You are using an outdated browser. Upgrade your browser today or install Google Chrome Frame to better experience this site.
- Section 5 - Tetanus
- Section 5 - Perspectives : Testing Travelers for Mycobacterium Tuberculosis Infection

Tuberculosis
Cdc yellow book 2024.
Author(s): John Jereb
Infectious Agent
Transmission, epidemiology, clinical presentation.
INFECTIOUS AGENT: Mycobacterium tuberculosis complex
Worldwide, but with wide variations by region and social context
TRAVELER CATEGORIES AT GREATEST RISK FOR EXPOSURE & INFECTION
PREVENTION METHODS
Avoid high-risk social contexts
Obtain pre- and posttravel testing and preventive treatment for new infections
Get fit-tested and use respiratory protection (e.g., N95 respirators) in high-risk occupational settings
Consider vaccination with bacillus Calmette-Guérin (no longer available in the United States)
DIAGNOSTIC SUPPORT
Mycobacterium tuberculosis complex is a group of closely related rod-shaped, nonmotile, slow-growing, acid-fast bacteria, which includes M. bovis and M. tuberculosis hominis, the most common cause of human tuberculosis (TB), usually referred to as M. tuberculosis .
TB transmission occurs when a patient with a contagious form of the infection coughs, spreading bacilli through the air. People can acquire bovine TB (caused by M. bovis ) by consuming unpasteurized dairy products from infected cattle.
The risk for M. tuberculosis transmission on an airplane is low, but instances of in-flight TB transmission have occurred. The risk of transmission is dependent on the contagiousness of the person with TB, seating proximity, flight duration, and host factors. To prevent transmission, people with contagious TB should not travel by commercial airplanes or other commercial conveyances. Typically, only TB of the lung or airway is contagious in community contexts, and health department authorities determine whether TB is contagious based on a person’s chest radiograph, sputum tests, symptoms, and treatment received. The World Health Organization (WHO) issued guidelines for notifying passengers potentially exposed to TB on airplanes. Passengers concerned about possible TB exposure should see their primary health care provider or visit their local health department clinic for evaluation.
Bovine TB is a risk for travelers who consume unpasteurized dairy products in countries (e.g., Mexico) where M. bovis in cattle is common. M. bovis risk in some African countries has been postulated, but human M. bovis statistics are unavailable for those countries.
According to the World Health Organization, ≈10 million new TB cases and ≈1.2 million TB-related deaths occurred in 2019. TB occurs throughout the world, but the incidence varies (see Map 5-02 ). In some countries in sub-Saharan Africa and Asia, the annual incidence is several hundred per 100,000 population. In the United States, the annual incidence is <3 per 100,000 population, but immigrants from countries with a high TB burden and long-term residents of high-burden countries have a 10× greater incidence of TB than the US national average. Of note, US surveillance does not capture travel-related cases of TB.
Drug-resistant TB is an increasing concern. Multidrug-resistant (MDR) TB is resistant to at least the 2 most effective drugs, isoniazid and rifampin. MDR TB is less common than drug-susceptible TB, but globally ≈363,000 cases of MDR TB were diagnosed in 2019, and MDR TB accounts for >25% of TB cases in some countries ( Table 5-06 ). MDR and higher-order resistance are of particular concern among HIV-infected or other immunocompromised people.
Map 5-02 Estimated tuberculosis incidence rates per 100,000 population
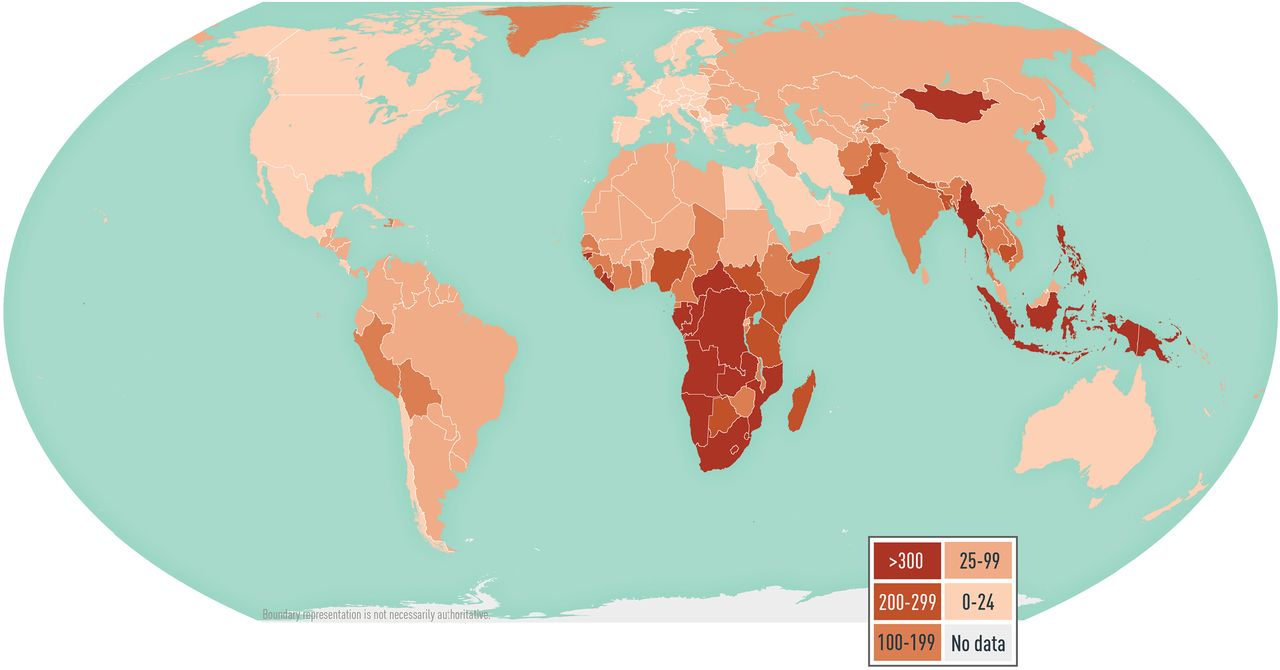
View Larger Figure
Disease data sources: World Health Organization. Global tuberculosis report 2020 ( https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf [PDF]); for French Guiana, Tableau 5: Taux de déclaration de tuberculose maladie par Nouvelles régions (taux pour 100 000), France entière, 2015–2020; Santé publique France; La tuberculose: données (Table 5: Tuberculosis disease reporting rate by New regions [rate per 100,000], Whole France, 2015–2020, Public Health France, Tuberculosis: data; www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/tuberculose/donnees/#tabs ); for Taiwan, Statistics of Communicable Diseases and Surveillance Report 2019, Centers for Disease Control, Ministry of Health and Welfare, R.O.C. (Taiwan), November 2020 ( www.cdc.gov.tw/En/File/Get/0nnMQjC37VAuhzVY3Vuq-A ).
Table 5-06 Estimated proportion of multidrug-resistant (MDR) tuberculosis (TB) cases in countries with high MDR TB burden, 2019
M. tuberculosis infection can be detected by a positive tuberculin skin test (TST) or interferon-γ release assay (IGRA) 8–10 weeks after exposure. Overall, only 5%–10% of otherwise healthy people who are infected progress to TB disease during their lifetimes. Progression to TB disease can take weeks to decades after initial infection. People with TB disease have symptoms or other manifestations of illness (e.g., an abnormal chest radiograph). For most people who become infected, M. tuberculosis remains in an inactive state (latent TB infection or LTBI) in which the infected person has no symptoms and cannot spread the infection to others.
TB disease can affect any organ, but affects the lungs in 70%–80% of cases. Typical TB symptoms include prolonged cough, fever, hemoptysis, night sweats, decreased appetite, and weight loss. The most common sites for TB outside the lungs (i.e., extrapulmonary TB) are the bladder, bones and joints, brain and meninges, genitalia, kidneys, lymph nodes, and pleura.
The risk for progression to disease is much higher in immunosuppressed people; for example, progression is 8%–10% per year in HIV-infected people not receiving antiretroviral therapy. People receiving tumor necrosis factor blockers to treat rheumatoid arthritis and other chronic inflammatory conditions also are at increased risk for disease progression.
Pretravel & Posttravel Testing
Before leaving the United States, travelers who anticipate possible prolonged exposure to TB (e.g., people who will care for patients, or who will work in health care facilities, prisons or jails, refugee camps, or homeless shelters) and those planning prolonged stays in TB-endemic countries should have a pretravel IGRA (e.g., QuantiFERON-TB Gold Plus, T-SPOT.TB, 2-step tuberculin skin test [TST]). For details, see the following chapter in this section, . . . perspectives: Testing Travelers for Mycobacterium tuberculosis Infection .
If the predeparture test is negative, repeat IGRA or single TST 8–10 weeks after the traveler returns. The predeparture test and follow-up test should be the same test type to facilitate interpretation of results. People with HIV infection or other immunocompromising conditions are more likely to have an impaired response to either a skin or a blood test; be sure to ask travelers about such underlying conditions.
Travelers who suspect they have been exposed to TB should inform their health care provider of the possible exposure and receive a medical evaluation. Because drug resistance is relatively common in some parts of the world, consult with experts in infectious diseases or pulmonary medicine regarding proper management and coordinate consultations with input from the public health department.
Diagnostic Testing Recommendations
The Centers for Disease Control and Prevention (CDC), the American Thoracic Society (ATS), and the Infectious Diseases Society of America (IDSA) jointly published diagnostic recommendations for both TB disease and LTBI. Collect sputum or other respiratory specimens for culture and smears for acid-fast bacilli (AFB) from people being examined for pulmonary TB.
Although diagnosis of TB disease can be made using clinical criteria in the absence of microbiologic confirmation, perform laboratory testing to confirm the diagnosis, guide treatment decisions, and provide bacterial DNA for molecular epidemiology. Molecular tests for mutations that confer drug resistance can be performed directly on specimens and can guide initial treatment while culture results are pending. Culture-based susceptibility testing is recommended for all patients with a positive culture result, to help determine the appropriate drug regimen.
Culture Methods
Culture methods, with referral to a public health reference laboratory in some instances, are necessary to identify the M. tuberculosis complex species responsible for infection. Culture and identification of M. tuberculosis takes ≈2 weeks, even with rapid culture techniques.
A preliminary diagnosis of TB can be made when AFB are seen by microscopy on a sputum smear or in other body tissues or fluids. Microscopy cannot distinguish M. tuberculosis from nontuberculous mycobacteria, however, which is particularly problematic in countries like the United States, where the prevalence of infections with nontuberculous mycobacteria is greater than that of TB.
Nucleic Acid Amplification Tests
Less sensitive than culture but more sensitive than AFB smear, nucleic acid amplification tests (NAAT) are specific for the M. tuberculosis complex. NAAT methods detect all members of the M. tuberculosis complex. Thus, a positive NAAT result can rapidly confirm a diagnosis and help guide initial treatment until culture results return.
The availability of NAAT methods and the policies for ordering these tests are locally determined, and clinicians should consult their state health department. Diagnosis of extrapulmonary TB disease can be confirmed with a NAAT positive for M. tuberculosis complex or a culture positive for M. tuberculosis from affected body tissues or fluids.
Diagnostic Support
TB disease is a nationally notifiable condition in the United States. LTBI is also notifiable in many jurisdictions. LTBI is diagnosed by a positive result from an IGRA or TST after further examinations (e.g., chest radiograph, symptom review) have excluded TB disease.
Expertise in the diagnosis of TB and its specialty laboratory services, or local referral for such expertise, is available from the health departments of cities, counties, and states. In most settings, contact tracing is managed by public health officials. General information and expert medical consultation also are available from the CDC-sponsored US TB Centers of Excellence for Training, Education, and Medical Consultation .
Latent Tuberculosis Infection
People with LTBI can be treated, and treatments are effective at preventing progression to TB disease. Clinicians must exclude TB disease before starting LTBI treatment. In the United States, several regimens exist for the treatment of drug-susceptible LTBI, including 3 months of once-weekly isoniazid and rifapentine; 4 months of daily rifampin; 3 months of daily isoniazid and rifampin; and 6–9 months of daily isoniazid. Given the low completion rates of the 6- to 9-month isoniazid regimen, shorter duration regimens are preferred.
Choose a regimen for patients based on coexisting medical conditions, potential for drug interactions, and drug-susceptibility results of the presumed source of exposure, if known. For example, rifampin has interactions with oral contraceptives and certain antiretroviral medications taken by people with HIV/AIDS. Individuals at especially high risk for TB disease who might have difficulty adhering to treatment, or who are given an intermittent dosing regimen, might be candidates for directly observed therapy for LTBI.
Tuberculosis Disease
CDC/ATS/IDSA published guidelines for treating drug-susceptible TB disease with a multiple-drug regimen administered by directly observed therapy for 6–9 months. Usually, the regimen is isoniazid, rifampin, ethambutol, and pyrazinamide for 2 months, then isoniazid and rifampin for an additional 4 months. Drug-resistant TB is more difficult to treat, historically requiring 4–6 drugs for 18–24 months and best managed by an expert. In a randomized controlled trial, a newer 6-month all-oral regimen of bedaquiline, pretomanid, and linezolid was effective in treating highly drug-resistant TB or patients who could not tolerate other regimens. This and other new regimens are being used in the United States.
Travelers should avoid exposure to people with TB disease in crowded and enclosed environments (e.g., health care facilities, prisons or jails, or homeless shelters). Advise travelers who will be caring for patients, or who will be working in health care facilities where people with TB are likely to be patients, to consult infection control or occupational health experts about baseline LTBI screening, procedures for obtaining personal respiratory protective devices (e.g., N95 respirators), and recommendations for respirator selection and training.
Based on WHO recommendations, bacillus Calmette-Guérin (BCG) vaccine is used once, at birth, in countries with higher TB burdens to reduce the severe consequences of TB in infants and children. BCG vaccine has low and variable efficacy in preventing TB in adults, however. Some experts advocate vaccinating health care providers likely to be exposed to drug-resistant TB in settings where infection control measures like those recommended in the United States are not fully implemented; US Food and Drug Administration–approved vaccine formulations of BCG are no longer available in the United States. All people, including those who have received BCG vaccination, must follow recommended TB infection control precautions to the greatest extent possible. IGRA is preferred over the TST for pretravel and posttravel testing in those vaccinated with BCG, because BCG might induce false-positive TST results. No BCG effects on IGRA results have been detected in multiple studies.
To prevent infections from M. bovis and other foodborne pathogens, travelers should avoid consuming unpasteurized dairy products.
CDC website: Tuberculosis
The following authors contributed to the previous version of this chapter: Neela D. Goswami, Philip A. LoBue
Bibliography
Brown ML, Henderson SJ, Ferguson RW, Jung P. Revisiting tuberculosis risk in Peace Corps volunteers, 2006–13. J Travel Med. 2016;23(1):tav005.
Centers for Disease Control and Prevention. Availability of an assay for detecting Mycobacterium tuberculosis , including rifampin resistant strains, and considerations for its use—United States, 2013. MMWR Morb Mortal Wkly Rep. 2013;62(41):821–7.
Conradie F, Diacon AH, Ngubane N, Howell P, Everitt D, Crook AM, et al. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med. 2020;382(10):893–902.
Jensen PA, Lambert LA, Iademarco MF, Ridzon R. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54(RR-17):1–141.
Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):111–5.
Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63(7):e147–95.
National Society of Tuberculosis Clinicians and National Tuberculosis Controllers Association. Testing and treatment of latent tuberculosis infection in the United States: clinical recommendations. Smyrna (GA): The Association; 2021. Available from www.tbcontrollers.org/resources/tb-infection/clinical-recommendations .
Seaworth BJ, Armitige LY, Aronson NE, Hoft DF, Fleenor ME, Gardner AF, et al. Multidrug resistant tuberculosis. Recommendations for reducing risk during travel for healthcare and humanitarian work. Ann Am Thorac Soc. 2014;11(3):286–95.
Sterling TR, Njie G, Zenner D, Cohn DL, Reves R, Ahmed A, et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep 2020;69(RR-1):1–11.
World Health Organization. Global tuberculosis report 2020. Geneva: The Organization; 2020. Available from: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf .
World Health Organization. Tuberculosis and air travel: guidelines for prevention and control, 3rd edition. Geneva: The Organization; 2008. Available from: www.who.int/publications/i/item/9789241547505 .
File Formats Help:
- Adobe PDF file
- Microsoft PowerPoint file
- Microsoft Word file
- Microsoft Excel file
- Audio/Video file
- Apple Quicktime file
- RealPlayer file
- Zip Archive file
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Tuberculosis and Air Travel: Guidelines for Prevention and Control
- PMID: 23785743
- Bookshelf ID: NBK143719
The emergence of MDdR-TB and extensively drug-resistant TB (XDdR-TB) has raised special concerns in relation to the international spread of particularly dangerous strains of Mycobacterium tuberculosis. Since the 2006 edition was published, several incidents have occurred involving air travel and potential transmission of TB. The revision of the International Health Regulations (IHR), which entered into force in June 2007, provides for the introduction of new measures that might potentially apply to international events involving TB. The IHR provide a legal framework for a more effective and coordinated international response to public health emergencies and risks, including those caused by outbreaks of communicable diseases. Several IHR provisions are relevant to the detection and control of TB during air travel, strengthening the role of WHO and of national public health authorities in this domain.
Following these important recent developments, WHO has prepared this third edition to address current public health risks that may arise from the potential transmission of TB during air travel, and new approaches to international collaboration. This edition builds upon the 2006 edition and adds to it in providing: (i) greater clarity in the definition of infectious index cases; (ii) procedures for the follow-up of contacts of infectious cases; and (iii) a more detailed definition of the roles and responsibilities of the agencies involved. The recommendations recognize that the response needs to be proportional to the risk, so that public confidence is preserved and unnecessary restrictions are avoided.
The guidelines were developed with the collaboration of public health authorities and international experts in the prevention and control of TB, travel medicine and air travel. Implementing the recommendations will help to reduce the international spread of TB and decrease the risk of infection among individual travellers. Although the role of air travel-related transmission of TB is minimal compared with the overall transmission of TB worldwide, these guidelines may nevertheless be useful for national authorities, especially in countries with a low TB burden, and for the airline industry, to facilitate procedures involving multiple actors.
Copyright © 2008, World Health Organization.
- Acknowledgements
- Methodology
- Glossary and abbreviations
- 1. Background information
- 2. Tuberculosis on aircraft
- 3. Aircraft ventilation
- 4. Cabin air quality
- 5. Reducing the risk of exposure to M. tuberculosis on aircraft
- 6. Contact investigation following potential exposure to M. tuberculosis
- 7. Legal and regulatory issues
- 8. Airline employee health
- 9. Role of WHO in prevention and control of tuberculosis associated with air travel
- 10. Recommendations
- Appendix 1 Literature search strategy
- Annex 1 International Health Regulations (2005): Selected provisions
- Annex 2 Sample letter from a national public health authority to an airline company requesting information for contact identification after possible exposure to M. tuberculosis
- Annex 3 Proposed procedure for contact investigation following exposure to tuberculosis from an infectious source during air travel
Publication types
- Practice Guideline
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Travel Med
Risk of latent and active tuberculosis infection in travellers: a systematic review and meta-analysis
Tanya r diefenbach-elstob.
Centre for Clinical Epidemiology, Lady Davis Institute, 3755 Côte Ste-Catherine Road, Montreal, Quebec H3T 1E2, Canada
Department of Medicine, McGill University, 1001 Decarie Boulevard, Suite D05-2212, Montreal, Quebec H4A 3J1, Canada
Balqis Alabdulkarim
Department of Internal Medicine, McGill University, 1001 Decarie Boulevard, Rm D05.5840, Montreal, Quebec H4A 3J1, Canada
Paromita Deb-Rinker
Public Health Agency of Canada, 130 Colonnade Road, A.L. 6501H, Ottawa, Ontario K1A 0K9, Canada
Jeffrey M Pernica
Department of Pediatrics, McMaster University, 1280 Main Street West, Hamilton, Ontario L8S 4L8, Canada
Guido Schwarzer
Institute of Medical Biometry and Statistics, Faculty of Medicine and Medical Center, University of Freiburg, Stefan-Meier-Straβe 26, 79104 Freiburg, Germany
Dick Menzies
McGill International TB Centre, 1001 Decarie Boulevard, Room EM3.3212, Montreal, Quebec, H4A 3J1, Canada
Montreal Chest Institute, 1001 Decarie Boulevard, Montreal, Quebec H4A 3J1, Canada
Research Institute of the McGill University Health Centre, 2155 Guy Street, Suite 500, Montreal, Quebec, H3H 2R9, Canada
Department of Family Medicine, McGill University, 5858 Côte-des-Neiges Road, 3rd floor, Montreal, Quebec H3S 1Z1, Canada
Kevin Schwartzman
Christina greenaway.
Division of Infectious Diseases, SMBD-Jewish General Hospital, 3755 Côte Ste-Catherine Road, Montreal, Quebec H3T 1E2, Canada
Associated Data
Introduction.
Achieving tuberculosis (TB) elimination in low TB incidence countries requires identification and treatment of individuals at risk for latent TB infection (LTBI). Persons travelling to high TB incidence countries are potentially at risk for TB exposure. This systematic review and meta-analysis estimates incident LTBI and active TB among individuals travelling from low to higher TB incidence countries.
Five electronic databases were searched from inception to 18 February 2020. We identified incident LTBI and active TB among individuals travelling from low (<10 cases/100 000 population) to intermediate (10–100/100 000) or high (>100/100 000) TB incidence countries. We conducted a meta-analysis and meta-regression using a random effects model of log-transformed proportions (cumulative incidence). Subgroup analyses investigated the impact of travel duration, travel purpose and TB incidence in the destination country.
Our search identified 799 studies, 120 underwent full-text review, and 10 studies were included. These studies included 1 154 673 travellers observed between 1994 and 2013, comprising 443 health care workers (HCW), 1 068 636 military personnel and 85 594 general travellers/volunteers. We did not identify any studies that estimated incidence of LTBI or active TB among people travelling to visit friends and relatives (VFRs). The overall cumulative incidence of LTBI was 2.3%, with considerable heterogeneity. Among individuals travelling for a mean/median of up to 6 months, HCWs had the highest cumulative incidence of LTBI (4.3%), whereas the risk was lower for military (2.5%) and general travellers/volunteers (1.6%). Meta-regression did not identify a difference in incident LTBI based on travel duration and TB incidence in the destination country. Five studies reported cases of active TB, with an overall pooled estimate of 120.7 cases per 100 000 travellers.
Conclusions
We found that travelling HCWs were at highest risk of developing LTBI. Individual risk activities and travel purpose were most associated with risk of TB infection acquired during travel.
Tuberculosis (TB) is a serious global health threat that in 2018 caused an estimated 10 million disease cases and 1.5 million deaths worldwide. 1 Diagnosis and treatment of latent TB infection (LTBI) is key to reaching TB elimination targets in low-incidence countries, where risk groups for LTBI and active TB include immigrants, Indigenous populations and marginalized populations such as prisoners and people who are homeless. 2 Travellers from low TB incidence settings to intermediate or high TB incidence settings are at risk of exposure to Mycobacterium tuberculosis (MTB), and may represent an additional risk group for developing LTBI and active TB.
Collectively, travellers represent a diverse group of individuals. Travel duration, purpose and destination are variable, including tourists undertaking short trips, business travellers making recurrent trips, individuals visiting friends and relatives (VFR), and visits of extended duration made by volunteers, health care workers (HCW) and military personnel. Previous reviews on TB infection in international travellers have noted that risk of LTBI is likely highest in individuals travelling for extended periods to areas of higher risk, but that individual-level factors (such as medical comorbidities) may increase the risk of progression to active TB if LTBI has been acquired. 3 , 4 A previous systematic review on the topic of TB infection in travellers found a cumulative incidence of LTBI of 2.0% (99% CI 1.6–2.4%) among long-term military and civilian travellers in studies undertaken from 1995 to 2007. 4 In addition, numerous observational studies have identified VFRs at increased risk of LTBI and active TB. 5–15 VFRs may already be at increased risk of LTBI if they were born or previously lived in an intermediate or high TB incidence region. However, VFRs may also be at increased risk of travel-associated disease due to pre- and during-travel factors including planning trips at shorter notice, and increased risk of exposure through staying with family and undertaking daily activities similar to those of the local population. 16 , 17 As such, VFRs are a key risk group for travel-associated LTBI and/or active TB.
Guidelines generally recommend pre- and/or post-travel LTBI screening using a tuberculin skin test (TST) or interferon-gamma release assay (IGRA) for travellers who anticipate or have had prolonged exposure to high-risk populations, such as in hospitals, prisons or homeless shelters. 18 , 19 Both pre- and post-travel screening are necessary to confirm incident LTBI and/or active TB attributable to travel, which can be challenging given the number of clinic visits required (at least four for TST and two for IGRA). Therefore, the number of studies reporting incident data are limited.
Travellers to countries with an intermediate or high TB incidence may risk exposure to MTB and development of LTBI and/or active TB. Although travellers are not generally considered a high-risk group for TB, individual-level risk based on travel duration or purpose may result in high-risk situations for some travellers. Furthermore, large numbers of travellers to higher risk destinations may represent a substantial risk group in otherwise low TB incidence settings. The aim of this systematic review and meta-analysis was to determine the risk of LTBI and active TB among individuals travelling from low to intermediate or high TB incidence countries, and to estimate the impact of travel duration, purpose and destination.
Study selection, and inclusion and exclusion criteria
The systematic review and meta-analyses were prepared and reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines. 20 The population of interest was persons travelling from low TB incidence countries (<10 cases per 100 000 population) to intermediate (10–100 cases per 100 000) or high (>100 cases per 100 000) TB incidence countries. Outcomes measured were development of incident LTBI or active TB. For LTBI, only studies that described pre- and post-travel testing using TST and/or IGRA were included (i.e. studies describing prevalent LTBI, based only on post-travel testing results, were excluded). Pre-travel screening was assumed to have ruled out active TB even if this was not explicitly stated. Only studies that explicitly mentioned active TB ascertainment were included in the meta-analysis for active TB.
Search strategy and screening
Five databases were searched (Medline via OVID, EMBASE, Global Health, ProQuest Public Health, Scopus) from inception to 18 February 2020. A combination of key search terms was used ([‘tuberculosis’ OR ‘active TB’ or ‘latent TB’] AND [‘travel’ OR ‘touris*’]) (Appendix 1). No language restrictions were applied in the search, but articles were restricted to English and French in screening. Additional studies were identified by hand searching reference lists from relevant articles.
No methodological restrictions were applied in screening. We included retrospective and prospective studies, and a variety of study designs were identified, including cohort studies, surveys and retrospective medical reviews. No minimum or maximum number of participants were required for inclusion.
Two reviewers (BA and JMP) screened the identified titles and abstracts for eligibility, and then screened identified full-text articles for inclusion, with disagreement resolved by consensus. Two reviewers (BA and JMP) extracted the data from the included studies, and one reviewer (TDE) confirmed the extracted data.
Study definitions
Studies were stratified by duration of travel as a surrogate for duration of exposure. Stratifications were based on the mean or median duration of travel reported, with studies grouped into those with an average of up to 6 months of travel, 7–12 months of travel and 13–24 months of travel. Travel duration classifications were developed post hoc, guided by the extracted data to reflect short-, intermediate- and long-term periods of travel.
Travellers were stratified by travel purpose as a surrogate for exposure risk. Subgroups included HCWs (individuals who travelled for a defined period to a non-home destination for medical work); military personnel; and general travellers/other volunteers (including tourists, Peace Corps Volunteers, other volunteers, etc.). The latter group was combined given the small number of non-volunteer general travellers (575/85 594), and the fact that many of them participated in higher risk activities such as using local transport and staying in local homes. 21
Travel destinations were stratified into intermediate incidence (10–100 TB cases/100 000 population annually) and high incidence (>100 TB cases/100 000 population) countries/regions. In general, destination countries in Latin America and the Caribbean, North Africa, the Middle East and Europe (not including low TB incidence countries) and Central Asia were classified as intermediate incidence, whereas countries in Sub-Saharan Africa, South or East Asia and the Pacific region were classified as high incidence.
Quality assessment
The Quality in Prognosis Studies (QUIPS) tool was used to assess risk of bias in the included studies. 22 Separate assessments were undertaken for studies reporting LTBI conversions and active TB. Two reviewers (TDE and PDR) independently completed the assessment tool for each study and outcome, with disagreements resolved through consensus with a third reviewer (BA).
Meta-analysis methodology
Some assumptions were made in defining numerators and denominators for the meta-analyses. The average number of months of travel among Peace Corps Volunteers was not reported in the study by Brown et al . 23 The studies by Brown et al . and Jung et al . were undertaken among Peace Corps Volunteers, during the periods 2006–2013 and 1996–2005, respectively; with data sourced from the same surveillance system. 23 , 24 To estimate the total number of participants in Brown et al . we used the reported volunteer-months (i.e. total months of travel), and assumed the same average number of months of travel (i.e. 18.193) calculated from the participants and volunteer-months reported in Jung et al . 24 Cumulative incidence was then calculated based on the estimated denominator. For both studies, the numbers of participants in intermediate and high TB incidence regions were estimated by dividing the total person-months (PM) of travel in these regions by the calculated average number of months of travel (18.193). In the study by Cobelens et al ., the numbers of LTBI and active TB diagnoses were not explicitly stated for HCWs with and without direct patient contact. 21 There were 100 HCWs reported in the paper, of whom 81 had direct patient contact. Overall, 12 cases of MTB infection were identified, including four HCWs with direct patient contact. The results did not state whether any of the remaining eight cases of MTB infection occurred among the 19 HCWs who did not have direct patient contact. To ensure that the remaining numerator (i.e. eight LTBI cases) was part of the denominator (travellers who were not HCWs with direct patient contact), we included the 19 HCW travellers without direct patient contact in the general traveller/volunteer group. For active TB, two cases were described in the results without mention that they were HCWs, therefore we assumed they were not HCWs. In the studies of HCWs by Gardner et al . and Szep et al . the outcomes were reported for HCWs both with and without direct patient contact, therefore all participants from these studies were included in the HCW analysis. 25 , 26 In the study by Kortepeter and Krauss, we excluded 25 individuals who were TST-positive prior to travel, classified as ‘reactors’ in the study. 27
Information about travel duration was not available for individual travellers in the included studies, so we were not able to estimate the risk of LTBI per unit time (e.g. per additional week or month of travel). We instead stratified studies by their mean or median travel duration, and undertook analyses using the cumulative incidence (proportion) of events.
Cumulative incidences (proportions) of LTBI conversion events were estimated as the number of events per 100 individuals. In the meta-analyses, data were stratified by travel duration (mean or median of up to 6, 7–12 or 13–24 months of travel), travel purpose (HCWs, military, general travellers/volunteers), and TB incidence in the destination country (intermediate incidence, high incidence). Post hoc pairwise comparisons were undertaken to compare subgroups within the forest plots when the overall test for subgroup differences was significant ( P < 0.05). Pairwise comparisons were considered significant where P < 0.05. Meta-regression was used to examine the effect of travel duration and TB incidence in the destination country as covariates. For the meta-regression the 7–12 month group (one study) was combined with the 13–24 month group, as the average travel duration of that study was 11.9 months. 4 HCWs were excluded from the meta-regression as no studies reported on HCWs travelling for more than 6 months. For active TB, cumulative incidence was estimated as the number of events per 100 000 individuals. Data were stratified by travel duration (up to 6 months and 13–24 months of travel). Meta-analysis and meta-regression were undertaken using a random effects model with log transformation, using the metaprop, metareg and forest commands in the meta package in R (version 3.6.1). 28 The I 2 statistic was used to describe between-study heterogeneity. Thresholds for heterogeneity can be misleading, and we used the guide provided in the Cochrane Handbook of Systematic Reviews of Interventions, as follows: not important (I 2 of 0–40%), moderate (30–60%), substantial (50–90%) and considerable (75–100%). 29
A total of 799 studies were identified after duplicates were removed, as shown in the PRISMA diagram (Appendix 2). Following screening, 120 full-text articles were assessed and 110 were excluded, most often because they were not relevant to the study question or their design was unsuitable. A total of 10 studies were included in the systematic review and meta-analyses. We included one systematic review that contained original data for LTBI conversion events sourced from military departments in Canada, the USA and Germany. 4 In our results, original data from this study are considered separately for each of the four subgroups included (Canadian military, German military, USA Air Force and USA Army).
The characteristics of studies reporting incident LTBI are summarized in Table 1 . There were 1 154 673 travellers included, comprising 85 594 general travellers/volunteers, 1 068 636 military personnel and 443 HCWs. For active TB, characteristics of included studies are presented in Table 2 . These five studies included 85 373 travellers, comprising 85 025 general travellers/volunteers and 348 HCWs.
Summary of LTBI data from included studies, presented in chronological order by the first year of each study
¶ estimated; *expressed as a median; ^expressed as an interquartile range.
HCW, healthcare workers; SW, southwest; TB, tuberculosis.
Summary of active TB data from included studies, including stratification by travel purpose and TB incidence in the destination country
¶ estimated; *expressed as a median; ^expressed as an interquartile range
HCW, healthcare workers; TB, tuberculosis.
Results for the risk of bias assessments are shown in Appendix 3. No studies were excluded from the meta-analyses as a result of these assessments. Overall, there was low to moderate risk of bias across all six risk domains for most studies. Risk of bias due to participant attrition was high in five of the eight LTBI studies. When risk of bias was high in the other domains this was usually due to lack of information about methodology and/or potential study confounders.
Cumulative incidence
The overall cumulative incidence of LTBI was 2.3% (95% CI 1.7–3.1%), with considerable heterogeneity (I 2 = 99%) ( Figure 1 ). Subgroups defined by travel duration and purpose explained some of the heterogeneity ( P < 0.0001), however residual heterogeneity was still considerable (residual I 2 = 88%). For travel duration up to 6 months, the pooled cumulative incidence of LTBI was significantly higher for HCWs (4.3%, 95% CI 2.8–6.7%) compared to general travellers/volunteers (1.6%, 95% CI 1.0–2.5%) and military personnel (2.5%, 95% CI 2.0–2.9%) (post hoc pairwise comparisons: P = 0.002 and P = 0.02, respectively). Only one study in military personnel reported data for 7–12 months of travel (cumulative incidence of 0.96%, 95% CI 0.94–0.98%). Three studies among general travellers/volunteers reported travel of 13–24 months, with a pooled cumulative incidence of 2.1% (95% CI 1.6–2.8%). There was not a significant difference in cumulative incidence between general travellers/volunteers travelling for up to 6 months compared to those travelling for 13–24 months (post hoc pairwise comparison: P = 0.29). Cumulative incidences of LTBI stratified by travel duration and purpose are also presented as a scatter plot in Figure 2 .

Meta-analysis of cumulative incidence of LTBI for mean/median travel durations of up to 6 months, 7–12 months and 13–24 months, stratified by travel purpose.

Scatter plot of cumulative incidence of LTBI and average travel duration in included studies.
A table presenting data stratified by travel duration and purpose is provided in Appendix 4. In meta-regression, no difference in incident LTBI was identified based on travel duration [risk ratio for 13–24 months: 0.82, 95% CI 0.48–1.39, P = 0.46 (up to 6 months as reference group)], or TB incidence in the destination country [risk ratio for high TB incidence: 0.97, 95% CI 0.55–1.69, P = 0.91 (intermediate TB incidence as reference group)] (Appendix 5). When excluding the single study with travel duration of 7–12 months the results were similar (Appendix 5).
A table outlining the type of testing (TST or IGRA) for each study is provided in Appendix 6. No differences in pooled estimates of cumulative incidence between groups were found based on stratification by type of test (TST only vs TST or IGRA) or by type of TST (1-step vs 2-step) (Appendix 7).
Five of the included studies provided data on individuals who developed active TB during or after travel. 21 , 23 , 24 , 26 , 30 Two studies were undertaken in the 6-month travel group, including HCWs and general travellers/volunteers, and three studies were undertaken among general travellers/volunteers in the 13–24 month travel group. The pooled cumulative incidence of active TB for all travel durations was 120.7 per 100 000 (95% CI 50.4–289.3), with considerable heterogeneity (I 2 = 81%) ( Figure 3 ). Of the three active TB cases in the 6-month travel group, two were identified among general travellers/volunteers ( n = 575), and one was identified among HCWs ( n = 348). No difference in cumulative incidence was found based on stratification by travel duration ( P = 0.07).

Meta-analysis of cumulative incidence of active TB stratified by mean/median travel duration.
In this review, we found that individuals travelling from low TB incidence countries to intermediate and high TB incidence countries were at risk of developing LTBI, and that risk varied by travel purpose, but not duration of travel (up to 24 months of travel) or TB incidence in the destination country. HCWs travelling for up to 6 months had the highest risk of developing LTBI compared to other groups travelling for up to 6 months. Individual risk behaviours are likely to be the key factor in acquisition of LTBI during travel.
Our study updates the earlier systematic review by Freeman et al . that analysed data from the period 1994–2007. 4 Our review includes five additional studies, extends the study period to 2013 (including 41 311 additional travellers) and stratified the data to include HCWs as a travel purpose. We also excluded one study among Navy and Marine Corps personnel which included travel on ships. 31 For military and civilian travellers only (i.e. all travellers except HCWs), we found a similar overall cumulative incidence compared to that in the Freeman study (2.1% vs 2.0%), 4 providing supporting evidence of a small and unchanged risk of LTBI for military and civilian travellers over a 20-year period (1994–2013) (Appendix 8).
We found that HCWs were the highest risk travellers, with a cumulative incidence of LTBI of 4.3% for up to 6 months of travel. This finding likely reflects the high risk of LTBI among HCWs in intermediate and high TB incidence countries. 32–34 The annual risk of LTBI among HCWs in low-, middle- and high TB incidence countries has been estimated at 2.9%, 8.7% and 7.2%, respectively; with an overall average annual risk of 4.6%. 35 The higher risk of LTBI seen among HCWs in our review may have been due to the substantial loss to follow-up of participants in the HCW group, with only 53% completing post-travel follow-up overall in the two studies undertaken exclusively among HCWs (Appendix 6). 25 , 26 This may have biased our estimate if follow-up was differential and only included HCWs who believed they had a significant TB exposure. Our results should therefore be interpreted with caution, but highlight the risk of LTBI among HCWs travelling for work, and the importance of pre- and post-travel screening and assessment in this group.
For travel of up to 6 months, the risk of LTBI was about half as likely for military personnel and general travellers/volunteers compared to HCWs. Individuals travelling for reasons other than health care work are likely to have variable exposure to people with TB depending on their individual activities or circumstances. In the military studies included in our review risk estimates varied widely. The cumulative incidence of LTBI in the US Army population reported by Freeman et al . was 0.96% (95% CI 0.94–0.98%), whereas it was 3.7% (95% CI 2.7–4.9%) in the US Army population studied by Kortepeter and Krauss. 4 , 27 This is likely due to different risk of exposure in these two settings. For example, the study by Kortepeter and Krauss was conducted among soldiers deployed to refugee camps housing Cuban and Haitian refugees in Guantanamo Bay, Cuba. 27 Although TB incidence was relatively low in Cuba during the study period (14.2 cases per 100 000 people per year), it was much higher in Haiti (79.4 per 100 000), and ~3% of Haitian refugees screened at Guantanamo Bay during 1991–1993 were diagnosed with and treated for presumptive active TB. 36–38 In other settings, military personnel may have very limited exposure to the local population. In the study of the US Army population by Freeman et al ., there was danger in off-base travel as personnel were primarily deployed to Iraq and Afghanistan. 4 This may have limited exposure to the local population with resultant lower risk of MTB exposure. 4
Increasing duration of travel is generally considered a risk factor for MTB exposure. 3 A study of Dutch-born travellers found that TST reactivity increased significantly with duration of reported travel history, based on no prior travel (1 TST-positive/389 participants), <3 months (1/323), 3–12 months (2/203) and >12 months (3/99) of travel to high TB incidence countries. 39 In our study travel duration up to 24 months for general travellers/volunteers was not associated with an increased risk of LTBI. This may be due to the fact that information about travel duration was not available for individual travellers in the studies included in our review, so we could not estimate the risk of LTBI per unit time (e.g. per additional week or month of travel). Future research should evaluate the impact of incremental increases in travel duration on risk of LTBI.
There was methodological heterogeneity among the included studies in the use of pre-travel 1-step or 2-step TST testing, and the interval between the end of travel and the post-travel test. As shown in Appendix 6, only three studies used pre-travel 2-step testing (including one military group from the included systematic review), and five studies did not state the approach used. If only a 1-step TST is used pre-travel, positive tests post-travel may be due to boosting, but would be misclassified as a TST conversion. Use of a 2-step TST pre-travel eliminates this problem by identifying false-negative pre-travel results. However, 2-step testing pre-travel may reduce adherence with study protocols by increasing the number of clinic visits required for the placement and reading of all three tests. Some studies used IGRA testing, and challenges associated with reproducibility of this assay have been described previously, especially when used for serial testing. 40–42 Factors such as tube handling, incubation conditions and analytical processing variability may affect IGRA results, and thus when used in serial testing may contribute to higher rates of conversions and reversions, particularly when assay results are borderline. 40–42 Thus, for travellers who underwent serial testing using IGRA there is the possibility of false-positive and/or false-negative results.
We identified five studies that described cases of active TB occurring among people who were TST- or IGRA-negative in pre-travel screening, with 63 cases of active TB identified among 85 373 travellers. The meta-analysis results were suggestive of higher TB incidence among individuals travelling up to 6 months compared to those travelling 13–24 months, but the test for subgroup differences was not significant. However, only small absolute numbers of active TB diagnoses were identified, thus we had limited precision in our estimates. The results for active TB are likely to reflect similar risk factors to those identified for LTBI, with risk of infection and disease most driven by individual risk activities while travelling.
In our included studies, post-travel testing periods were variable and sometimes poorly defined (Appendix 6). It is possible that some testing was undertaken too early to detect LTBI, given that contact investigations recommend definitive TST or IGRA testing 8–10 weeks post-exposure. 43 Conversely, the long post-travel follow-up periods of some studies (up to 3 years) raise the theoretical possibility of non-travel-associated LTBI occurring between the end of travel and the time of post-travel testing, although this is unlikely for travellers who returned to their low-incidence home countries. Furthermore, the highest risk of progression from LTBI to active TB is in the first 2 years following MTB exposure. 44 Therefore, for studies with relatively short follow-up times it is possible that additional cases of active TB developed post-travel, but were missed because they occurred after the follow-up period.
Observational studies have described LTBI and active TB among VFR travellers, 5–15 but we could not investigate TB risk among VFRs in our review as no studies provided data on incident infections in this population group. This lack of data represents an important knowledge gap in the understanding of TB risk among travellers, and studies investigating the risk of TB among VFRs are needed.
Our systematic review has some limitations. We did not have individual travel time for travellers in each study, so could not estimate LTBI rates per unit time (e.g. per additional week or month of travel). We therefore stratified studies by the mean or median travel duration. Travel duration categorizations were assigned post hoc, and there may have been overlap in exposure time of travellers in different studies. Follow-up time was variable, and in some cases may have decreased the ability to detect LTBI or active TB post-travel. Finally, no studies investigated incident LTBI or active TB among HCWs travelling for more than 6 months, so we could not evaluate TB risk for longer travel durations among this group. There was considerable residual heterogeneity for the overall estimates despite stratification into purpose of travel and travel duration, suggesting that there is unmeasured confounding.
Our review identified travelling for health care work as the strongest risk factor for travel-associated LTBI, suggesting that individual risk activities are the most important drivers of exposure, rather than duration of travel. However, there is a need for further study to better understand the impact of travel duration on MTB exposure risk. HCWs travelling for work in intermediate and high TB incidence countries face a substantial risk of MTB infection, and should be prioritized for LTBI and active TB screening, with the choice of pre- and/or post-travel screening dependent on the risk of pre-travel infection, and the need to distinguish newly incident infection in making treatment decisions. For other travellers, risk assessment should be undertaken in the context of intended travel duration and purpose, as screening may only be warranted if there is a higher likelihood of MTB exposure.
Supplementary Material
Tb_in_travelers_sr_and_ma_manuscript_appendices_r2_final_taaa214, acknowledgements.
The authors thank Gregory Traversy (Public Health Agency of Canada/Agence de la santé publique du Canada) and Beverley Shea (The Ottawa Hospital Research Institute) for methodological support. Tanya Diefenbach-Elstob is supported by a postdoctoral training award from the Fonds de recherche du Québec—Santé (FRQS). Dick Menzies holds a Tier 1 Canada Research Chair (CRC) awarded by the Canadian Institutes of Health Research (CIHR). No grant or financial support was received for this study.
Meetings at which this paper has been presented: This paper was accepted as an oral abstract at the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) 2020. The paper was not presented due to cancellation of the conference because of the COVID-19 pandemic.
Contributor Information
Tanya R Diefenbach-Elstob, Centre for Clinical Epidemiology, Lady Davis Institute, 3755 Côte Ste-Catherine Road, Montreal, Quebec H3T 1E2, Canada. Department of Medicine, McGill University, 1001 Decarie Boulevard, Suite D05-2212, Montreal, Quebec H4A 3J1, Canada.
Balqis Alabdulkarim, Department of Internal Medicine, McGill University, 1001 Decarie Boulevard, Rm D05.5840, Montreal, Quebec H4A 3J1, Canada.
Paromita Deb-Rinker, Public Health Agency of Canada, 130 Colonnade Road, A.L. 6501H, Ottawa, Ontario K1A 0K9, Canada.
Jeffrey M Pernica, Department of Pediatrics, McMaster University, 1280 Main Street West, Hamilton, Ontario L8S 4L8, Canada.
Guido Schwarzer, Institute of Medical Biometry and Statistics, Faculty of Medicine and Medical Center, University of Freiburg, Stefan-Meier-Straβe 26, 79104 Freiburg, Germany.
Dick Menzies, McGill International TB Centre, 1001 Decarie Boulevard, Room EM3.3212, Montreal, Quebec, H4A 3J1, Canada. Montreal Chest Institute, 1001 Decarie Boulevard, Montreal, Quebec H4A 3J1, Canada. Research Institute of the McGill University Health Centre, 2155 Guy Street, Suite 500, Montreal, Quebec, H3H 2R9, Canada.
Ian Shrier, Centre for Clinical Epidemiology, Lady Davis Institute, 3755 Côte Ste-Catherine Road, Montreal, Quebec H3T 1E2, Canada. Department of Family Medicine, McGill University, 5858 Côte-des-Neiges Road, 3rd floor, Montreal, Quebec H3S 1Z1, Canada.
Kevin Schwartzman, McGill International TB Centre, 1001 Decarie Boulevard, Room EM3.3212, Montreal, Quebec, H4A 3J1, Canada. Montreal Chest Institute, 1001 Decarie Boulevard, Montreal, Quebec H4A 3J1, Canada. Research Institute of the McGill University Health Centre, 2155 Guy Street, Suite 500, Montreal, Quebec, H3H 2R9, Canada.
Christina Greenaway, Centre for Clinical Epidemiology, Lady Davis Institute, 3755 Côte Ste-Catherine Road, Montreal, Quebec H3T 1E2, Canada. Department of Medicine, McGill University, 1001 Decarie Boulevard, Suite D05-2212, Montreal, Quebec H4A 3J1, Canada. Division of Infectious Diseases, SMBD-Jewish General Hospital, 3755 Côte Ste-Catherine Road, Montreal, Quebec H3T 1E2, Canada.
Authors’ contributions
CG conceived the study design. BA conducted the searches. BA and JMP screened the search results and extracted data, and TDE confirmed the extracted data. TDE, PDR and BA completed the risk of bias assessments. TDE conducted the analyses, with methodological guidance from GS, IS and CG. TDE and BA wrote the manuscript. All authors (BA, PDR, JMP, GS, DM, IS, KS and CG) provided input on the study analysis and results, and critical review of the manuscript.
Conflict of interest
None declared.
AITC Immunization & Travel Clinic
We provide travel health visits, vaccinations, TB testing, and blood tests. Appointment only.
Attention! starting April 15, 2024
Our new website address is SF.GOV/AITC

AITC Services, Price, and Forms
Book an appointment on line and get clinic forms
See our services and prices
Donate to AITC
Welcome to AITC
AITC is a non-profit clinic that is part of the San Francisco Department of Public Health (SFDPH). As public health providers, our mission is to prevent disease and protect the health of all.
We are open to the public, and serve all members of the community, including:
- Teens and adults seeking recommended vaccinations
- School age children needing vaccines required for school
- Adults who need vaccines for work or school
- Immigrants to the US requiring vaccines for Change of Status
- Individuals and families planning international travel
Our services are by appointment only.
AITC is unable to accept insurance. Fees must be paid at the time of service. Low-cost or free services are available to those who qualify.
Message about our MPOX vaccine (JYNNEOS) supply
Mpox vaccine at AITC is still supplied free of charge by the government. Later in 2024 we may need to purchase the vaccine and charge a fee for it. We will post more information when it becomes available.
Getting here
Metered street parking or Civic Center Garage
Public transportation
Southwest corner, Civic Center Plaza Across from City Hall BART / MUNI : Civic Center Station
Make an appointment online by clicking the left link. It is highly recommended; it is simpler and faster.
If you need assistance, please call us.
AITC Immunization & Travel Clinic
Mon to Fri, 9:00 am to 4:00 pm Closed for lunch 12 pm - 1pm
We are closed weekends and holidays .
Find more information about how to get to our clinic .
Departments
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- Hosted content
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 77, Issue 4
- BTS Clinical Statement on air travel for passengers with respiratory disease
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Robina Kate Coker 1 ,
- Alison Armstrong 2 ,
- Alistair Colin Church 3 ,
- Steve Holmes 4 ,
- Jonathan Naylor 5 ,
- Katharine Pike 6 ,
- Peter Saunders 7 ,
- Kristofer John Spurling 8 ,
- Pamela Vaughn 9
- 1 Respiratory Medicine , Hammersmith Hospital, Imperial College Healthcare NHS Trust , London , UK
- 2 The Newcastle upon Tyne Hospitals NHS Foundation Trust , Newcastle upon Tyne , UK
- 3 Scottish Pulmonary Vascular Unit , Golden Jubilee Hospital , Clydebank , UK
- 4 The Park Medical Practice , Shepton Mallet , UK
- 5 Queen Elizabeth Hospital , Birmingham , UK
- 6 Department of Paediatric Respiratory Medicine , Bristol Royal Hospital for Children , Bristol , UK
- 7 Churchill Hospital , Oxford , UK
- 8 Respiratory Physiology Department , North Middlesex University Hospital , London , UK
- 9 Glasgow Royal Infirmary , Glasgow , UK
- Correspondence to Dr Robina Kate Coker, Respiratory Medicine, Hammersmith Hospital, Imperial College Healthcare NHS Trust, London, London, UK; robina.coker{at}imperial.ac.uk
https://doi.org/10.1136/thoraxjnl-2021-218110
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
- pleural disease
- lung physiology
Introduction
BTS recommendations for managing passengers with stable respiratory disease planning air travel were published in Thorax in 2011. 1 This followed original guidance published in 2002 2 and an online update in 2004. 3 The 2011 recommendations provided an expert consensus view based on literature reviews, aimed at providing practical advice for lung specialists in secondary care. Recognising that knowledge in this area has grown since 2011, and that updated, pragmatic advice regarding which respiratory patients need specialist assessment is required, the Society has commissioned a new clinical statement.
European and North American regulatory authorities limit maximum cabin altitude to 2438 m (8000 ft) under normal operating conditions. 5–7 The choice of 2438 m was based on the oxyhaemoglobin dissociation curve, which shows that up to this level arterial oxygen saturations (SaO 2 ) remain >90% in the average healthy individual. 8 Some newer commercial aircraft have a lower normal cabin altitude, for example, the Boeing 787 Dreamliner. However, passengers booking such flights should note that airlines may, for operational reasons, switch at short notice to an aircraft with a higher normal cabin altitude.
Besides the passenger’s respiratory condition and significant comorbidities, a decision regarding suitability for air travel should consider flight duration and timings, destination (especially if at altitude or subject to extreme weather conditions), equipment and medications, and whether equipment will operate effectively and safely at altitude.
There have been developments in three key areas over the last decade. The first is an attempt, with research from several groups, to define more precisely the value and role of the hypoxic challenge test (HCT). This has included examining the accuracy of other, more routinely available lung function parameters, in predicting hypoxaemia during air travel. HCT can be expensive in terms of equipment and consumables; and demands additional staff time. A ‘negative’ HCT (where in-flight oxygen is not considered necessary) takes around 30 min; if oxygen titration is needed it takes around 60 min. In contrast, spirometry requires 20 min, a walk test 30 min, and ‘full’ lung function testing 45 min. 9 Results of such assessments may already be available as part of routine clinical care.
The second development has been increasing recognition that, although early research in this area focused on patients with chronic obstructive pulmonary disease (COPD), other patient groups may respond differently to altitude-related hypoxaemia. Although data remain limited, available evidence no longer appears to support a ‘one size fits all’ approach.
Finally, the equipment used to deliver oxygen has changed significantly over the last decade, with much greater availability of portable oxygen concentrators (POCs). For overseas travel, patients usually need to lease a POC privately, since UK companies do not generally allow their equipment to be taken out of the country. If a POC is to be used in-flight, the equipment must be approved by the airline before travel. There are now a wide variety of such devices, providing varying flow rates and modes of delivery (continuous flow vs pulse-dose), and not all are suitable for all individual patients.
Attention has, therefore, been drawn in this Statement to newer data, especially those published since the 2011 BTS recommendations. 1 Readers wanting more detailed background information on physiology and the flight environment should consult the 2002 and 2011 BTS documents. 1 2
The clinical statement provides practical advice for healthcare professionals in primary and secondary care managing passengers with pre-existing respiratory conditions planning commercial air travel, including those recovering from an acute event/exacerbation. It provides information for patients and carers; and is also intended to be helpful to patient support groups, airlines and associated medical services. Passengers returning home with a new diagnosis should be reviewed in the light of the presenting condition and individual circumstances. The document does not cover emergency aero-medical evacuation, or travel on non-commercial flights. Pregnant passengers with respiratory disease should also consult Royal College of Obstetricians and Gynaecologists guidance (see online supplemental appendix 1 ).
Supplemental material
The Statement addresses adults and children with the following conditions or undergoing the following procedures:
Airflow obstruction including asthma and COPD.
Bronchopulmonary dysplasia.
Cystic fibrosis (CF).
Non-CF bronchiectasis.
Restrictive respiratory disease including interstitial lung disease (ILD), respiratory muscle and chest wall disorders.
Thoracic surgery or other interventional procedures.
Pleural disease including pneumothorax and pleural effusion.
Respiratory infections.
Obstructive sleep apnoea syndrome (OSAS) and obesity hypoventilation syndrome (OHS).
Venous thromboembolism (VTE).
Pulmonary hypertension (PH).
Lung cancer and mesothelioma.
Hyperventilation and dysfunctional breathing (DB).
Preflight assessment is described. Appendix A provides information on logistics for air travel with equipment (nebulisers, oxygen and ventilators); Appendix B provides technical information for respiratory physiologists. Sources of useful information, Information for primary care healthcare practitioners and for patients are provided in online supplemental appendices 1–3 .
Heart disease and HIV are excluded, as are emergency repatriation and travel on military or other non-commercial flights including helicopter travel. The Terrence Higgins Trust and British Heart Foundation provide advice on travel with HIV and heart conditions respectively (see online supplemental appendix 1 ).
Methodology
Dr Robina Coker chaired the clinical statement group (CSG). Membership was drawn from respiratory medicine, paediatrics, nursing, respiratory physiology, physiotherapy and primary care. The CSG identified key areas requiring Clinical Practice Points. The group reviewed previous BTS recommendations on this topic 1–3 and supplemented the evidence with up-to-date literature searches. The overall content was developed to reflect the scope approved by the BTS Standards of Care Committee (SOCC). Following discussions of broad statement content, individual sections were drafted by group members. A final edited draft was reviewed by the BTS SOCC before posting for public consultation and peer review on the BTS website in January 2020. The document was revised in the light of consultation feedback and approved by the BTS Standards of Care Committee in July 2021 before final publication.
Summary of clinical practice points
Preflight screening.
All patients should undergo careful initial evaluation with history and physical examination by a clinician who is competent. The history should include:
Review of symptoms, baseline exercise capacity, recent exacerbation history, treatments and previous experience of air travel.
Consideration of the logistics of the intended journey, to include (if known):
Number and duration of flights, including whether daytime or overnight,
Location of stop-over(s) and destination: these determine air quality, altitude and available medical facilities,
Time away from home
Return journey.
Further assessment by a respiratory specialist is advised for those in whom screening raises concerns, and HCT may be advised.
The following clinical practice points are specific to infants and children
For infants born at term (>37 weeks) it is prudent to delay flying for 1 week after birth to ensure they are healthy.
Infants born prematurely (<37 weeks) with or without a history of respiratory disease who have not reached their expected date of delivery at the time of flying should have in-flight oxygen available. HCT may not be a reliable guide of oxygen requirement in this group. If air travel is essential, they should travel with oxygen at a tolerable low flow, recognising that this may be a minimum of 1 L/min depending on equipment.
Infants under 1 year with a history of chronic respiratory problems should be discussed with a respiratory paediatrician and HCT considered. Those with SpO 2 <85% on HCT should have in-flight oxygen available; paediatrician discretion should be used for infants with SpO 2 85%–90% recognising that sleep or respiratory infection may further reduce saturations in this group.
In children with chronic lung disease able to perform spirometry whose forced expiratory volume in 1 s (FEV 1 ) is consistently <50% predicted, HCT should be considered. This includes children with CF and primary ciliary dyskinesia (PCD). Children with chronic lung disease who are too young to perform spirometry reliably should have a clinical assessment of disease severity and their likely tolerance of hypoxia. In children with CF the disease is rarely severe enough to compromise lung function significantly at this age.
Infants and children who have required long-term oxygen in the last 6 months should be discussed with a respiratory paediatrician and HCT considered.
Patient selection for HCT
See figures 1 and 2 .
- Download figure
- Open in new tab
- Download powerpoint
Preflight assessment of patients with chronic airflow obstruction.
Preflight assessment of patients with restrictive respiratory disease.
The following patients should not require HCT
Those with stable disease who have previously undergone HCT (no recent hospital admissions, exacerbations, or significant changes to treatment).
Patients with COPD with baseline SpO2 ≥95% and either MRC score 1–2 or desaturation to no less than 84% during 6 min walk test (6MWT) or shuttle walking test (SWT), should be able to travel without in-flight oxygen.
Those with previous significant intolerance to air travel, such as mid-air emergency oxygen or diversion. These should have in-flight oxygen available at 2 L/min provided there is no history of hypercapnia.
Preterm infants who have not reached their due date at the time of travel, as testing is not a reliable guide of oxygen requirement in these infants. These should have in-flight oxygen available, delivered at 1–2 L/min if they develop tachypnoea, recession, or other signs of respiratory distress.
HCT should be considered for the following patients
Patients with COPD with resting SpO 2 ≤95%, MRC score 3 or greater, or desaturation to <84% on 6MWT or SWT, and in whom there are concerns about hypercapnia.
Infants and children with a history of neonatal respiratory problems, or existing severe chronic lung disease including those with FEV1 persistently <50% predicted.
Adults and children with severe asthma, evidenced by persistent symptoms and/or frequent exacerbations despite optimal treatment regardless of resting sea level SpO 2 .
Patients with ILD in whom SpO 2 falls to <95% on exercise, and whose resting sea level arterial oxygen tension (PaO 2 ) is ≤9.42 kPa or whose TLCO is ≤50%.
Those with severe respiratory muscle weakness or chest wall deformity in whom forced vital capacity (FVC) is <1 L.
Those with existing or previous hypercapnia and those at risk of hypercapnia, including those taking medication(s) which can cause respiratory depression.
Patients with a history of type 2 respiratory failure already on LTOT at sea level. However, if there is no evidence of hypercapnia, it seems reasonable to recommend an increase in flow rate by 2 L/min in-flight, provided the equipment can provide it (see Appendix A)
HCT results
PaO 2 ≥6.6 kPa (≥50 mm Hg) or SpO 2 ≥85%: in-flight oxygen not required.
PaO 2 <6.6 kPa (<50 mm Hg) or SpO 2 <85%: in-flight oxygen recommended.
Where required, titrate oxygen to maintain PaO 2 ≥6.6 kPa or SpO 2 ≥85% in adults, SpO 2 90% in children aged 1 year or more.
The patient’s condition should be optimised before travel, with attention paid to inhaler technique and smoking cessation referral as required.
All medications and spacer devices should be carried in hand luggage to mitigate the risk of lost or missing hold baggage.
Emergency medications, including salbutamol inhalers and spacers, must be immediately accessible.
Individuals prescribed epinephrine auto-injectors should have them readily available.
For acute exacerbations on board, the passenger’s own bronchodilator inhaler should be given, with a spacer if needed.
The passenger should alert the cabin crew if symptoms do not respond rapidly to use of the inhaler, or if they recur after a short interval.
If the passenger does not have their own inhaler with them, or if it is inaccessible, the airline may carry an inhaler in the emergency medical kit. Spacers are not commonly available.
Those with severe asthma should consult their respiratory specialist beforehand and consider taking an emergency supply of oral corticosteroid in their hand luggage in addition to their usual medication.
Passengers with severe asthma are advised to carry copies of their asthma management plan and/or relevant clinic letters. Information can be held securely as scanned copies on a mobile phone, or on a digital platform such as the National Health Service (NHS) App.
Food allergy affects up to 8.5% of children and adults with asthma and asthma is a risk factor for severe or fatal anaphylaxis. Appropriate precautions for those affected include wiping tray tables and hands, informing the airline beforehand and the cabin crew of allergies, and not eating during flights or bringing known ‘safe’ foods from home.
Chronic obstructive pulmonary disease
The patient’s condition should be optimised before travel, with attention paid to inhaler technique and smoking cessation referral where appropriate.
All medications and spacer devices should be carried in hand luggage to mitigate the risk of missing hold baggage.
For acute exacerbations on board, the passenger’s own bronchodilator inhaler should be given, with a spacer if appropriate.
Passengers with severe COPD are advised to carry a copy of their COPD management plan and/or relevant clinic letters. This information can be held securely as scanned copies on their mobile phone A history of previous pneumothorax or bullous lung disease necessitates assessment by a respiratory specialist to determine the potential risk of complications from reduced cabin pressure.
Patients with COPD are at greater risk of VTE as a direct consequence of the underlying condition, as well as after an exacerbation. They should be advised accordingly, especially if planning longer flights when the risk is further enhanced.
Patients requiring long-term oxygen therapy should also plan for oxygen supplementation at their destination (see online supplemental appendix 1 ).
Wherever possible, those who have had a recent exacerbation of their condition should not fly until their condition is stable and use of reliever therapy has returned to their usual baseline. If their condition deteriorates while overseas, medical advice should be sought before undertaking the return flight.
Cystic fibrosis
Patients with CF under the age of 6 are likely to be well enough to fly at the paediatrician’s discretion.
In those with CF who are old enough for spirometry and whose FEV1 is <50% predicted, HCT is recommended. If SpO2 falls below the 90% cut-off, as outlined above, in-flight oxygen is advised.
In children with chronic lung disease able to perform spirometry whose FEV 1 is consistently <50% predicted, HCT should be considered. This includes children with CF and non-CF bronchiectasis. Children with chronic lung disease who are too young to reliably perform spirometry should have a clinical assessment of assess disease severity and their likely tolerance of hypoxia. For children with CF disease is rarely severe enough to severely compromise lung function at this age.
Non-CF bronchiectasis
Regular airway clearance is essential for those dealing with overproduction of mucus.
Advice from a respiratory physiotherapist on adapting airway clearance techniques should be sought for long-haul flights.
Portable nebulisers and positive expiratory pressure (PEP) devices may be considered, but use of these devices in-flight must be approved by the airline before travel.
Interstitial lung disease
In patients with comorbidity, including PH and/ or cardiovascular disease, attention should also be paid to the impact of air travel on these conditions.
Physicians may wish to consider HCT in those whom SpO2 falls to <95% on exercise, and/or in those in whom either Transfer Factor Carbon Monoxide (TLCO) ≤50% or PaO 2 ≤9.42 kPa (if available).
Patients with TLCO <50% of predicted or PaO 2 ≤9.42 kPa are likely to need in-flight oxygen. If there are no concerns about hypercapnia it may be reasonable to recommend 2 L/min without recourse to HCT. In those in whom there are concerns about CO2 retention, titration HCT is advised to determine the oxygen flow rate.
Thoracic surgery
The opinion of the surgeon or interventionalist should be obtained before the patient travels by air. Patients, professionals and their carers should be aware that this may result in a delay of 4 weeks for non-essential air travel and 2 weeks for essential air travel.
Careful clinical assessment of the patient is required. This should include consideration of their baseline status including comorbidities, SpO2, postprocedure complications such as infection and/or pain, flight duration and destination.
Other interventional procedures
The opinion of the interventionalist should be obtained before the patient travels by air.
Careful clinical assessment of the patient is required. This should include consideration of baseline status including co-morbidities, SpO2, postprocedure complications such as infection or pain, flight duration and destination.
Patients with no pneumothorax seen on the postprocedure chest X-ray should wait for 1 week before air travel.
Patients with a pneumothorax seen on the post-procedure chest X-ray should wait for one1 week after resolution on chest X-ray before air travel.
Trapped lung
Patients should be assessed carefully and advised on a case-by-case basis.
Patients should be clinically stable before air travel.
Bronchoscopic procedures
Patients should be clinically stable before they travel.
After interventional bronchoscopy including Transbronchial Needle Aspiration (TBNA), Transbronchial Lung Biopsy (TBB), Endobronchial Ultrasound Bronchoscopy (EBUS) and endobronchial valve insertion, those with no pneumothorax seen on the postprocedure chest X-ray should wait for 1 week before air travel.
After interventional bronchoscopy including TBNA, TBB and EBUS, those with a pneumothorax seen on the post-procedure chest X-ray should wait for 1 week after resolution on chest X-ray before air travel.
Pneumothorax
Passengers should not travel by air until 7 days after full resolution on chest X-ray.
Those at higher risk of recurrent pneumothorax should be advised accordingly.
Higher-risk groups, including those with cystic lung disease such as lymphangioleiomyomatosis (LAM) and Birt-Hogg-Dubé (BHD) syndrome, should be advised accordingly.
Patients with trapped lung and a chronic air space thought to present a low risk should be evaluated in secondary care before travel.
Upper respiratory infection including otitis media and sinusitis
In passengers who develop sinus barotrauma after flying, it may be helpful to consider topical and oral decongestants as well as appropriate analgesia. Prolonged use of decongestants is not advised owing to the risk of rebound congestion on withdrawal.
If there is an allergic component, intranasal steroids used for a week prior to travel, and/or oral corticosteroids may be considered.
Symptoms and signs of barotrauma should have resolved before flying again. This usually takes between 1 and 6 weeks.
After an episode of acute otitis media, patients are usually advised not to fly for 2 weeks.
Viral infections
Patients with highly contagious infections including measles, chickenpox, mumps, SARS, Middle East respiratory syndrome (MERS) or COVID-19 should not be allowed to travel until they are considered non-infectious.
Passengers should familiarise themselves with current national and international regulations regarding air travel, which should always be observed.
Tuberculosis
Smear positive patients must not fly until they have provided two smear negative samples on treatment.
Those starting treatment for pulmonary tuberculosis (TB), where not all the information is yet available, should not travel by air for the first 2 weeks.
For those who are smear negative and have a fully sensitive organism, treatment would be expected to render them non-infectious after 2 weeks.
For patients with multidrug resistant/extensive drug resistant (MDR/XDR) TB, travel is prohibited until two negative culture samples have been produced and there is clinical evidence of improvement on treatment.
Extrapulmonary TB does not usually warrant additional precautions before air travel.
All but essential travel should be postponed for 7 days in those who have reduced baseline sea level SpO2 (<94%).
Obstructive Sleep Apnoea (OSAS) and Obesity Hypoventilation Syndrome (OHS)
Daytime flights are advised wherever possible.
The patient should be advised to carry their continuous positive airway pressure (CPAP) device as hand luggage, and a hospital letter to advise that the patient uses CPAP.
Careful planning and preparation are required, and use of the patient’s own CPAP device is advised.
Alcohol and sedatives should be avoided in the 12 hours before, and during, airline travel.
Patients should use their CPAP device on board if they are travelling overnight, and avoid sleeping during daytime flights.
Consideration should be given to device settings and whether adjustment is required for operation at altitude.
Airline approval for carriage and use of device, including battery specification, must be gained before travel.
Consideration should be given to the whole journey. If driving is required the following day, an overnight stay at destination may be advisable. Patients are advised to refrain from driving if tired and sleepy.

Respiratory muscle and chest wall disorders
HCT is recommended for all adult patients with FVC <1 L, pending further data, and may be considered in others thought to be at particular risk, including children with reduced FVC due to respiratory muscle or chest wall disorders.
If patients are unable to perform spirometry reliably, a walk test may be considered as an alternative.
Patients should be advised to take daytime flights where possible.
Further planning and support are required for those established on non-invasive ventilation (NIV) (see Appendix A). ( online supplemental appendix 2
Prevention of VTE during air travel
See table 1 .
- View inline
Summary of risk factors for VTE during air travel
Limit the risk of dehydration with adequate fluid intake.
Avoid alcohol.
Keep mobile, if possible, by walking around or doing seat-based exercises once an hour.
Consider graduated compression stockings (class 1 with 15–30 mm Hg).
Low molecular weight heparin (LMWH) or a Direct Acting Oral Anticoagulant (DOAC) are advised for both outward and return long haul flights (long haul defined as flights of 6–12 hours) in high-risk patients including those with a history of VTE; local policy should be followed regarding liaison with primary care and/or haematology services to teach the patient how to administer the injection and dispose safely of the equipment. There is no formally recommended dose, but enoxaparin at a dose of 40 mg or weight based 1 mg/kg injected once 4–5 hours before the flight has been suggested.
The prophylactic doses of the DOAC may also be used.
All patients with a recent (<6 weeks) history of VTE, especially any who presented with significant right ventricular strain and decompensation should be reassessed before air travel.
Air travel after VTE
Air travel should be delayed for 2 weeks after a diagnosis of DVT or pulmonary embolism (PE).
Pulmonary hypertension
Those in New York Heart Association (NYHA) WHO functional class 3 or 4 are usually advised to have in-flight oxygen. If there is no evidence of hypercapnia it seems reasonable to suggest 2 L/min by nasal cannulae. If there are concerns about hypercapnia, HCT should be considered if available.
Those eligible for LTOT (sea level PaO 2 <8 kPa at rest on air) should have in flight oxygen at double the flow rate recommended at sea level, provided there is no evidence of hypercapnia.
Lung cancer and mesothelioma
Patients undergoing chemotherapy should not travel while they are at increased risk of infection or suffering from significant side effects, such as vomiting.
Hyperventilation and DB
Patients with DB, inducible laryngeal obstruction (ILO) and/or vocal cord dysfunction (VCD) should be referred to a respiratory physiotherapy specialist for advice on symptom management before travel.
Those with anxiety disorders should be reviewed before travel; compliance with medication assessed; and use of short acting anxiolytics encouraged.
Other life-threatening conditions presenting with dyspnoea should be excluded on board as far as possible.
Supplemental oxygen should be given on board if the cause of breathlessness is unclear
Rebreathing via a paper bag is not recommended.
HCT outcomes
Preflight respiratory screening.
Medical incidents have been reported in around 1 in 600 flights, 10 or 1 in 30 000 passengers. 11 12 Estimates vary and reliable data are difficult to obtain, but respiratory events account for around 12% of in-flight incidents. In a recent study of 1260 healthy volunteers, no significant changes occurred in pulse oximetry (SpO 2 ) during a simulated 8-hour flight at cabin altitudes up to 2438 m (8000 ft). 13 However, if cabin altitude exceeds 3048 m (10 000 ft), hypoxaemia becomes more prominent and SaO 2 falls to∼89% in healthy individuals. 14 Other potential hazards for passengers with respiratory conditions include low relative humidity, and altitude-related expansion of gases within enclosed pulmonary parenchymal spaces. It follows from Boyle’s Law that a cabin altitude of 2438 m (8000 ft) will result in a 38% expansion of humidified gas.
There is no good-quality evidence to determine who should have a formal respiratory review before air travel. Experts generally advise preassessment or screening for the following adults, children and infants:
Those with a respiratory condition with the potential to deteriorate acutely resulting in incapacitation and/or the need for medical intervention. This includes (but is not exclusive to):
Severe (FEV1 <50% predicted 15 or poorly controlled obstructive airway disease (evidenced by symptoms, oxygen requirements, severe and/or frequent exacerbations).
Symptomatic restrictive lung or chest wall conditions, or known respiratory muscle weakness causing breathlessness and exercise limitation.
Comorbid conditions which may be worsened by hypoxaemia (cerebrovascular or cardiac disease).
Recent (<6 weeks) hospital treatment for a respiratory condition.
Requirement for CPAP or ventilator support such as NIV.
Active cancer with lung involvement.
Patients requiring domiciliary oxygen.
Recent (<6 weeks) pneumothorax and those at higher risk of pneumothorax (cystic lung disease or recurrent pneumothorax), and patients with trapped lung and a chronic air space.
Recent (<6 weeks) pulmonary embolus or deep venous thrombosis, or increased risk of VTE.
Anyone who has experienced significant symptoms during previous air travel, or whose condition is of concern to their physician.
The following are generally considered contraindications to air travel:
Untreated respiratory failure.
Untreated pneumothorax.
Active infection representing a risk to others for example, TB, SARS, MERS, COVID-19.
Bronchogenic cysts. Cerebral air embolism, in some cases fatal, has been reported in aircraft passengers after rupture of a bronchogenic cyst. 16
Patients with severe hypoxaemia requiring >4 L/min in-flight oxygen were previously advised against air travel, because 4 L/min was the maximum fixed flow rate routinely available on commercial aircraft. With the availability of flight approved POCs delivering a range of continuous and intermittent flow rates, this cut-off no longer applies. In-flight oxygen delivery is more varied, and maximum flow rate is determined by the equipment available. Pulse-dose delivery systems can however complicate determination of the flow delivered and may not be well tolerated. The effects of mouth-breathing, speech, snoring and/or sleeping should be considered. High-flow nasal oxygen (HFNO) cannot be delivered on board commercial aircraft.
In-flight oxygen may be contraindicated in adults and children with a history of type 2 respiratory failure. 17 18 Hypoxic challenge with arterial carbon dioxide tension (PaCO 2 ) measurement was advised for this group in 1996 17 but there has been little research since. This document, therefore, follows the 2015 BTS Guideline for Home Oxygen Use in Adults 19 when making recommendations for managing patients with previously documented hypercapnia.
Clinical practice points
Number and duration of flights, including whether daytime or overnight.
Location of stop-over(s) and destination: these determine air quality, altitude and available medical facilities.
Time away from home.
Further assessment by a Respiratory Specialist is advised for those in whom screening raises concerns, and hypoxic challenge testing may be advised.
Infants and children
In general, similar considerations apply to both adults and children if they have severe chronic airway disease, or require chronic supplementary oxygen, or non-invasive or tracheostomy ventilation. Both children and adults with these conditions require a preflight assessment. Similarly, unless otherwise stated, recommendations for individuals with previous thoracic surgery, pneumothorax or empyema apply to both adults and children. There are, however, some specific considerations for infants and younger children since several factors place infants at greater risk of developing hypoxia. These factors include left shift of the oxygen dissociation curve (due to the presence of foetal haemoglobin), smaller airway diameter, relatively fewer alveoli, compliant rib cage and increased tendency to pulmonary vasoconstriction and bronchoconstriction and thus ventilation–perfusion mismatch under hypoxic conditions. Moreover, preterm infants and infants under 2 months of age may develop apnoea/hypoventilation in response to hypoxia or infection. 20 21 Beyond 3 months of age there is no evidence that ex-preterm infants, without bronchopulmonary dysplasia, are at significantly greater risk of desaturation during a HCT than term infants. 22
In addition to very young and ex-preterm infants, the children most at risk of hypoxia are those with anaemia, congenital heart disease with an actual or potential right to left shunt, 23 neuromuscular disorders or chronic or acute lung disease. Low humidity during air travel can also present a problem for children with respiratory conditions such as CF. Those most at risk of complications associated with reduced air pressure are children with upper respiratory tract infections, or trapped intrathoracic air, including those with recent pneumothorax or cystic lung disease. 24
For infants born at term (>37 weeks) it is prudent to delay flying for 1 week after birth to ensure they are healthy. 25 In view of their greater risk of apnoea and hypoxia, infants born prematurely (<37 weeks) with or without a history of respiratory disease who have not reached their expected date of delivery at the time of flying should have in-flight oxygen available. HCT may not be a reliable guide of oxygen requirement in this group. 26 If air travel is essential, they should travel with oxygen at a tolerable low flow, recognising that this may be a minimum of 1 L/min depending on equipment.
The following Clinical Practice Points are specific to infants and children.
Infants born prematurely (<37 weeks) with or without a history of respiratory disease who have not reached their expected date of delivery at the time of flying should have in-flight oxygen available. HCT may not be a reliable guide of oxygen requirement in this group. If air travel is essential, they should travel with oxygen at a tolerable low flow rate, recognising that this may be a minimum of 1 L/min depending on equipment.
In children with chronic lung disease able to perform spirometry whose FEV 1 is consistently <50% predicted, HCT should be considered. This includes children with CF and PCD. Children with chronic lung disease who are too young to perform spirometry reliably should have a clinical assessment of disease severity and their likely tolerance of hypoxia. In children with CF the disease is rarely severe enough to compromise lung function significantly at this age.
Pulse oximetry is the easiest and usually the first screening test. 4 It has generally been accepted in the past that those with resting SpO 2 >95% at sea level should not require in-flight oxygen. 2 25 27–30 Spirometry results may already be available in patients with known acute or chronic lung disease, or with symptoms suggesting lung disease. 31 32 However, lung function parameters are in many cases poor at predicting hypoxaemia or complications. 28 33–35
Many airlines have historically considered that those able to walk 50 m or climb up 10–12 steps without distress have sufficient cardiopulmonary reserve to fly. 2 36 The role of the 6MWT in preflight evaluation, widely used to assess functional capacity and exercise-induced hypoxaemia in COPD 37–40 and ILD including IPF, 41–43 has also been examined. Current data suggest that the 50 m walk test is an insensitive assessment of ‘fitness to fly’ 38 44 45 although still sometimes referenced. 36 46 47 Several studies show no correlation between walking distance and HCT outcome in patients with COPD, ILD or extrapulmonary restriction. 38 44 45 48 One study showed no correlation between exertional dyspnoea and HCT outcome. 38 The 50 m walk test alone thus appears unsuitable for preflight assessment.
The 6MWT and externally paced incremental SWT may be of value. Baseline values do not reliably predict in-flight hypoxaemia in a number of respiratory conditions 1 4 33 34 44 49–51 but changes in SpO 2 during 6MWT and SWT may correlate with HCT outcome in COPD, ILD and chest wall deformity. 30 38 44 45 Walk tests cannot predict the in-flight oxygen flow rate required, but they may help inform the decision as to who needs further assessment.
A walk test is not always practical. Data from one small study in COPD suggest that MRC scores may help predict the likelihood of exercise desaturation. 52 From this it appears that patients with COPD, MRC score 1 or 2 and resting oxygen saturations >95% do not usually need further testing before air travel. If there are still concerns, a walk test may help decide whether HCT is required. In those with COPD who do undergo 6MWT or SWT and do not desaturate below 84%, in flight oxygen should not be required and they should not need HCT.
If resting oxygen saturations are SpO2 92%–95% and they desaturate <84% but have no evidence of CO 2 retention, data from Edvardsen et al 30 suggest it is reasonable to recommend in-flight oxygen at 2 L/min without proceeding to HCT. Patients in whom there are concerns about hypercapnia should proceed to HCT.
Data are much more limited in restrictive disease, including ILD, and baseline SpO2 does not appear to predict outcome. In general, it seems reasonable to suggest that if baseline saturations are >95% at rest and there is no desaturation below 95% on 6MWT or SWT, HCT should not be required. Those with ILD and TLco ≤50% of predicted and PaO 2 ≤9.42 kPa are likely to need in-flight oxygen or HCT. If there are no concerns about hypercapnia it may be reasonable to recommend 2 L/min without recourse to HCT. If there are concerns about CO 2 retention, titration HCT will be required to determine the oxygen flow rate.
Hypoxic challenge testing
HCT is performed using an inspired gas mixture containing 15% oxygen, which gives an approximate similar inspired oxygen tension (PO 2 ) to breathing air at the maximum allowable cabin pressure altitude (2438 m or 8000 ft). 53 54 HCT is usually performed in a specialist respiratory physiology unit. The provision of a 15% oxygen gas mixture can be achieved using one of the methods described in online supplemental appendix B .
The closest approximation to aircraft cabin conditions entails exposure to simulated altitude in a hypobaric chamber, but such chambers are not available for clinical assessment. A reasonable substitute is the normobaric HCT, described by Gong et al 55 in patients with chronic airflow obstruction. This assesses the response to hypoxaemia achieved by breathing a hypoxic gas mixture at sea level. Various methods of hypoxic gas delivery produce equivalent results to tests in a hypobaric chamber or during real flights in adults with COPD. 1 39 56–58 Data are limited in other conditions as well as for children and neonates.
The HCT is used to help decide whether passengers with respiratory disease need in-flight oxygen and at what flow rate. It does not assess fitness for air travel, despite its reputation as a ‘fitness to fly’ test. If healthcare providers give this impression in patient information, they must manage patient and carer expectations accordingly. A ‘preflight oxygen test’ is a more accurate description. Most patients do not require HCT as part of preflight medical assessment, and there should not be pressure on physicians to arrange, or healthcare professionals to perform, unnecessary HCT.
The physiological response to hypobaric hypoxia (PaO 2 <8 kPa) is increased ventilation. 59 Alterations in respiratory pattern may adversely impact on lung mechanics, 60 which may be further impaired by gas expansion, reducing vital capacity and increasing residual volume. 61 The increase in ventilatory drive is likely to be limited on commercial flights, 62 but a modest increase in ventilation can exhaust an already reduced ventilatory reserve. 16 60 63
The usual consensus is to recommend in-flight oxygen if PaO 2 is predicted to fall below 6.6 kPa (50 mm Hg) or SpO2 below 85% in flight. There is little high-quality evidence supporting these cut-off values, but this PaO 2 value ensures that SpO2 remains above the steep portion of the oxyhaemoglobin dissociation curve. 64 Some authors consider 6.6 kPa to represent the lower safe limit for hypoxaemia, 65 66 as PVR increases sharply in response to arterial pO2 below this level, 67 with the potential for an acute increase in right ventricle afterload and right ventricular dysfunction. 16 29 As many patients with COPD have cardiac comorbidity, 68 hypoxaemia in these patients could precipitate cardiac ischaemia; this is unlikely in those with stable disease in NYHA functional class I or II (no or mild limitation of physical activity). 69 , 70 In the absence of new evidence to the contrary, the cut-off PaO 2 of 6.6 kPa during HCT appears reasonable.
HCT outcomes do not predict respiratory symptoms during air travel. 71 , 72 , 73 Such symptoms do not appear to result directly from hypoxaemia, 62 but from a combination of poor respiratory mechanics and reduced respiratory reserve impairing the response to hypoxaemia. Symptoms are more likely to occur in those with more severe breathlessness at sea level. 4 72 Limited evidence suggests that those who desaturate during HCT and have previously experienced respiratory symptoms during air travel can avoid these by using in-flight oxygen. 29 71 Symptoms may also result from anxiety regarding air travel (see section on hyperventilation and DB).
Those with stable respiratory disease without history of air travel intolerance, normal resting and exercise SpO2 at sea level and no significant cardiac comorbidity, are unlikely to need in-flight oxygen and should not require HCT. Those who have had HCT in the past should not need it repeated unless their clinical condition has changed. The patient’s plans should, however, be discussed with the patient’s respiratory physician, paediatrician or specialist nurse.
Those already using LTOT will need in-flight oxygen. Ideally, the flow rate required at cruising altitude should be determined using HCT. If HCT is not readily available and there are no concerns about hypercapnia, passengers already on LTOT should be advised that they will need a flow rate 2 L/min greater than their baseline flow rate. This should be sufficient to compensate for the relative hypoxia at normal cabin altitude. However, current POCs do not routinely offer continuous flow rates above 3 L/min, and a pulse-dose delivery mode at higher levels may not always be suitable. 74
As noted above, it is not practical for all patients with COPD who want to fly to undergo 6MWT. Respiratory physicians may however wish to consider 6MWT if there has been a significant change in the patient’s condition since the last assessment, or in new patients previously unknown to the service. Those who desaturate below 84% may then be referred for HCT at the discretion of the respiratory physician.
Some data are available in smaller numbers of patients with restrictive lung disease, but there is currently no consensus regarding the best walk test or cut-off values. In a study of 14 patients with primary thoracic scoliosis, Bandyopadhyay et al found that resting SpO2 >95% did not accurately identify those who do not desaturate during HCT, and recommend a low threshold for performing HCT on patients with thoracic scoliosis. 44 Likewise, in a study of 13 patients with OHS, baseline SpO2 did not predict HCT outcome. 49 In a study including 42 patients with ILD and 20 with extra-pulmonary restriction 35 before and after ‘2 min of moderate exercise’, Ling et al proposed that a postexercise SpO2 of no less than 95% could be used to exclude the need for HCT. Further research is required to determine the most appropriate assessments for patients with a variety of restrictive lung diseases, including which (if any) can reliably eliminate the need for HCT.
Clinical practice points: patient selection for HCT
See figures 1 and 2
Patients with COPD with baseline SpO2 ≥95% and either MRC score 1–2 or desaturation to no less than 84% during 6MWT or SWT, should be able to travel without in-flight oxygen.
HCT should be considered for the following
Patients with COPD with resting SpO 2 ≤95%, MRC score 3 or greater, or desaturation to <84% on 6MWT or SWT, and in whom there are concerns about hypercapnia
Infants and children with a history of neonatal respiratory problems, or existing severe chronic lung disease including those with FEV1 persistently <50% predicted (see page 7).
Adults and children with severe asthma, evidenced by persistent symptoms and/or frequent exacerbations despite optimal treatment (see BTS/SIGN Asthma Guideline 75 ) regardless of resting sea level SpO 2 .
Patients with ILD in whom SpO 2 falls to <95% on exercise, and whose resting sea level PaO 2 is ≤9.42 kPa or whose TLCO is ≤50%.
Those with severe respiratory muscle weakness or chest wall deformity in whom FVC is <1 L.
Patients with a history of type 2 respiratory failure already on LTOT at sea level. However, if there is no evidence of hypercapnia, it seems reasonable to recommend an increase in flow rate by 2 L/min in-flight, provided the equipment can provide it (see Appendix A).
Clinical practice points: HCT results
Air travel may be contraindicated in infrequent cases when supplementary oxygen, at the flow rate needed to maintain PaO 2 ≥6.6 kPa or SpO 2 ≥85%, causes significant changes to pH and pCO 2 . The 2015 BTS Guidelines for Home Oxygen Use in Adults 19 consider that a pH <7.35, and a PaCO 2 increase >1 kPa from baseline (within 20 min) is significant. Specialist respiratory physicians should use their discretion to determine the risk in individual cases and advise accordingly. Where hyperventilation is suspected, especially in response to anxiety rather than hypoxaemia, results should be interpreted with caution as there is a risk of false negative results. 76
HCT methods
These are described in Appendix B.
Exertion on board
Studies in adults with COPD 33 77 78 or CF 79 have shown that patients can develop profound hypoxaemia when exercising under hypoxic conditions, whether on board a commercial aircraft at cruising altitude or during HCT. This has been reported in patients whose HCT results would otherwise not warrant oxygen. In some cases, PaO 2 values as low as 3.9 kPa have been recorded. 33 78
The combination of further hypoxaemia and increased ventilatory demand from exertion while flying may challenge those already approaching the limits of their respiratory reserve. It therefore seems prudent to recommend that passengers with significant respiratory limitation, regardless of whether they travel with in-flight oxygen, should request an aisle seat near a toilet to avoid long periods of walking. 1 29 80 Passengers should keep active by undertaking seat-based exercises and/or standing at intervals if flight conditions permit.
Patients who cannot tolerate withdrawal of supplemental oxygen for even a short period of time should not travel by air, as there will be periods of time when oxygen cannot be supplied. POC use below 10 000 ft may in some circumstances be prohibited by cabin crew. The reduction in cabin pressure between an aircraft taking off and reaching 10 000 ft is small (10%) and unlikely to have any clinical impact on those who do not usually require oxygen at rest at sea level. Aircraft descent may however take longer than ascent, and the time to landing may be less predictable.
Disease/condition-specific advice
Chronic airflow obstruction including asthma and copd.
Most adults and children with well-controlled mild or moderate airflow obstruction and no other co-morbidities should have no problem with commercial air travel, but they should be prepared for the possibility of an exacerbation of their condition. Air travel presents a theoretical risk of bronchospasm because of mucosal water loss due to low cabin humidity.
Cigarette smokers are at a physiological disadvantage during exposure to altitude. 81 Every opportunity should be taken, when reviewing travel plans, to take a smoking history and offer brief intervention and smoking cessation referral as appropriate.
While asthma is prevalent and has the potential to be life-threatening, most episodes are not. 82 83
Most passengers with asthma will have relatively mild disease and do not require HCT. HCT should however be considered for those with severe asthma, regardless of baseline sea level oxygen saturation. In a retrospective study of 37 adults with severe asthma (as defined in the BTS/SIGN Asthma guideline 75 ) undergoing HCT, two-thirds who fulfilled the criteria for in-flight oxygen on HCT had baseline sea level oxygen saturations of >95%. 84
The role of HCT has not been studied in children with severe stable asthma. A study in 51 children aged 2–12 years requiring transient oxygen therapy during an acute asthma attack (SpO2 <92%) showed that although 5% failed HCT within 24 hours of discontinuing oxygen therapy, all passed the HCT when retested at 48 hours. 85
Food allergy affects up to 8.5% of children and adults with asthma, 86 and asthma is a risk factor for severe or fatal anaphylaxis. 87 Appropriate precautions for those affected include wiping tray tables and hands, informing the airline beforehand and the cabin crew of allergies, and not eating during flights or bringing known ‘safe’ foods from home 88
Passengers with severe asthma are advised to carry copies of their asthma management plan and/or relevant clinic letters. Information can be held securely as scanned copies on a mobile phone, or on a digital platform such as the NHS App.
Patients with COPD planning air travel need careful evaluation, not only because of their respiratory disease, but also because of their high levels of comorbidity. 71 89
Respiratory symptoms in those with COPD are common during air travel, but Edvardsen et al have shown that HCT does not predict respiratory symptoms during air travel in patients with moderate to very severe COPD. 71 They suggest that exacerbation of comorbidities such as cardiovascular disease (the most common cause of death in COPD) is the most threatening consequence of severe hypoxaemia. This is consistent with data showing a risk of cardiac arrhythmias and ischaemic chest pain in patients with COPD unable to respond to the physiological stressors of air travel. 55 70 Work by Robson et al shows that resting sea level saturations alone do not predict HCT outcome. 28
Spirometry does not reliably predict hypoxaemia or complications in COPD. 29 It seems prudent to avoid air travel within 6 weeks of an exacerbation although there are few data to support this recommendation. Patients with COPD are at greater risk of VTE as a direct consequence of the underlying condition, as well as after an exacerbation. They should be advised accordingly, especially if planning longer flights when the risk is further enhanced (see section on VTE). 90–92 See Figure 1.
CF (adults and children)
The risks associated with air travel are greater for those with CF than for healthy individuals. 93 This is despite the fact that people with CF have been shown to tolerate PaO 2 values below 6.6 kPa (50 mm Hg) for several hours without cardiac decompensation or cerebral symptoms 94 ; do not usually have cardiovascular comorbidities; and are generally younger than patients with other respiratory conditions. Hypoxaemia results mainly from ventilation/perfusion mismatch attributable to chronic inflammation and mucus plugging. It is not clear which physiological values measured at sea level best predict hypoxaemia or complications during flight.
In 1 study of 30 adults with CF undergoing HCT, four fulfilled the study’s criteria for supplemental oxygen (PaO 2 <6.6 kPa) at rest and a further 11 dropped below this threshold while walking slowly. Variables obtained during CPET (including SpO 2 and PaO 2 ) showed a stronger correlation with arterial oxygen tension (Pao 2 ) during HCT than baseline SpO 2 or spirometry. 79 However, in children with CF the sensitivity and specificity of preflight HCT have been reported as 20% and 99% (using a cut-off of SpO 2 <90% during HCT with FiO2 0.15), compared with 70% and 96% for spirometry (cut-off FEV1 <50% predicted). 95 Combining spirometry and HCT increased sensitivity to 80%. Spirometry may, therefore, usefully predict who may desaturate during flight, and a cut-off of FEV1 50% has been used to recommend HCT.
Passengers with CF should practise good hand hygiene using soap and water or an alcohol-based hand gel, and avoid touching their face, particularly after touching arm rests, food trays or toilet doors to minimise risk of infection. These measures are included within recommendations from the European Centres of Reference Network for Cystic Fibrosis project, endorsed by the European Cystic Fibrosis Society. 93 These also advise checking the relevant airline policy and levels of CF healthcare provision at the proposed destination before travel (see online supplemental appendix 1 ).
Passengers with bronchiectasis should not necessarily be discouraged from flying, but air travel can pose challenges.
Portable nebulisers and PEP devices may be considered, but use of these devices in-flight must be approved by the airline before travel.
Like individuals with airflow limitation, patients with ILD, including pulmonary fibrosis, respond to hypoxaemia at altitude with increased heart rate and minute ventilation. In severe disease the ability to increase minute ventilation is limited and the resulting hypoxaemia may be marked. However, unlike COPD, where many patients appear to be able to tolerate marked hypoxia, 65 patients with ILD may have acute or subacute disease and be less able to withstand marked hypoxia. There are fewer relevant studies available in ILD, and patient numbers are smaller than in COPD studies.
Two studies in patients with ILD (n=15 and 10, respectively) have shown that sea level oxygen saturations do not reliably predict HCT outcome, and that oxygen saturations fall significantly after light exercise performed under conditions of normobaric hypoxia. 73 96 These findings are consistent with those from the UK Flight Outcomes Study, 4 a prospective observational study of 431 patients including 186 with ILD. This showed that neither FEV1 nor sea level SpO 2 reliably predict desaturation at altitude, and that patients with ILD were more likely than others to require unscheduled healthcare for respiratory events within 4 weeks of air travel.
In a study including 15 patients with ILD, Martin et al found that predictive equations overestimated the need for in-flight oxygen in patients with ILD, as they did for those with COPD and CF. 97
More recently, Barratt et al examined the predictive value of various parameters for HCT outcome in 106 ILD patients (69 with IPF). 98 Only the combined parameters of TLCO >50% predicted and sea level PaO 2 >9.42 kPa independently predicted a successful HCT outcome. From analysis of a subset of 88 patients with a complete dataset available the authors propose a new prelight algorithm for patients with ILD with a sensitivity of 86% and specificity of 84%. In patients with both sea level PaO 2 ≤9.42 kPa and TLCO ≤50% predicted, in-flight oxygen is recommended without recourse to an initial diagnostic HCT. HCT for titration of the oxygen flow rate required on board is still advised. For patients in whom either TLCO ≤50% or PaO 2 ≤9.42 kPa, diagnostic HCT is advised. This promising approach requires further validation in a larger, prospective cohort of patients with ILD, preferably supported by patient reported outcomes from actual flight(s).
In patients with comorbidity, including PH and/or cardiovascular disease, attention should also be paid to the impact of air travel on these conditions.
Physicians may wish to consider HCT in those whom SpO2 falls to <95% on exercise, and/or in those in whom either TLCO ≤50% or PaO 2 ≤9.42 kPa (if available).
Patients with TLco <50% of predicted or PaO 2 ≤9.42 kPa are likely to need in-flight oxygen. If there are no concerns about hypercapnia it may be reasonable to recommend 2 L/min without recourse to HCT. In those in whom there are concerns about CO2 retention, titration HCT is advised to determine the oxygen flow rate.
Thoracic surgery and other interventional procedures
There is no high-quality evidence in this area and further research and/or data collection are needed. The following are suggested time periods before which a medically unaccompanied commercial flight can safely be undertaken after the specific thoracic interventions described below. The advice is conservative. Shorter recovery periods may be appropriate in individual cases, but only if approved by the doctor/surgeon carrying out the procedure. It is also important to note that the potential risks of travel are not just those associated with a postprocedure pneumothorax, but include wound infection and pain, which could require medical attention at destination and would need approval by the travel insurer.
Thoracic surgery, including VATS procedures
In the absence of published evidence, we advocate a conservative and safe minimum time interval, with the caveat that flying sooner after such procedures may be possible and/or desirable, but that this should be agreed with the surgeon and discussed with the airline. At least two UK centres independently advise against non-essential air travel for 4 weeks after removal of drains (Jon Naylor, personal communication). If air travel is essential, a minimum delay of 2 weeks is advised, depending on the type of surgery and the surgeon’s advice.
The opinion of the surgeon or interventionalist should be obtained before the patient travels by air. Patients, professionals, and their carers should be aware that this may result in a delay of 4 weeks for non-essential air travel and 2 weeks for essential air travel.
Percutaneous lung biopsy, pleural procedures (including thoracocentesis, medical thoracoscopy and insertion of indwelling pleural catheter)
A North American study of 179 patients, who between them underwent 183 percutaneous transthoracic needle biopsies, suggested that air travel was safe within 24 hours of procedure, even in the 65 patients (35%) who developed a small, stable postbiopsy pneumothorax. 99 Fifty (77%) of them flew within 4 days of the final postbiopsy chest radiograph. During a brief telephone survey after the flight, 14 (8%) reported worsening of pre-existing respiratory symptoms or new respiratory symptoms. There were no reported events requiring in-flight medical attention or flight diversion. These were, however short, internal North American flights over land, where diversion is relatively straightforward if required. The situation for a UK-based patient travelling on a long-haul flight to the Middle East, USA, Far East or Australasia is quite different.
Careful clinical assessment of the patient is required. This should include consideration of baseline status including comorbidities, SpO2, postprocedure complications such as infection or pain, flight duration and destination.
Patients with a pneumothorax seen on the postprocedure chest X-ray should wait for 1 week after resolution on chest X-ray before air travel.
‘Trapped lung’ after drainage of pleural space
Only very limited data are available, from a report of two patients with a small chronic pneumothorax. 100 This suggests that such patients may be able to travel safely by air, but require thorough clinical assessment, CT imaging and HCT as a minimum beforehand.
The risks associated with air travel are not only those of a possible pneumothorax, but also the effects of sedation, exacerbation of pre-existing or new symptoms such as cough, hoarse voice haemoptysis and dyspnoea, respiratory infection and the consequences of arrhythmias observed during the procedure.
After interventional bronchoscopy including TBNA, TBB, EBUS and endobronchial valve insertion, those with no pneumothorax seen on the postprocedure chest X-ray should wait for 1 week before air travel.
After interventional bronchoscopy including TBNA, TBB and EBUS, those with a pneumothorax seen on the postprocedure chest X-ray should wait for 1 week after resolution on chest X-ray before air travel.
Pleural disease
Pleural effusion.
Patients with stable pleural disease and normal resting oxygen saturations should be able to fly without further precautions. Those who have indwelling long-term drainage catheters should be reminded that the manufacturers do not advise air travel. Those who choose to travel should be encouraged to take a supply of drainage bottles for their time away.
In those with a recent onset pleural effusion, investigation should be delayed if air travel is planned within 2 weeks, since intervention may increase the risk of pneumothorax. The risk of delaying investigation should be discussed with the individual to determine whether travel plans can be modified.
The prevalence of in-flight pneumothorax in passengers with existing lung disease appears low overall, being zero in the UK Flight Outcomes Study. 4 It increases, however, in those at increased risk: 3.6% in a study of 276 patients with LAM 101 ; and 9% within 1 month of air travel in a retrospective survey of 145 patients with BHD syndrome. 102
Most individuals with an untreated, closed pneumothorax should not travel by air. In exceptional cases where the pneumothorax is long-standing and thought to present a low risk, secondary care evaluation is strongly advised before travel.
In individuals with a treated pneumothorax, exposure to altitude poses a risk of recurrence. The 2010 BTS Pleural Disease guidelines state that patients ‘…should be cautioned against commercial flights … until full resolution of the pneumothorax has been confirmed by a chest X-ray”. 103 These guidelines state that patients should wait a week after pneumothorax resolution before flying. There is limited, more recent evidence to suggest that in the case of traumatic pneumothorax, air travel as early as 72 hours after chest drain removal with full lung inflation may be safe. 104 A prospective observational study of 20 patients with a small residual traumatic pneumothorax, exposed to hypobaric hypoxia for 2 hours suggested no significant clinical effects despite expansion of up to 171%. 105 The data should, however, be interpreted with caution given the small numbers involved, the small size of the pneumothorax in each case, and the limited duration of hypobaric exposure. They are not sufficiently robust to justify overriding current BTS guidance.
In those who have undergone thoracotomy and surgical pleurodesis, the recurrence rate is so low that no subsequent restriction on travel is necessary. 1 The recurrence rate has been reported to be four times greater after video-assisted thoracoscopy, 106 suggesting that this procedure may not be as definitive. The risk of recurrence is greatest in those with pre-existing lung disease, cigarette smokers and taller men. 1
Higher-risk groups, including those with cystic lung disease such as LAM and BHD syndrome, should be advised accordingly.
Respiratory tract infections
During air travel with acute infection of the upper airway, the main risks are unpredictable, but may reflect previous experience. They are of pain and potential rupture of the tympanic membrane. Those with significant symptomatic viral upper respiratory tract infection may wish to delay travel because of the risk of pain and disseminating infection to others.
Barotrauma, characterised by otalgia, is a consequence of inability to equilibrate the pressure differential between the external and middle ear. This is usually more severe during landing than take-off. Most passengers, including older children, can equilibrate the pressure through yawning, swallowing, chewing or a Valsalva manoeuvre (eg, pinching the nose and blowing). Infants and young children may be unable to perform these manoeuvres, but swallowing may be encouraged by drinking. It is also more frequent when the child is awake and/or crying.
It has been estimated that 10% of adults and 22% of children may have changes to the ear drum after a flight, although perforation is rare and symptoms usually resolve spontaneously. 107 108
Historically, oral (and to a lesser extent topical) decongestants have been recommended for adults with risk factors for sinus or middle ear barotrauma. 1 The evidence is very weak, and there is no evidence in children. Treatment with intranasal steroids (commenced at least a week before the flight) can however improve symptoms, as for inflammatory rhinosinusitis. After an episode of acute otitis media, patients are usually advised not to fly for 2 weeks 107
Although viral infections may be transmitted on board, as in any environment where people are in proximity for prolonged periods, available data suggest this is not common on modern commercial aircraft. This may reflect lack of face-to-face contact, the barriers afforded by seat backs, and the characteristics of cabin airflow on board, which is not front to back. Viruses are within the particle size range captured by HEPA filters on modern commercial aircraft, which are like those used in hospitals. Transmission by droplet spread, including via fomites, is applicable to all environments. 100 101 This may be reduced by passengers wearing masks, frequent use of hand sanitiser and disinfectant wipes for hard surfaces, and by regular deep cleaning of the aircraft cabin. The risk of infection in airport facilities on departure, during stopovers, and on arrival should also be considered. More general hygiene practices, such as handwashing and covering the mouth and nose when coughing or sneezing, have also been shown to reduce spread of viral infections. 109 110
Some respiratory viral infections may be more infectious than others. A review of passengers on a flight carrying a confirmed case of SARS in 2003 reported 16 cases of SARS developing in fellow passengers, 111 but it seems likely that affected individuals were in close proximity in the airport lounge, so transmission may have occurred before boarding. To date there is just one reported case of possible aircraft transmission of COVID-19, 112 but the literature is clearly evolving.
The principal public health concern around air travel is the role it plays in carrying infected persons (who may be asymptomatic and are not always contagious) long distances within a short space of time, with the associated risk of disseminating novel contagious disease to new locations. This has been especially evident during the COVID-19 pandemic. Special attention should therefore be paid to the clearance of people wishing to fly who have respiratory tract symptoms during outbreaks of such infections. At any time, and not just during outbreaks of serious infectious respiratory disease, airport screening measures may be implemented and travellers with a fever can be refused boarding by the airline. In cases of serious epidemics and/or pandemics such as MERS and COVID-19, even urgent travel may be prohibited. The Centers for Disease Control and Prevention website has regular updates on air travel ( www.cdc.gov ). The 2020 BTS COVID-19 Statement on Air Travel contains practical advice for potential passengers with lung disease during the COVID-19 pandemic. 113
Patients with highly contagious infections including measles, chickenpox, mumps, SARS, MERS or COVID-19 should not be allowed to travel until they are considered non-infectious.
WHO provides comprehensive information about the risk of air travel with TB. 114 Risk is determined by two factors: whether acid fast bacilli are present on smears of respiratory samples, or a sputum smear is culture positive; and whether drug resistance is present. Patients with sputum smear or culture positivity are considered potentially infectious.
Those starting treatment for pulmonary TB, where not all the information is yet available, should not travel by air for the first 2 weeks.
For patients with MDR/XDR TB, travel is prohibited until two negative culture samples have been produced and there is clinical evidence of improvement on treatment.
Patients suffering from acute lobar bacterial pneumonia present a low risk to other passengers. They are, however, more likely to experience in-flight desaturation during flight.
OSAS and OHS
The most recent available guidance states that for patients with OSAS, the potential risks during commercial airline travel are worsening hypoxaemia when asleep, and exacerbation of jet lag with potential adverse effects on driving. 1
Data are sparse regarding risks for passengers with OSAS during air travel. Some data suggest there is a risk of cardiovascular and other adverse events in this group when staying at high altitude destinations. Hypobaric hypoxia can promote central apnoeas in addition to obstructive events, which may cause tachycardia, cardiac arrhythmia and systemic hypertension. 115 It is not, however, clear how quickly this response develops, and therefore whether the findings are relevant to air travel.
Using hypobaric chamber simulation testing, studies have shown that there is an association between hypoxaemia, decreased sleep time and an increased frequency of hypopneas for patients with OSAS who are acutely exposed to high altitude. 116 There are further adverse effects on sleep and OSAS if alcohol 117 or sedatives 118 are taken.
Many patients with OSAS are already established on CPAP. Some studies have shown that patients with OSAS have lower oxygen saturations at baseline and at cabin altitude simulation than normal subjects. 119 The changes are more marked in those with severe OSAS. Use of CPAP at altitude is associated with decreased central sleep apnoea and increased sleep efficiency. 116
Consideration must be given to the whole journey including the return flight. This is especially important if the flight involves an overnight element and patients expect to drive the next day. Studies have identified that not using CPAP for one night during the flight increases the risk of drowsiness at destination the following day. 120
Careful planning is required. A retrospective survey of 394 patients who undertook air travel with CPAP reported that over a third encountered problems with their equipment, power cord, adapter or transport of the CPAP machine. 121 These findings highlight the need for clinical teams to understand the logistics so that they can support safe patient travel (see Appendix A).
In summary, the potential physiological risks for this group include cardiac stress; increased frequency of hypopnoeas; possible central apnoeas; hypoxaemia and exacerbation of jet lag.
There are no data relating specifically to air travel in OHS, which is considered a restrictive disorder. For these patients, physicians should refer to guidance around the use of NIV in those with respiratory muscle and chest wall disorders.
The patient should be advised to carry their CPAP device as hand luggage, and a hospital letter to advise that the patient uses CPAP.
There is little good evidence to guide decision-making around the need for oxygen or NIV during air travel for patients with severe extrapulmonary restriction resulting from chest wall disorder or respiratory muscle weakness. One case report suggests that a long-haul flight may have precipitated a first episode of PH and right heart failure requiring intubation and ventilator support in a man aged 59 with congenital kyphoscoliosis and apparently stable cardiorespiratory function before travel. FVC was documented as 0.98 L on recovery. 122
Some work has been conducted to understand which patients require HCT. The authors of a study of 21 adults with idiopathic kyphoscoliosis or neuromuscular disease 123 concluded that those with FVC <1 L, even with resting SpO2 >95%, are likely to desaturate significantly at cabin altitude. In some restrictive conditions, for example, bulbar MND, FVC is difficult to reproduce. Walk tests may aid decision-making in patients with scoliosis, 35 but may also be inaccessible to those with MND and similar conditions where spirometry is a challenge.
Since the 2011 BTS recommendations, 1 several studies have tried to identify factors that may predict the need for in-flight oxygen for patients with neuromuscular disease. One study aimed to identify parameters that predict HCT outcome in 40 patients with MND. Baseline PaCO2 was the only independent predictor of hypoxaemia during HCT. 124 This appears to be supported by a more recent study examining baseline PaCO2 as a predictor of HCT outcome. 125 Patients likely to fail HCT have a higher baseline PaC02, but the authors were unable to determine an absolute threshold PaC02 value that could identify patients needing in-flight supplementary oxygen.
Another study in 36 patients with MND examined baseline lung function as a predictor of hypoxaemia in response to altitude simulation. 126 The authors concluded that maximuminspiratory pressure (MIP) and sea level SpO2 may help identify MND patients who will develop hypoxaemia at altitude. Despite the small numbers, none of the patients with an MIP >30 mm Hg or with sea level SpO2 >96% desaturated below 85% during HCT. Preliminary data from a smaller study of 12 patients with MND suggested that sniff nasal inspiratory pressure (SNIP) may more accurately predict the risk of hypoxia during air travel in those with neuromuscular disease and respiratory muscle weakness. 127
While evidence to date addresses specific patient groups, the principles may be applied to any individual with a restrictive disorder resulting from respiratory muscle weakness or chest wall deformity. Further research on the value of FVC, PaCO2, MIP and/or SNIP in predicting HCT outcome in this group is desirable. In the meantime, it seems reasonable to recommend that individuals with severe respiratory muscle weakness or chest wall deformity (FVC <1 L) should undergo HCT before air travel. Physicians should use their discretion for considering HCT if there are additional reasons for concern, such as a history of previous travel intolerance, hypoxaemia or hypercapnia.
In those with respiratory muscle weakness, the possibility of respiratory failure should also be considered. For patients established on NIV, further planning and advice are required to support the use of NIV during flight. If continuous flow oxygen cannot be provided by the airline or by POC, oxygen and NIV cannot be used simultaneously. A decision around whether NIV or supplementary oxygen is of greatest physiological importance to the patient is then required on an individual basis. The previous travel history, current clinical condition and the presence or absence of overnight travel should also be considered. (see Appendix B, table 2 ).
Clinical practice points: Hypoxic challenge test (HCT) outcomes
In summary, the potential physiological risk for patients with restrictive respiratory disease is respiratory failure resulting from inadequate ventilation. It is therefore essential to assess ventilatory requirements before deciding whether supplementary oxygen is required. The risk of respiratory failure must be understood and assessed before travel, and there are currently no absolute predictors to guide which patients are likely to require supplementary oxygen.
Further planning and support are required for those established on NIV (see Appendix A).
VTE (DVT and PE)
The risk of VTE during air travel appears low overall, and prophylaxis is unnecessary for most travellers. Prolonged travel (exceeding 6 hours) and/or the coexistence of another risk factor for VTE increase the risk. The incidence of symptomatic VTE has been estimated at 0.5% on flights over 12 hours, but asymptomatic rates may be higher. 128–130 The reasons for the increased risk are not entirely clear. Potential contributory factors include prolonged immobility and dehydration, but these are not conclusively proven. Other risk factors for VTE such as obesity, recent surgery, pregnancy, malignancy and previous VTE all increase the overall risk for travel-related VTE and may necessitate additional prophylaxis.
General measures, including getting up and walking around where possible every 2–3 hours; ankle and calf exercises and avoidance of alcohol or sedating drugs; are advisable for most travellers. Although there is no conclusive evidence that flying causes dehydration, the fall in cabin humidity along with alcohol consumption and reduced fluid intake, may increase the risk on long haul flights. Remaining well hydrated is, therefore, advisable. Wearing graduated compression stockings during travel may reduce the incidence of deep venous thrombosis. 131 Data are sparse regarding the method or duration of pharmacological prophylaxis, and recommendations rely on consensus expert opinion. Physicians may wish to recommend pharmacological prophylaxis for those at higher risk of VTE, for example an obese patient planning a flight exceeding 6 hours with a history of recent surgery. The risks of prophylaxis are thought to be low. There is limited evidence for LMWH as prophylaxis. 132 There is no formally recommended dose, but enoxaparin 40 mg at a dose of 40 mg or weight based 1 mg/kg injected once 4–5 hours before the flight has been suggested. 132 The use of factor Xa inhibitors is off-licence in this situation and currently has no evidence base.
LMWH or a DOAC are advised for both outward and return long haul flights (long haul defined as flights of 6–12 hours) in high-risk patients including those with a history of VTE; local policy should be followed regarding liaison with primary care and/or haematology services to teach the patient how to administer the injection and dispose safely of the equipment. There is no formally recommended dose, but enoxaparin at a dose of 40 mg or weight based 1 mg/kg injected once 4–5 hours before the flight has been suggested.
Although the risks of prolonged air travel and development of VTE are well known, there are fewer data on the risks associated with flying after a diagnosis of VTE.
A patient with a confirmed diagnosis of PE is highly likely to start anticoagulation, with the aim of preventing the formation of new deep venous thrombi and further PEs. There is a general acceptance that flying immediately after a diagnosis of PE/DVT should be avoided. It appears reasonable to assume that the sooner air travel occurs after a PE the greater the likelihood that hypoxic pulmonary vasoconstriction will exacerbate ventilation-perfusion mismatch and raise pulmonary pressures, affecting cardiac output.
Some authors, but not all, suggest that most clots are resolved after 14–21 days. 133 Consensus opinion is to delay air travel, if possible, usually for at least 2 weeks, although there are no concrete data to support a safe time interval. Clearly the risk-benefit ratio needs to be assessed if more urgent air travel is needed. Clot resolution depends principally on in vivo fibrinolysis. Consideration should be given to the severity of the initial presentation. It is good practice, before any proposed air travel, to reassess clinically a patient who has presented with significant right ventricular strain and decompensation.
The probability of recurrent VTE while anticoagulated is extremely low. Recovered, stable patients who remain on anticoagulation should be reassured accordingly and advised to follow the above general measures.
Clinical practice point
Air travel should be delayed for 2 weeks after a diagnosis of DVT or PE.
Data are sparse, and recommendations are largely based on expert consensus opinion. The concern in PH is the risk of hypoxia causing increased pulmonary arterial pressure and right ventricular strain. Most studies have employed HCT. They have shown that most patients with PH can tolerate this degree of hypoxia with minor increases in dyspnoea. 134 Furthermore, the effect on the right ventricle in one study has been shown to be minimal. 134 However, most of these studies only covered a short time period. 134 Longer exposure to hypoxia on long haul flights may have more significant effects.
One study has monitored patients during commercial flights. 135 This showed that up to a quarter of patients with PH desaturate during short haul flights, with higher altitude, ambulation and longer flights correlating with desaturations. Most tolerated this well, with fewer than 40% of participants reporting symptoms. A larger questionnaire based retrospective study has also confirmed that in most patients with stable PAH, flight is well tolerated with minimal clinical effects. 136 Around half those surveyed travelled with supplementary oxygen.
Hypoxia reduces exercise capability; breathing oxygen-enriched air improves exercise capacity. 134 It therefore appears logical to give patients with impaired functional capacity supplemental oxygen on board the aircraft. Whether patients should have oxygen while walking around as well as when sitting is unknown; ambulatory oxygen on board presents obvious logistical challenges.
The 2011 BTS Recommendations advised that patients in NYHA WHO functional class 3 or 4 should have supplemental oxygen during air travel. 1 This recommendation is pragmatic rather than evidence based; and may result in over-prescribing of in-flight oxygen. One study suggests that more than double the number of patients would be recommended in-flight oxygen based on functional class rather than HCT outcome. 134
If HCT is not readily available and there are no concerns about hypercapnia, passengers already on LTOT should be advised that they will need a flow rate 2 L/min greater than their baseline flow rate. This should be sufficient to compensate for the relative hypoxia at normal cabin altitude.
Those in NYHA WHO functional class 3 or 4 are usually advised to have in-flight oxygen. If there is no evidence of hypercapnia it seems reasonable to suggest 2 L/min by nasal cannulae. If there are concerns about hypercapnia, HCT should be considered if available.
When evaluating those with lung cancer or mesothelioma it is important to consider the nature and extent of their condition as well as their treatment. A pragmatic approach is to evaluate their risk of haemorrhage, pneumothorax, pleural effusion, VTE and any recent surgical and/or bronchoscopic interventions.
If taking medication, and particularly controlled drugs, patients must be aware of the appropriate documentation required for the countries they are visiting. Advance-planning is essential.
There are few data on the implications of functional breathing disorders for air travel, whether DB, VCD or ILO. Asthma should not be overlooked as a possible association in those with DB. 137
Acute shortness of breath is one of several symptoms for which flight diversion is advised. 138 Diversions are costly, typically ranging from £10 000–£80 000 depending on aircraft size and diversion destination. 139 Dyspnoea caused by DB or hyperventilation is unlikely to have serious clinical consequences; but it must be distinguished from dyspnoea attributable to life-threatening acute medical conditions such as acute coronary syndrome or PE. 1 140
Data from the last two decades suggest that 65% of in-flight medical emergencies were due to exacerbations of pre-existing conditions and that respiratory problems were most common; half were due to asthma or ‘asthma-like’ presentations. 141–143
Air travel can be stressful. Physiological or psychological stress may precipitate acute breathlessness in patients with respiratory disease. 144 Acute hyperventilation can be a response to stress independent of lung pathology, usually in those with known panic and anxiety disorders. 145
Hyperventilation can cause bronchoconstriction resulting in ‘asthma-like’ symptoms 146 which are unresponsive to standard asthma medication. 147 148 A perception that the usual ‘rescue’ medication is ‘not working’ may worsen an individual’s breathing pattern, causing concern to them, other passengers and air crew. Similar situations can arise with ILO or VCD, and onset of symptoms is often sudden. 149
Where DB is linked to respiratory conditions, particularly asthma, national and international guidelines endorse breathing exercise programmes provided by a specialist respiratory physiotherapist as an adjuvant to pharmacological treatment. Patients should be advised to use breathing techniques in situations where breathlessness may become problematic. 75 150 ILO and VCD, which can present with acute respiratory distress and stridor, may be treated with breathing exercises taught by a respiratory physiotherapist or a speech therapist with specialist expertise in paradoxical vocal cord movement. 151
Paper bag rebreathing is no longer recommended, because inspired oxygen concentration decreases sufficiently to endanger hypoxic patients. There are data reinforcing that significant harm to patients can result from acute myocardial infarction, pneumothorax and PE being misdiagnosed as hyperventilation. 152 , 153
Patients with DB, ILO and/or VCD should be referred to a Respiratory Physiotherapy Specialist for advice on symptom management before travel.
Supplemental oxygen should be given on board if the cause of breathlessness is unclear.
Appendix A Logistics of air travel with equipment
Passengers requiring oxygen and travelling overseas will usually need to lease a POC privately, since UK companies do not generally allow equipment provided through the NHS to be taken out of the country. Furthermore, most airlines have moved away from supplying routine medical oxygen.
Where battery powered mechanical devices are required in-flight, including a POC, sufficient batteries should be carried for 1.5 times the anticipated duration of the journey to cope with delays or diversions. For example, a patient using a POC on a 4-hour flight should have 6 hours of battery life. Any spare batteries must be correctly packaged and should be carried in cabin baggage. The airline must be notified in advance of these plans, or airline staff can refuse to allow the equipment to be taken on board.
Pulse-dose oxygen may not be suitable for patients with a fast and shallow respiratory pattern, or during sleep. 154 155 Pulse-dose settings do not equate to the equivalent continuous flow rates, 74 and not every POC functions well at altitude. 156 In contrast, pulse-dose oxygen functions reliably when provided by a cylinder and conserving device. 156 One author found significantly lower PaO 2 values when using a POC, compared with compressed oxygen with a conserving device. Acceptable in-flight values are achievable with POCs, but the dose may need to be increased. 56
Pulse-dose delivery systems can complicate determination of the flow delivered; and may not be well tolerated. The effects of mouth-breathing, speech, snoring and/or sleeping should be considered. HFNO cannot be delivered on board commercial aircraft.
Pulse-dose oxygen has not been studied in infants and children; and should not be used unless they have been shown to trigger the device’s inspiratory flow.
Currently available POCs that do supply continuous flow oxygen cannot provide flow rates above 3 L/min. Passengers must refer to POC documentation to check that the equipment meets their requirements before they lease it for air travel.
For all these reasons, assessments would ideally take place using the same equipment as that which will be used on board the aircraft. This is only likely to be possible when the patient has leased or purchased a POC for their own long term, private use.
Continuous positive airway pressure
Few airlines, if any, allow any medical device to be powered via the aircraft power supply. An appropriate battery must, therefore, be used. The device and battery specifications must be approved for use by the airline before travel. Battery performance should be checked by the user beforehand, so there is an understanding of operating times on their usual settings.
Further consideration needs to be given to CPAP use during flight and at high altitude destinations, as it requires a machine that will perform adequately at low ambient pressure. The 2011 BTS guidance 1 reported that a fixed-pressure CPAP machine without pressure compensation, set to deliver a pressure of 12 cm H 2 O at sea level, may deliver only 9 cm H 2 O at 8000 ft. The machine may therefore require adjustment to ensure a safe level of treatment throughout the flight. If continuous flow oxygen cannot be provided by the airline or by POC, oxygen and CPAP cannot be used simultaneously. The availability of distilled water for humidifiers may be restricted.
Non-invasive ventilation
For patients established on NIV, further planning and advice are required to support the use of NIV during flight. If continuous flow oxygen cannot be provided by the airline or by POC, oxygen and NIV cannot be used simultaneously. A decision around whether NIV or supplementary oxygen is of greatest physiological importance to the patient is then required on an individual basis. Previous travel history, current clinical condition and the presence or absence of overnight travel should also be considered. The level of clinical and personal dependency must be considered in the context of requirements for trained supervision and assistance by the caregiver.
Appendix B Quick reference guide for respiratory physiologists
Most patients with respiratory conditions are able to fly safely without any additional support. The following guide provides specific information for respiratory physiologists regarding patients who do need further investigation before embarking on air travel. See figure 3 .
HCT for physiologists.
The HCT uses an inspired gas mixture containing 15% oxygen, which gives an approximate similar PO 2 to breathing air at the maximum allowable cabin pressure altitude (2438 m or 8000ft). 53 54 HCT is usually performed in a specialist respiratory physiology unit. The provision of a 15% oxygen gas mixture can be achieved as follows:
A premixed cylinder containing a 15% oxygen gas mixture can be obtained from medical gas providers, or Douglas bags can be mixed with air and nitrogen to reduce the percentage of inspired oxygen to 15%, both to supply a tight-fitting face mask in a closed circuit. 1
Pure nitrogen can be introduced into a sealed chamber such as a body plethysmograph for paediatric or mask-intolerant patients, removing the need for a face mask. 17 Paediatric patients can be sat in a body plethysmograph on an adult’s lap throughout; 1 the adult should also undergo SpO 2 monitoring to avoid excessive hypoxaemia. A body box is generally used for children, although some paediatric laboratories use masks
A 40% Venturi oxygen mask can be used with pure nitrogen as the driving gas, giving a resultant gas mixture containing approximately 15% oxygen. 11 58
A hypoxic gas generator, like an oxygen concentrator, can be used to provide a continuous supply of variable hypoxic gas mixtures to supply a mask or closed chamber. This can be the most cost-effective method for centres with a high demand for HCT. 12
The patient usually breathes the hypoxic gas mixture for 20 min, or until SpO 2 reaches 85%. Although this is shorter than the briefest commercial flight, oxygenation equilibrium is usually reached within this time. 134 157 The patient is advised to have in-flight oxygen if PaO 2 falls below 6.6 kPa (<50 mm Hg) or SpO 2 remains <85% 1 17 (see page 11).
HCT with oxygen
If PaO 2 or SpO 2 values meet the criteria, in-flight oxygen is recommended. 1 The flow rate required can be assessed as part of the HCT. 17 Previous BTS recommendations advised in-flight oxygen to be supplied at two or 4 L/min via nasal cannulae, which were for many years the only fixed flow rates routinely available on commercial aircraft. As airline-supplied in-flight oxygen becomes less common and greater numbers of patients travel with flight-approved POCs delivering a wide range of continuous and intermittent flow rates, these figures are less critical. The HCT should ideally be performed with the modality that is intended for use in-flight.
Most airlines have moved away from supplying routine medical oxygen. In-flight oxygen is thus now likely to be supplied by an FAA approved POC, leased by the patient. These are mostly pulse-dose delivery. It is, therefore, advisable to conduct a titrated HCT with pulsed dose oxygen to maintain PaO 2 at ≥6.6 kPa or SpO2 ≥85%, using setting 2 as the starting point. This approximates to the 2 L/min originally stated. If pulse-dose oxygen at higher settings is insufficient to maintain PaO 2 at ≥6.6 kPa or SpO2 ≥85%, then continuous oxygen should be considered. It should be noted that POC models supplying continuous flow are limited, and they do not currently supply >3 L/min.
Pulse-dose delivery is not suitable for young children, for use during sleep 154 155 or for certain adults. Not all POCs function as expected under conditions of simulated altitude 156 and pulse-dose settings may not equate to equivalent continuous flow rates 74 (see Appendix A).
Patients with hypercapnia
Previous BTS advice was to err on the side of recommending oxygen if in doubt, 1 and other authors have recommended doubling oxygen flow rates for patients with a pre-existing oxygen requirement. 1 29 However, there is a potential risk of developing hypercapnia and respiratory acidosis from oxygen during HCT in patients with type 2 respiratory failure. 18 Patients with a history of hypercapnia should ideally undergo HCT with blood gas sampling. In these cases, the minimum amount of oxygen should be delivered to maintain PaO 2 ≥6.6 kPa while monitoring PaCO 2 and pH. If blood gas sampling is not available then care should be taken not to raise SpO 2 above the resting level with supplementary oxygen in this group of patients . 17 In some cases it may be unsafe to undertake air travel even if good oxygenation can be achieved, if adverse PCO 2 and pH changes are evident. 18
An incidental finding of an elevated COHb during HCT represents an important opportunity to take a smoking history and offer smoking cessation referral as appropriate.
Ethics statements
Patient consent for publication.
Not applicable.
Acknowledgments
The assistance of the British Thoracic Society Standards of Care Committee is gratefully acknowledged. The Society and the Air Travel Clinical Statement Group is also grateful to the organisations that provided feedback as part of the consultation process.
- Ahmedzai S ,
- Balfour-Lynn IM ,
- Bewick T , et al
- British Thoracic Society Standards of Care Committee
- Shiner RJ ,
- Partridge MR
- Federal Aviation Administration
- Aerospace Medical Association
- Peterson DC ,
- Martin-Gill C ,
- Guyette FX , et al
- Spurling KJ ,
- ↵ Health effects of airline cabin environments in simulated 8-Hour flights . Aerosp Med Hum Perform 2017 ; 88 : 651 – 6 . doi:10.3357/AMHP.4366.2017 pmid: http://www.ncbi.nlm.nih.gov/pubmed/28641682 OpenUrl PubMed
- Aiello M , et al
- Moonsie IK ,
- Hardinge M ,
- Annandale J ,
- Bourne S , et al
- Gonzalez F ,
- Bossley CJ ,
- Mason B , et al
- Doughty VL ,
- Starling L , et al
- Campbell A ,
- Vyas J , et al
- Aerospace Medical Assocation
- Resnick SM ,
- Simmer KN , et al
- Josephs LK ,
- Thomas M , et al
- Robson AG ,
- Pacilli AMG , et al
- Edvardsen A ,
- Christensen CC , et al
- Nicholson TT ,
- Sznajder JI
- Christensen CC ,
- Refvem OK , et al
- Hartung TK ,
- James AL , et al
- Castagnetti C ,
- Swanney MP ,
- Seccombe LM , et al
- du Bois RM ,
- Weycker D ,
- Albera C , et al
- Holland AE ,
- Fiore J , et al
- Lancaster LH
- Bandyopadhyay D ,
- Oscroft NS ,
- Shneerson JM , et al
- Edvardsen A
- British Airways
- Civil Aviation Authority
- Faughnan ME ,
- Stanbrook MB , et al
- Gulati A , et al
- Schwartz JS ,
- Bencowitz HZ ,
- Edvardsen A , et al
- Hussein K ,
- Farouk Alkarn A ,
- El-Sokkary R , et al
- ↵ Far code of US federal regulations Washington DC: Federal Aviation Administration , 2019 . Available: https://www.ecfr.gov/cgi-bin/text-idx?SID=82d757ba11a578e04151050a42c48741&mc=true&tpl=/ecfrbrowse/Title14/14cfrv1_02.tpl#0
- Tashkin DP ,
- Lee EY , et al
- Dillard TA ,
- Moores LK ,
- Bilello KL , et al
- Gradwell DP
- Rajagopal KR ,
- Slivka WA , et al
- Soffler MI ,
- Schwartzstein RM
- Driscoll P ,
- O'Driscoll R
- Beasley R ,
- Douglas J , et al
- Sadatsafavi M , et al
- Hammadah M ,
- Kindya BR ,
- Allard-Ratick MP , et al
- Derderian SS , et al
- Akerø A , et al
- Hardie JA , et al
- Seccombe LM ,
- Wong CK , et al
- Chatburn RL ,
- Williams TJ
- ↵ BTS/SIGN. British guideline on the management of asthma . SIGN 2019 . July 2019 .
- McGoldrick VP
- Naughton MT ,
- Rochford PD ,
- Pretto JJ , et al
- Edvardsen E ,
- Skjønsberg OH , et al
- Johnson AOC
- Nesthus T ,
- Garner R SM
- Cummins RO ,
- Schubach JA
- George PM ,
- Ward S , et al
- Peña-Zarza JA ,
- Sailer S , et al
- Jaramillo R ,
- Sicherer SH , et al
- Muñoz-Furlong A ,
- Greenhawt M ,
- MacGillivray F ,
- Batty G , et al
- Vanfleteren LEGW ,
- Spruit MA ,
- Groenen M , et al
- Shale DJ , et al
- Bertoletti L ,
- Mismetti P , et al
- Brækkan SK ,
- Enga K , et al
- Hirche TO ,
- Bradley J ,
- d'Alquen D , et al
- Fischer R ,
- Buchdahl RM ,
- Babiker A ,
- Bush A , et al
- Martin SE ,
- Bradley JM ,
- Buick JB , et al
- Barratt SL ,
- Jones R , et al
- Ensor JE , et al
- Currie GP ,
- Kennedy A-M ,
- Paterson E , et al
- Pollock-BarZiv S ,
- Downey GP , et al
- Johannesma PC ,
- van de Beek I ,
- van der Wel JWT , et al
- MacDuff A ,
- Arnold A JH
- Elterman J ,
- Burns C , et al
- Majercik S ,
- Van Boerum DH , et al
- Maratos EC ,
- Edmonds L , et al
- Stangerup SE ,
- Tjernström O ,
- Klokker M , et al
- Roguski K , et al
- Bin Abdulrahman AK ,
- Bin Abdulrahman KA ,
- Almadi MK , et al
- Chang H-L ,
- Cheung TY-Y , et al
- Lagier J-C ,
- Mailhe M , et al
- ↵ British thoracic Society: points to consider when planning air travel by passengers with respiratory disease during the endemic phase of COVID-19 London: BTS , 2020 . Available: https://www.brit-thoracic.org.uk/about-us/covid-19-resumption-and-continuation-of-respiratory-services/#planning-air-travel/
- Latshang TD ,
- Nishida K ,
- Lanspa MJ ,
- Cloward TV , et al
- Sullivan CE ,
- Alcohol SCE
- Liistro G ,
- Rodenstein DO
- Bazurto Zapata MA ,
- Dueñas Meza E ,
- Jaramillo C , et al
- Kribbs NB ,
- Kline LR , et al
- Bodington R ,
- Johnson O ,
- Carveth-Johnson P , et al
- Davidson JA
- Thirumaran M ,
- Tuggey JM , et al
- Crawford E ,
- Brazzale D ,
- Ruehland W ,
- Humphreys J ,
- Agarwal S ,
- Cliff I , et al
- Pérez-Rodríguez E ,
- Jiménez D ,
- Díaz G , et al
- Machin SJ ,
- Bailey-King S , et al
- Clarke MJ ,
- Broderick C ,
- Hopewell S , et al
- Cesarone MR ,
- Belcaro G ,
- Nicolaides AN , et al
- Peacock AJ ,
- Johnson MK , et al
- Roubinian N ,
- Elliott CG ,
- Barnett CF , et al
- Voswinckel R ,
- Tiede H , et al
- Heaney LG ,
- Robinson DS
- Ruskin KJ ,
- Hernandez KA ,
- Balkissoon R ,
- Matsumoto K ,
- Porsbjerg C ,
- Menzies-Gow A
- Bahrainwala AH ,
- Parsons JP ,
- Benninger C ,
- Hawley MP , et al
- Pichelin M , et al
- McDonald CF ,
- Hazard A , et al
- Einhaeupl F , et al
- Okawa S , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
- Data supplement 2
- Data supplement 3
Twitter @RobinaCoker1
Contributors RKC was the lead author responsible for the final document. All authors agreed the outline and content of the document and authored sections of the document.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer A Clinical Statement reflects the expert views of a group of specialists who are well versed in the topic concerned, and who carefully examine the available evidence in relation to their own clinical practice. A Clinical Statement does not involve a formal evidence review and is not developed in accordance with clinical practice guideline methodology. Clinical Statements are not intended as legal documents or a primary source of detailed technical information. Readers are encouraged to consider the information presented and reach their own conclusions.
Competing interests AA declared funding from Fisher Paykel and Breas. CC declared funding from Pfizer, GSK, Janssen, MSD. SH declared funding from Astra Zeneca, GSK, Roche, Chiesi, Trudell, Boehringer Ingelheim, Mylan, Teva.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Linked Articles
- Airwaves Highlights from this issue The Triumvirate Thorax 2022; 77 319-319 Published Online First: 15 Mar 2022. doi: 10.1136/thoraxjnl-2022-218902
Read the full text or download the PDF:
How TB Spreads
TB bacteria spread through the air from one person to another. When a person with TB disease of the lungs or throat coughs, speaks, or sings, TB bacteria can get into the air. People nearby may breathe in these bacteria and become infected.
TB is NOT spread by
- shaking someone’s hand
- sharing food or drink
- touching bed linens or toilet seats
- sharing toothbrushes
When a person breathes in TB bacteria, the bacteria can settle in the lungs and begin to grow. From there, they can move through the blood to other parts of the body, such as the kidney, spine, and brain.
TB disease in the lungs or throat can be infectious. This means that the bacteria can spread to other people. TB in other parts of the body, such as the kidney or spine, is usually not infectious.
People with TB disease are most likely to spread it to people they spend time with every day. This includes family members, friends, and coworkers or schoolmates.
Learn about latent TB infection and TB disease .
To receive email updates about this page, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- United Arab Emirates
- Switzerland
- The Netherlands
- Puerto Rico
- United States
- New Zealand
- ➨ Choose from World Map
- Budget Travel
- Family Travel
- Getting Around
- Visas & Passports
- Work with Us
Browsing Category
- Czech Republic
- Saint Martin
- Uncategorized

Moscow Travel Guide: Best Things to Do + More [2023]
· everything to know about visiting moscow, including the best things to do and how to get around. ·.

Moscow is Russia’s vibrant capital city, and it also happens to be the largest city in all of Europe. The city’s long and infamous history makes it one of the most unique places we have ever visited.
The architecture ranges from centuries-old palaces to uniform, gray concrete buildings. The people range from cold and private to warm and welcoming. Moscow is a city is strong juxtapositions, and we learned a lot during our time there.
This post will break down all you need to know about visiting Moscow, including the best things to do, how to get there, how to get around, and more.

The Best Things to Do in Moscow
1. explore the red square.
The Red Square is the heart of Moscow. Most of the city’s top attractions can be found here, including just about everything on this list. The Kremlin, St. Basil’s Cathedral, and Lenin’s Mausoleum are all located here, and the State Historical Museum and GUM are not far from here, either.
The Red Square is a common home for parades, protests, and seasonal celebrations. There are massive Christmas celebrations here, with food vendors and carnival rides set up in numbers.

2. Check Out the Ziferblat
The Ziferblat is a café in Moscow that is unlike any café we have ever been to. While most cafes charge you for your drinks and food, the Ziferblat charges you for your time.
Upon arrival, you are given a clock. When you leave, the barista calculates how much time you spent in the café and charges you accordingly. This concept was created to help visitors to be more intentional with their time, and the cafe itself is incredibly charming.
For a detailed look at everything you need to know before you visit, make sure you read my post about visiting the Ziferblat Cafe in Moscow .

3. Marvel at St. Basil’s Cathedral
St. Basil’s Cathedral is one of the most iconic churches in the world, and it was the single thing we were most excited to see while in Moscow. Built almost 500 years ago, St. Basil’s Cathedral is recognized by its colorful domes and whimsical style. The church is of the Russian Orthodox faith, and the inside is just as wondrous as the outside.
St. Basil’s Cathedral is located on the edge of the Red Square, making it incredibly convenient to visit. Entrance for non-worshippers costs 800 rubles, and tickets can be bought at the church

4. Explore the Kremlin
The Kremlin is the largest active fortress in Europe, and it is the site of most of Russia’s government affairs. In addition to government buildings, the Kremlin Complex is filled with courtyards, towers, and museums that are open to the public. If you have the time, you could spend a couple of days fully exploring all that there is to see in the Kremlin.

5. Walk Through Lenin’s Mausoleum
Vladimir Lenin is one of the most important figures in Russian history, and his body is located perfectly embalmed in a mausoleum in the Red Square. The Mausoleum is open to the public to visit, and as long as you are willing to go through a few security checks, it is easily one of the best things to do in Moscow. Its convenient location in the Red Square makes it a can’t miss attraction.
There is absolutely no photography allowed inside the Mausoleum. Do not test this rule.

6. Wander Along Arbat Street
The Arbat is a very popular street in Moscow that is lined with stores, cafes, and other touristy attractions. It is one of the oldest streets in the city, dating back to the 1400s. This street is both quaint and trendy, and there are many walking tours that introduce tourists to the neighborhood’s wonders and highlights.

7. Catch a Show at the Bolshoi Theatre
As a lover of the arts, it is hard to think of Moscow and not think of ballet. Russia has always been a top dog in the world of fine arts, and Bolshoi Theater is one of the best places to catch a performance. We were lucky enough to attend an Opera here, and it is a venue that you don’t want to miss out on if you enjoy opera, ballet, or orchestral performances.
8. Visit the State Historical Museum
The State Historical Museum is one of the most respected museums in Moscow. Despite its name, it is not really focused on the history of Russia as a nation. Rather, it contains a collection of artifacts from all throughout Russia’s history.
The museum’s collection is very broad in nature. It houses some items from indigenous tribes that used to occupy the region, pieces collected by the Romanov family, and more.
9. Wander Around GUM
GUM is an absolutely massive mall within walking distance of the Red Square. It isn’t just the size that draws visitors here; it’s the sense of luxury. The mall is so beautiful inside, much like the metro stations.
While visiting a mall might not sound like it belongs on a bucket list, this mall does. You will not want to miss out on visiting GUM while in Moscow.

10. Admire the Cathedral of Christ the Saviour
While St. Basil’s Cathedral is the most iconic church in Moscow, it isn’t the only one. The Cathedral of Christ the Saviour is absolutely stunning, with massive golden domes. It is the tallest Orthodox church in the world, and it is the seat of the Orthodox Patriarch of Moscow.
It is located just about a mile from the Red Square, just south of the Kremlin Complex. You can walk to it from the Red Square in about 20 minutes.
How to Get to Moscow
Flying to moscow.
Moscow has three major international airports: Sheremetyevo (SVO) , Domodedovo (DMO) , and Vnukovo (VKO) . All three of them are directly connected to downtown Moscow by the Aeroexpress trains, which leave every 30 minutes throughout the day. By Aeroexpress train, you can expect to get to the city center in 25-45 minutes depending on the airport that you fly into.
Sheremetyevo is the biggest and busiest of the three airports, and it is the one you are most likely to fly into – especially if you are coming from outside of Europe or the Caucus region. We flew into Sheremetyevo on a direct flight from New York City.
I usually provide backup airport options, because flying right into the city isn’t always the cheapest way to get where you’re going. Unfortunately, when it comes to Moscow, don’t really have a choice other than to fly right into Moscow. It is a very remote city, and it is usually the cheapest place to fly into in Russia as a whole.
Since Sheremetyevo is so busy, you will probably find a great flight option anyway. I wrote in my post about finding cheap flights that using hub airports will lead to more affordable airfare, and the same logic applies here. Even though Russia’s national airline, Aeroflot, is no longer a member of the SkyTeam Alliance, Moscow is still a major hub connecting passengers from all over the world.

READ OUR CHEAT SHEET
Train or Bus to Moscow
Trains and buses are one of the most popular ways to get around Europe. However, they’re of very little use when you’re trying to get to Moscow.
Moscow is hundreds of miles from the nearest major cities. The only major European city that can even be reached within 8 hours on the ground is St. Petersburg, and even the Baltic capitals of Riga, Vilnius, and Tallinn are over 12 hours away.
If you want to get to Moscow, the best option is almost always to fly. While the train routes to Moscow are scenic, they simply take forever.
How to Get Around Moscow
METRO | TROLLEYS | TRAMS | BUSES
Moscow has one of the most memorable metro systems in the world. Its metro lines are very deep underground, and the stations are absolutely stunning. Each station has its own unique style, but all of them contain escalators that seem to go on forever.

The system was built in an effort to showcase the power of the Soviet Union and its bright future. The plans were a form of propaganda, but they resulted in what is still one of the most visually appealing subway systems on earth.
Moscow’s metro system isn’t just pretty. It is also very useful and accessible. The system has 17 lines that connect the city and its surrounding area.
But wait; there’s more!
The Moscow metro system is also incredibly affordable, with each ride costing less than a dollar. The metro is by far the best way to get around Moscow, as it is almost impossible to beat the connection times and the low cost to ride.
Tickets can be bought at electronic, English-speaking kiosks in stations, or directly from ticket counters at certain larger stations. There are also day passes available, which are a very solid option if you plan on riding the metro several times per day.

The metro is by far the best way to get around Moscow.
In addition to the metro system, Moscow also has a network of buses, trams, and trolleys. This system is nowhere near as convenient or well-connected as the metro, though, and is likely of little use to you during your trip. There is no Uber in Moscow, but a similar app named Yandex is available if you need a ride in a pinch.
How Many Days Do You Need in Moscow?
Moscow is the biggest city in all of Europe, and it is absolutely loaded with things to do. You could spend weeks in Moscow and still find new things to do. Of course, most travelers don’t have that kind of time to spend in one place!
I recommend spending no less than three full days in Moscow, and ideally closer to five or seven.
Moscow is very spread out, and it can take some time to get from one major point to another. There are also so many places that are nice to just sit back and relax, which is hard to do when you’re in a hurry trying to cram activities into just a few days.
If you only have a week to visit Russia, I’d advise spending all of the time in one city. If you decide to split your time between Moscow and St. Petersburg, I recommend not trying to squeeze in any day trips beyond those two cities.

When Is the Best Time of the Year to Visit Moscow?
There are two different ways to approach this question. Personally, I think the best time to visit Moscow is around Christmas and New Year’s Day. While the weather will be absolutely freezing, Moscow is a surreal winter wonderland in December and January.
We were in Moscow right before Christmas. While it was very cold, you can always bundle up. Exploring the Christmas markets and pop-up ice skating rinks throughout Moscow is one of my favorite memories from anywhere I’ve traveled, and I dream of going back to do it again.
If you aren’t fond of the cold, Moscow is beautiful in the summer. It tends to get pretty cold in the shoulder seasons, so if you want warm weather, you should plan to visit in the summer. Moscow actually gets pretty warm in July and August, and there are a bunch of fantastic places to soak up the sun within the city.
The best time to visit Moscow is either around Christmas or from late May to August.

Is Moscow Safe to Visit?
While Moscow is a truly wonderful city, there’s no denying that visiting Russia comes with risks. As the country is run by an infamous communist dictator, concerns about visiting are valid. While we didn’t experience any sort of threat or negative treatment during our time in Moscow, we visited in a peaceful time.
In our experience, Russia doesn’t seem to detain normal Americans or Westerners to use as pawns. As a regular person, as long as you don’t commit any crimes, there is a slim chance you will run into any issues. However, Russia will not hesitate to enforce its laws against foreigners, and illegal behaviors will likely land you in a very compromising position.
Russia will not hesitate to enforce its laws against foreigners, and illegal behaviors will likely land you in a very compromising position.
To make matters worse, Russia has a bad reputation for gang violence. While the Russian mafia has very little interest in normal Western tourists, they won’t hesitate to pick a fight with anyone who ventures into their sphere of influence. If you seek out illegal substances or activities, you could be a target of the mafia.
If you seek out illegal substances or activities, you could be a target of the mafia.
Finally, since Russia’s invasion of Ukraine, things are all very different. Russia is currently at war, and there are battles raging within 8 hours of Moscow. While it is still relatively safe to visit, that could change at any time as the war with Ukraine continues.
Is Moscow Worth Visiting?
Without a doubt, Moscow is worth visiting. It is one of the most unique major cities we have ever visited, and we hope to make it back one day. The Russian Orthodox churches are stunning, the city’s history is unlike any other, and the food is to die for.
While many visitors prefer St. Petersburg to Moscow, I think Moscow deserves a lot of hype of its own. Moscow is the beating heart of Russian culture and history, and it’s a place I highly recommend checking out if you have the chance.

That’s all we have for you about Moscow! I hope this post was helpful as you plan your trip to Russia’s capital.
Have you been to Moscow? Or is this your first time visiting? Comment below if you have anything to add to our travel guide!
Hi, I'm Greg. I'm an avid traveler who has traveled to over 50 countries all around the world with my wife and kids. I've lived in Italy, Mexico, China, and the United States, and I dream of moving abroad again in the future. With this blog, I provide my audience with detailed destination guides to my favorite places and pro-tips to make travel as stress-free as possible.
Leave a comment
Save my name, email, and website in this browser for the next time I comment.
Meet The Author - Greg
Recent Post

How Much Does a Trip to Egypt Cost: Budget Breakdown
March 10, 2024

Best Time to Visit the India Gate in Delhi [2024]
March 1, 2024

Flying with a Sinus Infection: Tips to Avoid Pain
February 20, 2024

11 Best Things to Do in Breckenridge Besides Skiing
February 12, 2024

10 Best Beaches in Mexico for Families (We Lived Here)
February 3, 2024

IMAGES
COMMENTS
Tuberculosis and Air Travel: Guidelines for Prevention and Control (PDF). View Page In: English Spanish. Page last reviewed: December 17, 2020. Content source: Division of Tuberculosis Elimination, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention.
Travelers who anticipate possible close contact or prolonged exposure to people with TB should have a TB skin test or a TB blood test before leaving the United States. If the test reaction is negative, they should have a repeat test 8 to 10 weeks after returning to the United States. Additionally, annual testing may be recommended for those who ...
Tuberculosis (TB) is a disease caused by bacteria called Mycobacterium tuberculosis. People with TB can spread it in the air to others when they cough, speak, or sing. You can get sick when you breathe TB bacteria into your lungs. TB bacteria in the lungs can move through the blood to infect other parts of the body, such as the kidney, spine ...
The guidelines were developed with the collaboration of public health authorities and international experts in the prevention and control of TB, travel medicine and air travel. Implementing the recommendations will help to reduce the international spread of TB and decrease the risk of infection among individual travellers. Although the role of ...
You were born in or frequently travel to countries where TB disease is common, including Mexico, the Philippines, Vietnam, India, China, Haiti, and Guatemala, and other countries where TB is common. You currently live, used to live, or are employed in a large group setting where TB is more common, such as a homeless shelter, prison, jail, or ...
Travel and Tuberculosis. In 1882, the German doctor Robert Koch discovered the bacteria Mycobacterium Tuberculosis which causes Tuberculosis (TB). Despite important advances to cure the disease, TB continues to be a major global health concern - three persons die every minute.
Infectious Agent. Mycobacterium tuberculosis complex is a group of closely related rod-shaped, nonmotile, slow-growing, acid-fast bacteria, which includes M. bovis and M. tuberculosis hominis, the most common cause of human tuberculosis (TB), usually referred to as M. tuberculosis.. Transmission. TB transmission occurs when a patient with a contagious form of the infection coughs, spreading ...
The revised guidelines address the concerns about transmission of TB during air travel and provide the following: (i) information on transmission of TB on aircraft; (ii) a summary of the practices adopted for the management of patients with infectious TB associated with air travel, and of commonly encountered diffi culties; (iii) suggestions on practical ways to reduce the risk of exposure to ...
Overview. The guidelines were developed with the collaboration of public health authorities and international experts in the prevention and control of TB, travel medicine and air travel. Implementing the recommendations will help to reduce the international spread of TB and decrease the risk of infection among individual travellers.
2. Physicians should inform all infectious and potentially infectious TB patients that they must not travel by air on any commercial flight of any duration until they are sputum smear-negative on at least two occasions (additional steps are required for MDR-TB and XDR-TB, see recommendation 3). 3. Physicians should inform all MDR-TB and XDR-TB ...
The emergence of MDdR-TB and extensively drug-resistant TB (XDdR-TB) has raised special concerns in relation to the international spread of particularly dangerous strains of Mycobacterium tuberculosis. Since the 2006 edition was published, several incidents have occurred involving air travel and pot …
Background. Tuberculosis (TB) is a serious global health threat that in 2018 caused an estimated 10 million disease cases and 1.5 million deaths worldwide. 1 Diagnosis and treatment of latent TB infection (LTBI) is key to reaching TB elimination targets in low-incidence countries, where risk groups for LTBI and active TB include immigrants, Indigenous populations and marginalized populations ...
WHO international guidelines for the control of tuberculosis in relation to air travel require—after a risk assessment—tracing of passengers who sat for longer than 8 h in rows adjacent to people with pulmonary tuberculosis who are smear positive or smear negative. A further recommendation is that all commercial air travel should be prohibited until the person has two consecutive negative ...
Tuberculosis (TB) is a disease caused by bacteria that are spread from person to person through the air. TB usually afects the lungs, but it can also afect other parts of the body, such as the brain, the kidneys, or the spine. In most cases, TB is treatable and curable; however, persons with TB can die if they do not get proper treatment.
For the most part, the travel experience for people with latent TB is identical to the experience enjoyed by TB-free travelers, but some people with latent TB must pay extra attention to their ...
We provide travel health visits, vaccinations, TB testing, and blood tests. Appointment only. Attention! starting April 15, 2024. Our new website address is SF.GOV/AITC. ... AITC Immunization & Travel Clinic 101 Grove Street, Room 102 San Francisco, CA 94102. Mon to Fri, 9:00 am to 4:00 pm Closed for lunch 12 pm - 1pm.
BTS recommendations for managing passengers with stable respiratory disease planning air travel were published in Thorax in 2011.1 This followed original guidance published in 20022 and an online update in 2004.3 The 2011 recommendations provided an expert consensus view based on literature reviews, aimed at providing practical advice for lung specialists in secondary care. Recognising that ...
tuberculosis (TB) epidemic and 53 million lives having been saved between 2000 and 2016, the challenge of this ancient disease remains significant. Tuberculosis (TB) is the leading infectious disease killer and one of the top ten causes of death worldwide. With a timely diagnosis and correct treatment, most people who develop TB disease can be ...
As the incidence of TB has decreased, the MDR-TB rate has increased, leading to a prediction that every second patient will have MDR-TB by 2015. Figure 3. Prevalence of bacilli-positive (smear- or culture-positive) tuberculosis ([TB] with and without multidrug resistance [MDR]) in Russia, 2005-2012 (per 100,000 population).
Walking tour around Moscow-City.Thanks for watching!MY GEAR THAT I USEMinimalist Handheld SetupiPhone 11 128GB https://amzn.to/3zfqbboMic for Street https://...
The Do Not Board and Lookout lists have been used for people with suspected or confirmed infectious tuberculosis (TB), including multidrug-resistant tuberculosis (MDR-TB), and measles. During 2020-2022, CDC used these authorities to restrict travel of people with COVID-19 and close contacts who were recommended to quarantine.
Game 1: Sunday, at Florida (12:30 p.m. ET; ESPN, SN, TVAS, BSSUN, BSFL). The Florida Panthers will try to make a return trip to the Stanley Cup Final and their first step is the Eastern Conference ...
TB bacteria spread through the air from one person to another. When a person with TB disease of the lungs or throat coughs, speaks, or sings, TB bacteria can get into the air. People nearby may breathe in these bacteria and become infected. TB is NOT spread by. When a person breathes in TB bacteria, the bacteria can settle in the lungs and ...
3. Marvel at St. Basil's Cathedral. St. Basil's Cathedral is one of the most iconic churches in the world, and it was the single thing we were most excited to see while in Moscow. Built almost 500 years ago, St. Basil's Cathedral is recognized by its colorful domes and whimsical style.