipal Academy of Higher Education, Bejai, Mangalore-575004, India.
- 2 Department of Radiodiagnosis, Kasturba Medical College Mangalore, Manipal Academy of Higher Education, Mangalore-575004, India.
- 3 Department of Pulmonary Medicine, Kasturba Medical College, Manipal Academy of Higher Education, Mangalore-575004, India.
- PMID: 30719351
- PMCID: PMC6335861
- DOI: 10.1155/2019/6364376
Background: Chronic Obstructive Pulmonary Disease (COPD) impairs the function of the diaphragm by placing it at a mechanical disadvantage, shortening its operating length and changing the mechanical linkage between its various parts. This makes the diaphragm's contraction less effective in raising and expanding the lower rib cage, thereby increasing the work of breathing and reducing the functional capacity.
Aim of the study: To compare the effects of diaphragmatic stretch and manual diaphragm release technique on diaphragmatic excursion in patients with COPD.
Materials and methods: This randomised crossover trial included 20 clinically stable patients with mild and moderate COPD classified according to the GOLD criteria. The patients were allocated to group A or group B by block randomization done by primary investigator. The information about the technique was concealed in a sealed opaque envelope and revealed to the patients only after allocation of groups. After taking the demographic data and baseline values of the outcome measures (diaphragm mobility by ultrasonography performed by an experienced radiologist and chest expansion by inch tape performed by the therapist), group A subjects underwent the diaphragmatic stretch technique and the group B subjects underwent the manual diaphragm release technique. Both the interventions were performed in 2 sets of 10 deep breaths with 1-minute interval between the sets. The two outcome variables were recorded immediately after the intervention. A wash-out period of 3 hours was maintained to neutralize the effect of given intervention. Later the patients of group A and group B were crossed over to the other group.
Results: In the diaphragmatic stretch technique, there was a statistically significant improvement in the diaphragmatic excursion before and after the treatment. On the right side, p=0.00 and p=0.003 in the midclavicular line and midaxillary line. On the left side, p=0.004 and p=0.312 in the midclavicular and midaxillary line. In manual diaphragm release technique, there was a statistically significant improvement before and after the treatment. On the right side, p=0.000 and p=0.000 in the midclavicular line and midaxillary line. On the left side, p=0.002 and p=0.000 in the midclavicular line and midaxillary line. There was no statistically significant difference in diaphragmatic excursion in the comparison of the postintervention values of both techniques.
Conclusion: The diaphragmatic stretch technique and manual diaphragm release technique can be safely recommended for patients with clinically stable COPD to improve diaphragmatic excursion.
Publication types
- Comparative Study
- Randomized Controlled Trial
- Cross-Over Studies
- Diaphragm / diagnostic imaging
- Diaphragm / physiopathology*
- Middle Aged
- Musculoskeletal Manipulations / methods*
- Pulmonary Disease, Chronic Obstructive / physiopathology
- Pulmonary Disease, Chronic Obstructive / therapy*
- Ultrasonography
- Work of Breathing*

IMAGES
VIDEO
COMMENTS
Diaphragmatic excursion. Diaphragmatic excursion is the movement of the thoracic diaphragm during breathing. Normal diaphragmatic excursion should be 3-5 cm, but can be increased in well-conditioned persons to 7-8 cm. This measures the contraction of the diaphragm. It is performed by asking the patient to exhale and hold it.
3. To measure diaphragmatic excursion, ask your patient to inhale and hold it. Percuss from the lower edge of his right scapula down toward the diaphragm (see Technique for percussion). When the note changes from resonant to dull, you've located your first landmark. Tell him to breathe, then mark the landmark with a skin marker.
Diaphragmatic excursion: Can be evaluated via percussion. Is 4-6 centimeters between full inspiration and full expiration. May be abnormal with hyperinflation, atelectasis, the presence of a pleural effusion, diaphragmatic paralysis, or at times with intra-abdominal pathology.
Pulmonary Exam: Percussion & Inspection. The pulmonary exam is one of the most important and often practiced exam by clinicians. While auscultation is most commonly practiced, both percussion and inspection are equally valuable techniques that can diagnose a number of lung abnormalities such as pleural effusions, emphysema, pneumonia and many ...
The ratio of right to left diaphragmatic excursion during quiet breathing was (1.009±0.19); maximum 181% and minimum 28%. Only 19 cases showed a right to left ratio of less than 50% (5 men and 14 women). The diaphragmatic excursion was higher in males than females. There was a significant difference in diaphragmatic excursion among age groups.
Check us out on Facebook for DAILY FREE REVIEW QUESTIONS and updates! (https://www.facebook.com/medschoolmadeeasy) Check out our website for TONS OF FREE REV...
The mean diaphragmatic excursions of the two hemidiaphragms have been determined for men and women (Table (Table1). 1). Furthermore, in 1995, Houston et al have reported that in healthy volunteers, the right-to-left ratio of hemidiaphragmatic excursion during deep inspiration was in the range of 0.5-1.6. Consequently, this ratio has been ...
Diaphragmatic excursion is a promising novel tool to quantify improved pain and respiratory function after serratus anterior nerve block and possibly other blocks. ... Documentation on file. Conflicts of Interest: By the CPC-EM article submission agreement, all authors are required to disclose all affiliations, funding sources and financial or ...
Demonstration of diaphragm excursion using B-mode. M-mode can then be applied as perpendicular to the diaphragm as possible and activated to obtain a quantitative value. The image was obtained using a 3.5-MHz transducer. The transducer is in longitudinal orientation and placed perpendicular to the chest wall to scan through the 6th intercostal ...
Background Although the pathophysiological mechanisms involved in the development of dyspnoea and poor exercise tolerance in patients with COPD are complex, dynamic lung hyperinflation (DLH) plays a central role. Diaphragmatic excursions can be measured by ultrasonography (US) with high intra- and interobserver reliability. The objective of this study was to evaluate the effect of ...
The mean percentage changes between A/C and SBT in diaphragm excursion, T i, and E-T index between the two patient groups are compared in Table 4. A decrease in diaphragmatic E-T index less than 3.8% during transition from A/C to SBT had a sensitivity of 79.2% and a specificity of 75% in predicting successful extubation (AUC, 0.77) ( Fig 2 C).
a Probe position for B and M mode diaphragmatic excursion measurements with 3.5-5 MHz probe.b B-mode diaphragm sonography. The bright line reflects the diaphragm.c M-mode diaphragm sonography.Arrows indicate the beginning and the end of the diaphragmatic contraction. The distance between the arrows, indicate an excursion (displacement) of 1.9 cm.The inspiratory time (Tinsp) is measured at 1. ...
Diaphragmatic excursion was calculated as the difference between axial slices through the lungs on inspiration and expiration, using the lung apex as the cranial bound, and the hemidiaphragm caudally. Inspiratory and expiratory lung and tracheal volumes were calculated through volumetric segmentation. Tracheal morphology was assessed at 1 cm ...
Diaphragmatic excursion ranged from -0.6 cm-6.8 cm, with an average change in diaphragmatic excursion of 2.5 cm between inspiratory and expiratory scans (2.3 cm in women, and 2.7 cm in men, p = .5). There was a strong positive correlation between diaphragmatic excursion and change in lung volume between inspiratory and expiratory scans (r = 0 ...
We report that diaphragmatic excursion can perfectly predict successful weaning in patients with COVID-19. The diaphragm is the key respiratory muscle, which is responsible for ≈ 70% of the tidal volume during inspiration . Diaphragmatic dysfunction is common in critically ill patients and a pivotal factor in failure of weaning from MV.
c. assess respiratory excursion (expansive movements of the. chest during breathing) d. assess skin condition (temperature, etc.) Percussion a. assess any areas of dullness, flatness, tympany. b. assess areas found to be abnormal from previous examinations. Auscultation a. assess quality and intensity of breath sounds.
Diaphragmatic excursions were assessed by M-mode ultrasonography, using an approach perpendicular to the posterior part of the diaphragm. Anatomical M-mode was used for the recording of the complete excursion during deep breathing. The study included 270 men and 140 women. The diaphragmatic motions during quiet breathing and voluntary sniffing ...
Figure 7. Exhalation time of diaphragmatic excursion 3.1 Specific results of diaphragmatic excursion by ultrasound. The mean diaphragmatic thickness measured during expiration was 3.4 mm, while in inspiration it was 2.58 mm; with a thickening fraction of approximately 43 %. The mean diaphragmatic excursion
Percuss along the posterior chest to get a rough idea of where the diaphragm lies under normal breathing. The lungs will be tympanic on percussion whereas the retroperitoneum below the diaphragm will be dull. Ask the patient to fully inspire. Percuss the new level of dullness and mark this as the inferior level of diaphragmatic excursion.
Diaphragmatic excursion and lung function were analyzed by an independent t-test. To analyze the relationship between lung function and diaphragmatic excursion, Karl Pearson's correlation coefficient test was used. The level of significance was <0.05 (P < 0.05). Results. Forty-eight participants were included in the study. Out of those, 30 were ...
The assessment of the respiratory system includes assessing the thorax, lungs, ventilatory function and oxygenation of the body. Focused assessment techniques will be applied intensively in this system: inspect level of consciousness, agitation, skin color, clubbing fingers, shortness of breath, use of accessory muscles, position and alignment ...
Results: In the diaphragmatic stretch technique, there was a statistically significant improvement in the diaphragmatic excursion before and after the treatment. On the right side, p=0.00 and p=0.003 in the midclavicular line and midaxillary line. On the left side, p=0.004 and p=0.312 in the midclavicular and midaxillary line.
The mean weight at the time of collection was 2,307.0 ± 672.76 grams and the gestational age was 35.7 ± 3.3 weeks. Diaphragmatic excursion increased with increasing gestational age (p = 0.01, df = 0.21) in term infants (p = 0.17, df = 0.35). Conclusion There was a positive correlation between diaphragmatic excursion and gestational age.