Advertisement
Notes: pga tour q-school 2nd stage, final results, share this article, southern hills plantation, brooksville, fla..
Winner: Robert Karlsson (18 under). The two-time Ryder Cupper closed with a 5-under 67 to win by two shots over Derek Fathauer and Billy Hurley.
Number of qualifying spots: 19 and ties
Full results
NOTABLES WHO ADVANCED
• Billy Hurley (16 under, T-2), who made $474,130 on the PGA Tour in 2012, shot 6-under 66 on Saturday, the second-best round of the day. Hurley had two top-10s on Tour this season.
• Arjun Atwal (12 under, T-7) used his second round in the 60s – a 4-under 68 – to give himself some cushion moving into Saturday’s final round. Atwal had five birdies on his front nine, but picked up three bogeys on the back.
• Patrick Sheehan (10 under, T-10), who had 21 starts on the PGA Tour and seven more on the Web.com Tour in 2012, closed with a 1-under 71 to advance. He was just 1 under over his final two rounds, but a second-round 65 is the main reason he’s moving on.
• Daniel Chopra (10 under, T-10) shot 6-under 66 on Saturday to move inside the cut line and advanced to the Q-School finale. Chopra had 21 starts on the PGA Tour in 2012, making only six cuts and making $192,198. He had two top-25 finishes.
NOTABLES NOT MOVING FORWARD
• Frank Lickliter (6 under, T-30), who has two career wins on the PGA Tour, shot 1-under 71 on Saturday to miss out on advancing by two shots.
• Lee Janzen (5 under, T-34) matched his total from Friday, shooting 3-under 69 on Saturday, but he also failed to advance.
• Sam Saunders , the grandson of Arnold Palmer, shot 1-over 73 on Saturday thanks to four bogeys and a double bogey. Saunders (3 under, T-42) did record an eagle, but he’ll miss out on a spot in the Q-School finale.
• Ryuji Imada , who made only 12 cuts in 28 starts on the PGA Tour this season and picked up only one top 10, withdrew.

Hombre Golf Club (Bad/Ugly), Panama City Beach, Fla.
Winner: Matt Fast (13 under) used a stretch of six birdies in eight holes to card a 4-under 67 and win by three shots.
• Erik Compton (8 under, T-8) followed a third-round, 5-under 66 with a 2-under 69 on Saturday. Compton made 16 cuts in 26 starts on the PGA Tour this season, but had only one top 25.
• Len Mattiace (6 under, T-12), a two-time winner on the PGA Tour, survived a final-round, 1-over 72 to advance.
• Two-time U.S. Open participant Scott Langley (5 under, 19th) got the last spot in the Q-School finale after an even-par 71 that was highlighted by three birdies and three bogeys.
• Former LSU star John Peterson (3 under, T-21) made a run with a 3-under 68, but it wasn’t enough as Peterson fell two shots short.
• Eugene Wong (2 under, T-23) posted his best round of the tournament – a 2-under 69 – but he, too, missed out on advancing, finishing three shots behind Langley.
• Hunter Hamrick (Even, T-33) shot 3-under 68 on Saturday, but it wasn’t enough to erase a third-round 76. The former Alabama player shot 66 in the second round, but was a combined 8 over in the first and third rounds.
Bear Creek GC, Murrieta, Calif.
Winner: Si Kim (19 under) shot a 1-under 71 on Saturday and held off a charge from Bhavik Patel (6-under 66) to win by a shot.
• Danny Lee (10 under, T-4), who had three top-25 finishes in 2012 and made the cut in 13 of his 26 starts, shot 1-under 71 to safely advance.
• Former UCLA star Patrick Cantlay (7 under, T-8) will move on after a 3-under 69 on Saturday. It was Cantlay’s first round in the 60s this tournament.
• Tom Pernice (7 under, T-8) advanced behind a final-round, 5-under 67, which was fueled by six birdies.
• Former UNLV standout Derek Ernst (5 under, T-17) carded four bogeys en route to a 1-over 73, but he was one of six players tied at 5 under who just made it into the Q-School finale.
• Duffy Waldorf (4 under, T-23), who has four career wins on the PGA Tour, missed out on advancing by one stroke despite a final-round, 4-under 68.
• K.T. Kim (3 under, T-26), a member of the International team at the 2011 Presidents Cup, finished two shots outside the cut line after a final-round, 1-under 71.
• Jason Gore (7 over, T-58) followed a 6-over 78 with a 1-over 73 to end his hopes of advancing.
• Stanford senior Andrew Yun (8 over, T-61) wrapped up his tournament with a 2-under 70, but rounds of 76 on Wednesday and 78 on Friday kept him from advancing.
FROM FRIDAY . . .
Tpc craig ranch, mckinney, texas.
Winner: Scott Gutschewski of Proper, Texas, shot 20-under 268 (67-66-67-68) to finish one shot ahead of PGA Tour player Scott Dunlap. Gutschewski finished 49th in earnings on this year’s Web.com Tour.
Number of qualifying spots: 20 and ties
NOTABLES WHO MADE IT
• Norway’s Joakim Mikkelsen , who won the 2012 Big 12 Championship while at Baylor, finished T-6 at 15 under par.
• Andrew Putnam , the younger brother of PGA Tour player Michael Putnam, shot a final-round 67 to finish T-9 at 14 under.
• Todd Hamilton , former winner of the Open Championship, tied for 15th.
• Andrew Loupe shot a final-round 64, the day’s low round by three shots. Loupe made seven birdies in an eight-hole stretch from Nos. 6-13 and made a 6-foot birdie putt on the final hole. He finished at 11-under 277 to advance on the number.
• Kevin Tway , son of former PGA Championship winner Bob Tway, shot 71 and advanced on the number. Bob Tway was on his bag.
• Bio Kim made eagle on the par-5 18th hole, but was one shot too high.
• Texas sophomore Jordan Spieth shot 71 and finished T-26, three shots too high.
• Daniel Miernicki, a 2012 first-team All-American at Oregon, shot 73 Friday and was five shots too high.
• North Florida senior Sean Dale finished T-45.
Read Sean Martin’s story on Spieth failing to make it to the final stage here.
Redstone Golf Club, Humble, Texas
Winner: Franklin Corpening of Ft. Worth, Texas, and Patrick Reed of Spring, TX, won by one shot at 14 under. Reed shot 3-under 69 on Friday to move on to California.
• 2011 U.S. Amateur champion Kelly Kraft (12 under, 4th) saved his best for last, posting a 6-under 66 on Friday to get comfortably inside the top 19.
• Mathew Goggin (9 under, T-9) made a big move on Friday behind a 5-under 67. Goggin made only 10 cuts in 23 starts on the PGA Tour in 2012.
• Mark Anderson (8 under, T-12), coming off his best PGA Tour finish of the season at the Children’s Miracle Network Hospitals Classic (T-9), bookended 1-over 73s, but his second-round 65 was enough to move him forward.
• Alexander Noren (7 under, T-18) used a bogey-less, 2-under 70 on Friday to squeak inside the top 19, despite a 2-over 74 on Thursday. Noren is No. 49 in the Official World Golf Ranking. He has three career wins on the European Tour.
• Shaun Micheel (7 under, T-18) birdied the par-5 18th to get inside the top 19, erasing four bogeys on the front nine. Micheel shot 1-over 73 Friday, after posting 69-69-70 in the first three rounds. Micheel is the 2003 PGA Championship winner.
• Joe Durant (5 under, T-26) fired a final-round, even-par 72 to finish two shots outside the top 19. Durant is a four-time PGA Tour winner and has earned more than $13 million in his career. He had only one top-10 in 2012 and earned $427,678.
• University of Missouri senior Jace Long (4 under, T-32) tried to make a run on Friday, but his two birdies to open the back nine were wasted by a bogey on No. 12 and he finished three shots back of the top 19. Long failed to break 70 over the four rounds.
Plantation Preserve Golf Course & Club, Plantation, Fla.
Winner: Rob Oppenheim of Orlando shot 63-67-66-67 to win by four shots. Oppenheim was 42nd on this year’s Web.com Tour money list.
• Georgia Tech star James White overcame an opening-round 73 with rounds of 65-64-66 to finish T-3 at Plantation and move forward.
• Kris Blanks (16 under, T-3), who made only $390,059 on the PGA Tour in 2012, fired a final-round 67 to cruise into the final stage.
• Michael Sims (12 under, T-11), who shot 59 earlier this year on the eGolf Professional Tour, used a pair of birdies and a single bogey on Friday to card a 1-under 70.
• Englishman Ross Fisher (11 under, T-13) birdied his final hole (a par-5) after bogeys at Nos. 15 and 16 to safely advance. Fisher has four career European Tour wins and is currently No. 91 in the Official World Golf Ranking.
• Stephan Jaeger (10 under, T-17), a first-team All-American at Chattanooga in 2012, earned one of the final spots behind a final-round 68.
• Oliver Fisher (10 under, T-17) birdied his final two holes to move inside the top 19 and ties to move forward. Fisher has one career European Tour victory (2011 Czech Open). Fisher represented Great Britain & Ireland at the 2005 Walker Cup.
• Hank Kuehne (9 under, T-20) made double-bogey 7 at the finishing par-5 18th to fall outside the top 19. Kuehne had five birdies, two bogeys and a double in his 1-under 70.
• Jamie Lovemark (9 under, T-20) missed by a shot in spite of four final-round birdies. He was the 2010 Web.com Tour player of the year.
• Sam Osborne (8 under, T-28) is a journeyman from England that earned a spot in the 2012 U.S. Open, but came up two shots short of a trip back to California.
Get the latest British Open leaderboard updates , news, and more.
Most Popular
Here are five notable teams that missed the cut at the 2024 zurich classic of new orleans, photos: lpga's amy olson announces retirement, rickie fowler makes hole-in-one with star-studded group at one of the most exclusive golf clubs in the country, it's a wild scene again at liv golf adelaide. here are the photos to prove it, the list of top 18 money winners in pga tour history has plenty of surprises, is jon rahm having an existential crisis he's certainly going off his liv golf script a bunch, lynch: rory mcilroy thinks he can help the pga tour’s board. bless the lad’s optimism.

Follow Playing Through online:
- Follow Playing Through on Twitter
- Follow Playing Through on Instagram
- Follow Playing Through on Facebook
Site search
- Champions Tour
- DP World Tour
- Latest News
Filed under:
PGA TOUR Q-school 2012 leaderboard: Dong-hwan Lee takes over with 8-under day
Dong-hwan Lee is the new leader at the Q-school tournament, shooting 8-under on Saturday to pass up Meen Whee Kim.
Share this story
- Share this on Facebook
- Share this on Twitter
- Share this on Reddit
- Share All sharing options
Share All sharing options for: PGA TOUR Q-school 2012 leaderboard: Dong-hwan Lee takes over with 8-under day
/cdn.vox-cdn.com/uploads/chorus_image/image/4244301/146222562.0.jpg)
The second half of the PGA Tour 2012 Q-school got under way on Saturday, and we've got a new leader in Dong-hwan Lee. Moving up 16 spots with his 19-under mark, Lee finished the day 8-under, his best day thus far. Meen Whee Kim was the leader heading into the day, but he finished 17-under with a 1-over mark on Saturday.
A tie for second place with Kim rounds out the top five, as Edward Loar, Vaughn Taylor and Richard H. Lee all occupy that spot at 17-under overall. Other players with big games on the day include Michael Letzig, who finished tied for 10th, with a 6-under for the day and a 15-under overall, and Richard H. Lee, who was 8-under on the day, with a whopping 28 spots jumped.
Robert Karlsson, perhaps the most recognizable name in the tournament, had a poor day, dropping 15 spots and finishing the day at No. 17, tied with two others, and 3-over on the day (14-under overall).
With two rounds left, there's still plenty chance for some of the lower-ranked guys to punch their tickets into the 2013 tour. Round 5 will get under way on Sunday.
Next Up In Golf
- Grace Kim taking advantage of Nelly Korda absence at LA Championship
- LIV Golf Adelaide sees fan rocket water bottle of caddie’s head, sending him to the ground
- Tommy Fleetwood caddies stepson past Challenge Tour cut, beats out pros
- PGA Tour Canadian duo taking Zurich Classic of New Orleans by storm again
- Rory McIlroy, Shane Lowry maintain Zurich Classic lead, ready to “stroll” Bourbon Street
- Joel Dahmen, Keith Mitchell prioritize fun over winning Zurich Classic of New Orleans
Sign up for the newsletter Sign up for the Playing Through Daily Roundup newsletter!
Thanks for signing up.
Check your inbox for a welcome email.
Oops. Something went wrong. Please enter a valid email and try again.
Zurich Classic of New Orleans
TPC Louisiana
The ordeal that is Q school to begin its final chapter
By Bill Fields
It made it to middle age and caused a lot of gray hair in the process.
PGA Tour Qualifying School, 1965-2012.
The beginning of the end of a sports institution commences Tuesday morning with the first stage of qualifying school at six locations after pre-qualifiers last month. First stage will be conducted at 14 venues, followed by six second-stage qualifiers next month, with the 108-hole finals scheduled Nov. 28-Dec. 3 at PGA West, where the top 25 finishers and ties will earn 2013 PGA Tour privileges.

Anthony Kim is one of many to earn PGA Tour playing privileges through Q School.
Q school is out for good after this fall, its 47-year role as a gateway to the PGA Tour over. Starting in 2013, as part of a drastic makeover of the PGA Tour's competitive calendar -- the new season will start in October -- Q school will only award cards to the Web.com Tour.
Instead, a three-tournament series blending golfers who have done well on the Web.com Tour and not so well on the PGA Tour will take place. Fifty golfers will earn PGA Tour exemptions for 2014 through the series, the top 25 on the Web.com circuit guaranteed status as is currently the case, their pecking order up for grabs.
Related: The most historic Q School grads
Q school, the place with the loudest silence in golf, where shots that mean so much are witnessed by so few, will drift into golf's closet of what-has-been.
While the closed-shop argument put forth when the demise of Q school was first percolating has merit (although golfers who have apprenticed on the Web.com Tour have historically done better on the PGA Tour), the only people who have ever loved Q school are those who have never played in one.
A golfer can become infamous at Q school but not famous. It is a pass-fail exam, and the failures have tended to get more attention than the successes. Most of the blunders that have become grisly lore happen late -- during the back nine on Monday of the finals -- but pressure builds, and games crack, in anonymity much earlier.
"Due to the sheer scale of the event, the stories that get passed around are of players who get through 99 or 100 holes in great position, then fall apart for keeps, scattering strokes all over the final homeward nine," David Gould writes in his richly-detailed 1999 book, Q School Confidential: Inside Golf's Cruelest Tournament . "Behind these easily spotted tragedies are the untold stories of players whose courage ran dry at an isolated moment, doing swift but permanent damage that the player alone can appreciate."
But plenty of golfers also show sporting bravery at Q school, coming through in the clutch despite sweaty palms and nervous stomachs to attain -- or, often, regain -- the chance to live out a dream.
Related: Frank Nobilo on why changing Q School is good
Golf, especially Q school, is about keeping on and plugging away. No one showed more perseverance at Q school than Mac O'Grady who qualified 30 years ago on his 17th attempt despite shooting 79-76 in the opening two of his six rounds at the finals.
Donnie Hammond won that Q school at TPC Sawgrass and Sawgrass CC by a record 14 shots, but Q school is one tournament where winning really doesn't matter. A golfer just needs to be "inside the number." That fall those who got PGA Tour cards along with Hammond and O'Grady were Nick Price, Dan Forsman, Ken Green, Russ Cochran, Loren Roberts, Tom Lehman and Jeff Sluman.
Among the competitors in 2012's first stage are a Wadkins (Travis), a Tway (Kevin) and a Sindelar (Jamie).
There is also a Nicklaus.
That's Nicklaus Newcomb, a young Kentuckian in the field at Madison, Miss.
At Q school, it is about the game not the name, but a little karma never hurt.
Follow @BillFields1
( Photo by AP Images )
More from Golf Digest
Trending now.

WITB at 2012 PGA Tour Q-School
The annual grind of PGA Tour Q School. Come in and see WITB of the players.
Click here to see 1 of 10 threads of hundreds of photos… http://www.golfwrx.com/forums/topic/548047-2011-pga-tour-q-school-finals-pga-west-tuesday-pics-part-3/
Click here to see all the links to all the galleries of 2011 PGA Tour school pics… http://www.golfwrx.com/forums/topic/546951-2011-pga-tour-q-school-final-stage-picture-comment-thread/page__hl__pga+tour+school

John Feinstein’s latest hit piece on Tiger
Brandel Chamblee goes off on Tiger Woods and Sean Foley
GolfWRX is the world's largest and best online golf community. Expert editorial reviews, breaking golf tour and industry news, what to play, how to play and where to play. GolfWRX surrounds consumers throughout the buying, learning and enrichment process from original photographic and video content, to peer to peer advice and camaraderie, to technical how-tos, and more. As the largest online golf community we continue to protect the purity of our members opinions and the platform to voice them. We want to protect the interests of golfers by providing an unbiased platform to feel proud to contribute to for years to come. You can follow GolfWRX on Twitter @GolfWRX and on Facebook .
Jan 7, 2012 at 9:46 pm
sorry about that. I fixed it so you can click the links above
Jan 7, 2012 at 5:42 pm
How do I view the gallery?
Your email address will not be published. Required fields are marked *
This site uses Akismet to reduce spam. Learn how your comment data is processed .

You may like
Tour photo galleries, photos from the 2024 zurich classic of new orleans.
GolfWRX is live on site this week at the Zurich Classic of New Orleans for the PGA Tour’s one-and-only two-man team event.
As usual, general galleries, WITBs, and pullout albums — including some pretty spicy custom putters and headcovers — await your viewing.
Be sure to check back for more photos from the Big Easy, as we’ll continue to update this page with additional galleries throughout the week.

General Albums
- 2024 Zurich Classic – Monday #1
- 2024 Zurich Classic – Monday #2
- 2024 Zurich Classic – Tuesday #1
- 2024 Zurich Classic – Tuesday #2
WITB Albums
- Alex Fitzpatrick – WITB – 2024 Zurich Classic
- Austin Cook – WITB – 2024 Zurich Classic
- Alejandro Tosti – WITB – 2024 Zurich Classic
- Davis Riley – WITB – 2024 Zurich Classic
- MJ Daffue – WITB – 2024 Zurich Classic
- Nate Lashley – WITB – 2024 Zurich Classic
- James Nicholas – WITB – 2024 Zurich Classic
- Kevin Streelman – WITB – 2024 Zurich Classic
- Rasmus Hojgaard – WITB – 2024 Zurich Classic
- Tom Whitney – WITB – 2024 Zurich Classic
- SangMoon Bae – WITB – 2024 Zurich Classic
- Daniel Berger – WITB – 2024 Zurich Classic
- Rory McIlroy – WITB – 2024 Zurich Classic
- Russ Cochrane – WITB – 2024 Zurich Classic
- Aldrich Potgieter – WITB – 2024 Zurich Classic
- Steve Stricker WITB – 2024 Zurich Classic
- Drew Brees WITB (Legendary New Orleans Saints QB) – 2024 Zurich Classic
- Derek Carr (New Orleans Saints QB) – 2024 Zurich Classic
- Thriston Lawrence WITB – 2024 Zurich Classic

Pullout Albums
- MJ Daffue’s custom Cameron putter – 2024 Zurich Classic
- Cameron putters – 2024 Zurich Classic
- Swag covers ( a few custom for Nick Hardy) – 2024 Zurich Classic
- Custom Bettinardi covers for Matt and Alex Fitzpatrick – 2024 Zurich Classic
- Doug Ghim’s custom Cameron putter – 2024 Zurich Classic
- Patrick Cantlay spotted testing a Scotty Cameron blade putter – 2024 Zurich Classic

See what GolfWRXers are saying about our photos from the Zurich Classic of New Orleans in the forums.
Photos from the 2024 RBC Heritage

GolfWRX is on site this week at Harbour Town Golf Links on Hilton Head Island for the RBC Heritage. Plenty of golfers who competed in the Masters last week will be making the quick turnaround in the Lowcountry of South Carolina as the Heritage is again one of the Tour’s Signature Events.
We have general albums for you to check out, as well as plenty of WITBs — including Justin Thomas and Justin Rose.
We’ll continue to update as more photos flow in from SC.
Check out links to all our photos, below.

- 2024 RBC Heritage – Monday #1
- 2024 RBC Heritage – Monday #2
- 2024 RBC Heritage – Tuesday #1
- 2024 RBC Heritage – Tuesday #2

- Justin Thomas – WITB – 2024 RBC Heritage
- Justin Rose – WITB – 2024 RBC Heritage
- Chandler Phillips – WITB – 2024 RBC Heritage
- Nick Dunlap – WITB – 2024 RBC Heritage
- Thomas Detry – WITB – 2024 RBC Heritage
- Austin Eckroat – WITB – 2024 RBC Heritage
- Xander Schauffele – WITB – 2024 RBC Heritage
- Jason Day – WITB – 2024 RBC Heritage
- Will Zalatoris – WITB – 2024 RBC Heritage
- Patrick Cantlay – WITB – 2024 RBC Heritage
- Ludvig Aberg – WITB – 2024 RBC Heritage
- Collin Morikawa – WITB – 2024 RBC Heritage
- Sam Burns – WITB – 2024 RBC Heritage
- Stephen Jaeger – WITB – 2024 RBC Heritage
- Wyndham Clark’s Odyssey putter – 2024 RBC Heritage
- JT’s new Cameron putter – 2024 RBC Heritage
- Justin Thomas testing new Titleist 2 wood – 2024 RBC Heritage
- Cameron putters – 2024 RBC Heritage
- Odyssey putter with triple track alignment aid – 2024 RBC Heritage
- Scotty Cameron The Blk Box putting alignment aid/training aid – 2024 RBC Heritage

- Cameron putter – 2024 RBC Heritage
- Odyssey Ai One Eleven T putters – 2024 RBC Heritage
- Christian Bezuidenhout – testing new Callaway Ti 340 mini driver – 2024 RBC Heritage
- Rory McIlroy testing the new TaylorMade BRNR Mini Driver Copper – 2024 RBC Heritage
- Xander Schauffele testing the Callaway Ti 340 mini driver & the DUW – 2024 RBC Heritage
- Byeong Hun An, two new L.A.B. Golf putter builds with “T” alignment – 2024 RBC Heritage
See what GolfWRXers are saying and join the discussion in the forums.
Photos from the 2024 Valero Texas Open

GolfWRX is on site this week at the Valero Texas Open.
The event has been around since 1922, making it one of the oldest on the PGA Tour calendar. Over the years, it’s been held at a variety of courses across the Lone Star State, but it’s found its home at TPC San Antonio in recent years. Some of the biggest names in golf have taken home the title here, including Arnold Palmer, Ben Hogan, Lee Trevino, and Ben Crenshaw.
GolfWRX has its usual assortment of general galleries, WITBs and special pull-out albums. As always, we’ll continue to update the links below as more photos come in from TPC San Antonio.

- 2024 Valero Texas Open – Monday #1
- 2024 Valero Texas Open – Tuesday #1
- Ben Taylor – WITB – 2024 Valero Texas Open
- Paul Barjon – WITB – 2024 Valero Texas Open
- Joe Sullivan – WITB – 2024 Valero Texas Open
- Wilson Furr – WITB – 2024 Valero Texas Open
- Ben Willman – SoTex PGA Section Champ – WITB – 2024 Valero Texas Open
- Jimmy Stanger – WITB – 2024 Valero Texas Open
- Rickie Fowler – WITB – 2024 Valero Texas Open
- Harrison Endycott – WITB – 2024 Valero Texas Open
- Vince Whaley – WITB – 2024 Valero Texas Open
- Kevin Chappell – WITB – 2024 Valero Texas Open
- Christian Bezuidenhout – WITB (mini) – 2024 Valero Texas Open
- Scott Gutschewski – WITB – 2024 Valero Texas Open
- Cameron putter – 2024 Valero Texas Open
- Ben Taylor with new Titleist TRS 2 wood – 2024 Valero Texas Open
- Swag cover – 2024 Valero Texas Open
- Greyson Sigg’s custom Cameron putter – 2024 Valero Texas Open
- Davis Riley’s custom Cameron putter – 2024 Valero Texas Open
- Josh Teater’s custom Cameron putter – 2024 Valero Texas Open
- Hzrdus T1100 is back – – 2024 Valero Texas Open
- Mark Hubbard testing ported Titleist irons – 2024 Valero Texas Open
- Tyson Alexander testing new Titleist TRS 2 wood – 2024 Valero Texas Open
- Hideki Matsuyama’s custom Cameron putter – 2024 Valero Texas Open
- Cobra putters – 2024 Valero Texas Open

See what GolfWRXers are saying in the forums .

Dave Portnoy places monstrous outright bet for the 2024 Masters

John Daly stuns fans into silence with brutal opening tee shot on PGA Tour Champions

Things got heated at the Houston Open between Tony Finau and Alejandro Tosti. Here’s why

Justin Thomas on the equipment choice of Scottie Scheffler that he thinks is ‘weird’

Tiger Woods arrives at 2024 Masters equipped with a putter that may surprise you

‘Absolutely crazy’ – Major champ lays into Patrick Cantlay over his decision on final hole of RBC Heritage

Report: Tiger Woods has ‘eliminated sex’ in preparation for the 2024 Masters

Two star names reportedly blanked Jon Rahm all week at the Masters

2-time major champ announces shock retirement from the sport at age of 33

Report: LIV Golf identifies latest star name they hope to sign to breakaway tour

Steve Stricker WITB 2024 (April)
Steve Stricker WITB accurate as of the Zurich Classic. More photos from the event here. Driver: Titleist TSR3 (9 degrees, C4...

Alex Fitzpatrick WITB 2024 (April)
Alex Fitzpatrick what’s in the bag accurate as of the Zurich Classic. Driver: Ping G430 LST (10.5 degrees) Shaft: Fujikura...

Alejandro Tosti WITB 2024 (April)
Alejandro Tosti what’s in the bag accurate as of the Zurich Classic. Driver: Srixon ZX5 Mk II LS (9.5 degrees...

Drew Brees WITB 2024 (April)
View this post on Instagram A post shared by GolfWRX (@golfwrx) Driver: TaylorMade Stealth 2 Plus (10.5 degrees)...

Neal Shipley presser ends in awkward fashion after reporter claims Tiger handed him note on 8th fairway

Brandel Chamblee has ‘no doubt’ who started the McIlroy/LIV rumor and why
- Q-School Schedule
- 2023 KFT Schedule

Search for Tournaments

PGA TOUR Q-School
Filter by date.
- Detailed View Condensed View Card View Map View Calendar
- Messenger Messenger
- Gmail

Current Events
Quick links, contact info.
© 2024 Korn Ferry Qualifying Tournament

- Terms of Service Terms
- Accessibility
- Copyright © 2022 BlueGolf © 2022 BlueGolf
Create New List
- FanNation FanNation FanNation
- Swimsuit SI Swimsuit SI Swimsuit
- Sportsbook SI Sportsbook SI Sportsbook
- Tickets SI Tickets SI Tickets
- Shop SI Shop SI Shop
- Free Agency
- What's on TV
- Golf Golf Golf
- Home Home Home
- News News News
- Leaderboard Leaderboard Leaderboard
- Schedules Schedules Schedules
- SI Rankings SI Rankings SI Rankings
- Travel Travel Travel
- Instruction Instruction Instruction
- Gear Gear Gear
- Betting Betting Betting

Five PGA Tour Cards Are Up for Grabs This Week at Q-School
- Author: Gabrielle Herzig
For the first time this week, five players will earn full PGA Tour membership at the Final Stage of PGA Tour Q-School, otherwise known as “qualifying” school.
The culminating 72-hole event of the three-stage qualifying series gives a pool of players the opportunity to earn status on various tours for the upcoming year. For the first time since 2012, a direct path to the PGA Tour will be available for the top 5 finishers at Final Stage.
Played at TPC Sawgrass’s Dye’s Valley Course and Sawgrass Country Club, the tournament begins Thursday and finishes Sunday.
Here are a few key details to know as Q-School gets underway.
Who is playing?
As mentioned above, Q-School features a series of tournaments prior to Final Stage: pre-qualifying, First Stage and Second Stage. The fields of each of the varying stages are filled based on how players perform in 2023.
For example, the first 40 players ranked below the top 125 on the 2022-23 FedEx Cup points list automatically qualify for Final Stage. On the flip side, players can work their way from pre-qualifying to Final Stage with high finishes in each event.

Fan favorite Harry Higgs is looking to regain full status this week via Q-School.
Dan Hamilton/USA TODAY Sports
A few notable players include:
- James Nicholas: A former dual-athlete at Yale (golf and football), Nicholas already secured his DP World Tour card through Q-School this season and is looking to also gain PGA Tour status. Nicholas has amassed a significant following on social media by sharing the realities of mini-tour life.
- Sam Bennett: The U.S. Amateur champion and Texas A&M stand-out was exempt into Final Stage via the PGA Tour University program. He broke amateur records at the 2023 Masters , where he finished T16.
- Fred Biondi: The Florida product is looking for PGA Tour status for his rookie year as a pro. Biondi’s decision to turn professional meant he had forgo his invitation to the 2024 Masters as the NCAA D-I individual champion.
- Harry Higgs: The fan-favorite PGA Tour player dropped to 132nd in the FedEx Cup standings this season and is looking to regain full status.
- Wesley Bryan: The full-time pro golfer and part-time YouTuber (check out Bryan Bros Golf here ) is currently ranked 190th in the FedEx Cup. His best finish on Tour last season was a solo 6th at the Puerto Rico Open.
There is no cut at Q-School. All 168 players in the field will finish 72 holes in order for the results of the tournament to determine their 2024 status.
Qualification Breakdown
Depending on how players finish at Final Stage, they will receive a variety of benefits for the upcoming season. Here’s what’s at stake.
- Top 5: PGA Tour membership.
- Next 40: Exempt status on the Korn Ferry Tour.
- Next 20: Exempt status on PGA Tour Americas and conditional status on the Korn Ferry Tour.
- Remaining players: Conditional Korn Ferry Tour membership and conditional PGA Tour Americas membership.
How to Watch
Because players are competing for a career lifeline, Q-School is never short of drama. You can catch the action on Golf Channel on Saturday from 2:30- 4:30 p.m ET and Sunday from 1:30- 4:30 p.m ET.

Golf’s Q-School Explained
The players who earn their card at Q-School will join the “in the money” players from the previous years who have received exempt status for their play in the current year. For example, Ian Poulter finished in the top 125 this year in the number 45 spot, therefore he has automatically earned his TOUR card for next year and is exempt from further qualification. A player can finish outside the 125 and still be exempt under certain conditions such as winning specific tournaments like majors or the Tour Championship.
Q-School dates back to 1965. The 2012 edition involves four stages:
- Pre-Qualifying Stage: Five tournaments are held in September in warm-weather locations in the United States. All of these tournaments are played over three rounds. In each tournament, roughly 35 to 40 players, plus ties, advance to the next stage.
- First Stage: Thirteen tournaments held in October all in the United States and each are played over four rounds. The participants are a mixture of Pre-Qualifying Stage winners and players who were exempted from Pre-Qualifying. Approximately the top 25 players plus the players that tie in each tournament will advance to the Second Stage.
- Second Stage: Six tournaments which are held in November and played over four rounds. Like the First Stage, certain players can receive exemptions to this stage. The top 20 plus tied players in each tournament will advance.
- Final Stage: One tournament played over six rounds in late November-early December. The field consists of Second Stage winners and players who received exemptions into the Final Stage. In this stage, the top 25 players, plus ties, earn PGA TOUR cards for the following year.
There are other ways to earn exemptions, for example, Bubba Watson won the 2012 Masters, and so he automatically retains a TOUR card for 5 years. No matter how it’s achieved, earning one’s TOUR card is never an easy endeavor. The process ultimately ensures that only the world’s top players are permitted to compete on TOUR. Either way it certainly has us thinking… wouldn’t it be nice to earn a PGA TOUR card and quit your 9-5? For now, we can only dream.
( Photo Credit )
No Surprise: Rory McIlroy named 2012 PGA TOUR Player of the Year
Golf Officials Propose Ban on the Way Belly Putter is Used
DP World Tour Reveals a Way Rahm and Hatton Could Eligible for 2025 Ryder Cup

Even if LIV Goes to 72 Holes it May Not be Enough for OWGR

The Byron Nelson Has a New Sponsor and New Experiences — But It’s The Same Legendary Tournament
How I Fixed My Shanks — Advice From a Normal, Everday Golfer
Charlie Woods Shoots 81 in US Open Qualifier
Bryson DeChambeau is Using Custom 3D Printed Irons at The Masters

Fantasy Golf Picks, Odds, and Predictions – 2024 Masters Tournament
Bryson DeChambeau Tested “Rolled Back” Golf Balls with Surprising Results
PGA Tour Loyalty Payouts Revealed: Tiger Woods Stands to Make The Most
Malbon Has Jason Day Looking DAPPER for The Masters

We Played This Old Golf Course Where Thomas Edison Was A Member

Are You MEASURING Your Golf Swing Correctly? (This Can Help)

Zach Johnson Explains Why He Didn’t Pick Bryson DeChambeau for the Ryder Cup

Justin Thomas FIRES Coach before Ryder Cup…Who’s Next?

What Nobody Tells You About Great Ball Striking

‘It’s Quite Nauseating’: Max Homa on Current State of Men’s Pro Golf

Fantasy Golf Picks, Odds, and Predictions – 2024 Zurich Classic

The Masters TV Ratings Were Down 20%, But What Does it Mean?

The PGA Championship is Set to Return to Kiawah Island!

PGA Tour Players Who Rejected LIV Golf Offers Set to Find Out How Much Their Loyalty is Worth

Report: Rory McIlroy Set to Rejoin PGA Tour Policy Board

Golf’s Best Kept Secret: Like You’ve Never Seen it Before
- Skip to Navigation
- Skip to Main Content
- Skip to Related Content
- Today's news
- Reviews and deals
- Climate change
- 2024 election
- Fall allergies
- Health news
- Mental health
- Sexual health
- Family health
- So mini ways
- Unapologetically
- Buying guides
Entertainment
- How to Watch
- My watchlist
- Stock market
- Biden economy
- Personal finance
- Stocks: most active
- Stocks: gainers
- Stocks: losers
- Trending tickers
- World indices
- US Treasury bonds
- Top mutual funds
- Highest open interest
- Highest implied volatility
- Currency converter
- Basic materials
- Communication services
- Consumer cyclical
- Consumer defensive
- Financial services
- Industrials
- Real estate
- Mutual funds
- Credit cards
- Credit card rates
- Balance transfer credit cards
- Business credit cards
- Cash back credit cards
- Rewards credit cards
- Travel credit cards
- Checking accounts
- Online checking accounts
- High-yield savings accounts
- Money market accounts
- Personal loans
- Student loans
- Car insurance
- Home buying
- Options pit
- Investment ideas
- Research reports
- Fantasy football
- Pro Pick 'Em
- College Pick 'Em
- Fantasy baseball
- Fantasy hockey
- Fantasy basketball
- Download the app
- Daily fantasy
- Scores and schedules
- GameChannel
- World Baseball Classic
- Premier League
- CONCACAF League
- Champions League
- Motorsports
- Horse racing
- Newsletters
New on Yahoo
- Privacy Dashboard
- Yahoo Sports AM
- College Sports
- Fantasy Sports
- Horse Racing
- Leaderboard
- Masters Tournament
- NFL Draft Winners & Losers: Jim Harbaugh electric
- First-round grades
- Day 2 grades: Rounds 2-3
- Hailburton hits OT winner vs. Bucks
- Kawhi looks far from 100% in G3 loss
PGA Tour, Korn Ferry Tour cards on the line this week at Q-School
For the first time since 2012, PGA Tour cards will be handed out this week via the final stage of Q-School.
The top five finishers and ties in Ponte Vedra Beach, Florida, will collect Tour cards for the 2024 season. The event will feature 168 players and will be contested over two courses, the Dye Valley Course at TPC Sawgrass (par 70, 6,850 yards on the scorecard) and the East-West course at Sawgrass Country Club (par 70, 7,054 yards).
There is no cut in the 72-hole tournament, and players will compete on each course over the first two days.
There will be a regrouping after the third and fourth rounds. The top half of the field after 36 holes will play Round 3 at Sawgrass and Round 4 at Dye. The bottom half through 36 holes will Dye and then Sawgrass.
For the last decade, Q-School has been used to determine solely Korn Ferry Tour status. Prior to 2013, however, PGA Tour cards were awarded to the top finishers (top 25 and ties, most recently).
While the top five and ties this week will again get PGA Tour cards, the next 40 finishers and ties will earn KFT exempt status (subject to various reshuffles). The next 20 finishers and ties will earn exempt status for the Latin America Swing of the 2024 PGA Tour Americas season, while also earning conditional KFT membership. All remaining finishers will have at least conditional KFT and PGA Tour Americas status.
Golf Channel and Peacock will air live coverage of the final two rounds (all times ET):
Saturday 12:30-2:30PM (Peacock): PGA Tour Q-School, Rd. 3 2:30-4:30PM (GC/Peacock): PGA Tour Q-School, Rd. 3
Sunday 12:30-1:30PM (Peacock): PGA Tour Q-School, Rd. 4 1:30-4:30PM (GC/Peacock): PGA Tour Q-School, Rd. 4
Five things to know: ISPS HANDA - CHAMPIONSHIP
The DP World Tour visits a new course this week in Japan as the ISPS HANDA - CHAMPIONSHIP marks the penultimate regular event of the Asian Swing.

All to play for as Asian Swing continues
The ISPS HANDA – CHAMPIONSHIP returns for its second edition in Japan this week, marking the penultimate event in the Asian Swing.
As with the four other Global Swings, the Asian Swing will have its own champion who will earn $200,000 from an overall $1million bonus pool.
Swing champions will also qualify for each of the Back 9 events, where players will play for increased Race to Dubai ranking points.
The leading DP World Tour member (not otherwise exempt) will also qualify for the Genesis Scottish Open , the second of five Rolex Series events this season.
But in an another exciting reward for DP World Tour members, those who finish in positions one to three in the final Asian Swing standings (at the conclusion of the Volvo China Open) will be exempt into the U.S. PGA Championship at Valhalla Golf Club from May 16-19.
As it stands, Keita Nakajima, Jesper Svensson and Kiradech Aphibarnrat sit in the top three positions on the rankings, but with a tight leaderboard, anyone down to T-58 can go top with a win in Japan.
Mount Fiji provides backdrop to new venue on the DP World Tour
With views of Mount Fuji offering a spectacular backdrop to this year's tournament, Taiheiyo Club Gotemba Course will play host on the DP World Tour for the first time this week, following on from last year's event at PGM Ishioka GC.
The par 70 layout, which plays at 7262 yards and is described by the club as a 'true hillside course', was designed in 1977 by Shunsuke Kato and is the flagship layout of Taineiyo Club's 18 courses. Situated 100km southwest of Tokyo, the course underwent a renovation in 2018 overseen by Rees Jones with consultation from Major Champion Hideki Matsuyama.
No stranger to elite competitions, the course has staged the Taiheiyo Masters on the Japan Golf Tour every year since its opening in 1977. It has also played host to the 2001 World Cup of Golf, won by South African duo Ernie Els and Retief Goosen, and will also stage this year's Asia Pacific Amateur Championship in October.

Japanese stars in action
A host of players will be hoping to become the latest Japanese stars to make their mark after a record-breaking six months that has seen three champions crowned on the DP World Tour.
Keita Nakajima became the latest name to break through at the Hero Indian Open at the end of March, just 49 days after compatriot Rikuya Hoshino earned his own maiden title at the Commercial Bank Qatar Masters. Last season, Ryo Hisatsune became just the third player in history - following Hideki Matsuyama (2021 Masters) and Isao Aoki (1983 Panasonic European Open) to lift a DP World Tour title at the 2023 Cazoo Open de France.
With the event co-sanctioned with the Japan Golf Tour, Nakajima is joined in the field this week by 45 other players hoping to impress the home fans at a venue that is very well-known to them. Those include the likes of Takumi Kanaya, Kazuki Higa, Masahiro Kawamura, Ryo Ishikawa (a three-time champion on this course) and Hideto Tanihara (a two-time winner on this course)- who all have several previous top ten finishes in DP World Tour events.

ISPS HANDA continues support of golf
ISPS HANDA was founded by the Japanese philanthropist, Dr Haruhisa Handa, in 2006 in the belief that sport has the power to inspire, transform, and unite people and communities across social, racial and socio-economic barriers.
The DP World Tour and ISPS HANDA have collaborated on several events during a long-standing relationship, in turn raising the profile of golf as an accessible sport for all.
This was seen recently with ISPS HANDA backing the Australian All Abilities Championship – an event that features on the European Tour group’s G4D Tour schedule – at the end of last year.
Previous events ISPS HANDA has supported on the DP World Tour include the ISPS HANDA Wales Open (2012-14 and 2020), ISPS HANDA Perth International (2012-14 and 2016), ISPS HANDA World Cup of Golf (2013, 2016, 2018), (ISPS HANDA World Super 6 Perth (2017-19), ISPA HANDA Vic Open (2019-20), ISPS HANDA UK Championship (2020), ISPS HANDA World Invitational presented by Modest! Golf Management (2021), ISPS HANDA Championship in Spain (2022) and ISPS HANDA World Invitational presented by AVIV Clinics (2019, 21-23).
Inside the field
Matthieu Pavon is the top-ranked player in the field this week, having reached a career-high in the Official World Golf Ranking with a tie for 12th on his Masters debut earlier this month.
The French star, who won his first PGA TOUR title earlier this year, is joined in action by fellow ISPS HANDA ambassadors Rafa Cabrera-Bello and Christiaan Bezuidenhout, who are also fellow winners on the DP World Tour.
Jesper Svensson, who like Nakajima has won his first DP World Tour title during the Asian Swing, is aiming to strengthen his bid for a first Major start at next month’s U.S. PGA Championship, while Matteo Manassero and Jordan Gumberg are this season’s other winners teeing it up.
New Zealand’s Kazuma Kobori, who has guaranteed DP World Tour status for next season by finishing top of the Challenger PGA Tour of Australasia Order of Merit, is another rising star to watch out for.
Former DP World Tour winners Renato Paratore and Tom Lewis are among the Qualifying School graduates looking to make an impression, while could we see a third Challenge Tour graduate from the class of 2023 win this season after Matteo Manassero and Jesper Svensson?
DP World Tour Partners

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 26 April 2024
Apolipoprotein E controls Dectin-1-dependent development of monocyte-derived alveolar macrophages upon pulmonary β-glucan-induced inflammatory adaptation
- H. Theobald ORCID: orcid.org/0000-0003-4580-5542 1 na1 ,
- D. A. Bejarano ORCID: orcid.org/0000-0001-7804-0131 1 na1 ,
- N. Katzmarski 1 na1 ,
- J. Haub 1 na1 ,
- J. Schulte-Schrepping 2 , 3 ,
- K. Bassler ORCID: orcid.org/0000-0002-4780-372X 2 ,
- A. L. Ament 4 ,
- C. Osei-Sarpong 5 ,
- F. Piattini 6 ,
- L. Vornholz 7 , 8 ,
- W. T’Jonck 9 ,
- A. H. Györfi 10 , 11 ,
- H. Hayer 1 ,
- S. Sheoran 1 ,
- A. Al Jawazneh 13 , 14 ,
- S. Chakarov 15 ,
- K. Haendler 16 , 17 ,
- G. D. Brown ORCID: orcid.org/0000-0002-0287-5383 18 ,
- D. L. Williams 19 ,
- L. Bosurgi 13 , 14 ,
- J. H. W. Distler 10 , 11 ,
- F. Ginhoux ORCID: orcid.org/0000-0002-2857-7755 15 , 20 , 21 ,
- J. Ruland ORCID: orcid.org/0000-0002-8381-3597 7 , 8 , 22 , 23 ,
- M. D. Beyer ORCID: orcid.org/0000-0001-9704-148X 5 , 16 ,
- M. Greter ORCID: orcid.org/0000-0002-7220-5369 12 ,
- C. C. Bain ORCID: orcid.org/0000-0001-8884-327X 9 ,
- A. I. Vazquez-Armendariz 4 ,
- M. Kopf 6 ,
- J. L. Schultze ORCID: orcid.org/0000-0003-2812-9853 2 , 3 , 16 &
- A. Schlitzer ORCID: orcid.org/0000-0001-7662-3712 1
Nature Immunology ( 2024 ) Cite this article
74 Altmetric
Metrics details
- Acute inflammation
- Alveolar macrophages
- Chronic inflammation
The lung is constantly exposed to the outside world and optimal adaptation of immune responses is crucial for efficient pathogen clearance. However, mechanisms that lead to lung-associated macrophages’ functional and developmental adaptation remain elusive. To reveal such mechanisms, we developed a reductionist model of environmental intranasal β-glucan exposure, allowing for the detailed interrogation of molecular mechanisms of pulmonary macrophage adaptation. Employing single-cell transcriptomics, high-dimensional imaging and flow cytometric characterization paired with in vivo and ex vivo challenge models, we reveal that pulmonary low-grade inflammation results in the development of apolipoprotein E (ApoE)-dependent monocyte-derived alveolar macrophages (ApoE + CD11b + AMs). ApoE + CD11b + AMs expressed high levels of CD11b, ApoE, Gpnmb and Ccl6, were glycolytic, highly phagocytic and produced large amounts of interleukin-6 upon restimulation. Functional differences were cell intrinsic, and myeloid cell-specific ApoE ablation inhibited Ly6c + monocyte to ApoE + CD11b + AM differentiation dependent on macrophage colony-stimulating factor secretion, promoting ApoE + CD11b + AM cell death and thus impeding ApoE + CD11b + AM maintenance. In vivo, β-glucan-elicited ApoE + CD11b + AMs limited the bacterial burden of Legionella pneumophilia after infection and improved the disease outcome in vivo and ex vivo in a murine lung fibrosis model. Collectively these data identify ApoE + CD11b + AMs generated upon environmental cues, under the control of ApoE signaling, as an essential determinant for lung adaptation enhancing tissue resilience.
Similar content being viewed by others
Alveolar macrophage metabolic programming via a C-type lectin receptor protects against lipo-toxicity and cell death
A Lacticaseibacillus rhamnosus secretome induces immunoregulatory transcriptional, functional and immunometabolic signatures in human THP-1 monocytes
The impact of the lung environment on macrophage development, activation and function: diversity in the face of adversity
The lung is exposed to a variety of immunostimulatory agents shaping its immune responses 1 . How such environmental non-pathological immune activation is controlled at the cellular and molecular levels is poorly understood. β-glucans are integral components of environmental pathogenic and non-pathogenic fungi and are proposed as immune modulators 2 . Ambient concentrations of β-glucan oscillate during the year and, in combination with pathogen exposure, correlate with allergic rhinitis increases 3 . Recognition of β-glucan by Dectin-1 modulates systemic immune responses through a process termed innate immune memory 4 , characterized by increased cytokine responses of monocytes, upon a secondary heterologous stimulus, facilitated by a metabolic and epigenetic rewiring, allowing a more efficient first-line immune response 4 , 5 , 6 , 7 , 8 , 9 . Lung-specific mechanisms of β-glucan, the organ where it is most often recognized, and how it acts as a nongenetic modifier of immune responses to subsequent disease, remain unknown.
Immune cells residing in the alveolar space are the body’s respiratory first line of defense, ensuring efficient immune response induction against airborne pathogens, while regulating immune activation to ensure intact lung function 10 . AMs and monocytes constitute the major mononuclear phagocytes (MPs) found during homeostasis in humans and mice within the alveolus. AMs and monocytes express high amounts of Dectin-1 and are highly plastic 11 . Dectin-1 signaling critically depends on spleen tyrosine kinase (Syk) which upon activation triggers phospholipase C gamma 2 (PLCγ2)-dependent calcium release, downstream nuclear factor of activated T cells (NFAT) and extracellular signal-regulated kinase (ERK) activation. This cascade leads to production of interleukin (IL)-2 and IL-10. Furthermore, Syk activates caspase recruitment domain-containing protein 9 (CARD9) leading to nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling and the release of tumor necrosis factor (TNF) and IL-6. Additionally, Dectin-1 ligation directly induces reactive oxygen species (ROS) production via activation of phosphoinositide 3 kinase (PI3K) 12 , 13 . Murine homeostatic AMs are embryonically derived with only minor contribution of adult bone marrow (BM) homeostasis 14 , 15 . Pulmonary viral infection or radiation was shown to induce differentiation of Ly6c + monocytes into long-lived monocyte-derived alveolar macrophages (MoAMs) 16 , 17 , 18 . During viral infection, MoAM-derived IL-6 is crucial for the defense against subsequent Streptococcus pneumoniae infection 16 . Finally, viral-induced environmental adaptation affects resident AM-dependent CD8 + T cell rewiring, inducing efficient bacterial clearance 19 .
Chronic lung diseases, like asthma or pulmonary fibrosis, hinge on environmental factors. Thus, understanding environmental immune adaptation in bronchoalveolar MPs is crucial for insights into cellular, functional and molecular consequences 20 , 21 , 22 , 23 , 24 .
To investigate this, we developed a reductionist model of a single low-dose intranasal β-glucan exposure. Using single-cell transcriptomic, functional in vivo and ex vivo analysis of cellular development and function, this model allowed us to dissect acute and chronic molecular adaptations of macrophages upon environmental cues. We show that a single intranasal β-glucan exposure induces developmentally and functionally modified ApoE + CD11b + MoAMs, detected up to 21 days after β-glucan exposure. ApoE + CD11b + MoAMs are glycolytic, highly phagocytic and release, upon activation, high amounts of IL-6. Functional changes are cell intrinsic and upon subsequent infection with Legionella pneumophila or a challenge by bleomycin-induced fibrosis lead to improved in vivo outcomes. Molecularly, ApoE + CD11b + MoAMs are controlled by the Dectin-1–CARD9 pathway, whereas maintenance of ApoE + CD11b + MoAMs depends on paracrine ApoE and macrophage colony-stimulating factor (M-CSF). Taken together, we identify ApoE as a crucial checkpoint for low-grade inflammation-associated M-CSF-controlled monocyte-to-macrophage differentiation triggered by the Dectin-1–CARD9 pathway within the immune-adapted microenvironment of lung.
Intranasal β-glucan induces ApoE + CD11b + AMs
The lung is constantly exposed to pollutants, sterile and non-sterile pathogens, and components thereof. Our understanding of the cellular and molecular mechanisms of immune adaptation to these environmental cues is limited. To investigate this, we developed a simplified model involving a single low-dose intranasal exposure to β-glucan particles (200 µg), mimicking environmental exposure 25 . To assess the impact of this stimulation and investigate its lasting effects, we examined bronchoalveolar lavage fluid (BALF)-resident macrophages (CD45 + Lin − SSC int– hi ) of C57BL/6 mice 7 days after intranasal phosphate-buffered saline (PBS) or β-glucan treatment using single-cell transcriptomics (Fig. 1a and Supplementary Fig. 1a–c ). Dimensionality reduction using uniform manifold approximation and projection (UMAP) analysis and unsupervised clustering (Louvain) revealed five distinct transcriptional clusters within the BALF (Fig. 1a,b ). Here, high expression of AM signature genes Siglecf and Itgax identified all investigated cells as AMs (Supplementary Fig. 1d,e ) 26 . Further analysis identified clusters 0 and 1 as subsets of resident AMs expressing Ear2, Wfdc21 and Hmox1 (refs. 26 , 27 ). Cluster 2 (proliferating AM) expressed genes linked to proliferation such as Top2a , Mki67 and Birc5 (ref. 28 ). Cluster 3 (ApoE + AMs) expressed genes associated with a lipid-associated inflammatory monocyte-derived macrophage (MoMac) phenotype, including Apoe , Cd63 , Spp1 , Gpnmb and Trem2 (refs. 16 , 29 , 30 ). Cluster 4 (ISG + AMs), was characterized by expression of interferon-stimulated genes (ISGs), such as Ifit2 , Ifit3 , Ifi204 and Isg15 (Fig. 1b ). To determine which cluster was associated with β-glucan-induced environmental adaptation, the relative contribution of each stimulatory condition to individual clusters was examined (Fig. 1c–e and Supplementary Fig. 1f ). Here, ApoE + AMs were only present within the BALF of β-glucan-exposed mice 7 days prior, concomitant with a reduction in proliferating AMs. Previous studies suggested that CD11b expression on stimulated AMs serves as a marker for enhanced inflammatory potential 31 , 32 . Therefore, we used co-detection by indexing (CODEX)-enabled high-dimensional imaging to characterize the phenotype of ApoE + AMs at the protein level in the lung and in BALF 7 days after β-glucan stimulation (Fig. 1f and Supplementary Fig. 1g ) 33 . ApoE + AMs not only coexpressed the classical AM markers, CD11c and Siglec-F, but also expressed high amounts of CD11b, ApoE and GPNMB proteins (Fig. 1f and Supplementary Fig. 1g ). Furthermore, to confirm the overlap of Apoe mRNA expression and CD11b protein expression, we measured Apoe mRNA levels using PrimeFlow. Apoe mRNA signals were detectable only in BALF CD11b + AMs isolated from mice stimulated with β-glucan 7 days prior (Fig. 1g ). Consequently, we refer to this AM subpopulation as ApoE + CD11b + AMs. To investigate the β-glucan-induced cellular dynamics of ApoE + CD11b + AMs we monitored the BALF from day 0 to day 21 after β-glucan exposure using flow cytometry (Fig. 1h,i and Supplementary Fig. 1h,i ). This analysis revealed that, in line with the single-cell transcriptomic data, ApoE + CD11b + AMs peaked at day 7 after β-glucan inoculation and gradually declined until day 21 (Fig. 1h,i ). In agreement with this, we observed an overall increase in total AMs peaking at day 7 after β-glucan exposure (Supplementary Fig. 1j,k ). Generation of ApoE + CD11b + AMs was associated with a transient influx of neutrophils and eosinophils on days 1 and 3 (Supplementary Fig. 1l,m ). To confirm the macrophage identity of ApoE + CD11b + AMs, Siglec-F expression on CD11b − AMs, ApoE + CD11b + AMs and Ly6c + monocytes was assessed. Both CD11b − and ApoE + CD11b + AMs expressed high levels of Siglec-F, whereas Ly6c + monocytes did not, further establishing ApoE + CD11b + AMs as part of the AM compartment (Supplementary Fig. 1n ). Finally, to understand the abundance ApoE + CD11b + AMs in total lung single-cell suspensions, we quantified ApoE + CD11b + AMs using flow cytometry (Supplementary Fig. 1o ). ApoE + CD11b + AMs were also present in full lung suspensions, displaying similar quantity and marker profiles as in BALF. In summary, intranasal β-glucan induces ApoE + CD11b + AMs as a cellular response to environmental stimulation.
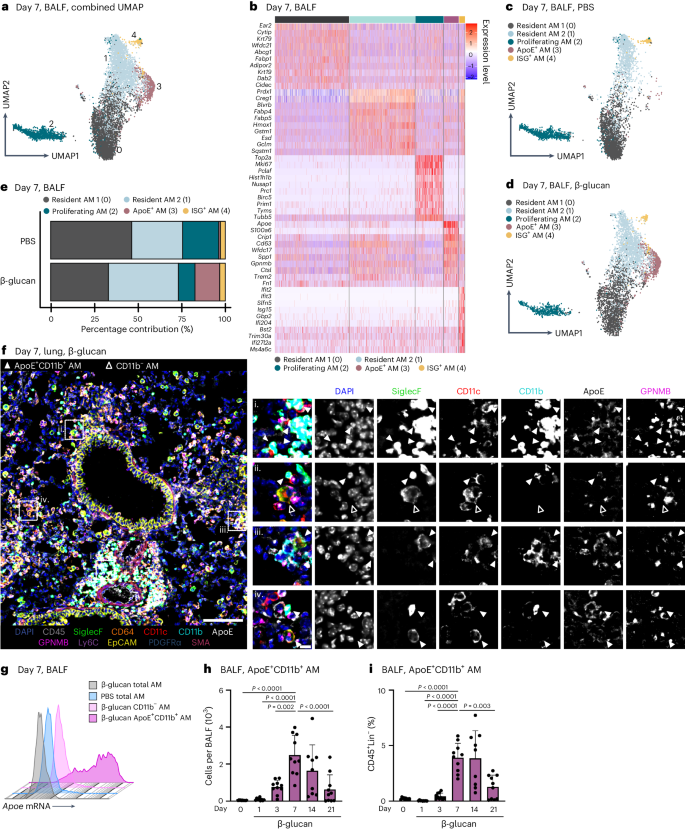
a – e , Single-cell RNA sequencing (scRNA-seq) of the BALF of male 8- to 12-week-old C57BL/6J (WT) mice after intranasal stimulation with 200 µg β-glucan or PBS ( n = 10,202 cells). Seven days after exposure, cells of three mice per condition were harvested and sorted for SSC hi , Lin − (B220, CD19, CD3ε, Nk1.1, Ter-119), DRAQ7 − singlets ( n = 3 mice, one independent experiment). a , UMAP analysis of both conditions combined shows five different clusters. b , Heat map of the top ten highly expressed genes for each of the five clusters. c , d , UMAP from a separated by PBS ( c ; n = 4,845 cells) or β-glucan ( d , n = 5,357 cells) condition. e , Percentage contribution of the five annotated clusters to overall cells split by conditions. f , 5-µm frozen section of the left lobe of the lung of a Ms4a3-cre Rosa26TOMATO mouse 7 days after β-glucan exposure stained with a 17-plex CODEX antibody panel. Overlaid images show the markers used to identify AM populations. Single stainings of these markers are shown in grayscale. Filled arrowheads indicate ApoE + CD11b + AMs, whereas open arrowheads indicate CD11b − AMs. Scale bar, 100 µm (large image) and 10 µm (enlargements; n = one representative mouse of two independent experiments). g , Detection of Apoe mRNA expression in the AM subsets of the BALF 7 days after intranasal PBS or β-glucan exposure in WT mice by PrimeFlow ( n = 3 mice pooled per group, one of two independent experiments shown). h , i , Flow cytometric quantification of absolute numbers ( h ) or frequency ( i ) of ApoE + CD11b + AM (CD45 + Siglec-F + CD64 + CD11c + CD11b + ) in the BALF in a time course from 1 to 21 days after β-glucan stimulation of WT mice ( n = 9–10 mice, two independent experiments). Data in h and i are depicted as the mean ± s.d. Significance was assessed using ordinary one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons.
Source data
Apoe + cd11b + ams are derived from monocytes and depend on ccr2.
Both acute and chronic inflammation induce the recruitment of MoMacs into the bronchoalveolar space 16 , 31 , 34 . To understand whether β-glucan-induced environmental adaptation induces a new resident AM cell state or results in the recruitment and differentiation of Ly6c + monocytes into ApoE + CD11b + AMs, we tracked the influx of Ly6c + monocytes into the BALF using flow cytometry. After β-glucan stimulation, Ly6c + monocytes were recruited to the bronchoalveolar space, peaking 3 days after stimulation, remaining elevated on day 7, and gradually declining from day 14 onwards (Fig. 2a and Supplementary Fig. 2a ). To connect these findings with the emergence of ApoE + CD11b + AMs, we utilized the Ms4a3 -cre Rosa26TOMATO mice, enabling genetic tracing of BM-derived granulocyte-macrophage progenitors (GMPs).
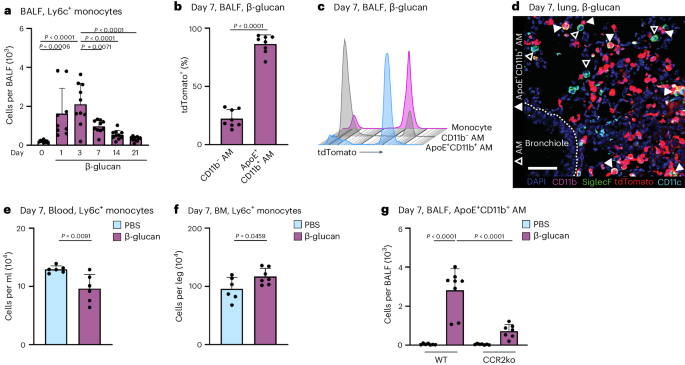
a , Flow cytometric quantification of absolute Ly6c + monocyte (CD45 + Ly6g − Siglec-F − CD64 int CD11b + Ly6c + ) numbers in the BALF 1 to 21 days after β-glucan stimulation of WT mice ( n = 9–10 mice, two independent experiments). b , c , Flow cytometric analysis of BALF from Ms4a3-cre Rosa26TOMATO mice 7 days after intranasal PBS or β-glucan stimulation ( n = 8, two independent experiments). Percentage of tdTomato + labeling in CD11b − and ApoE + CD11b + AMs ( b ) and proportion of tdTomato + labeling in CD11b − and ApoE + CD11b + AMs compared to monocytes (CD45 + Siglec-F − Ly6g − CD11b + F4/80 + ) ( c ). d , CODEX multiplexed immunostaining of the left lobe of a Ms4a3-cre Rosa26TOMATO mouse 7 days after β-glucan exposure (enlargement from Fig. 1f ). Filled arrowheads indicate ApoE + CD11b + AMs, whereas empty arrowheads indicate CD11b − AMs. tdTomato reporter signals are represented in red. Scale bar, 50 µm. e , f , Absolute counts of Ly6c + monocytes in the blood ( e ) or BM ( f ) of WT mice 7 days after PBS or β-glucan by flow cytometry ( n = 6 mice, two individual experiments). g , Flow cytometric quantification of absolute ApoE + CD11b + AM numbers in the BALF 7 days after β-glucan exposure in WT or CCR2 −/− mice ( n = 7–8 mice, two individual experiments). Data are depicted as the mean ± s.d. Significance was assessed using ordinary one-way ANOVA with Tukey’s multiple comparisons ( a and g ) and unpaired two-tailed student’s t -test ( b , e and f ).
Genetic lineage tracing revealed that 86% ± 7.8% of ApoE + CD11b + AMs were labeled with tdTomato, indicating a BM GMP lineage origin (Fig. 2b,c ). CODEX imaging showed coexpression of CD11b and tdTomato in Siglec-F + CD11c + AMs within tissue sections from mice stimulated with β-glucan 7 days prior, supporting their GMP and monocyte origin (Fig. 2d ). To determine if Ly6c + monocytes are systemically mobilized and recruited to the lung from the BM following β-glucan stimulation, we assessed the abundance of Ly6c + monocytes, common monocyte progenitors (cMOPs) and GMPs in the blood and BM (Fig. 2e,f and Supplementary Fig. 2b–g ). This revealed a reduction in Ly6c + monocytes in the blood, accompanied by a compensatory increase in BM Ly6c + monocytes 7 days after β-glucan stimulation. To confirm the monocytic BM origin of ApoE + CD11b + AMs, we utilized CCR2-deficient mice, in which the recruitment of Ly6c + monocytes into peripheral tissues is impaired 35 . To investigate dependence of ApoE + CD11b + AMs on CCR2, we intranasally inoculated control and CCR2-deficient mice with β-glucan and analyzed the BALF 7 days later using flow cytometry (Fig. 2g and Supplementary Fig. 2h ). This analysis revealed a significant reduction in ApoE + CD11b + AMs in CCR2-deficient mice following β-glucan inoculation. In summary, these results collectively demonstrate that ApoE + CD11b + AMs induced by β-glucan exposure originate from BM Ly6c + monocytes in a CCR2-dependent manner.
ApoE + CD11b + AMs exhibit an elevated release of IL-6
MoMacs have been linked to heightened inflammatory responses following high-grade inflammatory and infectious events, such as influenza A virus infection 16 . Specifically, the increased production of IL-6 is a hallmark feature of functionally modified MoMacs during acute inflammation. However, it remains unclear whether BALF-resident mononuclear cells functionally adapt to local low-grade inflammation. To address this, we investigated the functional profile of AMs 7 days after exposure to β-glucan. We isolated BALF AMs, and subsequently restimulated them with PBS or lipopolysaccharide (LPS) for 24 h in vitro. Analysis of IL-6 release in the supernatants using ELISA revealed that macrophages pre-exposed in vivo to β-glucan released significantly higher amounts of IL-6 upon LPS restimulation, compared to their PBS-pretreated counterparts (Fig. 3a ). To identify the cellular source responsible for increased IL-6 production, we purified BALF-resident CD11b − and ApoE + CD11b + AMs of intranasal β-glucan-treated mice 7 days earlier and restimulated them with LPS (Fig. 3b ). Only ApoE + CD11b + AMs released comparable amounts of IL-6 to those observed in complete BALF AM preparations (Fig. 3a ). Subsequently, intracellular flow cytometric analysis demonstrated a significant increase in IL-6 + CD11b + AMs following β-glucan exposure compared to PBS-exposed controls (Fig. 3c and Supplementary Fig. 3a ). Our data indicate that the generation of ApoE + CD11b + AMs in response to β-glucan exposure depends on CCR2 (Fig. 2g ). To confirm that the increased IL-6 observed in ex vivo restimulated AMs can be directly attributed to CCR2-dependent ApoE + CD11b + AMs, we exposed CCR2-deficient and control mice to β-glucan. Seven days later, BALF AMs were enriched, restimulated and restimulated with LPS for 24 h in vitro (Fig. 3d ). This analysis revealed that the elevated IL-6 levels observed in β-glucan-exposed BALF AMs are CCR2 dependent, providing evidence that ApoE + CD11b + AMs are the primary source of increased IL-6 during β-glucan-induced environmental adaptation. Finally, to causally establish whether the enhanced IL-6 production is an intrinsic cellular feature of ApoE + CD11b + AMs, we transferred CD45.2 + BALF-resident AMs into naive CD45.1 + mice 5 days after β-glucan-induced environmental adaptation. Two days later, we restimulated BALF AMs with LPS in vitro (Fig. 3e and Supplementary Fig. 3b,c ). Transfer of β-glucan-experienced BALF AMs led to an increased IL-6 production upon in vitro restimulation of AMs within the recipient mouse. These findings causally establish the intrinsic β-glucan-induced functional change in ApoE + CD11b + AMs.
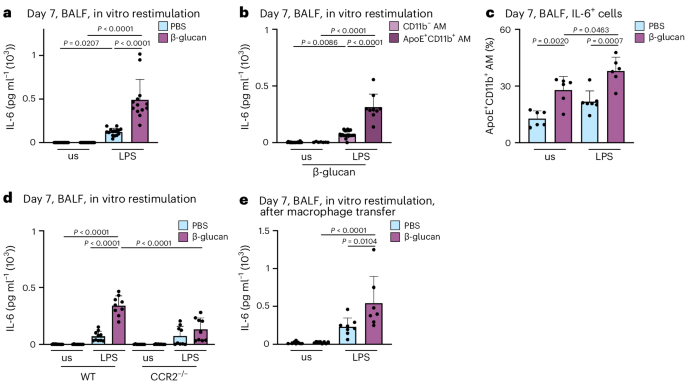
a – c , BALF cells were harvested from WT or Ms4a3-cre ROSA26TOMATO mice 7 days after intranasal exposure with PBS or β-glucan and subsequently restimulated in vitro with or without LPS (unstimulated, us) for 24 h. a , Quantification of IL-6 protein levels by ELISA in the cell culture supernatant 24 h after LPS restimulation of WT mice ( n = 13–15 mice, three individual experiments). b , CD11b + Ms4a3 + AMs and CD11b − Ms4a3 − AMs were sorted from the pooled BALF of PBS or β-glucan-stimulated Ms4a3-cre ROSA26TOMATO mice and seeded with 0.2 × 10 5 cells per well before LPS restimulation ( n = 9 mice for PBS, n = 31 mice for β-glucan; one dot represents the pooled supernatant of two technical replicate wells, minimum of nine data points per group, two individual experiments). c , Percentage of IL-6 + cells among ApoE + CD11b + AMs after restimulation with LPS for 6–8 h followed by intracellular staining and flow cytometric analysis ( n = 6–7 mice, two individual experiments). d , Quantification of IL-6 protein levels by ELISA in the cell culture supernatant 24 h after restimulation with LPS of WT and CCR2 −/− mice ( n = 9–10 mice, two individual experiments). e , BALFs of PBS or β-glucan-experienced CD45.2 WT mice were harvested and pooled 5 days after stimulation. 2 × 10 5 cells in 35 µl were intratracheally transferred into CD45.1 mice. Quantification of IL-6 protein levels by ELISA in the cell culture supernatant after restimulation with LPS 48 h after transfer ( n = 7–8 mice, two individual experiments). Data are depicted as the mean ± s.d. Significance was assessed using ordinary one-way ANOVA with Tukey’s multiple comparisons ( a – e ).
β-glucan aids lung bacterial defense and experimental fibrosis recovery
Previous studies associated altered cytokine responses following systemic β-glucan stimulation with increased glycolysis in MPs 7 , 8 . To investigate this in our system, we measured glycolysis. This revealed a significant increase in glycolysis, glycolytic capacity and glycolytic reserve 7 days after β-glucan exposure in BALF AMs (Fig. 4a,b and Supplementary Fig. 4a,b ). In addition, induction of glycolysis in MPs is associated with enhanced phagocytosis 36 , 37 . Thus, we assessed BALF AM phagocytosis of Staphylococcus aureus -coated particles in vitro isolated from mice adapted to PBS or β-glucan 7 days prior. This demonstrated a significant increase in phagocytic activity in BALF AMs isolated from β-glucan-adapted mice but not PBS-adapted mice (Fig. 4c,d and Supplementary Fig. 4c,d ). This prompted us to investigate the in vivo functional impact of intranasal β-glucan adaptation in response to acute bacterial challenge. We infected C57BL/6 mice that were β-glucan or PBS adapted 7 days earlier with L. pneumophila and analyzed the BALF’s bacterial burden and cellular composition 2 days after infection 38 . β-glucan-adapted mice exhibited a significant reduction in bacteria detected in BALF, along with an increased count of pro-inflammatory macrophages associated with bacterial clearance (Fig. 4e,f and Supplementary Fig. 4e ).
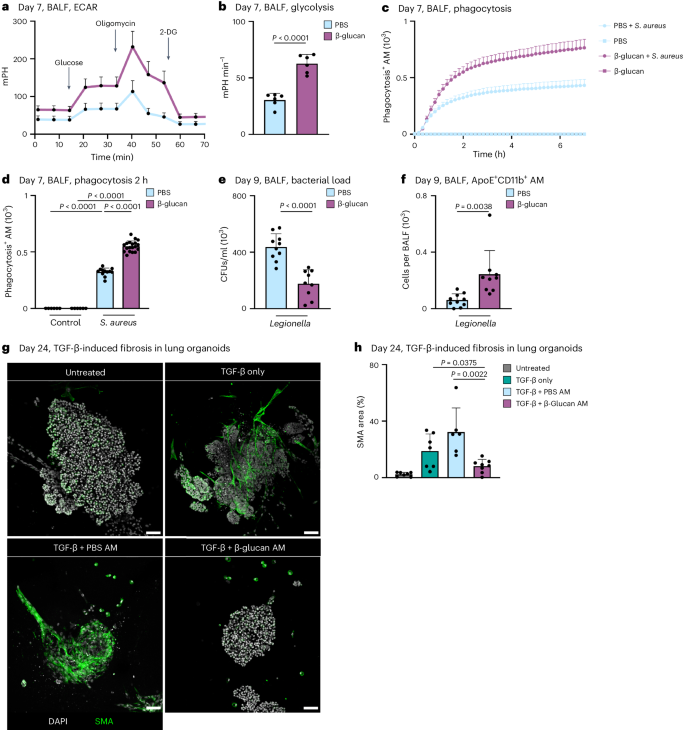
a , b , Extracellular acidification rate (ECAR; a ) and glycolysis ( b ) in BALF cells 7 days after PBS or β-glucan stimulation of WT mice measured by Seahorse ( n = 6 mice, one independent experiment). c , d , AM cells from day 7 PBS- or β-glucan-experienced WT mice were selected by adherence and subsequently treated with 2.5 µg pHrodo S. aureus bioparticles ( n = 3 mice pooled per condition, technical replicates: 6 control wells, 12–19 treated wells per group, one of two independent experiments shown). c , Representative curve of absolute phagocytosis + AM numbers over the time course of 7 h (here shown as mean ± s.d. of all technical replicates). d , Absolute numbers of phagocytosis + AMs 2 h after adding the pHrodo S. aureus bioparticles. e , f , C57BL/6J WT mice were intranasally stimulated with PBS or β-glucan followed by intratracheal infection with 5 × 10 6 colony-forming units (CFUs) L. pneumophilia at day 7 after primary stimulation and analysis at day nine ( n = 9–10 mice, two independent experiments). Quantification of bacterial load in BALF ( e ) and absolute numbers of ApoE + CD11b + AMs by flow cytometry 9 days after primary stimulation ( f ). g , h , Representative confocal images ( g ) and SMA area quantification ( h ) of BALOs co-cultured with PBS- or β-glucan-experienced AMs 48 h after induction of fibrosis via TGF-β. Seven days after stimulation, 2.5 × 10 4 AMs of PBS- or β-glucan-experienced WT mice were co-cultured with day 21 lung BALOs for 24 h. AM–organoid co-cultures were subsequently treated with 1.05 ng ml −1 TGF-β for 48 h before fixation and antibody staining. Myofibroblasts were stained for α-SMA ( n = 6–8 organoids per condition from two replicate wells; one of two independent experiments shown). Scale bars, 50 µm ( g ). Data are depicted as the mean ± s.d. Significance was assessed using unpaired two-tailed student’s t -test ( b , e and f ), ordinary one-way ANOVA with Tukey’s multiple comparisons ( d ) and two-tailed Mann–Whitney test ( h ).
Furthermore, we examined whether β-glucan adaptation had effects beyond the modulation of acute bacterial infection using bleomycin-induced experimental lung fibrosis. β-glucan-adapted mice showed significantly higher survival rates, lower disease burden and reduced weight loss over a 14-day observation period after bleomycin inoculation (Supplementary Fig. 4f–i ). Moreover, the pro-resolution associated effectors, IL-4 and IL-33, were enhanced on day 3 after bleomycin inoculation, while on day 14 after bleomycin thymic stromal lymphopoietin (TSLP) decreased in β-glucan-adapted mice (Supplementary Fig. 4j–l ). No difference in lung fibrotic area was observed (Supplementary Fig. 4m,n ). These findings highlight the substantial regulatory role of ApoE + CD11b + AMs elicited by environmental adaptation in the control and severity of acute and chronic inflammation. To elucidate the direct effect of ApoE + CD11b + AMs on the development of lung fibrosis, we generated bronchoalveolar lung organoids (BALOs) containing myofibroblasts 39 . We treated them with transforming growth factor-beta (TGF-β) to induce a fibrotic response. BALF AMs isolated from β-glucan or PBS-adapted mice were added to day 21 BALOs 24 h before TGF-β pro-fibrotic stimulation and co-cultured for 48 h. Adding TGF-β to BALOs led to the increased production of fibroblast smooth muscle actin (SMA), a hallmark of lung fibrosis. We quantified SMA production in β-glucan or PBS-adapted AM-supplemented fibrotic BALOs. We observed a significant reduction in SMA production when β-glucan-adapted AMs were added, while the addition of PBS-adapted AMs showed no effect (Fig. 4g,h and Supplementary Fig. 4o ). In conclusion, functional in vivo and in vitro data establish ApoE + CD11b + AMs as crucial environmentally induced modulators of lung inflammation, providing valuable insights into the molecular mechanisms underlying their role in mitigating fibrosis.
β-glucan induces ApoE + CD11b + AMs via Dectin-1/CARD9
β-glucan is recognized by various receptors, including CR3, Dectin-1 and CD5 (refs. 40 , 41 , 42 ). Dectin-1 is most prominently expressed on MPs. To understand how Dectin-1 regulates ApoE + CD11b + AMs, we used flow cytometry to profile its expression on BALF macrophages (Fig. 5a ). This revealed that homeostatic Dectin-1 expression is largely confined to resident AMs with only a small fraction of monocytes expressing Dectin-1. To assess the role of Dectin-1 for the development of ApoE + CD11b + AMs, we intranasally inoculated control or Dectin-1 −/− mice with β-glucan and used flow cytometry to analyze BALF-resident immune cells 7 days later. This revealed that generation of ApoE + CD11b + AMs is dependent on Dectin-1 expression, whereas initial inflammatory recruitment of Ly6c + monocytes to the BALF is not (Fig. 5b,c ). Next, to understand whether immune cell-intrinsic or stromal cell recognition via Dectin-1 is critical for the development of ApoE + CD11b + AMs and thus environmental adaptation, we transferred Dectin-1 −/− or control (CD45.2 + ) BM into lethally irradiated CD45.1 + control mice and analyzed their BALF 7 days after environmental adaptation by β-glucan. Here, generation of ApoE + CD11b + AMs was entirely dependent on hematopoietic expression of Dectin-1 (Fig. 5d and Supplementary Fig. 5a,b ). CARD9 mediates activation of NF-κB by Dectin-1 (refs. 12 , 13 , 43 ). To investigate if ApoE + CD11b + AMs require CARD9 for their development, we treated lethally irradiated mice reconstituted with Card9 −/− or control BM with β-glucan or PBS and flow cytometrically analyzed the BALF 7 days later. This revealed that development of ApoE + CD11b + AMs depends on Dectin-1-elicited CARD9-dependent signaling (Fig. 5e and Supplementary Fig. 5c,d ). Next, we investigated whether the loss of ApoE + CD11b + AMs by abrogating Dectin-1 or CARD9 signaling leads to a loss of increased IL-6 secretion upon in vitro LPS restimulation in BALF macrophages. In line with the data obtained in the CCR2 −/− mouse model, enhanced IL-6 secretion was abolished in the absence of Dectin-1 or CARD9 signaling and thus can be attributed to ApoE + CD11b + AMs (Fig. 5f,g ).
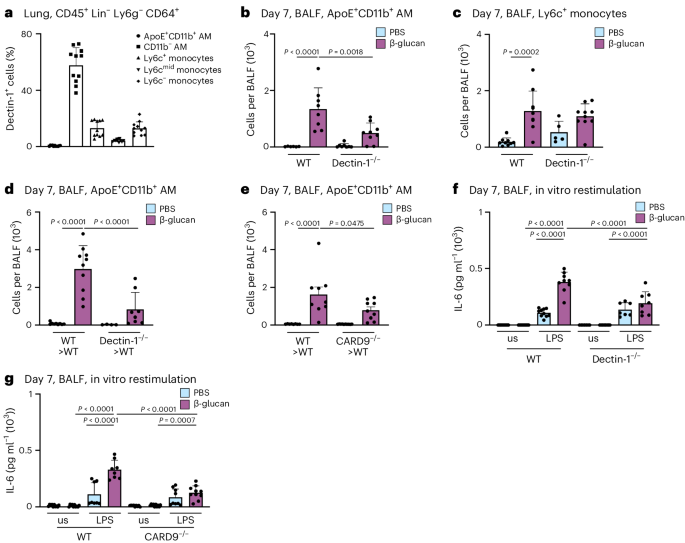
a , Percentage of monocyte and macrophage populations contributing to Dectin-1 + cells in the WT mouse lung pregated on CD45 + Lin − Ly6g − CD64 + cells ( n = 11 mice, two independent experiments) by flow cytometry. b , c , Absolute ApoE + CD11b + AM ( b ) and Ly6c + monocyte ( c ) numbers in the BALF 7 days after PBS or β-glucan exposure in WT or Dectin 1 −/− mice ( n = 5−9, two independent experiments) by flow cytometry. d , e , Absolute ApoE + CD11b + AM numbers in the BALF 7 days after PBS or β-glucan exposure in Dectin1 −/− ( d ; n = 4–10 mice, two independent experiments) or C ard9 −/− ( e ; n = 8–9 mice, two independent experiments) BM chimeras by flow cytometry. f , Quantification of IL-6 protein levels by ELISA in the cell culture supernatant 24 h after LPS restimulation of WT and Dectin1 −/− mice ( n = 7–10 mice, two independent experiments). g , Quantification of IL-6 protein levels by ELISA in the cell culture supernatant 24 h after LPS restimulation of WT and C ard 9 −/− mice ( n = 9–10 mice, two independent experiments). Data are depicted as the mean ± s.d. Significance was assessed using ordinary one-way ANOVA with Tukey’s multiple comparisons ( b – g ).
β-glucan triggers ApoE + CD11b + AM differentiation via myeloid ApoE
ApoE is expressed in various MoMac populations associated with different low-grade or chronic inflammatory diseases but its role in monocyte-to-macrophage differentiation and maintenance remains unexplored 16 , 29 . During β-glucan-induced environmental adaptation, ApoE was highly expressed in ApoE + CD11b + AMs and detectable at the protein level as early as 1 day after intranasal β-glucan stimulation, coinciding with BALF Ly6c + monocyte recruitment (Figs. 2a and 6a,b ). To elucidate the role of ApoE in the environmental adaptation of the lung MP repertoire, we intranasally inoculated Apoe fl Ly z2 Cre mice, which lack ApoE expression within the myeloid lineage, with β-glucan. Next, we used flow cytometry to analyze the composition of the BALF MP compartment 7 days later. β-glucan-stimulated Apoe fl Lyz2 Cre mice did not exhibit increased numbers of BALF Ly6c + monocytes and, as a consequence, failed to generate ApoE + CD11b + AMs (Fig. 6c,d and Supplementary Fig. 6a,b ).
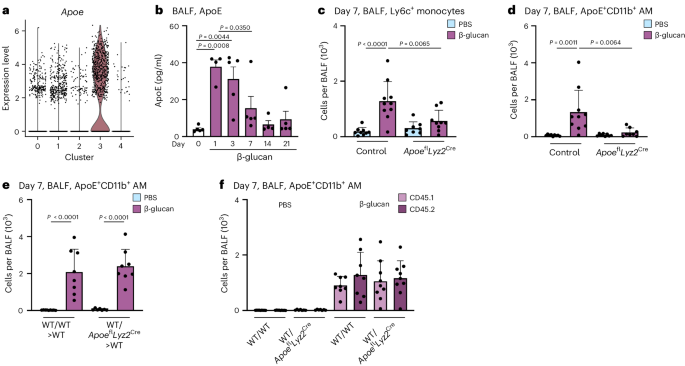
a , Violin plot of Apoe RNA expression levels in the BALF 7 days after β-glucan exposure by scRNA-seq. b , WT mice were stimulated with β-glucan, and BALF was harvested at different time points. The plot shows ApoE protein levels in the BALF measured by ELISA ( n = 4–5 mice, one independent experiment). c , d , Absolute numbers of ApoE + CD11b + AM ( c ) and Ly6c + monocytes ( d ) 7 days after intranasal β-glucan exposure of control or Apoe fl Lyz 2 Cre mice by flow cytometry ( n = 8–10 mice, three independent experiments). e , f , Lethally irradiated CD45.1 + /CD45.2 + male mice were reconstituted with 1.5 × 10 6 CD45.1 + mixed with CD45.2 + BM cells (WT/WT) or with CD45.1 + mixed with Apoe fl Lys2 Cre CD45.2 + BM cells (WT/ Apoe fl Lyz2 Cre ) for 12 weeks and subsequently intranasally stimulated with PBS or β-glucan ( n = 8–9 mice, two independent experiments). Flow cytometric quantification of ApoE + CD11b + AM numbers ( e ) and contribution of donor cells (CD45.1 + or CD45.2 + ) to the ApoE + CD11b + AM pool ( f ) 7 days after exposure. Data are depicted as the mean ± s.d. Significance was assessed using ordinary one-way ANOVA with Tukey’s multiple comparisons ( b – f ).
Next, we examined whether loss of myeloid ApoE influences the observed feedback on blood and BM Ly6c + monocytes. Myeloid ApoE deficiency abrogated the decrease in blood monocytes and the compensatory increase in BM Ly6c + monocytes observed in control mice. Myeloid ApoE deficiency showed no effect on cMOPs or GMPs 7 days after β-glucan stimulation (Supplementary Fig. 6c–f ). Subsequently, we investigated whether ApoE controls the generation of ApoE + CD11b + AMs through paracrine or autocrine signaling, as both modes have been described previously 44 , 45 . Here, we generated mixed BM chimeras with a 50:50 ratio of wild-type (WT; CD45.1 + ) and Apoe fl Lyz2 Cre (CD45.2 + ) cells or congenic mixed WT (CD45.1 + ):WT (CD45.2 + ) control chimeras. After reconstitution, we intranasally stimulated them with β-glucan and used flow cytometry to analyze their BALF MP repertoire 7 days later. This revealed that both WT/WT and WT/ Apoe fl Lyz2 Cre chimeras efficiently generated ApoE + CD11b + AMs 7 days after β-glucan exposure, supporting a paracrine signaling mode (Fig. 6e and Supplementary Fig. 6g,h ). Next, we examined the contribution of ApoE-deficient CD45.2 + cells to the pool of total ApoE + CD11b + AMs. We found that both ApoE-proficient (CD45.1) and ApoE-deficient (CD45.2) cells equally contributed to the pool of β-glucan-stimulated ApoE + CD11b + AMs (Fig. 6f ). This demonstrates that a paracrine myeloid cell-derived source of ApoE is sufficient to rescue the generation of ApoE + CD11b + AMs during environmental adaptation in the lung.
Myeloid-derived ApoE controls survival of ApoE + CD11b + AMs by regulation of cholesterol storage and M-CSF secretion
To elucidate ApoE’s role in the differentiation and survival of ApoE + CD11b + AMs, we conducted experiments to determine whether myeloid-derived ApoE influences the initial commitment of Ly6c + monocytes to or the maintenance and survival of MoMacs. We used control and Apoe fl Lyz2 Cre mice 3 days after intranasal β-glucan exposure. On day 3 after β-glucan exposure, similar numbers of BALF Ly6c + monocytes were present in control and Apoe fl Lyz2 Cre mice. This suggests that ApoE does not regulate the initial commitment to the macrophage lineage or the recruitment of monocytic precursors to the BALF. However, our results established a critical time window during which ApoE is essential for monocyte-to-macrophage differentiation following β-glucan inoculation (Fig. 7a,b and Supplementary Fig. 7a,b ). To investigate potential molecular dysregulation caused by the absence of ApoE in differentiating macrophages, we assessed intracellular cholesterol content and distribution of BALF ApoE + CD11b + AMs 3 days after β-glucan stimulation. This revealed that ApoE-deficient CD11b + AMs have increased intracellular cholesterol, as indicated by filipin staining (Fig. 7c,d ). Previous data linked dysregulated ApoE signaling to cholesterol accumulation in the endoplasmic reticulum and a reduction in protein synthesis 46 . To test these mechanisms in our experimental system of monocyte-to-macrophage differentiation, we analyzed the colocalization of BODIPY-cholesterol with the endoplasmic reticulum-associated protein calreticulin and early or late endosome markers (EEA1 and LAMP1) using confocal microscopy. This revealed that, in ApoE-deficient differentiating macrophages 3 days after β-glucan exposure, BODIPY-cholesterol colocalizes with calreticulin, accumulating at the endoplasmic reticulum (Fig. 7e,f ). M-CSF-releasing monocytes differentiating into macrophages have been described to be crucial for lung monocyte-to-macrophage differentiation 47 . To assess whether aberrant cholesterol accumulation affects the M-CSF–M-CSF receptor (CSF-1R) macrophage survival circuit, we monitored BALF and lung tissue intracellular and extracellular M-CSF secretion. Here, ApoE deficiency resulted in a reduction of both BALF and lung tissue extracellular and intracellular production of M-CSF on day 1 and 3 after β-glucan exposure (Fig. 7g,h and Supplementary Fig. 7c–e ), leading to an increase in TUNEL + ApoE-deficient CD11b + AMs in Apoe fl Lyz2 Cre mice (Fig. 7i ). To determine if the loss of M-CSF plays a crucial molecular role in the loss of differentiating monocytes to macrophages following β-glucan-induced environmental adaptation, we used antibody-mediated CSF-1R blockade on days 0 and 3 after β-glucan stimulation and analyzed treated and control animals 7 days later. Mice treated with anti-CSF-1R-blocking antibody exhibited significantly lower numbers of ApoE + CD11b + AMs 7 days after β-glucan inoculation, underscoring the importance of M-CSF in the monocyte-to-macrophage differentiation process following β-glucan stimulation (Fig. 7j and Supplementary Fig. 7f–i ). Taken together, this suggests ApoE as a central regulator of pulmonary monocyte-to-macrophage differentiation and survival via the M-CSF signaling axis upon β-glucan-induced environmental adaptation.
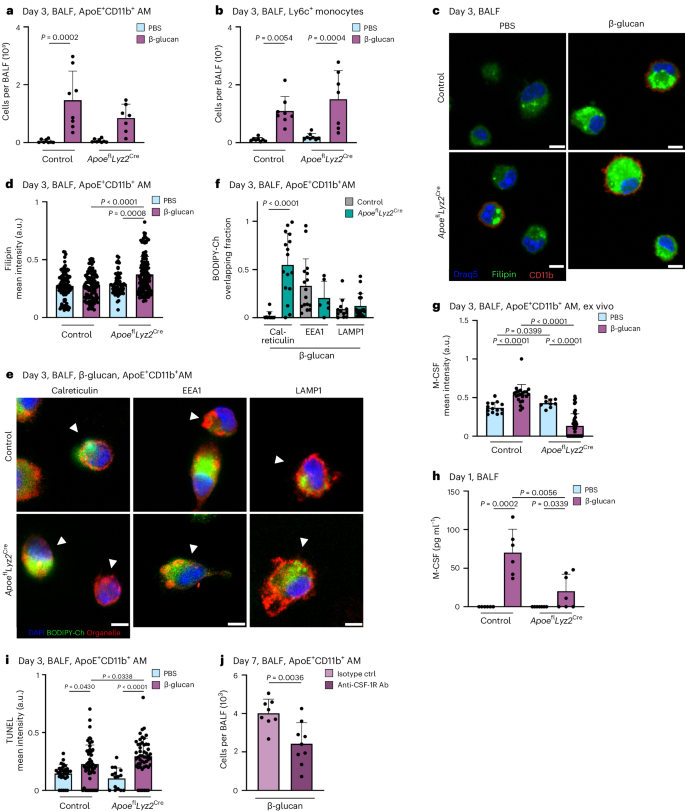
a , b , Absolute numbers of ApoE + CD11b + AMs ( a ) and Ly6c + monocytes ( b ) 3 days after intranasal β-glucan exposure in control or Apoe fl Lyz2 Cre mice ( n = 7–8, two independent experiments) by flow cytometry. c , d , BALF of PBS or β-glucan-stimulated control or Apoe fl Lyz2 Cre mice was harvested 3 days after exposure, seeded and fixed after 2 h. Filipin staining was performed followed by immunofluorescence analysis. Representative images are shown in c . Scale bars, 5 µm. Plot in d shows mean filipin signal intensities of individual ApoE + CD11b + AMs in the different conditions ( n = 3 mice per condition, two independent experiments). e , f , Three days after stimulation of PBS- or β-glucan-stimulated control or Apoe fl LysM Cre mice, AMs from the BALF were selected by adherence and afterwards incubated with 0.5 µM BODIPY-cholesterol overnight. Cells were fixed for 15 min the next day and immunofluorescence was performed. e , Representative confocal images. Scale bar, 5 µm. f , Quantification of overlapping signals of BODIPY-cholesterol and the organelle markers calreticulin (endoplasmic reticulum), EEA1 (endosomes) and LAMP1 (lysosomes) ( n = 2 mice per condition, two independent experiments). g , BALF of PBS- or β-glucan-stimulated control or Apoe fl Lyz2 Cre mice was harvested 24 h after exposure and seeded. Cells were fixed after 2 h and immunostained to detect Siglec-F, CD11b and M-CSF. Plot shows mean M-CSF signal intensities of individual ApoE + CD11b + AMs ( n = 3 mice per condition, two independent experiments). h , Quantification of M-CSF protein levels in the BALF 1 day after β-glucan exposure in control or Apoe fl Lyz2 Cre mice ( n = 6 mice, two independent experiments) measured by ELISA. i , BALF of PBS- or β-glucan-stimulated control or Apoe fl Lyz2 Cre mice was harvested 3 days after exposure, seeded and fixed after 2 h. TUNEL staining was performed, followed by conventional immunofluorescence to detect Siglec-F and CD11b. Plot shows mean TUNEL signal intensities of individual ApoE + CD11b + AMs ( n = 3 mice per condition, two independent experiments). j , Absolute numbers of ApoE + CD11b + AMs in the BALF of WT mice 7 days after intranasal β-glucan treatment together with 500 µg of CSF-1R antibody or the respective isotype control and follow-up treatment 12 h and 3 days later ( n = 8–9 mice, two independent experiments). Data are depicted as the mean ± s.d. Significance was assessed using ordinary one-way ANOVA with Tukey’s multiple comparisons ( a , b and g – i ), two-tailed Mann–Whitney test ( d and f ) and unpaired two-tailed student’s t -test ( j ). a.u., arbitrary units.
Within our modern-day environment, the lung is constantly exposed to a plethora of sterile immunostimulatory components. However, the developmental, functional and molecular consequences for lung-resident macrophages are incompletely understood. Here, we show that a single non-pathological intranasal β-glucan stimulus induces the development of MoAMs, which highly express CD11b and ApoE and are characterized by their superior IL-6 production capacity in response to secondary LPS stimulation. Additionally, ApoE + CD11b + AMs are glycolytic, highly phagocytic and modify the outcome of a secondary bacterial infection and of a chronic fibrotic response in vivo. Molecularly, this is instructed by Dectin-1-mediated recognition of β-glucan and its signaling adaptor protein CARD9. Further analysis revealed a crucial role of ApoE for the maintenance of BALF-resident ApoE + CD11b + AMs via the control of macrophage-derived M-CSF. This reveals that ApoE is a crucial checkpoint for monocyte-to-macrophage differentiation in the face of environmental adaptation and couples cellular cholesterol metabolism to differentiation and function.
Prior studies examined the development of MoAMs during viral, bacterial and fungal infections, radiation or bleomycin-induced fibrosis 16 , 18 , 38 , 48 , 49 , 50 , 51 . However, how bronchoalveolar macrophages adapt their transcriptome, metabolism and function to ambient immunostimulatory components beyond the effects of the acute recognition of such stimuli remains poorly understood 52 . Here, we show that although the initial pulmonary inflammation evoked by β-glucan is minimal, functionally modified MoAMs arise from Ly6c + monocytes within the BALF, a process previously affiliated to viral or bacterial infection, for example. This validates the model as suitable for low-grade environmentally induced inflammation. ApoE + CD11b + AMs are induced for up to 21 days after β-glucan stimulation leading to functional modification of the macrophage repertoire in the lung, demonstrating the importance of low-grade inflammatory sterile insults in shaping the overall immune competence of the lung-resident macrophage repertoire.
β-glucan, a cell wall component of many pathological and non-pathological fungi, can be found within ambient air 25 . During a fungal infection, monocytes and MoMacs are major antifungal effectors, via ROS and the activation of antifungal neutrophils, in mice and man 50 , 53 , 54 . Furthermore, during the later stages of fungal pathogenesis, monocyte descendants are important for induction of CD4 + T cell responses 51 .
Recently, studies evaluated the role of β-glucan for the induction of systemic innate immune training but did not examine its effects at the level of the tissue 4 , 55 . Systemically, β-glucan-induced functional modulations were accompanied by the induction of glycolysis and the enhanced release of the pro-inflammatory cytokines IL-6 and TNF in circulating Ly6c + monocytes, similarly to β-glucan-induced functional adaptation in the lung. Systemic β-glucan administration expands BM GMP/multipotent progenitor-like progenitors, resulting in enhanced pathogen clearance or tissue maladaptation 56 , 57 , 58 . We show that pulmonary β-glucan administration only minimally affects BM progenitors and that generation of ApoE + CD11b + AMs induces cMOP expansion and recruitment of CCR2-dependent Ly6c + monocytes to the lung. Dectin-1 recognizes β-glucan upon challenge; Dectin-1 downstream signaling is heterogeneous and determines the functional output. We show that tissue adaptation induces Dectin-1 CARD9-dependent signaling circuits, in concordance with increased IL-6 levels, most probably via activation of NF-κB signaling. Finally, β-glucan-induced macrophages upregulate ApoE, a protein demonstrated to be part of various disease-specific MoMac gene signatures, for example, during influenza infection, lung fibrosis or obesity 16 , 29 , 48 . Its functional role in MoMac development was not investigated. In hematopoietic stem cells, ApoE was shown to inhibit proliferation and subsequent progenitor maturation by controlling sensitivity towards granulocyte-macrophage stimulating factor and IL-3 (ref. 59 ). We show that myeloid-specific deletion of ApoE leads to the accumulation of cholesterol at the endoplasmatic reticulum, loss of M-CSF production and increased cell death ultimately inhibiting development of long-lived ApoE + CD11b + AMs upon intranasal β-glucan challenge. ApoE + MoMacs are also found in white adipose tissues during obesity, a condition conferring a training-like feature to MPs or during influenza-induced lung inflammation supporting the crucial role of ApoE for the development of functionally adapted macrophages during inflammation 29 , 60 . Other work has established functional NF-κB-response elements within the APOE gene, and CARD9 directly activates NF-κB thus linking activation of inflammatory NF-κB responses to the induction of inflammatory adaptation in MoMacs.
Collectively, we provide evidence that a single non-pathological environmental stimulation via the Dectin-1–CARD9 axis generates inflammation-experienced MoMacs, which modify subsequent pulmonary acute and chronic inflammation under the control of ApoE, thus molecularly linking macrophage inflammatory amplitude to lung resilience and disease susceptibility.

Animal studies and mouse models
All mice used in this study were bred in the animal facility of the LIMES Institute, University of Bonn, Germany or Center for Translational Cancer Research, Klinikum rechts der Isar, Technical University of Munich, Germany. Mice were housed in individually ventilated cages under conventional conditions (12-h/12-h light–dark cycle, 22 °C), with ad libitum access to food and water. All experiments were performed using C57BL/6J (WT) mice, which also served as controls for Ccr2 − / − , Dectin1 − / − and CARD9 −/− knockout mice. For Apoe fl Lyz2 Cre/+ mice, Cre-negative ApoE fl Lyz2 +/+ littermates were used as controls. Eight- to twelve-week-old male mice were used for experiments. All experiments were approved by the government of North Rhine-Westphalia (84-02.04.2017.A347, 81-02.04.2020.A454).
Intranasal stimulation
Mice were anesthetized by intraperitoneal injection of a ketamine–xylazine mixture and intranasally inoculated with endotoxin-free 1× PBS (EMD Millipore) or 200 µg β-glucan from Candida albicans . BALF was collected 1, 3, 7, 14 or 21 days after inoculation. For cell analysis, the lung was flushed three times with 1 ml cold 1× PBS with 10 mM EDTA. Afterwards, the fluid was centrifuged for 5 min at 365 g at 4 °C, and the supernatant was discarded. For cytokine and chemokine assessment, the lung was flushed three times with the same 1 ml of cold 1× PBS with 10 mM EDTA. Afterwards, the supernatant without cells was frozen in liquid nitrogen until analysis. Supernatants were thawed on ice and centrifuged for 5 min at 10,000 rpm at 4 °C to remove debris. M-CSF (R&D Systems) and ApoE (Abcam) protein levels were measured by ELISA according to manufacturer’s protocols.
Macrophage transfer
C57BL/6J CD45.2 WT donor mice were intranasally stimulated with PBS or β-glucan as described above. At day 5, BAL fluid was harvested, and cells of the same condition were pooled. After centrifugation, the supernatant was discarded, and cells were resuspended in LPS-free 1× PBS. Afterwards, 2 × 10 5 donor cells in a volume of 35 µl were intratracheally transferred into CD45.1 recipient mice. BALF was harvested 48 h after transfer and either analyzed by flow cytometry or used for ex vivo restimulation with LPS (see below).
In vivo CSF-1 receptor blockade
Before in vivo application, the amount of the anti-CSF-1R or the isotype control antibody was calculated per cohort. To reduce the application volume, a Concentrator Plus vacuum centrifuge (Eppendorf) was used in V-AQ mode to evaporate off excess liquid to yield a final product in one-quarter of the starting volume. C57BL/6J WT mice were intranasally stimulated with β-glucan mixed with 500 µg anti-CSF-1R (BioLegend) or 500 µg isotype control (BioLegend). At 12 h and 3 days after the initial treatment, intranasal application of 500 µg anti-CSF-1R or the respective isotype control was repeated before analysis at day 7.
Pulmonary fibrosis and L. pneumophila infection
Mice were intranasally stimulated with β-glucan or PBS 7 d before induction of pulmonary fibrosis or infection by L. pneumophila . For fibrosis induction, Streptomyces verticillus bleomycin (Sigma-Aldrich, 0.75 mg per kg body weight) was administered by intranasal installation. Body weight and health status were scored on a daily basis. Analysis was performed 3 or 14 days after bleomycin application. For bacterial infections, intratracheal application of L. pneumophila (5 × 10 6 colony-forming units per mouse) was performed, and mice were killed 2 days after induction. For bacterial load determination, BALF supernatant was plated in duplicates on CYE-plates and grown for 3–4 days at 37 °C in a non-CO 2 incubator.
BM chimeras
BM chimeras were generated by multiple intraperitoneal busulfan (Sigma-Aldrich) injections or irradiation of recipient mice with 10 Gy. Afterwards, 5 × 10 6 (busulfan-treated mice) or 1.5 × 10 6 (irradiated mice) freshly isolated BM cells from the donor animals were intravenously injected into the recipients. Peripheral blood chimerism was assessed 28 d after reconstitution by flow cytometry. BM chimeras were used in experiments after 8–12 weeks of reconstitution.
Flow cytometry and cell sorting
For flow cytometry, cells of the bronchoalveolar space were harvested by flushing the lungs with 3 × 1 ml ice-cold 1× PBS containing 10 mM EDTA. After centrifugation with 365 g for 5 min at 4 °C, cell pellets were resuspended in antibody mix and stained for 35 min at 4 °C. After washing with FACS buffer (1× PBS, 2 mM EDTA, 0.5% BSA (SERVA)), life/death stain was performed using DRAQ7 (BioLegend, 1:1,000 dilution in FACS buffer) for 5 min at room temperature (RT). Red blood cell lysis was performed only if necessary. For the lung tissue, the more segmented lobe was minced and enzymatically digested for 45 min at 37 °C in HBSS (PAN Biotech) supplemented with 10% FCS (Sigma-Aldrich), 0.2 mg ml −1 collagenase IV (Sigma-Aldrich) and 0.05 mg ml −1 DNase I (Sigma-Aldrich). Afterwards, the tissue pieces were homogenized with a 19G syringe and filtered through a 70-µm strainer. Red blood cell lysis was performed once for 5 min at RT before life/death stain and acquisition. For blood analysis, blood was collected in 1× PBS with 10 mM EDTA and stained in antibody mix for 35 min at 4 °C. Red blood cell lysis was performed twice for 5 min at RT before life/death stain and acquisition. For BM cells, femurs and tibias were flushed with 1× PBS and stained with antibody mix for 1 h, and red blood cell lysis and life/death stain were subsequently performed. Cells were washed and resuspended in FACS buffer and recorded using a FACS Symphony A5 (Becton Dickinson). FACS data were analyzed using FlowJo v10.8.1 (Becton Dickinson).
Cell sorting was performed using an ARIA III (Becton Dickinson) instrument. Briefly, cells from the lung lavage were stained with antibodies followed by life/death stain. Cell sorting was performed using a 100-µm nozzle into cooled 1.5-ml reaction tubes containing FACS buffer.
The following monoclonal anti-mouse antibodies anti-CD45R (clone RA3-6B2, BioLegend; 1:400 dilution), anti-CD117 (clone 2B8, BioLegend; 1:200 dilution), anti-CD11b (clone M1/70, BioLegend; 1:200 dilution), anti-CD11c (clone N418, BioLegend; 1:200 dilution), anti-CD11c (clone N418, BioLegend; 1:200 dilution), anti-CD135 (clone A2F10, BD Biosciences; 1:200 dilution), anti-CD192 (clone SA203G11, BioLegend or clone 475301, BD Biosciences; 1:200 dilution), anti-CD19 (clone 6D5, BioLegend; 1:200 dilution), anti-CD150 (clone 475301, BioLegend; 1:200 dilution), anti- CD3 (clone 17A2, BioLegend; 1:200 dilution), anti-CD131 (clone JORO 50, BD Biosciences; 1:200 dilution), anti-CD45 (clone I3/2.3, BioLegend or 30-F11, BioLegend/BD Biosciences; 1:200 dilution), anti-CD45.1 (clone A20, BD Biosciences; 1:200 dilution), anti-CD45.2 (clone 104, BD Biosciences; 1:200 dilution), anti-CD16/32 (clone 2.4G2, BD Horizon or clone 93, BioLegend; 1:100 dilution), anti-CD206 (clone C068C2, BioLegend; 1:200 dilution), anti-CD48 (clone HM48-1, BioLegend; 1:100 dilution), anti-CD90 (clone 53-2.1, BioLegend; 1:200 dilution), anti-CD64, (clone X54-5/7.1, BioLegend; 1:100 dilution), anti-CX3CR1 (clone SA011F11, BioLegend; 1:100 dilution), anti-F4/80 (clone BM8, BioLegend; 1:100 dilution), anti-IL-6 (clone MP5-20F3, BD Biosciences; 1:100 dilution), anti-Ly6C (clone HK1.4, BioLegend; 1:200 dilution), anti-Ly6G (clone 1A8, BioLegend/BD Biosciences; 1:200 dilution), anti-MERTK (clone 2B10C42, BioLegend; 1:200 dilution), anti-MHC2 (clone M5/114.15.2, BioLegend/BD Biosciences; 1:200 dilution), anti-NK-1.1 (clone PK136, BioLegend; 1:200 dilution), anti-Ly-6A/E (clone D7, Thermo Fisher Scientific; 1:200 dilution), anti-Siglec-F (clone E50-2440, BD Biosciences; 1:200 dilution), anti-mouse TCR beta chain (clone H57-597, BioLegend; 1:400 dilution), anti-TER-119, (clone TER-119, BioLegend; 1:200 dilution), anti-CD34 (clone SA376A4, BioLegend; 1:100 dilution), anti-CD335 (clone 29A1.4, BioLegend; 1:200 dilution), anti-CD115 (clone AFS98, BioLegend or clone T38-320, BD Biosciences; 1:100 dilution), anti-CD24 (clone M1/69, BioLegend/BD Biosciences; 1:100 dilution), anti-Sca-1 (clone D7, BioLegend; 1:100 dilution) and anti-CD43 (clone S7, BD Biosciences; 1:200 dilution) were used for flow cytometry or cell sorting.
PrimeFlow RNA detection by flow cytometry
BALF samples of three stimulated WT mice were pooled, centrifuged at 365 g for 5 min at 4 °C and resuspended in antibody mix for surface staining. Samples were transferred to 1.5 ml microcentrifuge tubes provided by the PrimeFlow RNA Assay Kit (Thermo Fisher Scientific) and stained for 35 min at 4 °C. Afterwards, life/death staining was performed using Zombie NIR Fixable Viability dye (1:1,000 dilution in PBS, BioLegend) for 10 min at RT. In the further steps, samples were handled according to the manufacturer’s instructions.
Ex vivo stimulation and assessment of cytokine production
BALF of stimulated mice was collected, centrifuged at 365 g for 5 min at 4 °C and resuspended in 1 ml RPMI 1640 (PAN Biotech) supplemented with 10% FCS (Sigma-Aldrich), 2 mM GlutaMAX (Gibco), 1% MEM non-essential amino acids (Sigma-Aldrich), 1 mM sodium pyruvate (Gibco), 50 U ml −1 penicillin–streptomycin (Gibco) and 0.1% β-mercaptoethanol. Cells were counted and seeded with 0.2 × 10 5 cells per well. After 2 h resting in 500 µl medium at 37 °C and 5% CO 2 , medium was exchanged to wash away non-adherent cells. Remaining macrophages were subsequently stimulated with 10 ng ml −1 LPS (Sigma-Aldrich) in a final volume of 500 µl. For intracellular cytokine stain, cells were restimulated for 4 h, then 2.5 µg brefeldin A (BioLegend) and 2 nM monensin (BioLegend) were added to each well and incubated for a further 2 h. Cells were harvested in 1× PBS using a cell scraper followed by staining of surface markers by antibodies for 30 min at 4 °C. Cells were washed and stained with Zombie NIR fixable viability dye (1:1,000 dilution in PBS, BioLegend) for 15 min. Afterwards, cells were permeabilized using the Cytofix/Cytoperm kit (Becton Dickinson, adapted from the manufacturer’s protocol). In brief, cells were resuspended in 200 µl Cytofix/Cytoperm solution per tube and incubated for 20 min at 4 °C. Cells were washed twice with Perm/Wash and intracellularly stained using 100 µl Perm/Wash containing IL-6 (MP5-20F3; 1:100 dilution) antibody or the corresponding isotype control for 30 min at 4 °C. Cells were washed twice with Perm/Wash and resuspended in 1× PBS before acquisition. For cytokine assessment from the supernatant, cell culture supernatant was harvested 24 h after LPS and snap frozen for further analysis. Supernatants were thawed on ice and centrifuged for 5 min at 10,000 rpm at 4 °C to remove debris. IL-6 (Thermo Fisher) protein levels were measured by ELISA. For multiplex cytokine and chemokine analysis, a customized 18-plex Procartaplex kit (Thermo Fisher) was used according to manufacturer’s protocols and run on a Luminex FLEXMAP 3D (Thermo Fisher) device.
In vitro phagocytosis assay
BALF cells of PBS- or β-glucan-inoculated mice were seeded with 0.2 × 10 5 cells per well in a 96-well plate and selected by adherence as before. Medium was exchanged to 100 µl medium or 100 µl medium containing 2.5 µg pHrodo S. aureus bioparticles (Sartorius) per well. Phagocytosis was monitored every 10 min for 7 h in total using the microscopy-based approach of the Incucyte instrument (Sartorius). Analysis was performed using the Incucyte basic analyzer software in standard mode with two channels (phase and orange).
Extracellular flux analysis
BALF of two mice was pooled and 0.5–1 × 10 5 cells were plated in a 96-well Seahorse plate (Agilent) in Seahorse XF base medium (Agilent) supplemented with 5% l -glutamine (Sigma-Aldrich), 10% FCS (Sigma-Aldrich) and 50 U ml −1 penicillin–streptomycin (Gibco) for 2 h at 37 °C and 5% CO 2 . Before acquisition, cells were washed and incubated in FCS and glucose-free Seahorse XF base medium with 5% l -glutamine (Sigma-Aldrich) and 50 U ml −1 penicillin–streptomycin (Gibco). During the run, 100 mM glucose (Sigma-Aldrich) solution was injected into port A leading to a final glucose concentration of 10 mM per well. This was followed by injection of 10 µM oligomycin A (Sigma-Aldrich, final concentration 1 µm) solution and 500 mM 2-desoxyglucose (Sigma-Aldrich, final concentration 50 mM). Glycolysis, glycolytic capacity and glycolytic reserve were calculated using the Agilent Wave software. After the Seahorse assay, cell numbers per well were determined for normalization using the CyQUANT NF Cell Quantification Assay (Thermo Fisher) and a TECAN plate reader.
Lung organoid generation, fibrosis induction by TGF-β and immunofluorescence staining
Organoid cultures were prepared as previously described and cultured at 37 °C with 5% CO 2 (ref. 39 ). In brief, lung single-cell suspensions were prepared from adult wild-type mice and CD31 + CD45 + CD16/32 + cells were depleted by antibody-coupled magnetic beads. From the CD31 − CD45 − CD16/32 − negative fraction, EpCAM hi CD24 lo Sca‐1 + bronchoalveolar stem cells (BASCs) and EpCAM − Sca‐1 + lung resident mesenchymal cells (rMCs) were isolated by FACS. 5 × 10 3 BASCs and 1.8 × 10 4 rMCs were pooled and mixed with growth factor-reduced Matrigel (Corning; 1:1 ratio) and seeded on 12-mm cell culture inserts in a 24-well plate. α-MEM medium (Thermo Fisher) supplemented with 10% FCS (Thermo Fisher), 50 U ml −1 penicillin–streptomycin (Thermo Fisher), 1× insulin–transferrin–selenium (Thermo Fisher) and 2 µg ml −1 heparin (Stemcell Technologies) was added to the wells to obtain an air–liquid interface. For the co-culture, AMs were obtained from the BALF of WT mice 7 days after PBS or β-glucan inoculation. Subsequently, 2.5 × 10 4 AMs were seeded on top of the Matrigel layer of the day 21 organoid cultures. Twenty-four hours later, the organoid–AM co-cultures were treated with medium containing either PBS or 1.05 ng ml −1 TGF-β (Miltenyi Biotech) to induce fibrosis. After 48 h of TGF-β treatment, cultures were fixed in 4% paraformaldehyde (PFA), permeabilized and blocked overnight with 1× PBS containing 0.5% Triton X-100 (Thermo Fisher Scientific) and 5% donkey serum (PAN Biotech; blocking buffer). Primary and secondary antibodies were incubated overnight in blocking buffer. The samples were cleared by glycerol–fructose clearing as recently described 61 .
Mice were anesthetized followed by transcardial perfusion with 10 ml ice-cold 1× PBS containing 10 mM EDTA using the lung–heart circulation. Lungs were removed and fixed in 4% PFA overnight at 4 °C (for paraffin-embedded tissue) or infiltrated with 1 ml 50% OCT compound (in 1× PBS), removed and fixed for 6 h in 1.3% PFA at 4 °C. For paraffin sections, lungs were dehydrated and paraffin embedded. For frozen sections, after fixation lungs were dehydrated in 10%, 20% and 30% sucrose (in 1× PBS) for 24 h at 4 °C. After dehydration, the left lobe was separated and embedded in OCT. Sections of 5 µm were prepared for immunohistochemistry.
Immunofluorescence and histology staining
Coverslips containing frozen tissue sections were left drying on Drierite beads for 5 min and subsequently fixed on ice-cold acetone for 10 min. Afterwards, sections were washed twice and permeabilized with 0.2% Triton X-100 for 20 min at RT. Afterwards, sections were washed twice with 1× PBS and photobleached as described before 62 . Following photobleaching, sections were blocked in 3% BSA for 1 h at RT. After blocking, primary antibodies were added and left incubating overnight at 4 °C. Sections were then washed three times with 1× PBS and secondary antibodies and nuclear staining solution were subsequently added and left to incubate for 1 h at RT. Samples were washed as before and coverslips were mounted using mounting medium. Fluorescently labeled primary antibodies were added after washing the secondary antibodies and left to incubate for 2 h at RT. For immunofluorescence of cultured cells, no acetone fixation and photobleaching were performed.
Histological evaluation of lung fibrosis was performed by Picrosirius red and Masson’s trichrome stainings of two consecutive paraffin-embedded 5-µm tissue sections as previously described 63 , 64 .
Filipin, TUNEL and BODIPY-cholesterol staining
In total, 5–8 × 10 4 BALF cells were seeded in complete RPMI medium in a 24-well plate containing sterile glass coverslips. Cells were left adhering for 3 h at 37 °C. For filipin and TUNEL stainings, cells were washed with 1× PBS and subsequently fixed with 4% PFA for 30 min. For filipin staining, after washing away the fixative, cells were incubated in 100 mM glycine for 10 min at RT, and subsequently blocked with 3% BSA supplemented with 50 µg ml −1 filipin (Sigma-Aldrich) for 2 h at RT. Cells were washed three times with 1× PBS and immunostained as indicated above, but DRAQ5 (Thermo Fisher Scientific) was used as a counterstain. For TUNEL staining, the manufacturer’s instructions were followed and immunofluorescence was performed after TUNEL (Thermo Fisher Scientific). For BODIPY-cholesterol staining, BALF cells were seeded in 8-well chamber slides, left as before and incubated overnight in complete RPMI 1640 medium supplemented with 0.5 µM BODIPY-cholesterol (Biomol). Cells were washed three times with 1× PBS, fixed, permeabilized and immunostained as described above.
Images of the Picrosirius red and Trichrome stainings were acquired using the OLYMPUS Slideview VS200 (Evident Corporation). Sections were analyzed at a magnification of ×20. Images of immunofluorescence of tissue sections and cultured cells were acquired using a Zeiss LSM 880 Airyscan system using a ×60 oil immersion objective (NA) with a z -spacing of 500 nm. Images were acquired using the 405, 488, 561 and 640-nm laser lines. During acquisition, nuclei showing the prototypical shape of neutrophils or eosinophils were excluded.
Image analysis
To quantify signal intensities from different markers from individual BALF cells, images were analyzed with a customized pipeline in CellProfiler. Briefly, Hoechst or DRAQ5 signals were used to segment the cells. A second primary detection step was added to create a mask of all Siglec-F + objects. This mask was subsequently merged onto the nuclei mask and only overlapping objects were further analyzed. A secondary object detection step was incorporated to distinguish between ApoE + CD11b + and CD11b − cells and create a mask of AMs. Analysis of immunofluorescence of tissue sections was performed in QuPath 65 . Nuclear signals were used to identify all objects using a radius of 2 µm. For each channel, an object classifier was created to set the detection threshold based on the mean signal intensity. Subsequently, these classifiers were combined to identify AMs. Mean intensities of individual cells were exported. To measure the area of fibrosis and the Ashcroft score, scoring was performed in four different areas of each slide as previously described 66 , 67 . Quantification of fibrotic area from total tissue area was performed with ImageJ. The Ashcroft score was quantified using scores ranging from 0 to 8 by two independent investigators, which were blinded to the treatments. To score fibrosis in cultured organoids, images were imported into QuPath, and the area of the organoid was annotated using DAPI as a reference. A pixel classifier to detect SMA-positive areas was trained using the control samples (that is, untreated and TGF-β treated). Around 5–10 exemplary regions of SMA and background spots were selected. The trained model was applied to analyze all the images. The total SMA + area of each organoid was quantified. To quantify the percentage of colocalization and overlapping area of BODIPY-cholesterol with cellular organelles, full z -stacks of single ApoE + CD11b + and CD11b − AMs were uploaded to Fiji and analyzed using the JACoP plugin, as described before 68 . Thresholds were established using five randomly selected images from each condition and then applied to all the images.
CODEX multiplexed imaging and analysis
Fresh frozen sections of the left lobe of the lung of 8-week-old Ms4a3-cre Rosa26TOMATO mice 7 days after intranasal PBS or β-glucan were prepared and stained following manufacturer’s instructions. Briefly, sections were fixed in ice-cold acetone for 10 min. Afterwards, samples were rehydrated and permeabilized for 20 min with 0.2% Triton X-100. Sections were photobleached twice for 1 h as indicated before 62 . After photobleaching, samples were equilibrated for 30 min in staining buffer (Akoya Biosciences), and subsequently stained with a 17-plex CODEX antibody panel overnight at 4 °C. After staining, samples were washed in staining buffer, fixed in ice-cold methanol and washed. A final fixation step with BS3 crosslinker (Sigma-Aldrich) was performed. Specimens were stored in CODEX storage buffer (Akoya Biosciences) at 4 °C for a maximum of 1 week before imaging. BALF cells from WT mice were seeded on CODEX coverslips after harvesting 7 days after intranasal stimulation. Two hours after seeding, cells were fixed with 4% PFA for 20 min, washed and stored in PBS at 4 °C until CODEX staining. Except for the initial drying step, the same staining protocol and CODEX panel as for the lung sections were used.
Antibody detection was performed in a multicycle experiment with the corresponding fluorescently labeled reporters, following the manufacturer’s instructions. Images were acquired with a Zeiss Axio Observer widefield microscope (Carl Zeiss AG) using a ×20 air objective (NA 0.85) and a z -spacing of 1.5 µm. The 405-nm, 488-nm, 568-nm and 647-nm fluorescence channels were used. After acquisition, images were exported using the CODEX Instrument Manager (CIM, Akoya Biosciences) and processed with the CODEX Processor v1.7 (Akoya Biosciences). Cells were segmented using DAPI signals and ATPase I membrane staining to define the cell borders. Cell classification to detect AMs and other MPs was performed in CODEX MAV (Akoya Biosciences), following a similar gating scheme to the one used for flow cytometry.
Preparation of Seq-Well arrays and libraries
Seq-Well arrays and libraries were generated as previously described 69 . Briefly, arrays were generated by pouring PDMS master mix into master molds and then functionalized by plasma treatment, washing with acetone, incubation with 0.2% chitosan solution and subsequent incubation in PGA buffer under vacuum pressure. For library generation, 1.1 × 10 5 barcoded mRNA-capture beads in Bead Loading Buffer were loaded onto the array. Around 2–3 × 10 4 BALF cells in RPMI 1640 medium (Gibco) with 10% FCS (Sigma-Aldrich) were loaded and rocked for 10 min. The loaded arrays were washed, sealed by polycarbonate membranes under mild vacuum, incubated for 30 min at 37 °C in Agilent clamps (Agilent) and then incubated in a guanidinium-based lysis buffer for 20 min. After incubation in hybridization buffer, the mRNA-capture beads were washed from arrays and collected. Reverse transcription was performed on the bead pellet using a Maxima Reverse Transcriptase reaction (Thermo Fisher) for 30 min at RT followed by 90 min of incubation at 52 °C before stopping the reaction with TE buffer supplemented with 0.01% Tween-20. Excess primers were digested by exonuclease ExoI (New England Biolabs). Beads were counted, and the reverse-transcribed cDNA libraries were amplified in a PCR reaction. After PCR, 2–4 × 10 4 beads were pooled and cleaned using AMPure XP beads (Beckman Coulter). The library integrity was assessed using a High Sensitivity D5000 assay (Agilent) for Tapestation 4200 (Agilent).
The cDNA libraries (1 ng) were tagmented with the prepared single-loaded Tn5 transposase mixed with pre-annealed linker oligonucleotides and afterwards cleaned using MinElute PCR kit (Qiagen) following the manufacturer’s instructions. The Illumina indices (Illumina) were added to the tagmented product by PCR and subsequently cleaned by AMPure XP beads (Beckman Coulter). The final library quality was assessed using a High Sensitivity DNA5000 assay (Agilent) and quantified using the Qubit high-sensitivity dsDNA assay (Thermo Fisher). Seq-Well libraries were pooled in equimolar amounts and clustered at a concentration of 1.4 pM with 10% PhiX using High Output v2.1 chemistry (Illumina) on a NextSeq 500 system (Illumina). Paired-end sequencing was performed as follows: custom Drop-Seq Read 1 primer for 21 cycles, 8 cycles for the i7 index and 61 cycles for read 2. Single-cell data were demultiplexed using bcl2fastq2 (v2.20; Illumina). Fastq files were loaded into a snakemake-based data pre-processing pipeline (version 0.31, available at https://github.com/Hoohm/dropSeqPipe ) 70 .
scRNA-seq data analysis
Sequencing reads were mapped to the mouse reference genome mm10 using STAR alignment from the Drop-seq pipeline (v2.0.0) as previously described 70 . Next, we assessed the quality of our libraries and excluded cells with low quality (<500 genes per cell), doublets (>3,000 genes per cell) or dead cells (>10% of mitochondrial content). All genes expressed in less than five cells were filtered out.
Cell clustering analysis was performed using the Seurat package (v4.1.1) according to instructions 71 . In brief, the expression data were log normalized with a scale factor of 10,000. After scaling, principal component analysis was performed using the top 2,000 variable genes for a linear dimensional reduction. The first 10 principal components were used to cluster cells by the Louvain algorithm. To obtain an optimal cluster resolution, we set the resolution parameter in the FindClusters function as 0.25 to generate five major clusters, which were visualized after nonlinear dimensional reduction with UMAP. Differentially expressed genes in each cluster were identified by using the default Wilcoxon rank-sum test in the FindAllMarkers function, and were defined with logfc.threshold > 0.25 and min.pct > 0.25.
Statistical analysis and comparison were performed using Prism 10 (GraphPad). Data are shown as the mean ± s.d. Statistical significance was assessed by student’s t -test (unpaired) or ordinary one-way ANOVA with Tukey’s multiple-comparisons test. Survival of animals is displayed in Kaplan–Meier survival curves. A P value < 0.05 was considered as statistically significant, exact P values are displayed in the figures. Mice were randomly allocated to the control or treatment groups by the investigator. Mouse numbers are indicated as ‘ n ’ in the figure legends, as well as the number of independent experiments.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The scRNA-seq raw reads and processed data were submitted to the NCBI Gene Expression Omnibus under accession number GSE211575 . Source data are provided with this paper.
Code availability
All code used for data visualization of the scRNA-seq data can be found via GitHub at https://github.com/SchlitzerLab/Trained_immunity_2022 (ref. 72 ).
Kopf, M., Schneider, C. & Nobs, S. P. The development and function of lung-resident macrophages and dendritic cells. Nat. Immunol. 16 , 36–44 (2014).
Article Google Scholar
Divangahi, M. et al. Trained immunity, tolerance, priming and differentiation: distinct immunological processes. Nat. Immunol. 22 , 2–6 (2021).
Article CAS PubMed PubMed Central Google Scholar
Shah, A. & Panjabi, C. Allergic aspergillosis of the respiratory tract. Eur. Respiratory Rev. 23 , 8–29 (2014).
Quintin, J. et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12 , 223–232 (2012).
Article CAS PubMed Google Scholar
Saeed, S. et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345 , 1251086 (2014).
Article PubMed PubMed Central Google Scholar
Arts, R. J. W., Joosten, L. A. B. & Netea, M. G. Immunometabolic circuits in trained immunity. Semin. Immunol. 28 , 425–430 (2016).
Arts, R. J. W. et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 24 , 807–819 (2016).
Bekkering, S. et al. Metabolic induction of trained immunity through the mevalonate pathway. Cell 172 , 135–146 (2018).
Cirovic, B. et al. BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe 28 , 322–334 (2020).
Hussell, T. & Bell, T. J. Alveolar macrophages: plasticity in a tissue-specific context. Nat. Rev. Immunol. 14 , 81–93 (2014).
Brown, G. D. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 6 , 33–43 (2005).
Vornholz, L. & Ruland, J. Physiological and pathological functions of CARD9 signaling in the innate immune system. Curr. Top. Microbiol. Immunol. 429 , 177–203 (2020).
CAS PubMed Google Scholar
Gross, O. et al. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 442 , 651–656 (2006).
Guilliams, M. et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 210 , 1977–1992 (2013).
Schneider, C. et al. Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat. Immunol. 15 , 1026–1037 (2014).
Aegerter, H. et al. Influenza-induced monocyte-derived alveolar macrophages confer prolonged antibacterial protection. Nat. Immunol. 21 , 145–157 (2020).
Gibbings, S. L. et al. Three unique interstitial macrophages in the murine lung at steady state. Am. J. Respir. Cell Mol. Biol. 57 , 66–76 (2017).
Machiels, B. et al. A gammaherpesvirus provides protection against allergic asthma by inducing the replacement of resident alveolar macrophages with regulatory monocytes. Nat. Immunol. 18 , 1310 1320 (2017).
Yao, Y. et al. Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell 175 , 1634–1650 (2018).
Park, Y., Ahn, C. & Kim, T. H. Occupational and environmental risk factors of idiopathic pulmonary fibrosis: a systematic review and meta-analyses. Sci. Rep. 11 , 4318 (2021).
Rabe, K. F. & Watz, H. Chronic obstructive pulmonary disease. Lancet 389 , 1931–1940 (2017).
Article PubMed Google Scholar
Lloyd, C. M. & Marsland, B. J. Lung homeostasis: influence of age, microbes, and the immune system. Immunity 46 , 549–561 (2017).
Kaur, M., Bell, T., Salek-Ardakani, S. & Hussell, T. Macrophage adaptation in airway inflammatory resolution. Eur. Respir. Rev. 24 , 510–515 (2015).
Murdoch, J. R. & Lloyd, C. M. Chronic inflammation and asthma. Mutat. Res. 690 , 24–39 (2010).
Rathnayake, C. M. et al. Influence of rain on the abundance of bioaerosols in fine and coarse particles. Atmos. Chem. Phys. 17 , 2459–2475 (2017).
Article CAS Google Scholar
Yu, X. et al. The cytokine TGF-β promotes the development and homeostasis of alveolar macrophages. Immunity 47 , 903–912 (2017).
Gautier, E. L. et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 13 , 1118–1128 (2012).
Cohen, M. et al. Lung single-cell signaling interaction map reveals basophil role in macrophage imprinting. Cell 175 , 1031–1044 (2018).
Jaitin, D. A. et al. Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell 178 , 686–698 (2019).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169 , 1276–1290 (2017).
McCubbrey, A. L. et al. Deletion of c-FLIP from CD11b hi macrophages prevents development of bleomycin-induced lung fibrosis. Am. J. Respir. Cell Mol. Biol. 58 , 66–78 (2018).
Halstead, E. S. et al. GM-CSF overexpression after influenza a virus infection prevents mortality and moderates M1-like airway monocyte/macrophage polarization. Respiratory Res. 19 , 3 (2018).
Goltsev, Y. et al. Deep profiling of mouse splenic architecture with CODEX multiplexed imaging. Cell 174 , 968–981 (2018).
Mould, K. J. et al. Cell origin dictates programming of resident versus recruited macrophages during acute lung injury. Am. J. Respir. Cell Mol. Biol. 57 , 294–306 (2017).
Serbina, N. V. & Pamer, E. G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7 , 311–317 (2006).
Wang, Y. T. et al. Metabolic adaptation supports enhanced macrophage efferocytosis in limited-oxygen environments. Cell Metab. 35 , 316–331 (2023).
Schilperoort, M., Ngai, D., Katerelos, M., Power, D. A. & Tabas, I. PFKFB2-mediated glycolysis promotes lactate-driven continual efferocytosis by macrophages. Nat. Metab. 5 , 431–444 (2023).
Ataide, M. A., Manin, G. Z., Oliveira, S. S., Guerra, R. O. & Zamboni, D. S. Inflammasome activation and CCR2-mediated monocyte-derived dendritic cell recruitment restrict Legionella pneumophila infection. Eur. J. Immunol. 53 , e2249985 (2023).
Vazquez-Armendariz, A. I. et al. Multilineage murine stem cells generate complex organoids to model distal lung development and disease. EMBO J. 39 , e103476 (2020).
Fesel, P. H. & Zuccaro, A. β-glucan: crucial component of the fungal cell wall and elusive MAMP in plants. Fungal Genet. Biol. 90 , 53–60 (2016).
Kalia, N., Singh, J. & Kaur, M. The role of dectin-1 in health and disease. Immunobiology 226 , 152071 (2021).
Taylor, P. R. et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat. Immunol. 8 , 31–38 (2007).
Deerhake, M. E. et al. Dectin-1 limits autoimmune neuroinflammation and promotes myeloid cell-astrocyte crosstalk via Card9-independent expression of Oncostatin M. Immunity 54 , 484–498 (2021).
Kemp, S. B. et al. Apolipoprotein E promotes immune suppression in pancreatic cancer through NF-κB-mediated production of CXCL1. Cancer Res. 81 , 4305–4318 (2021).
Dove, D. E., Linton, M. F. & Fazio, S. ApoE-mediated cholesterol efflux from macrophages: separation of autocrine and paracrine effects. Am. J. Physiol. Cell Physiol. 288 , C586–C592 (2005).
Blanchard, J. W. et al. APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. Nature 611 , 769–779 (2022).
Molgora, M. et al. TREM2 modulation remodels the tumor myeloid landscape enhancing anti-PD-1 immunotherapy. Cell 182 , 886–900 (2020).
Misharin, A. V. et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 214 , 2387–2404 (2017).
Li, F. et al. Monocyte-derived alveolar macrophages autonomously determine severe outcome of respiratory viral infection. Sci. Immunol. 7 , eabj5761 (2022).
Idol, R. A. et al. Neutrophil and macrophage NADPH oxidase 2 differentially control responses to inflammation and to Aspergillus fumigatus in mice. J. Immunol. 209 , 1960–1972 (2022).
Hohl, T. M. et al. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe 6 , 470–481 (2009).
Zahalka, S. et al. Trained immunity of alveolar macrophages requires metabolic rewiring and type 1 interferon signaling. Mucosal Immunol. 15 , 896–907 (2022).
Serbina, N. V. et al. Distinct responses of human monocyte subsets to Aspergillus fumigatus conidia. J. Immunol. 183 , 2678–2687 (2009).
Loeffler, J. et al. Interaction analyses of human monocytes co-cultured with different forms of Aspergillus fumigatus . J. Med. Microbiol. 58 , 49–58 (2009).
Novakovic, B. et al. β-glucan reverses the epigenetic state of LPS-induced immunological tolerance. Cell 167 , 1354–1368 (2016).
Mitroulis, I. et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 172 , 147–161 (2018).
Kalafati, L. et al. Innate immune training of granulopoiesis promotes anti-tumor activity. Cell 183 , 771–785 (2020).
Li, X. et al. Maladaptive innate immune training of myelopoiesis links inflammatory comorbidities. Cell 185 , 1709–1727 (2022).
Murphy, A. J. et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J. Clin. Invest. 121 , 4138–4149 (2011).
Christ, A. et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 172 , 162–175. (2018).
Dekkers, J. F. et al. High-resolution 3D imaging of fixed and cleared organoids. Nat. Protoc. 14 , 1756–1771 (2019).
Du, Z. et al. Qualifying antibodies for image-based immune profiling and multiplexed tissue imaging. Nat. Protoc. 14 , 2900–2930 (2019).
Zehender, A. et al. The tyrosine phosphatase SHP2 controls TGFβ-induced STAT3 signaling to regulate fibroblast activation and fibrosis. Nat. Commun. 9 , 3259 (2018).
Zehender, A. et al. TGFβ promotes fibrosis by MYST1-dependent epigenetic regulation of autophagy. Nat. Commun. 12 , 4404 (2021).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7 , 16878 (2017).
Hubner, R. H. et al. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques 44 , 507–511 (2008).
Ashcroft, T., Simpson, J. M. & Timbrell, V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 41 , 467–470 (1988).
Bolte, S. & Cordelieres, F. P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224 , 213–232 (2006).
Gierahn, T. M. et al. Seq-Well: portable, low-cost RNA sequencing of single cells at high throughput. Nat. Methods 14 , 395–398 (2017).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161 , 1202–1214 (2015).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184 , 3573–3587 (2021).
Yu, J. Trained immunity 2022. GitHub https://github.com/SchlitzerLab/Trained_immunity_2022 (2022).
Download references
Acknowledgements
We thank D. Hüsson and S. Weber (Beyer lab) for technical assistance. This study was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy – EXC2151 – 390873048 (to A.S. and J.L.S.), SFB 1454-P05-432325352 (to A.S.), SFB 1454-P16-432325352 (to M.B.), IRTG 2168 272482170 (to M.B. and J.L.S.), SCHU 950/8-1 (to J.L.S), RU 695/12 -1 (to J.R.), Emmy Noether research grant (SCHL2116/1 to A.S.), the BMBF-funded excellence project Diet–Body–Brain (DietBB; to J.L.S.) and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 834154 (to J.R.); SYSCID 733100 (to J.L.S.)). This research was partly funded by the Wellcome Trust (grant nos. 217163 and 102705), the Medical Research Council Centre for Medical Mycology at the University of Exeter for funding (MR/N006364/2; to G.B.) and a Sir Henry Dale Fellowship jointly awarded by the Wellcome Trust and by the Royal Society (grant no. 206234/Z/17/Z; to C.C.B.). This research was also partly funded by the Swiss National Science Foundation (grant nos. 310030_184915 to M.G. and 310030B_182829 to M.K. and F.P.) and by the National Institutes of Health (NIH; grant nos. GM119197, GM083016 and AI173607 to D.L.W.). For the purpose of open access, the author has applied a CC BY public copyright license to any author accepted manuscript version arising from this submission. W.T. is funded by a UKRI Postdoctoral Guarantee Fellowship (EP/X025071/1).
Author information
These authors contributed equally: H. Theobald, D. A. Bejarano, N. Katzmarski, J. Haub.
Authors and Affiliations
Quantitative Systems Biology, Life & Medical Sciences Institute, University of Bonn, Bonn, Germany
H. Theobald, D. A. Bejarano, N. Katzmarski, J. Haub, J. Yu, H. Hayer, S. Sheoran & A. Schlitzer
Genomics & Immunoregulation, Life & Medical Sciences Institute, University of Bonn, Bonn, Germany
J. Schulte-Schrepping, K. Bassler & J. L. Schultze
Systems Medicine, Deutsches Zentrum für Neurodegenerativen Erkrankungen (DZNE), Bonn, Germany
J. Schulte-Schrepping & J. L. Schultze
University of Bonn, Transdisciplinary Research Area Life and Health, Organoid Biology, Life & Medical Sciences Institute, Bonn, Germany
A. L. Ament & A. I. Vazquez-Armendariz
Immunogenomics & Neurodegeneration, German Center for Neurodegenerative Diseases, Bonn, Germany
C. Osei-Sarpong & M. D. Beyer
Institute of Molecular Health Science, Department of Biology, ETH Zürich, Zürich, Switzerland
F. Piattini & M. Kopf
Institute of Clinical Chemistry and Pathobiochemistry, School of Medicine and Health, Technical University of Munich, Munich, Germany
L. Vornholz & J. Ruland
TranslaTUM, Center for Translational Cancer Research, Technical University of Munich, Munich, Germany
Centre for Inflammation Research, Institute for Regeneration and Repair, University of Edinburgh, Edinburgh BioQuarter, Edinburgh, UK
W. T’Jonck & C. C. Bain
Department of Rheumatology, University Hospital Düsseldorf, Medical Faculty of Heinrich-Heine University, Düsseldorf, Germany
A. H. Györfi & J. H. W. Distler
Hiller Research Center, University Hospital Düsseldorf, Medical Faculty of Heinrich-Heine University, Düsseldorf, Germany
Institute of Experimental Immunology, University of Zurich, Zurich, Switzerland
X. Yu & M. Greter
I. Department of Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
A. Al Jawazneh & L. Bosurgi
Protozoa Immunology, Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany
Shanghai Institute of Immunology, Shanghai JiaoTong School of Medicine, Shanghai, China
S. Chakarov & F. Ginhoux
PRECISE Platform for Single Cell Genomics and Epigenomics at DZNE & University of Bonn and West German Genome Center, Bonn, Germany
K. Haendler, M. D. Beyer & J. L. Schultze
Institute of Human Genetics, University Medical Center Schleswig-Holstein, University of Luebeck & Kiel University, Luebeck, Germany
K. Haendler
MRC Centre for Medical Mycology, University of Exeter, Exeter, UK
G. D. Brown
Department of Surgery and Center for Inflammation, Infectious Disease and Immunity, James H. Quillen College of Medicine, East Tennessee State University, Johnson City, TN, USA
D. L. Williams
Singapore Immunology Network, Agency for Science, Technology and Research, Singapore, Singapore
INSERM U1015, Gustave Roussy Cancer Campus, Villejuif, France
German Center for Infection Research (DZIF), partner site Munich, Munich, Germany
German Cancer Consortium (DKTK), partner site Munich, Munich, Germany
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: H.T., D.A.B., J.H., N.K. and A.S.; formal analysis and investigation: H.T., D.A.B., J.H., N.K., J.S.-S., J.Y., K.B., A.L.A., C.O.-S., F.P., M.K., L.V., Y.X., M.G., L.B., W.T., C.C.B., S.S., A.A.J., H.H., A.I.V.-A., A.H.G., J.H.W.D., A.A., S.C., K.H., G.B., D.L.W., F.G., J.R., M.D.B. and J.L.S.; writing: H.T., D.A.B., J.H. and A.S.; supervision: A.S.
Corresponding author
Correspondence to A. Schlitzer .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Immunology thanks John Grainger, Daniel Saban and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: S. Houston in collaboration with the Nature Immunology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information.
Supplementary Figs. 1–7.
Reporting Summary
Peer review file, source data fig. 1.
Statistical source data flow cytometry.
Source Data Fig. 2
Source data fig. 3.
Statistical source data flow cytometry and ELISA.
Source Data Fig. 4
Statistical source data functional assays and infection.
Source Data Fig. 5
Source data fig. 6, source data fig. 7.
Statistical source data flow cytometry, ELISA and histology.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Theobald, H., Bejarano, D.A., Katzmarski, N. et al. Apolipoprotein E controls Dectin-1-dependent development of monocyte-derived alveolar macrophages upon pulmonary β-glucan-induced inflammatory adaptation. Nat Immunol (2024). https://doi.org/10.1038/s41590-024-01830-z
Download citation
Received : 15 November 2022
Accepted : 27 March 2024
Published : 26 April 2024
DOI : https://doi.org/10.1038/s41590-024-01830-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Skip to primary navigation
- Skip to main content
- 110 Baker St. Moscow, ID 83843
- 208.882.1226
A Classical & Christ-Centered Education

Former FedEx Cup, PGA Tour winners join Myrtle Beach Classic field
MYRTLE BEACH, S.C. (WMBF) - Three more players are joining the field for the inaugural Myrtle Beach Classic.
Tournament officials announced Friday that Brandt Snedeker, Jhonattan Vegas and Joel Dahmen will play at next month’s event at the Dunes Golf and Beach Club. The three join Cameron Champ, Daniel Berger and Charley Hoffman - who were announced on Thursday.
Joining the field! Brandt Snedeker– A nine-time winner on the PGA TOUR & 2012 PGA TOUR FedEx Cup winner Jhonattan Vegas– A three-time winner on the PGA TOUR Joel Dahmen– A one-time winner on the PGA TOUR Visit https://t.co/LBOjqs222p to get your tickets. pic.twitter.com/YbPrTX5LK6 — Myrtle Beach Classic (@MyrtleBeachCl) April 26, 2024
Snedeker is a nine-time winner on the PGA Tour and won the FedEx Cup in 2012. Vegas is a three-time winner on the tour while Dahmen has won once in his career.
Matt Atkins, a USC Aiken alum, also earned a spot at the event after winning a qualifier event.
PREVIOUS COVERAGE: USC Aiken alum wins Myrtle Beach Classic qualifier
The Myrtle Beach Classic will be held May 6-12.
Stay with WMBF News for updates.
Copyright 2024 WMBF. All rights reserved.

Students facing charges linked to hoax threats at Carolina Forest High School

Robeson County man identified in Friday shooting that also left an infant shot

Passenger killed in Galivants Ferry crash identified

LOOK: Dog the Bounty Hunter spotted in Myrtle Beach

First round of players announced for Myrtle Beach Classic
Latest news.

Coastal Carolina begins national search for new athletic director

World’s Strongest Man prepares for second year in Myrtle Beach
- Sport Betting
- Yearly calendar
- Latest results
- English Español French Italiano Nederlands
TheSports.org
All sports Site

Field hockey - Dinamo Elektrostal Moscow

Hockey Club Dinamo Elektrostal is a field hockey team from Russia, based in Moscow. The club was founded in 1994.
Dinamo Elektrostal Moscow - Results
2021/2022 2018/2019 2017/2018 2017 2015/2016 2013/2014 2011/2012 2007/2008
Men's Euro Hockey League - Final Round - 2021/2022
Dinamo elektrostal moscow - identity.
- Official name : Hockey Club Dinamo Elektrostal
- Country : Russia
- Location : Moscow
- Founded : 1994
- Wikipedia link : http://nl.wikipedia.org/wiki/Dinamo_Elektrostal
Dinamo Elektrostal Moscow - Titles, trophies and places of honor
- Best result : First Round in 2021/2022
- Best result : 1st
- 1 times first in 2010
- 1 times second in 2009
- 1 times third in 2017
Postal Address
Griffin Wood leads before play called for darkness Friday at Veritex Bank Championship
Daily Wrap Up
Change Text Size
Leaderboard
Things to know.
- Open qualifier Griffin Wood cards 11-under 60 and ties the second-lowest 36-hole score (any consecutive rounds) in Korn Ferry Tour history at 18-under 124
- Frankie Capan III , who held a three-stroke lead after tying the Korn Ferry Tour record for lowest 18-hole score relative to par with a first-round 13-under 58, trails Wood by one stroke with two holes remaining in his second round (par-4 eighth, par-5 ninth)
- The current projected 36-hole cut line of 7-under 135 (19 players at T55) would tie the Korn Ferry Tour record for the lowest 36-hole cut score (7-under 135/2023 NV5 Invitational presented by Old National Bank; 5-under 135/2023 Blue Cross & Blue Shield of Kansas Wichita Open Benefitting KU Wichita Pediatrics), in addition to tying the lowest 36-hole cut score relative to par (7-under/five times)
- The 36-hole cut will be made upon completion of the second round Saturday morning, while third-round tee times will run from 10:30 a.m. through 12:48 p.m. in groups of three off the first and 10th tees
Griffin Wood (1st/-18)
- Follows first-round 7-under 64 with 11-under 60 in second round to tie the second-lowest 36-hole score (any consecutive rounds) in Korn Ferry Tour history
- A 34-year-old open qualifier making Korn Ferry Tour debut and seventh PGA TOUR-sanctioned start (previous six all came in 2022 on PGA TOUR Canada)
- Earned spot in this week’s field with birdie on the first hole of a 5-for-2 playoff at the Waterchase Golf Club open qualifier site
- Best finish in six starts on PGA TOUR Canada in 2022 was T40/Quebec Open
- Does not currently have status on any PGA TOUR-sanctioned tour
- Played four seasons at Evansville University (2008-12), posting a 75.00 career scoring average, which ranked second in program history upon exhaustion of his eligibility
- Attended Boonville High School in his former hometown of Boonville, Indiana, where he grew up playing Quail Crossing Golf Club
Lowest 36-Hole Score (Consecutive Rounds) in Korn Ferry Tour History
Frankie Capan III (2nd/-17 thru 16*)
- Stands 17-under par and one stroke behind clubhouse leader Griffin Wood with two holes remaining in his second round
- In Thursday’s first round, carded 13-under 58 and tied the Korn Ferry Tour record for lowest 18-hole score relative to par, in addition to tying the second-lowest 18-hole score in Korn Ferry Tour history, and recording the 13th sub-60 round in Korn Ferry Tour history
- In line to establish a new career-high 36-hole position in what is his 31st career start on the Korn Ferry Tour (T3/2023 Memorial Health Championship presented by LRS/finished 4th)
- Finished T30 at 2023 Veritex Bank Championship, his only previous appearance at the event
- Finished No. 51 on the 2023 Korn Ferry Tour Points List as a rookie last season, earning fully exempt status for the 2024 season
- Finished T8 at Final Stage of 2022 Korn Ferry Tour Qualifying Tournament (now PGA TOUR Q-School presented by Korn Ferry); he was one of eight players who advanced through each stage of Q-School, beginning with pre-qualifying, in 2022
- Played two seasons at University of Alabama (2018-20) and two seasons at Florida Gulf Coast University (2020-22), earning one victory and garnering All-Atlantic Sun Conference Second Team honors in 2021 and 2022 while at Florida Gulf Coast
- Won back-to-back individual state titles at the Arizona Interscholastic Association Division III Championships in 2017 and 2018, winning the latter by 10 strokes off the strength of a second-round 11-under 59 on the Sonoran Desert Course at Omni Tucson National Resort
Griffin Wood on where he’s played most of his professional golf to this point… “I've got a lot of mini tours in the U.S., played on the Dakotas Tour, I played everywhere, APT a little bit, the Asher Tour out in Phoenix and I played (PGA TOUR) Canada two years ago. I had conditional, so I played there half a season. Last year I had conditional, but I didn't get into anything. Yeah, so mostly in the U.S. I've been playing full time for about five years in total even though I'm a little older. I had to take some time off. A little later progression.”
Wood on what was behind his extended break from professional golf… “ I had a back injury where I couldn't play for a couple years. When I came back, I was not shooting 60s on courses. I had to work to support myself and try and get my game back and try and play as much as I could. I played one year out of college. So I graduated in 2012, played for a year. Played pretty well, like was ‑‑ so I kind of proved to myself I could do it in small mini tours. Then I got hurt right before Q‑School right out of college, it would have been 2013. Then I was off for a couple years and was kind of pedaling around for a while. I've been back for the last four, I'd say, playing pretty full time and trying to get back.”
Wood on the playoff at Monday’s open qualifier… “I was playing solid golf and I bogeyed my last hole in regulation. It was a pretty easy hole, and I was pretty devastated because I thought that would be the difference. But I rebounded really well, because after I made that bogey, you know, you can go south quick. Me and another guy, Matt Atkins, birdied the first hole in a 5‑for‑2 (playoff). I had my head on straight, that was the big thing, and I was able to play right after I finished. I went right to the playoff hole, which helped. We finished in the dark, we didn't have any more time to play another hole and it worked out perfectly.”
Additional Player Notes
- Trent Phillips (3rd/-16) follows career-low 10-under 61 with 6-under 65 and shatters his previous career-low 36-hole score of 132/2023 BMW Charity Pro-Am presented by TD SYNNEX
- Tim Widing (4th/-15), who won last week’s LECOM Suncoast Classic, has four holes remaining in his second round and is one of three Korn Ferry Tour winners in the top 10 of the leaderboard
- The only other Korn Ferry Tour winners in the current top 10 of the leaderboard are Kyle Jones (T5/-12) and Andrew Kozan (T5/-12)
- Amateur Preston Stout (T11/-11), who attended J.J. Pearce High School in nearby Richardson, Texas, follows 4-under 67 with 7-under 64; he is a freshman at Oklahoma State University who earned co-medalist honors Wednesday at the 2024 Big 12 Conference Championship, and is making his first PGA TOUR-sanctioned start
- Arlington, Texas native, University of Texas-Arlington alum and conditional member Caleb Hicks (T39/-8), competing as a sponsor exemption, is 5-under through 10 holes in his second round and seeking his first made cut in what is his second career Korn Ferry Tour start (MC/2024 Astara Chile Classic)
- Reigning champion Spencer Levin (T90/-5) finishes two strokes off the current projected 36-hole cut line of 7-under 135
- Miles Russell (T102/-4), a 15-year-old amateur who became the youngest player to make a cut in Korn Ferry Tour history at last week’s LECOM Suncoast Classic, makes a late run at the cut line with birdies at the par-4 seventh and eighth, but closes with bogey at par-5 ninth for second-round 1-under 70

2024 Shore high school golf: Boys, girls preview, top players, rankings, schedule
T here was a seismic shift on the boys high school golf landscape locally last spring, as Wall ended a decade-plus of CBA dominance to emerge as the Shore’s top team, and one of the best in the state.
Now, with Monmouth commit Pat Scenna leading the way, the Crimson Knights look to repeat at the Monmouth County and Shore Conference Tournaments, and make a run at an overall state championship after finishing fourth a year ago.
“We knew last year how good a chance we had, and this year I feel we have an even better chance, so we’re excited to get back to it,” said Scenna, who won the individual MCT title and finished a shot back at the SCT last season. “We’ve been hitting the ball until it's dark out to get ready, to be ready when it’s time to play tournaments and play the best we can. Hopefully when TOC comes around we’re all playing well.”
How dominant had CBA been?
The Colts had won 10 straight Monmouth County Tournament titles, dating back to 2012, and 12 straight Shore Conference Tournament titles. But the Crimson Knights pulled off a sweep, shooting 299 at Charleston Springs to win the Shore Conference title.
Wall coach Matt Stefanski always puts together a challenging schedule, and this season is no different, with his team set to face top competition, including defending its title Wednesday at the Garden State Cup, after beating Don Bosco Prep, Hunterdon Central, the eventual state champion, CBA and Westfield there a year ago.
It all begins with Monday’s Wall Invitational at Jumping Brook Country Club in Neptune, with a strong field expected for the season-opening event.
“We’re not there yet, but we have the potential to be one of the best teams in the state,” said Stefanski, entering his 21 st season, with his program having won five of the last eight sectional titles, and Group 2 crowns in 2021 and 2023.
Scenna, who was top 3 at MCT, SCT and the state championship tournament as a sophomore in 2022, when he won the individual title at the Wall Invitational.
“I feel like in this preseason we seem we’re just as good as we were last year, even after losing Alex (Menges) and some other guys. Bo (Boden Pepe) and Charlie (Cormey) have gotten exponentially better over the last year. They’re going to be competing in all the events now to win, not just help the team out.”
Scenna and Holmdel star Patrick Sharpe, a runner-up at last year's SCT, will join Menges, and ex-CBA star Ethan Lee at Monmouth next season, as the Hawks’ seek to use local talent as a foundation for building the program up.
And for Scenna and other top Shore players, there’s motivation provided by the fact that two former Shore Conference champs, CBA’s Chris Gotterup and Mater Dei’s Ryan McCormick, are playing on the PGA Tour this season.
“I’ve heard stories about (Gotterup) from a lot of people, about how maybe he wasn’t on that Tour trajectory in high school but in college (at Rutgers) he worked harder than anyone else and now he’s on the Tour,” Scenna said. “I’m hoping to get to college and put the work in and try to do what he does.”
GIRLS GOLF: Holmdel wants more
It is exciting time for Holmdel girls golf, with the Hornets coming into the 2024 season as defending champions in both the Monmouth County and Shore Conference tournaments.
And at the top of the lineup is junior Sirina Ganne, the reigning NJSIAA individual champion, who beat her teammate, senior Minna Liang, in a playoff to win the Shore Conference individual crown.
And while the Hornets are preparing for what they hope will be a breakthrough season the state level, the optimism is somewhat tempered by what coach Kathy Bradley feels is the inequitable situation her team faces.
As it stands now, the NJSIAA has only three sections – North, Central and South – for girls golf, while boys golf has Groups 1 through 4 in North, Central and South, plus North and South Non-Publics.
“The one thing we’ve been trying to get through to the NJSIAA is that we need to make divisions (groups) for girls golf,” Bradley said. “When they started girls golf they didn’t have that many teams but it’s all still in one pool this year.
“We just want what the boys have. We’re not going to be able to win a state championship playing against schools that can recruit players – from all over the world.”
While Ganne and Liang qualified as individuals based on their performance during the season, the Hornets were unable to quality as a team for the state championship tournament.
Ganne was the first Shore Conference player to win the state title since Red Bank Catholic’s Taylor Totland in 2012. Her older sister, Megha, is a sophomore at Stanford who currently sits 52 nd in the World Amateur Golf Rankings.
Big changes for NJ high school golf
The way high school golf is administered changes dramatically this season, with every team in the state requiring players to do live scoring on a hole-by-hole basis on their phones. In the past, the scores in a tournament, for instance, were kept by a coach that accompanied each group. Now, coaches will be free to actually roam around the course and coach their players, with the ability to interact with them now when they’re not on the tee or green.
“I think adding the coaching component is very good,” said Stefanski, who, along with Southern head coach Jeff Reilly, was on the NJSIAA committee that helped usher in the changes, part of a two-year pilot program.
The statewide online scoring system will help calculate players who qualify for NJSIAA championships based on their performances during the season. It’s the only way players can qualify for the season-ending state championship at Raritan Valley, with the sections now team qualifying events. The only way a player not previously qualified as an individual for the state championship can make it into the field now is by winning their section.
Wall Invitational on tap
When teams gather at Jumping Brook Country Club in Neptune Monday for the season-opening Wall Invitational, it marks the traditional starting point for the high school golf season in New Jersey.
This year’s tournament features an expanded field, with each team limited to its top four players, not top 5, in an effort to bring more teams into the event.
The top group at this year’s event is a good one. It features Monmouth-bound Scenna, Notre Dame commit Liam Pasternak from Morristown, Monmouth Regional’s Jack Hennelly, bound for Saint Peter’s, and Southern freshman Paul Reilly, with the son of Rams’ long-time head coach Jeff Reilly playing his first high school tournament.
BOYS GOLF: PRESEASON PLAYERS TO WATCH
Jack o’connor, cba, jr..
No one was as consistent as O’Connor last season, finishing fifth at Shore Conference and seventh at Monmouth County, before winning the South Non-Public title and placing 19th at the Tournament of Champions.
Ethan Weinberg, Colts Neck, Jr.
He was fifth at the Shore Conference last year, four shots out of the top spot, after placing 13 th at Monmouth County. He closed his junior year placing eighth in Central-South Group 3.
Dan Cassidy, Middletown South, Sr.
Cassidy helped the Eagles to top-5 team finishes at Shore Conference and Monmouth County, individually placing sixth and 11th, respectively.
Christian Klemanowicz, Freehold Township, Sr.
As a junior for the Patriots, he finished third at the NJSIAA Central-South Group 4 tournament, going on to shoot 82 at the state championship tournament, after finishing seventh at Monmouth County and 11 th at Shore Conference.
Sam Landers, Ranney, So.
The Panthers’ young star made quite a first impression as a freshman, beginning with a seventh-place finish at Monmouth County. He followed it up by placing 14 th at Shore Conference, fifth at Non-Public South, and 50 th at the state championship.
GIRLS GOLF: PRESEASON PLAYERS TO WATCH
Kashish malik, marlboro, jr..
After winning the Shore Conference and NJSIAA South sectional titles as a freshman, Malik won Monmouth County last spring and placed fourth at Shore Conference. She closed the season with a strong 17 th place finish at the state championship tournament.
Minna Liang, Holmdel, So.
She helped push the Hornets to team titles at Monmouth County and Shore Conference by finishing fifth and second, respectively, at the two area championships, losing a playoff to teammate Sirina Ganne at the SCT. She then placed 14 th at the NJSIAA Central sectional, and 45 th at the state championship.
Peyton Cerminaro, Howell, Sr.
A fixture over the past three seasons, Cerminaro won the Shore Conference title as a freshman in 2021. Last spring she had another solid campaign, finishing eighth at the SCT, 12 th at the MCAT, 39 th at the state championship and 37 th at the boys’ Shore Conference Tournament.
Sabrina Liding, Freehold Township, Sr.
With top-10 finishes at both the Shore Conference and Monmouth County tournaments, including placing sixth at the SCT, Liding was an Asbury Park Press All-Shore second team selection as a junior for the Patriots.
Caroline Hickey, Rumson-Fair Haven, Sr.
Entering her third season in the Bulldogs’ lineup, Hickey comes off a solid season in which she placed 10 th at Shore Conference and 14 th at Monmouth County.
BOYS GOLF: PRESEASON TOP 10
4. Colts Neck
6. Middletown South
7. Freehold Township
8. Southern
10. Point Boro
GIRLS GOLF: PRESEASON TOP 5
2. Marlboro
3. Trinity Hall
4. St. Rose
5. Southern
2024 GOLF TOURNAMENT SCHEDULE
April 16 – Ocean County (LBI National)
April 18 – Monmouth County (Charleston Springs)
April 24 – Shore Conference (Charleston Springs)
May 6 - NJSIAA Central & South sectionals
Group 4 (Charleston Springs)
Group 3 (River Winds)
Group 1 (Cream Ridge)
Non-Public South (Mountain View)
May 7 – NJSIAA Central & South sectional
Group 2 (Twisted Dune)
May 13 – NJSIAA State Championship (Raritan Valley)
April 15 – Monmouth County (Howell Park)
April 15 – Ocean County (Bey Lea)
April 29 – Shore Conference (Jumping Brook)
May 6 – NJSIAA Central Sectional (Stanton Ridge)
May 7 – NJSIAA South Sectional (McCullough’s)
May 14 – NJSIAA State Championship (Raritan Valley)
This article originally appeared on Asbury Park Press: 2024 Shore high school golf: Boys, girls preview, top players, rankings, schedule

- Introduction
- Other Vocal Works
- Instrumental Works
- Piano Transcriptions
- Other Arrangements
- Performers Vocal
- Performers Instrumental
- General Topics
- Bach & Other Composers
- J.S. Bach Biography
- Lutheran Church Year
- Readings from Bible
- Texts & Translations
- Commentaries
- BWV & BWV Anh Lists
- Chorale Texts
- Chorale Melodies
- Guide to Bach Tour
- Bach Festivals & Series
- Arts & Memorabilia
- The Face of Bach
- Terms & Abbreviations
- Order of Discussion
- Schedule of Concerts
- TNT's J.S. Bach Pages
- Links to Other Sites
- Search Works/Movements
- What's New?
- New & Upcoming Recordings
- Join Mailing Lists/Contribute


IMAGES
COMMENTS
2012 PGA Tour Qualifying School graduates. This is a list of the 26 players who earned their 2013 PGA Tour card through Q School in 2012. [1] [2] 2012 was the final year Q School would grant direct access to the PGA Tour. Future Q Schools will only grant access to the second-tier Web.com Tour. Brad Fritsch previously earned his 2013 PGA Tour ...
The final stage of the PGA Tour's 2012 Q-school is set, and there will be plenty of big names attempting to earn their card at PGA West in California. After two rounds of qualifying stages, 172 ...
The annual PGA Tour Qualifying Tournament, also known as Qualifying School or Q-School, was historically the main method by which golfers earned PGA Tour playing privileges, commonly known as a Tour card. From 2013 to 2022, Q-School granted privileges only for the Korn Ferry Tour, the PGA Tour's official developmental circuit, but in 2023 it began to again award a small number of PGA Tour cards.
Brigman is best known for signing an incorrect scorecard at the 1999 Q-School. He missed his PGA Tour card by a stroke after signing for a score one stroke higher than he shot. Brigman has never earned a PGA Tour card. ... Patrick Reed, 17-under 271. Reed earned $302,977 on the PGA Tour in 2012, including four top-25 finishes, after making 11 ...
Compton made 16 cuts in 26 starts on the PGA Tour this season, but had only one top 25. • Len Mattiace (6 under, T-12), a two-time winner on the PGA Tour, survived a final-round, 1-over 72 to advance. • Two-time U.S. Open participant Scott Langley (5 under, 19th) got the last spot in the Q-School finale after an even-par 71 that was ...
Dong-hwan Lee shot 25-under for the six-round, Q School PGA tournament, and earned his 2013 Tour card, along with 24 others, in the process. By JP Starkey Dec 3, 2012, 6:51pm EST Share this story
PGA TOUR Q-school 2012 leaderboard: Dong-hwan Lee takes over with 8-under day. Dong-hwan Lee is the new leader at the Q-school tournament, shooting 8-under on Saturday to pass up Meen Whee Kim.
The PGA Tour's qualifying school was officially known as the PGA Tour Qualifying Tournament, but the organization also frequently refers to it as "Q-School". The system began in 1965. The 2012 edition (the final Q School that offered a direct path to the PGA Tour) involved four stages:
The ordeal that is Q school to begin its final chapter. By Alex Myers. October 16, 2012. By Bill Fields. It made it to middle age and caused a lot of gray hair in the process. PGA Tour Qualifying ...
For the first time since 2012, PGA TOUR cards are available via Q-School. The top five and ties at PGA TOUR Q-School presented by Korn Ferry's Final Stage will earn 2024 PGA TOUR membership ...
Golf Channel and Peacock will broadcast live weekend coverage at Final Stage of 2023 PGA TOUR Q-School presented by Korn Ferry. Coverage on Golf Channel will be live from 2:30-4:30 p.m. ET on ...
Feb 27, 2024. By. GolfWRX Staff. GolfWRX is on site this week at the Cognizant Classic —FKA the Honda Classic. The first leg of the PGA Tour's traditional "Florida swing," the Cognizant Classic continues to be contested at PGA National's Champion Course in Palm Beach Gardens, Florida, host course of the 1983 Ryder Cup and 1987 PGA ...
Q-School is back in session -- and with higher stakes. For the first time in a decade, PGA TOUR cards will be available at the PGA TOUR Q-School presented by Korn Ferry, which will be held Dec. 14 ...
PGA TOUR Q-School presented by Korn Ferry. Q-School Schedule; 2023 KFT Schedule; Search for Tournaments
Dec 14, 2023. For the first time this week, five players will earn full PGA Tour membership at the Final Stage of PGA Tour Q-School, otherwise known as "qualifying" school. The culminating 72 ...
The Golf Today crew share their reactions to the PGA Tour Qualifying School returning for the first time since 2012, which will allow the top five players to...
December 1, 2012. By. In professional golf, the term "qualifying school", or "Q-School" represents annual qualifying tournaments for the world's leading golf tours such as the PGA, LPGA, and European Tours. Here players have the opportunity to win membership for the TOUR for the following season, otherwise known as obtaining your ...
For the first time since 2012, PGA Tour cards will be handed out this week via the final stage of Q-School. The top five finishers and ties in Ponte Vedra Beach, Florida, will collect Tour cards ...
Q School Q School Destinations Full Schedule ... Previous events ISPS HANDA has supported on the DP World Tour include the ISPS HANDA Wales Open (2012-14 and 2020), ISPS HANDA Perth International (2012-14 and 2016), ISPS HANDA World Cup of Golf (2013, 2016, 2018), (ISPS HANDA World Super 6 Perth (2017-19), ISPA HANDA Vic Open (2019-20), ISPS ...
This year's Q-School ramps up the stakes - for the first time since 2012, PGA TOUR cards are available at Final Stage. The cut-and-dry nature of Second Stage is now magnified (if that were ...
303 See Other. openresty
The Moscow Philharmonic Orchestra (= MPO) is one of the leading symphony orchestras in the world. The Moscow Philharmonic Orchestra was founded in 1951 by Samuel Samosud, a distinguished conductor of the Bolshoy Opera. Originally part of the All-Union Radio Committee, the orchestra was established primarily for the broadcasting of operatic ...
110 Baker St. Moscow, ID 83843; 208.882.1226; Directions; A Classical & Christ-Centered Education
Snedeker is a nine-time winner on the PGA Tour and won the FedEx Cup in 2012. Vegas is a three-time winner on the tour while Dahmen has won once in his career.
Dinamo Elektrostal Moscow - Titles, trophies and places of honor. Men's Euro Hockey League since 2007/2008 (7 participations) . Best result : First Round in 2021/2022; EuroHockey Men's Club Trophy since 2008 . Best result : 1st
Finished T8 at Final Stage of 2022 Korn Ferry Tour Qualifying Tournament (now PGA TOUR Q-School presented by Korn Ferry); he was one of eight players who advanced through each stage of Q-School ...
Ganne was the first Shore Conference player to win the state title since Red Bank Catholic's Taylor Totland in 2012. Her older sister, Megha, is a sophomore at Stanford who currently sits 52 nd ...
The Rusian pianist, Natalia Zagalskaia, was born to a family of musicians and was already 4 years received her first piano lessons from her mother Tatiana Zagalskaia-Bron. In 1984 she began her musical education with Ljudmila Ljacker. From 1990 to 1996 she studied at the Central Music School of the Moscow Conservatory in the class of Professor ...