Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic

RELATED TOPICS
INTRODUCTION
The treatment and prevention of travelers' diarrhea are discussed here. The epidemiology, microbiology, clinical manifestations, and diagnosis of travelers' diarrhea are discussed separately. (See "Travelers' diarrhea: Epidemiology, microbiology, clinical manifestations, and diagnosis" .)
Clinical approach — Management of travelers’ diarrhea depends on the severity of illness. Fluid replacement is an essential component of treatment for all cases of travelers’ diarrhea. Most cases are self-limited and resolve on their own within three to five days of treatment with fluid replacement only. Antimotility agents can provide symptomatic relief but should not be used when bloody diarrhea is present. Antimicrobial therapy shortens the disease duration, but the benefit of antibiotics must be weighed against potential risks, including adverse effects and selection for resistant bacteria. These issues are discussed in the sections that follow.
When to seek care — Travelers from resource-rich settings who develop diarrhea while traveling to resource-limited settings generally can treat themselves rather than seek medical advice while traveling. However, medical evaluation may be warranted in patients who develop high fever, abdominal pain, bloody diarrhea, or vomiting. Otherwise, for most patients while traveling or after returning home, medical consultation is generally not warranted unless symptoms persist for 10 to 14 days.
Fluid replacement — The primary and most important treatment of travelers' (or any other) diarrhea is fluid replacement, since the most significant complication of diarrhea is volume depletion [ 11,12 ]. The approach to fluid replacement depends on the severity of the diarrhea and volume depletion. Travelers can use the amount of urine passed as a general guide to their level of volume depletion. If they are urinating regularly, even if the color is dark yellow, the diarrhea and volume depletion are likely mild. If there is a paucity of urine and that small amount is dark yellow, the diarrhea and volume depletion are likely more severe.
You are using an outdated browser. Upgrade your browser today or install Google Chrome Frame to better experience this site.
- Section 2 - Travelers’ Diarrhea
- Section 2 - Food & Water Precautions
Perspectives : Antibiotics in Travelers' Diarrhea - Balancing Benefit & Risk
Cdc yellow book 2024.
Author(s): Mark Riddle, Bradley Connor
For the past 30 years, randomized controlled trials have consistently and clearly demonstrated that antibiotics shorten the duration of illness and alleviate the disability associated with travelers’ diarrhea (TD). Treatment with an effective antibiotic shortens the average duration of a TD episode by 1–2 days, and if the traveler combines an antibiotic with an antimotility agent (e.g., loperamide), duration of illness is shortened even further. Emerging data on the potential long-term health consequences of TD (e.g., chronic constipation, dyspepsia, irritable bowel syndrome) might suggest a benefit of early antibiotic therapy given the association between more severe and longer disease and risk for postinfectious consequences.
Antibiotics commonly used to treat TD have side effects, some of which are severe but rare. Perhaps of greater concern is the recent understanding that antibiotics used by travelers can contribute to changes in the host microbiome and to the acquisition of multidrug-resistant bacteria. Multiple observational studies have found that travelers (in particular, travelers to South and Southeast Asia) who develop TD and take antibiotics are at risk for colonization with extended-spectrum β-lactamase–producing Enterobacteriaceae (ESBL-PE).
The direct effect of colonization on the average traveler appears limited; carriage is most often transient, but it does persist in a small percentage of colonized persons. Elderly travelers (because of the serious consequences of bloodstream infections in this population) and those with a history of recurrent urinary tract infections (because Escherichia coli is a common cause) might be at an increased risk for health consequences from ESBL-PE colonization. At a minimum, clinicians should make these travelers aware of the risk and counsel them to convey their travel exposure history to their treating providers if they become ill after travel. Of broader importance, international travel has been associated with subsequent ESBL-PE colonization among close-living contacts, suggesting potentially wider public health consequences from ESBL-PE acquisition during travel.
The Challenge
The challenge providers and travelers face is how to balance the health benefit of short-course antibiotic treatment of TD with the risk for colonization and global spread of resistance. The role played by travelers in the translocation of infectious disease and resistance cannot be ignored, but the ecology of ESBL-PE infections is complex and includes diet, environment, immigration, and local nosocomial transmission dynamics. ESBL-PE infections are an emerging health threat, and addressing this complex problem will require multiple strategies, including antibiotic stewardship.
An Approach
Health care providers need to have conversations with travelers about the multilevel (individual, community, global) and multifactorial risks of developing TD: travel, individual behaviors (e.g., hand hygiene), diet (e.g., safe selection of foods and beverages), and other risk avoidance measures. But then, knowing it is often difficult to prevent or even reduce the risk for TD through behaviors and diet alone, what is the most reasonable way to prepare travelers for empiric TD self-treatment before a trip? Clinicians can strongly emphasize reserving antibiotics for moderate to severe TD and using antimotility agents for self-treatment of mild TD.
When it comes to managing TD, we expect the traveler to be both diagnostician and health care provider. For even the most astute traveler, making an appropriately informed decision about their own health can be challenged by the anxiety-provoking onset of that first abdominal cramp in sometimes austere and inconvenient settings. Given that TD counseling is competing with numerous other pretravel health topics that need to be covered, travel medicine providers might want to develop and implement simple messaging, handouts, or easy-to-access electronic health guidance. Providing travelers with clear written guidance about TD prevention and step-by-step instructions about how and when to use medications for TD is crucial.
Though further studies are needed (and many are under way), a rational approach involves using a single-dose regimen of an antibiotic that minimizes microbiome disruption and risk for colonization. Additionally, as travel and untreated TD independently increase the risk for ESBL-PE colonization, nonantibiotic chemoprophylactic strategies (e.g., self-treatment with bismuth subsalicylate), can decrease both the acute and posttravel risk concerns. Strengthening the resilience of the host microbiota to prevent infection and unwanted colonization, as with the use of prebiotics or probiotics, are promising potential strategies but need further investigation.
The following authors contributed to the previous version of this chapter: Mark S. Riddle, Bradley A. Connor
Bibliography
Arcilla MS, van Hattem JM, Haverkate MR, Bootsma MCJ, van Genderen PJJ, Goorhuis A, et al. Import and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): a prospective, multicentre cohort study. Lancet Infect Dis. 2017;17(1):78–85.
Riddle MS, Connor BA, Beeching NJ, DuPont HL, Hamer DH, Kozarsky P, et al. Guidelines for the prevention and treatment of travelers’ diarrhea: a graded expert panel report. J Travel Med. 2017;24 (Suppl 1):S57–74.
. . . perspectives chapters supplement the clinical guidance in this book with additional content, context, and expert opinion. The views expressed do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
File Formats Help:
- Adobe PDF file
- Microsoft PowerPoint file
- Microsoft Word file
- Microsoft Excel file
- Audio/Video file
- Apple Quicktime file
- RealPlayer file
- Zip Archive file
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.

Issues by year
Advertising
Volume 44, Issue 1, January-February 2015
Advising travellers about management of travellers’ diarrhoea
How is td defined.
Classic, severe TD is usually defined as at least three unformed bowel movements occurring within a 24-hour period, often accompanied by cramps, nausea, vomiting, fever and/or blood in the stools. 5–7 Moderate TD is defined as one or two unformed bowel movements and other symptoms occurring every 24 hours or as three or more unformed bowel movements without additional symptoms. Mild TD is defined as one or two unformed bowel movements without any additional symptoms and without interference with daily activities. 8,9 TD generally resolves spontaneously, usually after 3–4 days, 8 but, in the interim, frequently leads to disruption of planned activities.
What are the causes of TD?
Approximately 50–80% of TD is caused by bacterial infections; enterotoxigenic Escherichia coli (ETEC) is the most common cause overall. Other bacterial causes include enteroinvasive E. coli (EIEC), enteroaggregative E. coli (EAEC), Shigella , Campylobacter and Salmonella species. The exact breakdown of organisms varies according to destination, season and other factors. Noroviruses cause 10–20% of TD cases. Protozoal parasites should be considered particularly in those with persistent diarrhoea (illness lasting ≥14 days) or when antibacterial therapy fails to shorten illness. 10
How can TD be prevented?
Methods for preventing TD include avoidance, immunisation, non-antibiotic interventions or antibiotic prophylaxis. 11
What avoidance measures are generally recommended and do they work?
Avoidance of TD has traditionally relied on recommendations regarding careful food and drink choices (avoiding untreated/unboiled tap water, including ice and water used for brushing teeth, and raw foods such as salads, uncooked vegetables or fruits that cannot be peeled). This underpins the saying ‘Boil it, cook it, peel it or forget it…. easy to remember, impossible to do’. Additional standard advice is that undercooked or raw meat, fish and shellfish are high-risk foods. However, whether deliberately or inadvertently, most people find it very difficult to adhere to dietary restrictions 12 and over 95% of people disobey the rules of ‘safe’ eating and drinking within a few days of leaving home. Additionally, there is minimal evidence for a correlation between adherence to dietary precautions and a reduced risk of TD, 13 although common sense nevertheless supports care with food selection. 4
Where people eat may be more important than what people eat. Risks are associated, in descending order, with street vendors, restaurants and private homes. Use of antibacterial handwash before eating is also recommended. 14
Which vaccines can be considered?
Immunisation has little practical role in the prevention of TD and the only potentially relevant vaccines are those against rotavirus (infants only) and the oral cholera vaccine.
The cholera vaccine has >90% efficacy for prevention of Vibrio cholera but travellers are rarely at risk of infection with this pathogen. 1 The vaccine contains a recombinant B subunit of the cholera toxin that is antigenically similar to the heat-labile toxin of ETEC; therefore, the cholera vaccine may also reduce ETEC TD. However, it is not licensed for TD prevention in Australia and, although initially thought to offer a 15–20% short-term (3 months) reduction in TD, a recent Cochrane review showed no statistically significant effects on ETEC diarrhoea or all-cause diarrhoea. 15 Overall, there is, therefore, insufficient evidence to support general use of the cholera vaccine for TD protection, but it may still be considered for individuals with increased risk of severe or complicated TD (eg immunosuppressed or underlying inflammatory bowel disease).
Other vaccines directed against organisms spread by the faecal–oral route are the vaccines for typhoid, hepatitis A and polio, but infection with these organisms rarely causes TD. 15
Do non-antibiotic interventions work?
Several probiotic agents have been studied for treatment and prevention of TD, including Lactobacillus and Saccharomyces preparations. However, their effectiveness for TD prevention has been limited, 11,16,17 and a consensus group has recommended against their use. 4 Other over-the-counter agents are also available (eg travelan, which contains bovine colostrum harvested from cows immunised with an ETEC vaccine) but data regarding overall efficacy of reducing all-cause TD are currently lacking.
Should antibiotic prophylaxis against TD be given?
Quinolone antibiotics are highly effective (80–95%) in preventing TD, but antibiotic prophylaxis is rarely indicated. 4 It may result in a false sense of security and hence less caution in dietary choices, it poses risks of side effects, diarrhoea associated with Clostridium difficile , and, more importantly, would lead to a vast amount of antibiotic use, thus predisposing to more rapid development of antibiotic resistance globally. 11 Therefore non-antibiotic options for prevention and a focus instead on empirical self-treatment if needed according to symptoms are the mainstay of management, aligning with the antimicrobial stewardship perspective of minimisation of antimicrobial overuse and reducing promotion of antimicrobial resistance.
In rare circumstances, it may be reasonable to consider short courses of antibiotic prophylaxis in individuals at very high risk of infection (eg severely immunocompromised). 11 Globally, one of the most commonly used agents in this regard is rifaximin, a non-absorbed semisynthetic rifamycin derivative, which has been shown to be effective and is approved for use for TD prevention in some countries, but it is not approved for this indication in Australia. Other options include the antibiotics discussed below for TD self-treatment.
How should self-treatment of TD be managed?
Because of the limitations of TD prevention measures, the pre-travel consultation should be viewed as an opportunity to ‘arm’ travellers with the knowledge and medication needed to appropriately self-treat, should TD occur during their trip.
The first goal of therapy is the prevention and treatment of dehydration, which is of particular concern for young children, pregnant women and the elderly. Commercial packets of oral rehydration salts are readily available in pharmacies and should be purchased before travel. The other element of TD self-treatment is to recommend travellers bring an antimotility agent plus an antibiotic with them. Loperamide is preferred over the diphenoxylate/atropine combination, as the latter agent is generally less effective and associated with a greater potential for adverse effects.
When should loperamide alone versus loperamide plus an antibiotic be taken?
For mild symptoms of watery diarrhoea, self-treatment with oral rehydration plus loperamide is recommended. Loperamide therapy alone has no untoward effects in mild TD 18 but if symptoms worsen, or do not improve after 24 hours, antibiotics should be added. If TD is moderate or severe at onset, then combination therapy with loperamide plus antibiotics should be started immediately, as this optimises the clinical benefit of self-treatment by providing more rapid relief and shortening the symptom duration. 10,19
The recommended dose of loperamide is two tablets (4 mg) stat, then one tablet after each bowel motion to a maximum of eight per 24-hour period until the TD has resolved. Despite warnings regarding the safety of antidiarrhoeal agents with bloody diarrhoea or diarrhoea accompanied by fever, the combination with antibiotics is likely to be safe in the setting of mild febrile dysentery, 18 and a number of studies have shown the combination to be more efficacious than use of either agent alone. 7,18–20 Rapid institution of effective treatment shortens symptoms to 30 hours or less in most people. 12 For example, the duration of diarrhoea was significantly ( P = 0.0002) shorter following treatment with azithromycin plus loperamide (11 h) than with azithromycin alone (34 h). 19
Which antibiotic should be recommended for empirical elf-treatment of TD?
The most commonly used antibiotics for empirical TD therapy are fluoroquinolones (either norfloxacin or ciprofloxacin) or azithromycin ( Table 1 ). Cotrimoxazole has been used but is no longer recommended because of widespread resistance. For TD caused by ETEC, the fluoroquinolones and azithromycin have similar efficacy; however, in Asia (particularly South and South-East Asia), Campylobacter is a common cause of TD and strains occurring in this part of the world show a high degree of resistance to fluoroquinolones. 10,21 Therefore, azithromycin is preferred for travellers to this region. Azithromycin remains generally efficacious despite emerging resistance, and is also the preferred treatment for diarrhoea with complications of dysentery or high fever, and for use in pregnant women or children under the age of 8 years, in whom avoidance of quinolones is preferred. Moreover, the 24-hour dosing of azithromycin may be preferable to the 12-hourly dosing schedule required with fluoroquinolones.
What is the optimal dosing schedule?
The fluoroquinolones and azithromycin have been administered as a single dose or for 3 days ( Table 1 ). Usually a single dose is adequate and there is no apparent clinically important difference in efficacy with either dosing schedule for TD. 10 However, for bacteria such as Campylobacter and Shigella dysenteriae , single-dose therapy may be inadequate. 11 It is reasonable, therefore, to give travellers a 3-day supply of antibiotics and tell them to continue taking the therapy (either 12- or 24-hourly, depending on which antibiotic is prescribed) only if their TD symptoms persist. If the TD has resolved, no further antibiotics need to be taken and any remaining antibiotic doses can be kept in case of a second bout of TD. It is prudent to specifically highlight that this advice differs from the usual instructions to take all tablets even if symptoms have resolved.
What is the optimal empirical TD management in children?
There are few data on empirical treatment of TD in children and limited options for therapy. The mainstay of therapy is oral rehydration solution, particularly for children <6 years of age. Antimotility agents are contraindicated for children because of the increased risk of adverse effects, especially paralytic ileus, toxic megacolon and drowsiness (narcotic effect) with loperamide. 1 The lower age limit recommended for avoiding loperamide varies by location; US guidelines state that loperamide should not be given to infants <2 years of age, the UK <4 years and Australian guidelines state <12 years. 14 However, most Australian practitioners are prepared to use loperamide in children aged 6 years or older, if needed to control symptoms.
A paediatric (powder) formulation of azithromycin is available and is the most commonly recommended agent for children. The usual dose is 10–25 mg/kg for up to 3 days. A practical tip is to ensure that the pharmacy does not reconstitute the powder into a solution, as once dissolved, the solution lasts only for 10 days. Instead, sterile water should be provided along with instructions on how to reconstitute the powder if needed. Fluoroquinolones (ciprofloxacin or norfloxacin 10mg/kg bd) are an alternative option if there are reasons for avoiding azithromycin, with previous concerns regarding potential effects on cartilage not substantiated in recent studies. 14,22
Does starting antibiotics early prevent the chances of developing prolonged symptoms?
Although TD symptoms are short-lived in most cases, 8–15% of affected travellers are symptomatic for more than a week and 2% develop chronic diarrhoea lasting a month or more. 11 Episodes of TD have been shown to be associated with a quintuple risk of developing irritable bowel syndrome (IBS), and post-travel IBS occurs in 3–10% of travellers. However, it is unknown whether IBS can be prevented by starting antimicrobial therapy earlier in the course of enteric infection. 4,18,23
Should tinidazole also be prescribed and, if so, for whom?
Tinidazole can be prescribed as a second antibiotic for empirical self‑treatment as it is effective against the protozoan parasitic enteric pathogen Giardia intestinalis . A dose of 2 g (4 x 500 mg tablets) stat is recommended. However, for most short-term travellers, tinidazole may be unnecessary and the complexity of the additional instructions required may be unwarranted. It is optimally recommended, therefore, for travellers departing on trips of significant duration (>2–3 weeks). If prescribed, the instructions should be to take tinidazole if the TD persists following the 3-day course of antibiotic therapy (fluoroquinolone or azithromycin). This will mean that the TD has lasted for at least 72 hours, thus increasing the likelihood of a parasitic cause.
When should medical care for acute symptoms be recommended?
While most episodes of TD are amenable to self-treatment, if there is a risk of dehydration due to intolerance of oral fluids or comorbidities, as well as in the setting of frank blood in the stool or unremitting fevers (>38.5°C for 48 hours), medical therapy should be sought. 18
How should TD be managed after return?
While a full description of TD management is beyond the scope of this article, for returning travellers with diarrhoea, at least one (preferably three) stool sample(s) should be taken, including specific requests for evaluation of parasites. For patients who are unwell, particularly those with fevers or dysentery, initiation of empirical antibiotic treatment with azithromycin or a quinolone may be needed while awaiting results. For those with prolonged symptoms, tinidazole as empirical therapy for protozoan parasites may be considered. Endoscopic evaluation may also be advisable if no infectious cause is found and symptoms do not resolve.
- Travellers’ diarrhoea continues to affect 20–50% of people undertaking trips to areas with under-developed sanitation and there is minimal evidence for beneficial effects of dietary precautions.
- Evidence for the benefit of cholera vaccine in reducing TD is limited, but it can be considered in people at high risk of infection.
- In 50–80% of TD cases, TD is caused by bacterial infection. Mild diarrhoea can be managed with an antimotility agent (loperamide) alone, but for moderate or severe diarrhoea, early self-treatment with loperamide in conjunction with antibiotics is advised.
- Recommended empirical antibiotics are fluoroquinolones (norfloxacin / ciprofloxacin) or azithromycin for up to 3 days, although in the setting of increasing resistance, the latter is preferred for travellers to South and South-East Asia.
Competing interests: Karin Leader received a consultancy fee from Imuron in relation to the C. difficile vaccine. She is also an ISTM board member and received a consultancy from ISTM to join the GeoSentinel leadership team. She received grants from Sanofi to develop a mobile phone app for splenectomised patients and from GSK to research the use of the HBV vaccine. GSK also paid her to lecture on travel risks at the Asia Pacific Travel Health Conference. She has received support from both GSK and Sanofi to attend travel medicine conferences.
Provenance and peer review: Commissioned, externally peer reviewed
- Diemert DJ. Prevention and self-treatment of travelers’ diarrhea. Prim Care 2002;29:843–55. Search PubMed
- Department of Health and Human Services. Centers for Disease Control and Prevention. Travelers’ Diarrhea. Available at www.cdc.gov/ncidod/dbmd/diseaseinfo/travelersdiarrhea_g.htm [Accessed 25 November 2014]. Search PubMed
- Paredes-Paredes M, Flores-Figueroa J, Dupont HL. Advances in the treatment of travelers’ diarrhea. Curr Gastroenterol Rep 2011;13:402–07. Search PubMed
- DuPont HL, Ericsson CD, Farthing MJ, et al. Expert review of the evidence base for prevention of travelers’ diarrhea. J Travel Med 2009;16:149–60. Search PubMed
- Nair D. Travelers’ diarrhea: prevention, treatment, and post-trip evaluation. J Fam Pract 2013;62:356–61. Search PubMed
- De Bruyn G, Hahn S, Borwick A. Antibiotic treatment for travellers’ diarrhoea. The Cochrane Database Syst Rev 2000:CD002242. Search PubMed
- Riddle MS, Arnold S, Tribble DR. Effect of adjunctive loperamide in combination with antibiotics on treatment outcomes in traveler’s diarrhea: a systematic review and meta-analysis. Clin Infect Dis 2008;47:1007–14. Search PubMed
- Steffen R. Epidemiology of traveler’s diarrhea. Clin Infect Dis 2005;41(Suppl 8):S536–40. Search PubMed
- Steffen R, Collard F, Tornieporth N, et al. Epidemiology, etiology, and impact of traveler’s diarrhea in Jamaica. JAMA 1999;281:811–17. Search PubMed
- DuPont HL, Ericsson CD, Farthing MJ, et al. Expert review of the evidence base for self-therapy of travelers’ diarrhea. J Travel Med 2009;16:161–71. Search PubMed
- Diemert DJ. Prevention and self-treatment of traveler’s diarrhea. Clin Microbiol Rev 2006;19:583–94. Search PubMed
- Travelers’ diarrhea. NIH Consensus Development Conference. JAMA 1985;253:2700–04. Search PubMed
- Shlim DR. Looking for evidence that personal hygiene precautions prevent traveler’s diarrhea. Clin Infect Dis 2005;41(Suppl 8):S531–35. Search PubMed
- Plourde PJ. Travellers’ diarrhea in children. Paediatr Child Health 2003;8:99–103. Search PubMed
- Ahmed T, Bhuiyan TR, Zaman K, Sinclair D, Qadri F. Vaccines for preventing enterotoxigenic Escherichia coli (ETEC) diarrhoea. Cochrane Database Syst Rev 2013;7:CD009029. Search PubMed
- Ritchie ML, Romanuk TN. A meta-analysis of probiotic efficacy for gastrointestinal diseases. PloS One 2012;7:e34938. Search PubMed
- Centers for Disease Control Prevention. Yellow Book. Chapter 2. Travelers’ Diarrhea. Available at wwwnc.cdc.gov/travel/yellowbook/2014/chapter-2-the-pre-travel-consultation/travelers-diarrhea [Accessed 25 November 2014]. Search PubMed
- Wingate D, Phillips SF, Lewis SJ, et al. Guidelines for adults on self-medication for the treatment of acute diarrhoea. Aliment Pharmacol Ther 2001;15:773–82. Search PubMed
- Ericsson CD, DuPont HL, Okhuysen PC, Jiang ZD, DuPont MW. Loperamide plus azithromycin more effectively treats travelers’ diarrhea in Mexico than azithromycin alone. J Travel Med 2007;14:312–19. Search PubMed
- Murphy GS, Bodhidatta L, Echeverria P, et al. Ciprofloxacin and loperamide in the treatment of bacillary dysentery. Ann Intern Med 1993;118:582–86. Search PubMed
- Tribble DR, Sanders JW, Pang LW, et al. Traveler’s diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis 2007;44:338–46. Search PubMed
- Yung A, Leder K, Torresi J, et al. Manual of Travel Medicine. 3rd edn. Melbourne: IP Communciations, 2011. Search PubMed
- Stermer E, Lubezky A, Potasman I, Paster E, Lavy A. Is traveler’s diarrhea a significant risk factor for the development of irritable bowel syndrome? A prospective study. Clin Infect Dis 2006;43:898–901. Search PubMed
- Expert Group for Antibiotic. Antiobiotic: gastrointestinal tract infections: acute gastroenteritis: acute diarrhoea in special groups: travellers’ diarrhoea. In: eTG Complete [Internet] Melbourne. Therapeutic Guidelines Ltd, 2014. Search PubMed
Also in this issue: Environmental
Professional
Printed from Australian Family Physician - https://www.racgp.org.au/afp/2015/january-february/advising-travellers-about-management-of-travellers © The Australian College of General Practitioners www.racgp.org.au
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
Travellers’ diarrhoea
Chinese translation.
- Related content
- Peer review
- Jessica Barrett , infectious diseases registrar 1 ,
- Mike Brown , consultant in infectious diseases and tropical medicine 1 2
- 1 Hospital for Tropical Diseases, University College London Hospitals NHS Trust, London WC1E 6AU, UK
- 2 Clinical Research Department, London School of Hygiene & Tropical Medicine, London, UK
- Correspondence to: J Barrett jessica.barrett{at}gstt.nhs.uk
What you need to know
Enterotoxic Escherichia coli (ETEC) is the most common cause of acute travellers’ diarrhoea globally
Chronic (>14 days) diarrhoea is less likely to be caused by bacterial pathogens
Prophylactic antibiotic use is only recommended for patients vulnerable to severe sequelae after a short period of diarrhoea, such as those with ileostomies or immune suppression
A short course (1-3 days) of antibiotics taken at the onset of travellers’ diarrhoea reduces the duration of the illness from 3 days to 1.5 days
Refer patients with chronic diarrhoea and associated symptoms such as weight loss for assessment by either an infectious diseases specialist or gastroenterologist
Diarrhoea is a common problem affecting between 20% and 60% of travellers, 1 particularly those visiting low and middle income countries. Travellers’ diarrhoea is defined as an increase in frequency of bowel movements to three or more loose stools per day during a trip abroad, usually to a less economically developed region. This is usually an acute, self limiting condition and is rarely life threatening. In mild cases it can affect the enjoyment of a holiday, and in severe cases it can cause dehydration and sepsis. We review the current epidemiology of travellers’ diarrhoea, evidence for different management strategies, and the investigation and treatment of persistent diarrhoea after travel.
We searched PubMed and Cochrane Library databases for “travellers’ diarrhoea,” and “travel-associated diarrhoea,” to identify relevant articles, which were added to personal reference collections and clinical experience. Where available, systematic reviews and randomised controlled trials were preferentially selected.
Who is at risk?
Variation in incidence 1 2 may reflect the degree of risk for different travel destinations and dietary habits while abroad. Destinations can be divided into low, medium, and high risk (see box 1). Rates of diarrhoea are likely to correlate closely with the quality of local sanitation.
Box 1: Risk of travellers’ diarrhoea according to destination 1 3
High risk destinations.
South and South East Asia*
Central America*
West and North Africa*
South America
East Africa
Medium risk
South Africa
North America
Western Europe
Australia and New Zealand
*Regions with particularly high risk of travellers’ diarrhoea
Backpackers have roughly double the incidence of diarrhoea compared with business travellers. 4 Travel in cruise ships is associated with large outbreaks of viral and bacterial gastroenteritis. 5 General advice is to avoid eating salads, shellfish, and uncooked meats. There is no strong evidence that specific dietary measures reduce incidence of diarrhoea, but studies examining this are likely to be biased by imperfect recall of what was eaten. 6 Risk factors for travellers’ diarrhoea are listed in box 2.
Box 2: Factors increasing risk of travellers’ diarrhoea 4 7 8 9
By increased dietary exposure.
Backpacking
Visiting friends and family
All-inclusive holidays (such as in cruise ships)
By increased susceptibility to an infectious load
Age <6 years
Use of H 2 receptor antagonists and proton pump inhibitors
Altered upper gastrointestinal anatomy
Genetic factors (blood group O predisposes to shigellosis and severe cholera infection)
What are the most important causes of travellers’ diarrhoea?
Most studies report a failure to identify the causative pathogen in between 40% and 70% of cases. 10 This includes multicentre studies based in high prevalence settings (that is, during travel). 3 10 11 12 This low diagnostic yield is partly due to delay in obtaining samples and partly due to the insensitivity of laboratory investigations. Older studies did not consistently attempt to identify enteroaggregative Escherichia coli (EAEC), and surveillance studies vary in reporting of other E coli species. 3 Where a pathogen is identified, bacteria are the commonest cause of acute travellers’ diarrhoea, with the remainder being caused by norovirus, rotavirus, or similar viruses (see table 1 ⇓ ). Protozoa such as Giardia lamblia can also cause acute diarrhoea, but they are more often associated with persistent diarrhoea, lasting more than two weeks. Cyclospora catayensis , another protozoan cause of diarrhoea, was identified in an increased number of symptomatic travellers returning from Mexico to the UK and Canada in 2015. 13
Frequency of pathogens causing travellers’ diarrhoea 2 3 10 11 12
- View inline
Table 1 ⇑ illustrates overall prevalence of causative agents in returning travellers with diarrhoea. However relative importance varies with country of exposure. Rates of enterotoxigenic E coli (ETEC) are lower in travellers returning from South East Asia than in those returning from South Asia, sub-Saharan Africa, and Latin America, whereas rates of Campylobacter jejuni are higher. Norovirus is a more common cause in travellers to Latin America and sub-Saharan Africa, and Giardia lamblia and Entamoeba histolytica are more common in travellers to South and South East Asia. 10
The importance of enterotoxigenic E coli as a cause for diarrhoea in travellers returning from Latin America has been decreasing over the past four decades. 10 A large scale analysis of EuroTravNet surveillance data shows increasing incidence of Campylobacter jejuni infection in travellers returning from India, Thailand, and Pakistan. 2
How does travellers’ diarrhoea present?
Most episodes of travellers’ diarrhoea start during the first week of travel, with the peak incidence on the second or third day after arrival. 8 14
Typically diarrhoea caused by enterotoxigenic E coli (“turista”) is watery and profuse, and preceded by abdominal cramps, nausea, and malaise. Symptoms are not a reliable guide to aetiology, but upper gastrointestinal manifestations such as bloating and belching tend to predominate with Giardia lamblia , while colitic symptoms such as urgency, bloody diarrhoea, and cramps are seen more often with Campylobacter jejuni and Shigella spp.
Most episodes will last between one and seven days, with approximately 10% lasting for longer than one week, 5% lasting more than two weeks, and 1% lasting more than 30 days. 8 During the illness, few patients will be severely incapacitated (in one large prospective cohort about 10% of 2800 participants were confined to bed or consulted a physician), but planned activities are often cancelled or postponed. 8
How can travellers’ diarrhoea be prevented?
Several controlled trials have failed to demonstrate an impact of food and drink hygiene advice on rates of diarrhoea. 15 However, the clear food-related source of most diarrhoeal pathogens means that general consensus among travel physicians is to continue to recommend boiling water, cooking food thoroughly, and peeling fruit and vegetables. 6 Other basic advice includes avoiding ice, shellfish, and condiments on restaurant tables, using a straw to drink from bottles, and avoiding salads and buffets where food may have been unrefrigerated for several hours. Travellers should be advised to drink bottled water where available, including in alcoholic drinks, as alcohol does not sterilise non-bottled water. If bottled water is not available, water can be purified by boiling, filtering, or use of chlorine based tablets. 16 There is some weak evidence that use of alcohol hand gel may reduce diarrhoea rates in travellers, 17 but, based on studies in non-travellers, it is reasonable to strongly encourage travellers to adhere to good hand hygiene measures. Two recent systematic reviews estimated hand washing with soap reduces the risk of diarrhoeal illness by 30-40%. 18 19
When is antibiotic prophylaxis recommended?
For most travellers antibiotic chemoprophylaxis (that is, daily antibiotics for the duration of the trip) is not recommended. While diarrhoea is annoying and distressing, severe or long term consequences from a short period of diarrhoea are rare, and routine use of chemoprophylaxis would create a large tablet burden and expose users to possible adverse effects of antibiotic therapy such as candidiasis and diarrhoea associated with Clostridium difficile .
Chemoprophylaxis should be offered to those with severe immune suppression (such as from chemotherapy for malignancy or after a tissue transplant, or advanced HIV infection), underlying intestinal pathology (inflammatory bowel disease, ileostomies, short bowel syndrome), and other conditions such as sickle cell disease or diabetes where reduced oral intake may be particularly dangerous (table 2 ⇓ ). 22 These patient groups may be unable to tolerate the clinical effects and dehydration associated with even mild diarrhoea, or the consequences of more invasive complications such as bacteraemia. For such patients, it is important to discuss the benefits of treatment aimed at preventing diarrhoea and its complications against the risks of antibiotic associated diarrhoea and other side effects. If antibiotics are prescribed then consideration should be given to any possible interactions with other medications that the patient is taking.
Antibiotic chemoprophylaxis options for immunosuppressed or other high risk travellers
A small comparative study in US soldiers showed that malaria prophylaxis with daily doxycycline has the added benefit of reducing rates of travellers’ diarrhoea caused by enterotoxigenic E coli and Campylobacter jejuni . 23
Do vaccines have a role in prevention of travellers’ diarrhoea?
Vaccines have been developed and licensed against Salmonella typhi , Vibrio cholerae , and rotavirus—all with reasonable efficacy. However, unlike enterotoxigenic E coli , none of these is a major cause of travellers’ diarrhoea, and only vaccines against S typhi are recommended for most travellers to endemic settings. Phase 3 trials of enterotoxigenic E coli toxin vaccines have been undertaken but have failed to demonstrate efficacy. 24 Studies suggest vaccines against enterotoxigenic E coli would have a major public health impact in high burden countries, and further candidate vaccines are in development. 25
What are the options for self administered treatment?
Table 3 ⇓ summarises the options for self treatment.
Summary of self treatment choices
Anti-motility agents and oral rehydration therapy
For most cases of travellers’ diarrhoea, oral rehydration is the mainstay of treatment. This can be achieved with clear fluids such as diluted fruit juice or soups. Young children, elderly people, and those at greater risk from dehydration (that is, those with medical comorbidities) are recommended to use oral rehydration salts (or a mixture of six level teaspoons of sugar and half a teaspoon of salt in a litre of clean water if rehydration salts are unavailable) (see http://rehydrate.org/rehydration/index.html ).
Anti-motility agents such as loperamide may be appropriate for mild symptoms, or where rapid cessation of diarrhoea is essential. Case reports of adverse outcomes such as intestinal perforation suggest anti-motility agents should be avoided in the presence of severe abdominal pain or bloody diarrhoea, which can signify invasive colitis. 26 Systematic review of several randomised controlled trials have demonstrated a small benefit from taking bismuth subsalicylate, but this has less efficacy in reducing diarrhoea frequency and severity than loperamide. 27
Antibiotics
Symptomatic treatment is usually adequate and reduces antibiotic use. However, some travellers will benefit from rapid cessation of diarrhoea, particularly if they are in a remote area with limited access to sanitation facilities or healthcare. Several systematic reviews of studies comparing antibiotics (including quinolones, azithromycin, and rifaximin) against placebo have shown consistent shortening of the duration of diarrhoea to about one and a half days from around three days. 28 29 30 Short courses (one to three days) of antibiotics are usually sufficient to effect a cure. 30
For some people travelling to high and moderate risk areas (see box 1) it will be appropriate to provide a short course of a suitable antibiotic, with advice to start treatment as soon as they develop diarrhoea and to keep well hydrated. Choice of antibiotic will depend on allergy history, comorbidities, concomitant medications, and destination. Avoid quinolones for both prophylaxis and treatment of travellers to South East and South Asia as levels of quinolone resistance are high. 31 Azithromycin remains effective in these areas, but resistance rates are likely to increase.
A meta-analysis of nine randomised trials showed that the addition of loperamide to antibiotic treatment (including azithromycin, ciprofloxacin, and rifamixin) resulted in statistically significantly higher rates of cure at 24 and 48 hours compared with antibiotic alone. 32 Travellers can be advised to add loperamide to their antibiotic treatment to reduce the time to symptomatic improvement as long as there are no features of invasive colitis such as severe pain, high fever, or blood visible in the diarrhoea. 30 If any of these symptoms develop, travellers are advised to seek medical advice immediately.
Returned travellers with persistent diarrhoea
Most bacterial causes mentioned do not cause persistent diarrhoea in immune competent adults. Travellers with diarrhoea persisting beyond 14 days may present in primary or secondary care on their return and require assessment for other underlying causes of persistent diarrhoea.
Table 4 ⇓ lists the important clinical history and symptoms that can point to the underlying cause.
Assessment of chronic diarrhoea
What investigations should be sent?
For diarrhoeal symptoms that persist beyond 14 days following travel (or sooner if there are other concerning features such as fever or dysentery), offer patients blood tests for full blood count, liver and renal function, and inflammatory markers; stool samples for microscopy and culture; and examination for ova, cysts, and parasites. Historically, advice has been to send three stool samples for bacterial culture, but this is unlikely to increase the diagnostic yield. Instead, stool microscopy can be used to distinguish inflammatory from non-inflammatory causes: a small observational study found presence of faecal leucocytes was predictive of a positive bacterial stool culture. 33 Yield from stool culture may be increased by dilution of the faecal sample, and the introduction of molecular tests such as polymerase chain reaction (PCR) for common gastrointestinal pathogens such as Campylobacter spp may decrease turnaround times and increase yield. 34
Additional tests should be offered according to symptoms and risk (table 4 ⇑ ). If the patient has eosinophilia and an appropriate travel history, the possibility of schistosomiasis, strongyloides, and other helminthic infections should be considered. While schistosomiasis can rarely cause diarrhoea in the context of acute infection, serology may be negative in the first few months of the illness.
Imaging is required only if the patient has signs of severe colitis or local tenderness, in which instances toxic megacolon, inflammatory phlegmon, and hepatic collections should be excluded. Patients with severe colitis or proctitis may need joint assessment with gastroenterology and consideration of endoscopy, or laparotomy if perforation has occurred.
Where infectious and non-infectious causes have been appropriately excluded, the most likely diagnosis is post-infectious irritable bowel syndrome, although diarrhoea can also herald underlying bowel pathology and anyone with red flags for malignancy should be referred by the appropriate pathway for assessment. Post-infectious irritable bowel syndrome has an incidence of around 30% after an acute episode of travel associated gastroenteritis. 35 36 It is more commonly a sequela of prolonged episodes of diarrhoea or diarrhoea associated with fever and bloody stools. 36 There is weak evidence from small randomised trials suggesting that exclusion of foods high in fermentable carbohydrates (FODMAP) may be helpful. 37 Exclusion of dietary lactose and use of loperamide, bile acid sequestrants, and probiotics can also be tried, but there is limited evidence for long term benefit. 35 37 38
How should giardiasis be managed?
The most common pathogen identified in returning travellers with chronic diarrhoea is Giardia lamblia, particularly among people returning from South Asia. 39 Use of G lamblia PCR testing has increased detection, 40 which potentially will identify infection in some patients previously labelled as having post-infectious irritable bowel syndrome and in those whose diarrhoea may have been attributed to non-pathogenic protozoa. Most patients respond to 5-nitroimidazoles (a systematic review of a large number of trials has shown similar cure rates with tinidazole 2 g once only or metronidazole 400 mg three times daily for five days 41 ), but refractory cases are increasingly common and require investigation, identification of underlying risk factors, and repeated treatment (various antimicrobials have been shown to be effective but may have challenging risk profiles). .
Questions for future research
What is the justification for using antibiotics to treat a usually self limiting illness, in the wider context of rising levels of global antimicrobial resistance rates? What is the clinical impact of resistant enterobacteriaciae found in stool samples from returning travellers? 42 43
To what extent do host genetic factors increase susceptibility to gastrointestinal pathogens, and can this help to identify at risk populations and tailor treatments to individual patients?
What is the long term efficacy of new pharmacological treatments such as selective serotonin reuptake inhibitors and rifaximin in post-infectious irritable bowel syndrome?
Tips for non-specialists
Include consideration of chemoprophylaxis for high risk individuals in pre-travel assessment
Advise all travellers on hygiene measures (such as hand washing and food consumption) and symptom management of diarrhoea
Avoid quinolones for prophylaxis or treatment in travellers to South East and South Asia
Where diarrhoea persists beyond 14 days, consider investigations to rule out parasitic and non-infectious causes. The presence of white blood cells on stool microscopy indicates an inflammatory cause
Additional educational resources
Resources for patients.
National Travel Health Network and Centre (NaTHNaC): http://travelhealthpro.org.uk/travellers-diarrhoea/
Provides pre-travel advice, as well as links to country-specific advice
Fit for Travel: www.fitfortravel.nhs.uk/advice/disease-prevention-advice/travellers-diarrhoea.aspx
Provides similar pre-travel advice on hygiene and disease prevention
Patient.co.uk: http://patient.info/doctor/travellers-diarrhoea-pro
Has patient leaflets and more detailed information about investigation and management of travellers’ diarrhoea
Resources for healthcare professionals
Centers for Disease Control and Prevention yellow book: http://wwwnc.cdc.gov/travel/yellowbook/2016/the-pre-travel-consultation/travelers-diarrhea
Provides a guide to pre-travel couselling
Rehydration Project website: http://rehydrate.org/rehydration/index.html
Has additional information about non-pharmacological management of diarrhoea
How patients were involved in the creation of the article
No patients were involved in the creation of this review.
Contributors: Both authors contributed equally to the preparation of this manuscript. MB is guarantor. We thank Dr Ron Behrens for sharing his extensive expertise on this subject.
Competing interests: We have read and understood BMJ policy on declaration of interests and have no relevant interests to declare.
Provenance and peer review: Commissioned; externally peer reviewed.
- ↵ Greenwood Z, Black J, Weld L, et al. GeoSentinel Surveillance Network. Gastrointestinal infection among international travelers globally. J Travel Med 2008 ; 15 : 221 - 8 . doi:10.1111/j.1708-8305.2008.00203.x pmid:18666921 . OpenUrl Abstract / FREE Full Text
- ↵ Schlagenhauf P, Weld L, Goorhuis A, et al. EuroTravNet. Travel-associated infection presenting in Europe (2008-12): an analysis of EuroTravNet longitudinal, surveillance data, and evaluation of the effect of the pre-travel consultation. Lancet Infect Dis 2015 ; 15 : 55 - 64 . doi:10.1016/S1473-3099(14)71000-X pmid:25477022 . OpenUrl CrossRef PubMed
- ↵ Health Protection Agency. Foreign travel-associated illness: a focus on travellers’ diarrhoea. HPA, 2010. http://webarchive.nationalarchives.gov.uk/20140714084352/http:/www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1287146380314 .
- ↵ Schindler VM, Jaeger VK, Held L, Hatz C, Bühler S. Travel style is a major risk factor for diarrhoea in India: a prospective cohort study. Clin Microbiol Infect 2015 ; 21 : 676.e1 - 4 . doi:10.1016/j.cmi.2015.03.005 pmid:25882361 . OpenUrl CrossRef
- ↵ Freeland AL, Vaughan GH Jr, , Banerjee SN. Acute gastroenteritis on cruise ships - United States, 2008-2014. MMWR Morb Mortal Wkly Rep 2016 ; 65 : 1 - 5 . doi:10.15585/mmwr.mm6501a1 pmid:26766396 . OpenUrl PubMed
- ↵ DuPont HL, Ericsson CD, Farthing MJ, et al. Expert review of the evidence base for prevention of travelers’ diarrhea. J Travel Med 2009 ; 16 : 149 - 60 . doi:10.1111/j.1708-8305.2008.00299.x pmid:19538575 . OpenUrl Abstract / FREE Full Text
- ↵ Bavishi C, Dupont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther 2011 ; 34 : 1269 - 81 . doi:10.1111/j.1365-2036.2011.04874.x pmid:21999643 . OpenUrl CrossRef PubMed
- ↵ Pitzurra R, Steffen R, Tschopp A, Mutsch M. Diarrhoea in a large prospective cohort of European travellers to resource-limited destinations. BMC Infect Dis 2010 ; 10 : 231 . doi:10.1186/1471-2334-10-231 pmid:20684768 . OpenUrl CrossRef PubMed
- ↵ Flores J, Okhuysen PC. Genetics of susceptibility to infection with enteric pathogens. Curr Opin Infect Dis 2009 ; 22 : 471 - 6 . doi:10.1097/QCO.0b013e3283304eb6 pmid:19633551 . OpenUrl CrossRef PubMed
- ↵ Shah N, DuPont HL, Ramsey DJ. Global etiology of travelers’ diarrhea: systematic review from 1973 to the present. Am J Trop Med Hyg 2009 ; 80 : 609 - 14 . pmid:19346386 . OpenUrl Abstract / FREE Full Text
- ↵ von Sonnenburg F, Tornieporth N, Waiyaki P, et al. Risk and aetiology of diarrhoea at various tourist destinations. Lancet 2000 ; 356 : 133 - 4 . doi:10.1016/S0140-6736(00)02451-X pmid:10963251 . OpenUrl CrossRef PubMed Web of Science
- ↵ Steffen R, Collard F, Tornieporth N, et al. Epidemiology, etiology, and impact of traveler’s diarrhea in Jamaica. JAMA 1999 ; 281 : 811 - 7 . doi:10.1001/jama.281.9.811 pmid:10071002 . OpenUrl CrossRef PubMed Web of Science
- ↵ Nichols GL, Freedman J, Pollock KG, et al. Cyclospora infection linked to travel to Mexico, June to September 2015. Euro Surveill 2015 ; 20 . doi:10.2807/1560-7917.ES.2015.20.43.30048 pmid:26536814 .
- ↵ Steffen R, Collard F, Tornieporth N, et al. Epidemiology, etiology, and impact of traveler’s diarrhea in Jamaica. JAMA. 1999 ; 281 : 811 - 7 . doi:10.1001/jama.281.9.811 pmid:10071002 . OpenUrl CrossRef PubMed Web of Science
- ↵ DuPont HL. Systematic review: prevention of travellers’ diarrhoea. Aliment Pharmacol Ther 2008 ; 27 : 741 - 51 . doi:10.1111/j.1365-2036.2008.03647.x pmid:18284650 . OpenUrl CrossRef PubMed Web of Science
- ↵ Clasen TF, Alexander KT, Sinclair D, et al. Interventions to improve water quality for preventing diarrhoea. Cochrane Database Syst Rev 2015 ; 10 : CD004794 . pmid:26488938 . OpenUrl PubMed
- ↵ Henriey D, Delmont J, Gautret P. Does the use of alcohol-based hand gel sanitizer reduce travellers’ diarrhea and gastrointestinal upset?: A preliminary survey. Travel Med Infect Dis 2014 ; 12 : 494 - 8 . doi:10.1016/j.tmaid.2014.07.002 pmid:25065273 . OpenUrl CrossRef PubMed
- ↵ Ejemot-Nwadiaro RI, Ehiri JE, Arikpo D, Meremikwu MM, Critchley JA. Hand washing promotion for preventing diarrhoea. Cochrane Database Syst Rev 2015 ; 9 : CD004265 . pmid:26346329 . OpenUrl PubMed
- ↵ Freeman MC, Stocks ME, Cumming O, et al. Hygiene and health: systematic review of handwashing practices worldwide and update of health effects. Trop Med Int Health 2014 ; 19 : 906 - 16 . doi:10.1111/tmi.12339 pmid:24889816 . OpenUrl CrossRef PubMed
- Hu Y, Ren J, Zhan M, Li W, Dai H. Efficacy of rifaximin in prevention of travelers’ diarrhea: a meta-analysis of randomized, double-blind, placebo-controlled trials. J Travel Med 2012 ; 19 : 352 - 6 . doi:10.1111/j.1708-8305.2012.00650.x pmid:23379704 . OpenUrl Abstract / FREE Full Text
- Alajbegovic S, Sanders JW, Atherly DE, Riddle MS. Effectiveness of rifaximin and fluoroquinolones in preventing travelers’ diarrhea (TD): a systematic review and meta-analysis. Syst Rev 2012 ; 1 : 39 . doi:10.1186/2046-4053-1-39 pmid:22929178 . OpenUrl CrossRef PubMed
- ↵ Sommet J, Missud F, Holvoet L, et al. Morbidity among child travellers with sickle-cell disease visiting tropical areas: an observational study in a French tertiary care centre. Arch Dis Child 2013 ; 98 : 533 - 6 . doi:10.1136/archdischild-2012-302500 pmid:23661574 . OpenUrl Abstract / FREE Full Text
- ↵ Arthur JD, Echeverria P, Shanks GD, Karwacki J, Bodhidatta L, Brown JE. A comparative study of gastrointestinal infections in United States soldiers receiving doxycycline or mefloquine for malaria prophylaxis. Am J Trop Med Hyg 1990 ; 43 : 608 - 13 . pmid:2267964 . OpenUrl Abstract / FREE Full Text
- ↵ Behrens RH, Cramer JP, Jelinek T, et al. Efficacy and safety of a patch vaccine containing heat-labile toxin from Escherichia coli against travellers’ diarrhoea: a phase 3, randomised, double-blind, placebo-controlled field trial in travellers from Europe to Mexico and Guatemala. Lancet Infect Dis 2014 ; 14 : 197 - 204 . doi:10.1016/S1473-3099(13)70297-4 pmid:24291168 . OpenUrl CrossRef PubMed
- ↵ Bourgeois AL, Wierzba TF, Walker RI. Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine 2016 ; S0264-410X(16)00287-5 . pmid:26988259 .
- ↵ McGregor A, Brown M, Thway K, Wright SG. Fulminant amoebic colitis following loperamide use. J Travel Med 2007 ; 14 : 61 - 2 . doi:10.1111/j.1708-8305.2006.00096.x pmid:17241255 . OpenUrl Abstract / FREE Full Text
- ↵ Heather CS. Travellers’ diarrhoea. Systematic review 901. BMJ Clin Evid 2015 www.clinicalevidence.com/x/systematic-review/0901/overview.html .
- ↵ De Bruyn G, Hahn S, Borwick A. Antibiotic treatment for travellers’ diarrhoea. Cochrane Database Syst Rev 2000 ;( 3 ): CD002242 . pmid:10908534 .
- ↵ Ternhag A, Asikainen T, Giesecke J, Ekdahl K. A meta-analysis on the effects of antibiotic treatment on duration of symptoms caused by infection with Campylobacter species. Clin Infect Dis 2007 ; 44 : 696 - 700 . doi:10.1086/509924 pmid:17278062 . OpenUrl Abstract / FREE Full Text
- ↵ Steffen R, Hill DR, DuPont HL. Traveler’s diarrhea: a clinical review. JAMA 2015 ; 313 : 71 - 80 . doi:10.1001/jama.2014.17006 pmid:25562268 . OpenUrl CrossRef PubMed
- ↵ Dalhoff A. Global fluoroquinolone resistance epidemiology and implictions for clinical use. Interdiscip Perspect Infect Dis 2012 ; 2012 : 976273 . pmid:23097666 . OpenUrl CrossRef PubMed
- ↵ Riddle MS, Arnold S, Tribble DR. Effect of adjunctive loperamide in combination with antibiotics on treatment outcomes in traveler’s diarrhea: a systematic review and meta-analysis. Clin Infect Dis 2008 ; 47 : 1007 - 14 . doi:10.1086/591703 pmid:18781873 . OpenUrl Abstract / FREE Full Text
- ↵ McGregor AC, Whitty CJ, Wright SG. Geographic, symptomatic and laboratory predictors of parasitic and bacterial causes of diarrhoea in travellers. Trans R Soc Trop Med Hyg 2012 ; 106 : 549 - 53 . doi:10.1016/j.trstmh.2012.04.008 pmid:22818743 . OpenUrl Abstract / FREE Full Text
- ↵ Public Health England. Investigations of faecal specimens for enteric pathogens. UK Standards for Microbiology Investigations. B 30 Issue 8.1. 2014. www.hpa.org.uk/SMI/pdf .
- ↵ DuPont AW. Postinfectious irritable bowel syndrome. Clin Infect Dis 2008 ; 46 : 594 - 9 . doi:10.1086/526774 pmid:18205536 . OpenUrl Abstract / FREE Full Text
- ↵ Schwille-Kiuntke J, Mazurak N, Enck P. Systematic review with meta-analysis: post-infectious irritable bowel syndrome after travellers’ diarrhoea. Aliment Pharmacol Ther 2015 ; 41 : 1029 - 37 . doi:10.1111/apt.13199 pmid:25871571 . OpenUrl CrossRef PubMed
- ↵ Halland M, Saito YA. Irritable bowel syndrome: new and emerging treatments. BMJ 2015 ; 350 : h1622 . doi:10.1136/bmj.h1622 pmid:26088265 . OpenUrl Abstract / FREE Full Text
- ↵ Orekoya O, McLaughlin J, Leitao E, Johns W, Lal S, Paine P. Quantifying bile acid malabsorption helps predict response and tailor sequestrant therapy. Clin Med (Lond) 2015 ; 15 : 252 - 7 . doi:10.7861/clinmedicine.15-3-252 pmid:26031975 . OpenUrl CrossRef PubMed
- ↵ Swaminathan A, Torresi J, Schlagenhauf P, et al. GeoSentinel Network. A global study of pathogens and host risk factors associated with infectious gastrointestinal disease in returned international travellers. J Infect 2009 ; 59 : 19 - 27 . doi:10.1016/j.jinf.2009.05.008 pmid:19552961 . OpenUrl CrossRef PubMed Web of Science
- ↵ Nabarro LE, Lever RA, Armstrong M, Chiodini PL. Increased incidence of nitroimidazole-refractory giardiasis at the Hospital for Tropical Diseases, London: 2008-2013. Clin Microbiol Infect 2015 ; 21 : 791 - 6 . doi:10.1016/j.cmi.2015.04.019 pmid:25975511 . OpenUrl CrossRef PubMed
- ↵ Pasupuleti V, Escobedo AA, Deshpande A, Thota P, Roman Y, Hernandez AV. Efficacy of 5-nitroimidazoles for the treatment of giardiasis: a systematic review of randomized controlled trials. PLoS Negl Trop Dis 2014 ; 8 : e2733 . doi:10.1371/journal.pntd.0002733 pmid:24625554 . OpenUrl CrossRef PubMed
- ↵ Tham J, Odenholt I, Walder M, Brolund A, Ahl J, Melander E. Extended-spectrum beta-lactamase-producing Escherichia coli in patients with travellers’ diarrhoea. Scand J Infect Dis 2010 ; 42 : 275 - 80 . doi:10.3109/00365540903493715 pmid:20121649 . OpenUrl CrossRef PubMed
- ↵ Kantele A. A call to restrict prescribing antibiotics for travellers’ diarrhea--Travel medicine practitioners can play an active role in preventing the spread of antimicrobial resistance. Travel Med Infect Dis 2015 ; 13 : 213 - 4 . doi:10.1016/j.tmaid.2015.05.005 pmid:26005160 . OpenUrl CrossRef PubMed
- Patient Care & Health Information
- Diseases & Conditions
- Traveler's diarrhea
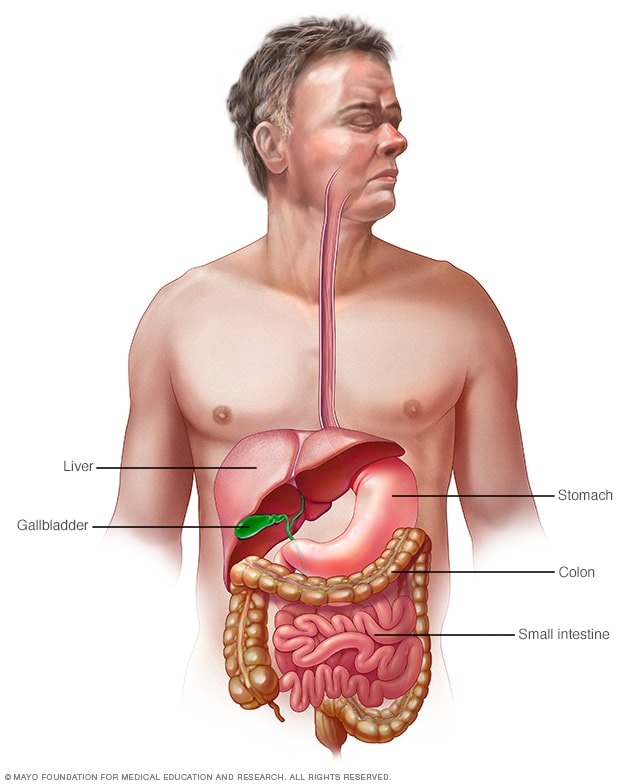
Gastrointestinal tract
Your digestive tract stretches from your mouth to your anus. It includes the organs necessary to digest food, absorb nutrients and process waste.
Traveler's diarrhea is a digestive tract disorder that commonly causes loose stools and stomach cramps. It's caused by eating contaminated food or drinking contaminated water. Fortunately, traveler's diarrhea usually isn't serious in most people — it's just unpleasant.
When you visit a place where the climate or sanitary practices are different from yours at home, you have an increased risk of developing traveler's diarrhea.
To reduce your risk of traveler's diarrhea, be careful about what you eat and drink while traveling. If you do develop traveler's diarrhea, chances are it will go away without treatment. However, it's a good idea to have doctor-approved medicines with you when you travel to high-risk areas. This way, you'll be prepared in case diarrhea gets severe or won't go away.
Products & Services
- A Book: Mayo Clinic Book of Home Remedies
- A Book: Mayo Clinic on Digestive Health
Traveler's diarrhea may begin suddenly during your trip or shortly after you return home. Most people improve within 1 to 2 days without treatment and recover completely within a week. However, you can have multiple episodes of traveler's diarrhea during one trip.
The most common symptoms of traveler's diarrhea are:
- Suddenly passing three or more looser watery stools a day.
- An urgent need to pass stool.
- Stomach cramps.
Sometimes, people experience moderate to severe dehydration, ongoing vomiting, a high fever, bloody stools, or severe pain in the belly or rectum. If you or your child experiences any of these symptoms or if the diarrhea lasts longer than a few days, it's time to see a health care professional.
When to see a doctor
Traveler's diarrhea usually goes away on its own within several days. Symptoms may last longer and be more severe if it's caused by certain bacteria or parasites. In such cases, you may need prescription medicines to help you get better.
If you're an adult, see your doctor if:
- Your diarrhea lasts beyond two days.
- You become dehydrated.
- You have severe stomach or rectal pain.
- You have bloody or black stools.
- You have a fever above 102 F (39 C).
While traveling internationally, a local embassy or consulate may be able to help you find a well-regarded medical professional who speaks your language.
Be especially cautious with children because traveler's diarrhea can cause severe dehydration in a short time. Call a doctor if your child is sick and has any of the following symptoms:
- Ongoing vomiting.
- A fever of 102 F (39 C) or more.
- Bloody stools or severe diarrhea.
- Dry mouth or crying without tears.
- Signs of being unusually sleepy, drowsy or unresponsive.
- Decreased volume of urine, including fewer wet diapers in infants.
It's possible that traveler's diarrhea may stem from the stress of traveling or a change in diet. But usually infectious agents — such as bacteria, viruses or parasites — are to blame. You typically develop traveler's diarrhea after ingesting food or water contaminated with organisms from feces.
So why aren't natives of high-risk countries affected in the same way? Often their bodies have become used to the bacteria and have developed immunity to them.
Risk factors
Each year millions of international travelers experience traveler's diarrhea. High-risk destinations for traveler's diarrhea include areas of:
- Central America.
- South America.
- South Asia and Southeast Asia.
Traveling to Eastern Europe, South Africa, Central and East Asia, the Middle East, and a few Caribbean islands also poses some risk. However, your risk of traveler's diarrhea is generally low in Northern and Western Europe, Japan, Canada, Singapore, Australia, New Zealand, and the United States.
Your chances of getting traveler's diarrhea are mostly determined by your destination. But certain groups of people have a greater risk of developing the condition. These include:
- Young adults. The condition is slightly more common in young adult tourists. Though the reasons why aren't clear, it's possible that young adults lack acquired immunity. They may also be more adventurous than older people in their travels and dietary choices, or they may be less careful about avoiding contaminated foods.
- People with weakened immune systems. A weakened immune system due to an underlying illness or immune-suppressing medicines such as corticosteroids increases risk of infections.
- People with diabetes, inflammatory bowel disease, or severe kidney, liver or heart disease. These conditions can leave you more prone to infection or increase your risk of a more-severe infection.
- People who take acid blockers or antacids. Acid in the stomach tends to destroy organisms, so a reduction in stomach acid may leave more opportunity for bacterial survival.
- People who travel during certain seasons. The risk of traveler's diarrhea varies by season in certain parts of the world. For example, risk is highest in South Asia during the hot months just before the monsoons.
Complications
Because you lose vital fluids, salts and minerals during a bout with traveler's diarrhea, you may become dehydrated, especially during the summer months. Dehydration is especially dangerous for children, older adults and people with weakened immune systems.
Dehydration caused by diarrhea can cause serious complications, including organ damage, shock or coma. Symptoms of dehydration include a very dry mouth, intense thirst, little or no urination, dizziness, or extreme weakness.
Watch what you eat
The general rule of thumb when traveling to another country is this: Boil it, cook it, peel it or forget it. But it's still possible to get sick even if you follow these rules.
Other tips that may help decrease your risk of getting sick include:
- Don't consume food from street vendors.
- Don't consume unpasteurized milk and dairy products, including ice cream.
- Don't eat raw or undercooked meat, fish and shellfish.
- Don't eat moist food at room temperature, such as sauces and buffet offerings.
- Eat foods that are well cooked and served hot.
- Stick to fruits and vegetables that you can peel yourself, such as bananas, oranges and avocados. Stay away from salads and from fruits you can't peel, such as grapes and berries.
- Be aware that alcohol in a drink won't keep you safe from contaminated water or ice.
Don't drink the water
When visiting high-risk areas, keep the following tips in mind:
- Don't drink unsterilized water — from tap, well or stream. If you need to consume local water, boil it for three minutes. Let the water cool naturally and store it in a clean covered container.
- Don't use locally made ice cubes or drink mixed fruit juices made with tap water.
- Beware of sliced fruit that may have been washed in contaminated water.
- Use bottled or boiled water to mix baby formula.
- Order hot beverages, such as coffee or tea, and make sure they're steaming hot.
- Feel free to drink canned or bottled drinks in their original containers — including water, carbonated beverages, beer or wine — as long as you break the seals on the containers yourself. Wipe off any can or bottle before drinking or pouring.
- Use bottled water to brush your teeth.
- Don't swim in water that may be contaminated.
- Keep your mouth closed while showering.
If it's not possible to buy bottled water or boil your water, bring some means to purify water. Consider a water-filter pump with a microstrainer filter that can filter out small microorganisms.
You also can chemically disinfect water with iodine or chlorine. Iodine tends to be more effective, but is best reserved for short trips, as too much iodine can be harmful to your system. You can purchase water-disinfecting tablets containing chlorine, iodine tablets or crystals, or other disinfecting agents at camping stores and pharmacies. Be sure to follow the directions on the package.
Follow additional tips
Here are other ways to reduce your risk of traveler's diarrhea:
- Make sure dishes and utensils are clean and dry before using them.
- Wash your hands often and always before eating. If washing isn't possible, use an alcohol-based hand sanitizer with at least 60% alcohol to clean your hands before eating.
- Seek out food items that require little handling in preparation.
- Keep children from putting things — including their dirty hands — in their mouths. If possible, keep infants from crawling on dirty floors.
- Tie a colored ribbon around the bathroom faucet to remind you not to drink — or brush your teeth with — tap water.
Other preventive measures
Public health experts generally don't recommend taking antibiotics to prevent traveler's diarrhea, because doing so can contribute to the development of antibiotic-resistant bacteria.
Antibiotics provide no protection against viruses and parasites, but they can give travelers a false sense of security about the risks of consuming local foods and beverages. They also can cause unpleasant side effects, such as skin rashes, skin reactions to the sun and vaginal yeast infections.
As a preventive measure, some doctors suggest taking bismuth subsalicylate, which has been shown to decrease the likelihood of diarrhea. However, don't take this medicine for longer than three weeks, and don't take it at all if you're pregnant or allergic to aspirin. Talk to your doctor before taking bismuth subsalicylate if you're taking certain medicines, such as anticoagulants.
Common harmless side effects of bismuth subsalicylate include a black-colored tongue and dark stools. In some cases, it can cause constipation, nausea and, rarely, ringing in your ears, called tinnitus.
- Feldman M, et al., eds. Infectious enteritis and proctocolitis. In: Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 11th ed. Elsevier; 2021. https://www.clinicalkey.com. Accessed May 25, 2021.
- LaRocque R, et al. Travelers' diarrhea: Microbiology, epidemiology, and prevention. https://www.uptodate.com/contents/search. Accessed May 26, 2021.
- Ferri FF. Traveler diarrhea. In: Ferri's Clinical Advisor 2023. Elsevier; 2023. https://www.clinicalkey.com. Accessed April 28, 2023.
- Diarrhea. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/digestive-diseases/diarrhea. Accessed April 27, 2023.
- Travelers' diarrhea. Centers for Disease Control and Prevention. https://wwwnc.cdc.gov/travel/yellowbook/2020/preparing-international-travelers/travelers-diarrhea. Accessed April 28, 2023.
- LaRocque R, et al. Travelers' diarrhea: Clinical manifestations, diagnosis, and treatment. https://www.uptodate.com/contents/search. Accessed May 26, 2021.
- Khanna S (expert opinion). Mayo Clinic. May 29, 2021.
- Symptoms & causes
- Diagnosis & treatment
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Make twice the impact
Your gift can go twice as far to advance cancer research and care!

GREGORY JUCKETT, M.D.
Am Fam Physician. 1999;60(1):119-124
See related patient information handout on traveler's diarrhea , written by the author of this article .
Common pathogens in traveler's diarrhea include enterotoxigenic Escherichia coli , Campylobacter, Shigella, Salmonella, Yersinia and many other species. Viruses and protozoa are the cause in many cases. Fortunately, traveler's diarrhea can usually be avoided by carefully selecting foods and beverages. Although drug prophylaxis is now discouraged, treatment with loperamide (in the absence of dysentery) and a fluoroquinolone, such as ciprofloxacin (500 mg twice daily for one to three days), is usually safe and effective in adults with traveler's diarrhea. Trimethoprim-sulfamethoxazole and doxycycline are alternatives, but resistance increasingly limits their usefulness. Antibiotic treatment is best reserved for cases that fail to quickly respond to loperamide. Antibiotic resistance is now widespread. Nonabsorbable antibiotics, immunoprophylaxis with vaccines and biotherapeutic microbes that inhibit pathogen infection may eventually supplant antibiotic treatment. In the meantime, azithromycin and new fluoroquinolones show promise as possible replacements for the older agents. Ultimately, the best solution is improvements in sanitary engineering and the development of safe water supplies.
Travel to destinations such as Latin America, Asia, Africa and the Middle East has never been more popular, with over 20 million travelers visiting a less developed country each year. 1 Approximately one third (20 to 50 percent) of travelers to less developed areas of the world become ill from ingesting fecally contaminated food or water. 2 , 3 In 10 to 20 percent of cases, fever and bloody stools (dysentery) occur. 2
Although traveler's diarrhea usually resolves within three to five days (mean duration: 3.6 days), in about 20 percent of persons the illness is severe enough to cause bed confinement and in 10 percent of cases the illness lasts more than one week. 3 , 4 In the very young and the very old, as well as in those who are immunocompromised, traveler's diarrhea can occasionally be life-threatening. It is important to realize, however, that traveler's diarrhea can be minimized by education about ways to prevent the disease. Physicians can do a great deal to ensure that their patients have a safe and enjoyable trip abroad.
Epidemiology
Traveler's diarrhea is defined as three or more unformed stools in 24 hours in a person from an industrialized nation traveling in a less developed country. Unlike in the United States, where most diarrheal disease is viral in origin, in developing countries, bacterial infection is the cause of diarrhea in at least 80 percent of cases. Viral, protozoal or undetermined etiologies account for the remainder of cases. Enterotoxigenic Escherichia coli is the chief pathogen, accounting for 40 to 50 percent of cases. 5 Other common bacterial causes include Campylobacter jejuni , Shigella, Salmonella, Aeromonas and Yersinia species, Plesiomonas shigelloides and Vibrio parahaemolyticus ( Table 1 ) . All of these agents are efficiently spread by the fecal-oral route and, in some cases, such as Shigella infections, a minute inoculum (as few as 10 to 100 organisms) is all that is necessary to produce disease.
Without stool cultures for identifying the pathogen in diarrheal illness, it is easy to confuse the symptoms of traveler's diarrhea with those of food poisoning produced by heat-stable, toxin-forming bacteria such as Staphylococcus aureus and Bacillus cereus or by the heat-labile toxin of Clostridium perfringens . In general, however, preformed toxins (Staphylococcus and Bacillus) produce symptoms within one to six hours, whereas infections (such as from Clostridium) that result in toxin formation in vivo cause symptoms within eight to 16 hours. Most invasive bacterial infections, on the other hand, become symptomatic after 16 hours. 6
Viruses that are responsible for traveler's diarrhea in the tropics include rotavirus and Norwalk agents. Diarrhea caused by viral agents is usually self-limited.
The three major protozoal causes of traveler's diarrhea are Entamoeba histolytica , Giardia duodenalis and Cryptosporidium parvum . Diarrheal disease caused by these organisms is notable for its longer duration and failure to respond to routine antibiotic therapy.
Risk factors for traveler's diarrhea are listed in Table 2 . Persons from a developing region who have relocated to an industrialized country and who then return to their country of origin are also at increased risk, especially since they seldom take precautions. Any “native resistance” is soon lost after relocation and subsequent alteration of the intestinal flora.
Traveler's diarrhea is fundamentally a sanitation failure, leading to bacterial contamination of food and water. It is best prevented through proper sewage treatment and water disinfection. In the absence of these amenities, the next best option is for the educated traveler to take precautions to prevent the disease.
Preventive measures include not drinking tap water, not using ice in beverages (even alcoholic drinks), not eating salads and other forms of raw vegetables, not eating fruits that can't be peeled on the spot and not eating mayonnaise, pastry icing, unpasteurized dairy products and undercooked shellfish.
Tying a ribbon around the faucet and keeping purified bottled water near the sink may serve as memory aids for travelers to remind them not to use tap water, even for tooth brushing. Hot cooked food, fresh bread, dry foods such as crackers, bottled carbonated beverages, coffee, tea and beer are usually safe, provided such food items are not obtained from street vendors. Helpful maxims to keep in mind include “boil it, cook it, peel it or forget it” and the “rule of P's”: food is safe if it is peelable, packaged, purified or piping hot. 3 Careful handwashing, most conveniently achieved with packaged wipes or antiseptic gel, is essential.
Active intervention involves boiling water for three to five minutes (depending on elevation), filtering water or using chlorine bleach (2 drops per quart) or tincture of iodine (5 drops per quart) in the water. Drawbacks with these methods of prevention include the need to allow sufficient time to disinfect the water, clogged filters, chlorine's incomplete effectiveness against some protozoal cysts and iodine's bad taste. 7 Antibiotics (i.e., tetracyclines) added to the water may not destroy resistant bacteria and protozoal cysts. Fortunately, the wide availability of safe bottled water makes these cumbersome interventions unnecessary for all but the most remote destinations.
Drug Prophylaxis
Table 3 summarizes the various drug therapies used for prophylaxis against traveler's diarrhea. Bismuth subsalicylate (Pepto-Bismol), in a dosage of two 262-mg tablets four times a day (taken with meals and in the evening) can prevent traveler's diarrhea. It has been shown to provide a 65 percent protection rate. 8 Bismuth subsalicylate can be taken for up to three weeks. Such long-term use can, however, darken the tongue and stool, produce tinnitus and cause reactions in salicylate-sensitive patients. Bismuth subsalicylate also interferes with the absorption of doxycycline and certain other medications. 8
Compared with bismuth subsalicylate, antibiotic prophylaxis with trimethoprim-sulfamethoxazole (Bactrim DS), in a dosage of 160 mg/800 mg daily, or doxycycline (Vibramycin), in a dosage of 100 mg daily, has been found to provide even better results for up to three weeks. 8 Increasingly, however, resistance to these antibiotics has become such a problem that their routine use is not recommended. For instance, trimethoprim-sulfamethoxazole continues to be effective prophylaxis for summertime travel in inland Mexico, but it is unreliable in many other situations. 2 Although fluoroquinolones such as ciprofloxacin (Cipro) may be the best alternatives to trimethoprim-sulfamethoxazole and doxycycline, resistance to fluoroquinolones is developing too rapidly to justify their continued use for prophylaxis against traveler's diarrhea.
Antibiotic prophylaxis for traveler's diarrhea, always a controversial topic, is now recommended only in specific situations, such as in the seriously immunocompromised patient or the seriously ill patient who would not be able to withstand a diarrheal illness. If antibiotic prophylaxis is used, the antibiotic should only be taken for a three-week period. Other exceptions may include persons who plan short-term critical travel, such as a diplomatic mission, or persons who are unable to practice prevention.
In most people, drug prophylaxis engenders a false sense of security. In addition, drug prophylaxis always carries the remote risk of a potentially life-threatening side effect such as pseudomembranous colitis or Stevens-Johnson syndrome. Nuisance side effects such as vaginal yeast infections and, with doxycycline, photosensitivity, are common. Therefore, the Centers for Disease Control and Prevention recommends preventive measures only and not drug prophylaxis for most travelers. If diarrhea occurs despite precautions, however, a self-treatment contingency plan is reasonable.
Mild traveler's diarrhea can usually be managed with the judicious use of antimotility agents such as loperamide (Imodium A-D), in a dosage of two 2-mg tablets initially, then one tablet after each loose stool (maximum 24-hour dosage: 8 mg). Additionally, a single dose of ciprofloxacin—750 mg; levofloxacin (Levaquin)—500 mg; or ofloxacin (Floxin)—400 mg, usually relieves mild cases of traveler's diarrhea in less than 24 hours. 9
The use of antimotility agents has traditionally been avoided in patients with dysentery, where decreased gut motility would be inadvisable. Moderate to severe traveler's diarrhea, including dysentery, can be empirically treated with a three-day course of a fluoroquinolone such as ciprofloxacin, norfloxacin (Noroxin) or ofloxacin ( Table 4 ) . Loperamide may also be taken if the patient does not have dysentery. Before beginning antibiotic therapy, however, patients should first take a dose of loperamide to see if the antimotility agent stops the diarrhea. Antibiotic therapy should be deferred until it is clear that the diarrheal illness requires antibiotic therapy, since dietary change and stress can cause transient gastrointestinal upset. 2 A single antibiotic dose may be effective for mild traveler's diarrhea, and patients should reassess their condition in 12 hours to determine if further doses are necessary. 10
Ciprofloxacin is not recommended in patients with seizure disorders, in patients who are pregnant and in children under 18 years of age. Children older than two years of age can be given trimethoprim-sulfamethoxazole. Children under two years of age and pregnant women can be treated with an oral rehydration solution. Older children and other adults with traveler's diarrhea also would benefit from oral rehydration, possibly supplemented with salted soda crackers. Commercially available packets of oral rehydration solution can be reconstituted with safe water.
If treatment with a fluoroquinolone fails to resolve the diarrhea, several other diagnostic possibilities should be considered. Protozoal infections and pseudomembranous colitis must be excluded. In addition, infection from an antibiotic-resistant organism is now a third and increasingly probable explanation for continued diarrhea. Azithromycin (Zithromax), in a dosage of 500 mg daily for three days, has been found to be very effective in treating resistant Campylobacter enteritis contracted in Thailand, and its usefulness in other situations of fluoroquinolone resistance merits investigation. 11
Patients should be told to consult a physician if diarrhea does not respond to the planned regimen, especially if high fever or bloody stools are present. Patients should also be warned to avoid over-the-counter anti-diarrheals such as iodochlorhydroxyquinoline (Entero-Vioform), which has been withdrawn from the U.S. market because of its association with myelooptic atrophy. This agent, however, may still be available outside this country.
Public Health Issues
Even with the emphasis now placed on pre-cautionary measures to prevent traveler's diarrhea rather than drug prophylaxis, the treatment of traveler's diarrhea will be increasingly hampered by antibiotic resistance. Multidrug-resistant Shigella and Salmonella strains are now so common that it is only a matter of time until they also become resistant to fluoroquinolones. Some respite may be provided by the most recently developed fluoroquinolones and azithromycin, but eventually a new approach will be necessary.
One future option might be nonabsorbable antimicrobial drugs such as bicozamycin, furazolidone (Furoxone), aztreonam and rifaximin, which have already shown some benefit in the treatment of traveler's diarrhea. Bicozamycin might also be an effective prophylactic agent that would treat only the gastrointestinal tract and, therefore, be more acceptable from the standpoint of safety. 2 Although not yet marketed in the United States, zaldaride, a new type of drug called a calmodulin inhibitor, is a promising treatment. 10
Use of biotherapeutic agents made of microorganisms that suppress pathogenic infection is another option. Lactobacillus casei and Saccharomyces boulardii , a nonpathogenic yeast, have been studied as prophylactic agents in travel scenarios, but the results have been inconsistent. The use of these organisms to treat other types of diarrhea has been encouraging, and improvements in bioengineered forms of these organisms may in the future yield an alternative to antibiotics. 12
Immunoprophylaxis by means of oral vaccines may become a third alternative to systemic antibiotics. Vaccines have already been developed for typhoid, enterotoxigenic E. coli and cholera (the latter two are not yet available in the United States). Shigella and Campylobacter vaccines are in the development stage, and a pediatric rotavirus vaccine has recently been labeled. A combination, or “super,” vaccine could be an effective way to reduce the most common types of traveler's diarrhea. The limiting factor is the vast array of potential pathogens that cannot be included in any single vaccine. 3 , 10
The most sensible, yet most problematic, approach to preventing traveler's diarrhea is to eliminate the basic problem of poor hygiene and water contamination through sanitary engineering, public education and, most importantly, the development of a safe water supply. Unfortunately, much of the developing world is in a “Catch-22” situation: poverty and unclean water hinder the tourism and investment money needed to correct these problems. Progress through international aid and economic development is difficult, but in an increasing number of transitional nations, such as Thailand, economic growth has reduced the country's risk profile in less than a decade. Ultimately, the elimination of poverty, and not new drugs, will resolve the problem of traveler's diarrhea.
Castelli I, Carosi G. Epidemiology of traveler's diarrhea. Chemotherapy. 1995;41(suppl 1):20-32.
DuPont HL, Ericsson CD. Prevention and treatment of traveler's diarrhea. N Engl J Med. 1993;328:1821-7.
Liu LX. Travel medicine part II: malaria, traveler's diarrhea, and other problems. Infect Med. 1993;10:24-8.
Steffen R. Epidemiologic studies of traveler's diarrhea, severe gastrointestinal infections, and cholera. Rev Infect Dis. 1986;8(suppl 2):S122-30.
Layton ML, Bia FJ. Emerging issues in travel medicine. Curr Opin Infect Dis. 1992;5:338-44.
Tauxe RV, Hughes JM. Food-borne disease. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas and Bennett's Principles and practice of infectious diseases. 4th ed. New York: Churchill Livingstone, 1995:1012–24.
Strum WB. Update on traveler's diarrhea. Postgrad Med. 1988;84(1):163-6.
DuPont HL, Ericsson CD, Johnson PC, Bitsura JA, DuPont MW, de la Cabada FJ. Prevention of traveler's diarrhea by the tablet formulation of bismuth subsalicylate. JAMA. 1987;257:1347-50.
Advice for travelers. Med Lett Drugs Ther. 1998;40(1025):47-50.
Ericsson CD. Travelers diarrhea. Epidemiology, prevention, and self-treatment. Infect Dis Clin North Am. 1998;12:285-303.
Kuschner RA, Trofa AF, Thomas RJ, Hoge CW, Pitarangsi C, Amato S, et al. Use of azithromycin for the treatment of Campylobacter enteritis in travelers to Thailand, an area where ciprofloxacin resistance is prevalent. Clin Infect Dis. 1995;21:536-41.
Elmer GW, Surawicz CM, McFarland LV. Biotherapeutic agents. A neglected modality for the treatment and prevention of selected intestinal and vaginal infections. JAMA. 1996;275:870-6.
Continue Reading
More in afp, more in pubmed.
Copyright © 1999 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
- GP practice services
- Health advice
- Health research
- Medical professionals
- Health topics
Advice and clinical information on a wide variety of healthcare topics.
All health topics
Latest features
Allergies, blood & immune system
Bones, joints and muscles
Brain and nerves
Chest and lungs
Children's health
Cosmetic surgery
Digestive health
Ear, nose and throat
General health & lifestyle
Heart health and blood vessels
Kidney & urinary tract
Men's health
Mental health
Oral and dental care
Senior health
Sexual health
Signs and symptoms
Skin, nail and hair health
- Travel and vaccinations
Treatment and medication
Women's health

Healthy living
Expert insight and opinion on nutrition, physical and mental health.
Exercise and physical activity
Healthy eating
Healthy relationships
Managing harmful habits
Mental wellbeing
Relaxation and sleep
Managing conditions
From ACE inhibitors for high blood pressure, to steroids for eczema, find out what options are available, how they work and the possible side effects.
Featured conditions
ADHD in children
Crohn's disease
Endometriosis
Fibromyalgia
Gastroenteritis
Irritable bowel syndrome
Polycystic ovary syndrome
Scarlet fever
Tonsillitis
Vaginal thrush
Health conditions A-Z
Medicine information
Information and fact sheets for patients and professionals. Find out side effects, medicine names, dosages and uses.
All medicines A-Z
Allergy medicines
Analgesics and pain medication
Anti-inflammatory medicines
Breathing treatment and respiratory care
Cancer treatment and drugs
Contraceptive medicines
Diabetes medicines
ENT and mouth care
Eye care medicine
Gastrointestinal treatment
Genitourinary medicine
Heart disease treatment and prevention
Hormonal imbalance treatment
Hormone deficiency treatment
Immunosuppressive drugs
Infection treatment medicine
Kidney conditions treatments
Muscle, bone and joint pain treatment
Nausea medicine and vomiting treatment
Nervous system drugs
Reproductive health
Skin conditions treatments
Substance abuse treatment
Vaccines and immunisation
Vitamin and mineral supplements
Tests & investigations
Information and guidance about tests and an easy, fast and accurate symptom checker.
About tests & investigations
Symptom checker
Blood tests
BMI calculator
Pregnancy due date calculator
General signs and symptoms
Patient health questionnaire
Generalised anxiety disorder assessment
Medical professional hub
Information and tools written by clinicians for medical professionals, and training resources provided by FourteenFish.
Content for medical professionals
FourteenFish training
Professional articles
Evidence-based professional reference pages authored by our clinical team for the use of medical professionals.
View all professional articles A-Z
Actinic keratosis
Bronchiolitis
Molluscum contagiosum
Obesity in adults
Osmolality, osmolarity, and fluid homeostasis
Recurrent abdominal pain in children
Medical tools and resources
Clinical tools for medical professional use.
All medical tools and resources
Traveller's diarrhoea
Peer reviewed by Dr Colin Tidy, MRCGP Last updated by Dr Toni Hazell Last updated 10 Feb 2023
Meets Patient’s editorial guidelines
In this series: Amoebiasis Giardia
Traveller's diarrhoea is diarrhoea that develops during, or shortly after, travel abroad. It is caused by consuming food and water, contaminated by germs (microbes) including bacteria, viruses and parasites. Other symptoms can include high temperature (fever), being sick (vomiting) and tummy (abdominal) pain. In most cases it causes a mild illness and symptoms clear within 3 to 4 days. Specific treatment is not usually needed but it is important to drink plenty of fluids to avoid lack of fluid in the body (dehydration). Always make sure that you get any advice that you need in plenty of time before your journey - some GPs offer travel advice but if yours doesn't then you may need to go to a private travel clinic.
In this article :
What is traveller's diarrhoea, what causes traveller's diarrhoea, are all travellers at risk, what are the symptoms of traveller's diarrhoea, how is traveller's diarrhoea diagnosed, when should i seek medical advice for traveller's diarrhoea, how is traveller's diarrhoea in adults treated, how is traveller's diarrhoea in children treated, side-effects of traveller's diarrhoea, how long does traveller's diarrhoea last, how can i avoid traveller's diarrhoea.
Continue reading below
Traveller's diarrhoea is diarrhoea that develops during, or shortly after, travel abroad. Diarrhoea is defined as: 'loose or watery stools (faeces), usually at least three times in 24 hours.'
Traveller's diarrhoea is caused by eating food, or drinking water, containing certain germs (microbes) or their poisons (toxins). The types of germs which may be the cause include:
Bacteria: these are the most common microbes that cause traveller's diarrhoea. Common types of bacteria involved are:
Escherichia coli
Campylobacter
Viruses: these are the next most common, particularly norovirus and rotavirus.
Parasites: these are less common causes. Giardia, cryptosporidium and Entamoeba histolytica are examples of parasites that may cause traveller's diarrhoea.
Often the exact cause of traveller's diarrhoea is not found and studies have shown that in many people no specific microbe is identified despite testing (for example, of a stool (faeces) specimen).
See the separate leaflets called E. Coli (VTEC O157) , Campylobacter, Salmonella, Cryptosporidium , Amoebiasis (dysentery information), Shigella and Giardia for more specific details on each of the microbes mentioned above.
Note : this leaflet is about traveller's diarrhoea in general and how to help prevent it.
Traveller's diarrhoea most commonly affects people who are travelling from a developed country, such as the UK, to a developing country where sanitation and hygiene measures may not meet the same standards. It can affect as many as 2 to 6 in 10 travellers.
There is a different risk depending on whether you travel to high-risk areas or not:
High-risk areas : South and Southeast Asia, Central America, West and North Africa, South America, East Africa.
Medium-risk areas : Russia, China, Caribbean, South Africa.
Low-risk areas : North America, Western Europe, Australia and New Zealand.
Sometimes outbreaks of diarrhoea can occur in travellers staying in one hotel or, for example, those staying on a cruise ship. People travelling in more remote areas (for example, trekkers and campers) may also have limited access to medical care if they do become unwell.
By definition, diarrhoea is the main symptom. This can be watery and can sometimes contain blood. Other symptoms may include:
Crampy tummy (abdominal) pains.
Feeling sick (nausea).
Being sick (vomiting).
A high temperature (fever).
Symptoms are usually mild in most people and last for 3 to 4 days but they may last longer. Symptoms may be more severe in the very young, the elderly, and those with other health problems. Those whose immune systems are not working as well as normal are particularly likely to be more unwell. For example, people with untreated HIV infection, those on chemotherapy, those on long-term steroid treatment or those who are taking drugs which suppress their immune system, for example after a transplant or to treat an autoimmune condition
Despite the fact that symptoms are usually fairly mild, they can often mean that your travel itinerary is interrupted or may need to be altered.
Traveller's diarrhoea is usually diagnosed by the typical symptoms. As mentioned above, most people have mild symptoms and do not need to seek medical advice. However, in some cases medical advice is needed (see below).
If you do see a doctor, they may suggest that a sample of your stool (faeces) be tested. This will be sent to the laboratory to look for any microbes that may be causing your symptoms. Sometimes blood tests or other tests may be needed if you have more severe symptoms or develop any complications.
As mentioned above, most people with traveller's diarrhoea have relatively mild symptoms and can manage these themselves by resting and making sure that they drink plenty of fluids. However, you should seek medical advice in any of the following cases, or if any other symptoms occur that you are concerned about:
If you have a high temperature (fever).
If you have blood in your stools (faeces).
If it is difficult to get enough fluid because of severe symptoms: frequent or very watery stools or repeatedly being sick (vomiting).
If the diarrhoea lasts for more than 5-7 days.
If you are elderly or have an underlying health problem such as diabetes, inflammatory bowel disease, or kidney disease.
If you have a weakened immune system because of, for example, chemotherapy treatment, long-term steroid treatment, or HIV infection.
If you are pregnant.
If an affected child is under the age of 6 months.
If you develop any of the symptoms listed below that suggest you might have lack of fluid in your body (dehydration). If it is your child who is affected, there is a separate list for children.
Symptoms of dehydration in adults
Dizziness or light-headedness.
Muscle cramps.
Sunken eyes.
Passing less urine.
A dry mouth and tongue.
Becoming irritable.
Symptoms of severe dehydration in adults
Profound loss of energy or enthusiasm (apathy).
A fast heart rate
Producing very little urine.
Coma, which may occur.
Note : severe dehydration is a medical emergency and immediate medical attention is needed.
Symptoms of dehydration in children
Passing little urine.
A dry mouth.
A dry tongue and lips.
Fewer tears when crying.
Being irritable.
Having a lack of energy (being lethargic).
Symptoms of severe dehydration in children
Drowsiness.
Pale or mottled skin.
Cold hands or feet.
Very few wet nappies.
Fast (but often shallow) breathing.
Dehydration is more likely to occur in:
Babies under the age of 1 year (and particularly those under 6 months old). This is because babies don't need to lose much fluid to lose a significant proportion of their total body fluid.
Babies under the age of 1 year who were a low birth weight and who have not caught up with their weight.
A breastfed baby who has stopped being breastfed during their illness.
Any baby or child who does not drink much when they have a gut infection (gastroenteritis).
Any baby or child with severe diarrhoea and vomiting. (For example, if they have passed five or more diarrhoeal stools and/or vomited two or more times in the previous 24 hours.)
In most cases, specific treatment of traveller's diarrhoea is not needed. The most important thing is to make sure that you drink plenty of fluids to avoid lack of fluid in your body (dehydration).
Fluid replacement
As a rough guide, drink at least 200 mls after each watery stool (bout of diarrhoea).
This extra fluid is in addition to what you would normally drink. For example, an adult will normally drink about two litres a day but more in hot countries. The above '200 mls after each watery stool' is in addition to this usual amount that you would drink.
If you are sick (vomit), wait 5-10 minutes and then start drinking again but more slowly. For example, a sip every 2-3 minutes but making sure that your total intake is as described above.
You will need to drink even more if you are dehydrated. A doctor will advise on how much to drink if you are dehydrated.
Note : if you suspect that you are becoming dehydrated, you should seek medical advice.
For most adults, fluids drunk to keep hydrated should mainly be water. However, this needs to be safe drinking water - for example, bottled, or boiled and treated water. It is best not to have drinks that contain a lot of sugar, such as fizzy drinks, as they can sometimes make diarrhoea worse. Alcohol should also be avoided.
Rehydration drinks
Rehydration drinks may also be used. They are made from sachets that you can buy from pharmacies and may be a sensible thing to pack in your first aid kit when you travel. You add the contents of the sachet to water.
Home-made salt/sugar mixtures are used in developing countries if rehydration drinks are not available; however, they have to be made carefully, as too much salt can be dangerous. Rehydration drinks are cheap and readily available in the UK, and are the best treatment. Note that safe drinking water should be used to reconstitute oral rehydration salt sachets.
Antidiarrhoeal medication
Antidiarrhoeal medicines are not usually necessary or wise to take when you have traveller's diarrhoea. However you may want to use them if absolutely necessary - for example, if you will be unable to make regular trips to the toilet due to travelling.You can buy antidiarrhoeal medicines from pharmacies before you travel. The safest and most effective is loperamide.
The adult dose of this is two capsules at first. This is followed by one capsule after each time you pass some diarrhoea up to a maximum of eight capsules in 24 hours. It works by slowing down your gut's activity.
You should not take loperamide for longer than two days. You should also not use antidiarrhoeal medicines if you have a high temperature (fever) or bloody diarrhoea.
Eat as normally as possible
It used to be advised to 'starve' for a while if you had diarrhoea. However, now it is advised to eat small, light meals if you can. Be guided by your appetite. You may not feel like food and most adults can do without food for a few days. Eat as soon as you are able but don't stop drinking. If you do feel like eating, avoid fatty, spicy or heavy food. Plain foods such as bread and rice are good foods to try eating.
Antibiotic medicines
Most people with traveller's diarrhoea do not need treatment with antibiotic medicines. However, sometimes antibiotic treatment is advised. This may be because a specific germ (microbe) has been identified after testing of your stool (faeces) sample.
Fluids to prevent dehydration
You should encourage your child to drink plenty of fluids. The aim is to prevent lack of fluid in the body (dehydration). The fluid lost in their sick (vomit) and/or diarrhoea needs to be replaced. Your child should continue with their normal diet and usual drinks. In addition, they should also be encouraged to drink extra fluids. However, avoid fruit juices or fizzy drinks, as these can make diarrhoea worse.
Babies under 6 months old are at increased risk of dehydration. You should seek medical advice if they develop acute diarrhoea. Breast feeds or bottle feeds should be encouraged as normal. You may find that your baby's demand for feeds increases. You may also be advised to give extra fluids (either water or rehydration drinks) in between feeds.
If you are travelling to a destination at high risk for traveller's diarrhoea, you might want to consider buying oral rehydration sachets for children before you travel. These can provide a perfect balance of water, salts and sugar for them and can be used for fluid replacement. Remember that, as mentioned above, safe water is needed to reconstitute the sachets.
If your child vomits, wait 5-10 minutes and then start giving drinks again but more slowly (for example, a spoonful every 2-3 minutes). Use of a syringe can help in younger children who may not be able to take sips.
Note : if you suspect that your child is dehydrated, or is becoming dehydrated, you should seek medical advice urgently.
Fluids to treat dehydration
If your child is mildly dehydrated, this may be treated by giving them rehydration drinks. A doctor will advise about how much to give. This can depend on the age and the weight of your child. If you are breastfeeding, you should continue with this during this time. It is important that your child be rehydrated before they have any solid food.
Sometimes a child may need to be admitted to hospital for treatment if they are dehydrated. Treatment in hospital usually involves giving rehydration solution via a special tube called a 'nasogastric tube'. This tube passes through your child's nose, down their throat and directly into their stomach. An alternative treatment is with fluids given directly into a vein (intravenous fluids).
Eat as normally as possible once any dehydration has been treated
Correcting any dehydration is the first priority. However, if your child is not dehydrated (most cases), or once any dehydration has been corrected, then encourage your child to have their normal diet. Do not 'starve' a child with infectious diarrhoea. This used to be advised but is now known to be wrong. So:
Breastfed babies should continue to be breastfed if they will take it. This will usually be in addition to extra rehydration drinks (described above).
Bottle-fed babies should be fed with their normal full-strength feeds if they will take it. Again, this will usually be in addition to extra rehydration drinks (described above). Do not water down the formula, or make it up with less water than usual. This can make a baby very ill.
Older children - offer them some food every now and then. However, if he or she does not want to eat, that is fine. Drinks are the most important consideration and food can wait until the appetite returns.
Loperamide is not recommended for children with diarrhoea. There are concerns that it may cause a blockage of the gut (intestinal obstruction) in children with diarrhoea.
Most children with traveller's diarrhoea do not need treatment with antibiotics. However, for the same reasons as discussed for adults above, antibiotic treatment may sometimes be advised in certain cases.
Most people have mild illness and complications of traveller's diarrhoea are rare. However, if complications do occur, they can include the following:
Salt (electrolyte) imbalance and dehydration .
This is the most common complication. It occurs if the salts and water that are lost in your stools (faeces), or when you are sick (vomit), are not replaced by you drinking adequate fluids. If you can manage to drink plenty of fluids then dehydration is unlikely to occur, or is only likely to be mild and will soon recover as you drink.
Severe dehydration can lead to a drop in your blood pressure. This can cause reduced blood flow to your vital organs. If dehydration is not treated, your kidneys may be damaged . Some people who become severely dehydrated need a 'drip' of fluid directly into a vein. This requires admission to hospital. People who are elderly or pregnant are more at risk of dehydration.
Reactive complications
Rarely, other parts of your body can 'react' to an infection that occurs in your gut. This can cause symptoms such as joint inflammation (arthritis), skin inflammation and eye inflammation (either conjunctivitis or uveitis). Reactive complications are uncommon if you have a virus causing traveller's diarrhoea.
Spread of infection
The infection can spread to other parts of your body such as your bones, joints, or the meninges that surround your brain and spinal cord. This is rare. If it does occur, it is more likely if diarrhoea is caused by salmonella infection.
Irritable bowel syndrome is sometimes triggered by a bout of traveller's diarrhoea.
Lactose intolerance
Lactose intolerance can sometimes occur for a period of time after traveller's diarrhoea. It is known as 'secondary' or 'acquired' lactose intolerance. Your gut (intestinal) lining can be damaged by the episode of diarrhoea. This leads to lack of a substance (enzyme) called lactase that is needed to help your body digest the milk sugar lactose.
Lactose intolerance leads to bloating, tummy (abdominal) pain, wind and watery stools after drinking milk. The condition gets better when the infection is over and the intestinal lining heals. It is more common in children.
Haemolytic uraemic syndrome
Usually associated with traveller's diarrhoea caused by a certain type of E. coli infection, haemolytic uraemic syndrome is a serious condition where there is anaemia, a low platelet count in the blood and kidney damage. It is more common in children. If recognised and treated, most people recover well.
Guillain-Barré syndrome
This condition may rarely be triggered by campylobacter infection, one of the causes of traveller's diarrhoea. It affects the nerves throughout your body and limbs, causing weakness and sensory problems. See the separate leaflet called Guillain-Barré syndrome for more details.
Reduced effectiveness of some medicines
During an episode of traveller's diarrhoea, certain medicines that you may be taking for other conditions or reasons may not be as effective. This is because the diarrhoea and/or being sick (vomiting) mean that reduced amounts of the medicines are taken up (absorbed) into your body.
Examples of such medicines are those for epilepsy, diabetes and contraception . Speak with your doctor or practice nurse before you travel if you are unsure of what to do if you are taking other medicines and develop diarrhoea.
As mentioned above, symptoms are usually short-lived and the illness is usually mild with most people making a full recovery within in few days. However, a few people with traveller's diarrhoea develop persistent (chronic) diarrhoea that can last for one month or more. It is also possible to have a second 'bout' of traveller's diarrhoea during the same trip. Having it once does not seem to protect you against future infection.
Avoid uncooked meat, shellfish or eggs. Avoid peeled fruit and vegetables (including salads).
Be careful about what you drink. Don't drink tap water, even as ice cubes.
Wash your hands regularly, especially before preparing food or eating.
Be careful where you swim. Contaminated water can cause traveller's diarrhoea.
Regular hand washing
You should ensure that you always wash your hands and dry them thoroughly; teach children to wash and dry theirs:
After going to the toilet (and after changing nappies or helping an older child to go to the toilet).
Before preparing or touching food or drinks.
Before eating.
Some antibacterial hand gel may be a good thing to take with you when you travel in case soap and hot water are not available.
Be careful about what you eat and drink
When travelling to areas with poor sanitation, you should avoid food or drinking water that may contain germs (microbes) or their poisons (toxins). Avoid:
Fruit juices sold by street vendors.
Ice cream (unless it has been made from safe water).
Shellfish (for example, mussels, oysters, clams) and uncooked seafood.
Raw or undercooked meat.
Fruit that has already been peeled or has a damaged skin.
Food that contains raw or uncooked eggs, such as mayonnaise or sauces.
Unpasteurised milk.
Drinking bottled water and fizzy drinks that are in sealed bottles or cans, tea, coffee and alcohol is thought to be safe. However, avoid ice cubes and non-bottled water in alcoholic drinks. Food should be cooked through thoroughly and be piping hot when served.
You should also be careful when eating food from markets, street vendors or buffets if you are uncertain about whether it has been kept hot or kept refrigerated. Fresh bread is usually safe, as is canned food or food in sealed packs.
Be careful where you swim
Swimming in contaminated water can also lead to traveller's diarrhoea. Try to avoid swallowing any water as you swim; teach children to do the same.
Obtain travel health advice before you travel
Always make sure that you visit your GP surgery or private travel clinic for health advice in plenty of time before your journey. Alternatively, the Fit for Travel website (see under Further Reading and References, below) provides travel health information for the public and gives specific information for different countries and high-risk destinations. This includes information about any vaccinations required, advice about food, water and personal hygiene precautions, etc.
There are no vaccines that prevent traveller's diarrhoea as a whole. However, there are some other vaccines that you may need for your travel, such as hepatitis A, typhoid, etc. You may also need to take malaria tablets depending on where you are travelling.
Antibiotics
Taking antibiotic medicines to prevent traveller's diarrhoea (antibiotic prophylaxis) is not generally recommended. This is because for most people, traveller's diarrhoea is mild and self-limiting. Also, antibiotics do not protect against nonbacterial causes of traveller's diarrhoea, such as viruses and parasites. Antibiotics may have side-effects and their unnecessary use may lead to problems with resistance to medicines.
Probiotics have some effect on traveller's diarrhoea and can shorten an attack by about one day. It is not known yet which type of probiotic or which dose, so there are no recommendations about using probiotics to prevent traveller's diarrhoea.
Further reading and references
- Bourgeois AL, Wierzba TF, Walker RI ; Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine. 2016 Mar 15. pii: S0264-410X(16)00287-5. doi: 10.1016/j.vaccine.2016.02.076.
- Travellers' diarrhoea ; Fitfortravel
- Riddle MS, Connor BA, Beeching NJ, et al ; Guidelines for the prevention and treatment of travelers' diarrhea: a graded expert panel report. J Travel Med. 2017 Apr 1;24(suppl_1):S57-S74. doi: 10.1093/jtm/tax026.
- Giddings SL, Stevens AM, Leung DT ; Traveler's Diarrhea. Med Clin North Am. 2016 Mar;100(2):317-30. doi: 10.1016/j.mcna.2015.08.017.
- Diarrhoea - prevention and advice for travellers ; NICE CKS, February 2019 (UK access only)
Article History
The information on this page is written and peer reviewed by qualified clinicians.
Next review due: 9 Feb 2028
10 feb 2023 | latest version.
Last updated by
Peer reviewed by

Feeling unwell?
Assess your symptoms online for free
Traveler’s Diarrhea
- Symptoms and Signs |
- Diagnosis |
- Treatment |
- Prevention |
- Key Points |
- More Information |
Traveler’s diarrhea is gastroenteritis that is usually caused by bacteria endemic to local water. Symptoms include vomiting and diarrhea. Diagnosis is mainly clinical. Treatment is with replacement fluids and sometimes antibiotics for moderate to severe diarrhea.
(See also Overview of Gastroenteritis and see the Center for Disease Control and Prevention’s [CDC] information for preparing international travelers for travelers’ diarrhea .)
Etiology of Traveler's Diarrhea
Traveler’s diarrhea may be caused by any of several bacteria, viruses, or, less commonly, parasites.
The most common cause of traveler's diarrhea is
Enterotoxigenic Escherichia coli ( E. coli )
E. coli is common in the water supplies of areas that lack adequate purification. Infection is common among people traveling to low-resource countries.
Norovirus gastroenteritis has been a particular problem on some cruise ships.
Both food and water can be the source of infection. Travelers who avoid drinking local water may still become infected by brushing their teeth with an improperly rinsed toothbrush, drinking bottled drinks with ice made from local water, or eating food that is improperly handled or washed with local water. People taking medications that decrease stomach acid (antacids, H2 blockers, and proton pump inhibitors) are at risk of more severe illness.
Symptoms and Signs of Traveler's Diarrhea
Nausea, vomiting, hyperactive bowel sounds, abdominal cramps, and diarrhea begin 12 to 72 hours after ingesting contaminated food or water. Severity is variable. Some people develop fever and myalgias. Diarrhea is rarely bloody.
Most cases are mild and self-limited, although dehydration can occur, especially in warm climates.
Diagnosis of Traveler's Diarrhea
Clinical evaluation
Specific diagnostic measures are usually not necessary. However, fever, severe abdominal pain, and bloody diarrhea suggest more serious disease and should prompt immediate evaluation.
Treatment of Traveler's Diarrhea
Fluid replacement
Sometimes antidiarrheal (antimotility) medications
Antibiotics (eg, ciprofloxacin , azithromycin ) for moderate to severe diarrhea
The mainstay of treatment of traveler's diarrhea is fluid replacement and an antidiarrheal medication such as loperamide
Antidiarrheal medications should not be used in adults with suspected C. difficile or E. coli O157:H7 infection (eg, with recent antibiotic use, bloody diarrhea, heme-positive stool, or diarrhea with fever) or in children, particularly those < 2 years. Iodochlorhydroxyquin, which may be available in some low- and middle-income countries, should not be used because it may cause neurologic damage.
Pearls & Pitfalls
Generally, antibiotics are not necessary for mild diarrhea. However, in patients with moderate to severe diarrhea ( ≥ Campylobacter 2017 guidelines for the prevention and treatment of travelers' diarrhea .)
Prevention of Traveler's Diarrhea
Travelers should dine at restaurants with a reputation for safety and avoid foods and beverages from street vendors. They should consume only cooked foods that are still steaming hot, fruit that can be peeled, and carbonated beverages without ice served in sealed bottles (bottles of noncarbonated beverages can contain tap water added by unscrupulous vendors); uncooked vegetables (particularly including salsa left out on the table) should be avoided. Buffets and fast food restaurants pose an increased risk.
Traveler's diarrhea is usually caused by enterotoxigenic E. coli , but viruses, parasites, and other bacteria may be involved.
Diagnosis is clinical and testing is not usually needed unless bloody diarrhea, fever, or abdominal pain is present.
Prevention is the best measure and involves careful selection of foods and beverages; prophylactic antibiotics are not routinely used except for patients with immunocompromise.
More Information
The following English-language resources may be useful. Please note that THE MANUAL is not responsible for the content of these resources.
Centers for Disease Control and Prevention: Preparing international travelers for travelers’ diarrhea
Expert panel: Guidelines for the prevention and treatment of travelers' diarrhea (2017)

- Cookie Preferences

Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- COVID-19 Vaccines
- Occupational Therapy
- Healthy Aging
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Best Traveler's Diarrhea Treatments for Symptom Relief
Sources of Bacteria, Prevention, and Medication Types
Complications
Frequently asked questions.
Traveler's diarrhea can turn a trip into a nightmare. Food and water contaminated by germs, also known as pathogens , is not uncommon in certain areas of the world that are popular travel destinations. Consuming even small amounts of these germs can cause loose, watery stool, the main sign of diarrhea , Luckily, treatment options are available.
This article explains the symptoms of traveler's diarrhea, how to treat it, and the best ways to prevent getting infected in the first place.
Luis Alvarez / Getty Images
Symptoms of traveler's diarrhea caused by bacteria or a virus usually appear six to 72 hours after eating or drinking something contaminated. With some types of pathogens, it may take a week or longer for stool to be affected.
Changes in your bowel habits it the main symptoms of diarrhea. At its mildest, diarrhea involves passing loose, watery stool three times a day. You may pass unformed stool 10 or more times a day in severe cases.
Other symptoms vary depending on the type of bacterial or virus you've been exposed to but may include:
- Stomach cramps
- Tenesmus , feeling you need to have a bowel movement even when your bowels are empty
- Mucous in stool
More severe cases of traveler's diarrhea may cause bloody stools .
Should You Go to a Doctor for Traveler's Diarrhea?
See a healthcare provider if your symptoms are accompanied by fever or bloody stools, or they last longer than 48 hours.
Traveler's Diarrhea Causes
The most common cause of traveler's diarrhea is probably poor hygiene (lack of cleanliness) in restaurants. You're most at risk when dining out in areas of Asia, the Middle East, Mexico, Africa, and South and Central America.
Pathogens are usually spread via the fecal-oral route . This means someone with the bacteria or virus excretes the germs in their feces. The feces may not be safely disposed of in a sanitary setting, or the infected person may not properly wash their hands before handling food and beverages. This allows germs to be transmitted to something you put into your mouth.
This cycle of contamination is most common in areas of the world that have specific conditions:
- Warmer climates that promote germ growth
- Poor sanitation (such as open sewage areas)
- Unreliable refrigeration
- Little education on safe food handling.
Common Bacterial Pathogens
The most common cause of traveler's diarrhea is bacteria, which are thought to lead to 80% to 90% of cases. These include:
- Escherichia coli or E-coli
- Campylobacter jejuni
Ingesting these bacterium causes gastroenteritis , which means the stomach and small intestines become inflamed. This leads to diarrhea.
Common Viral Pathogens
Viruses can also be transported via the fecal-oral route. The most common types of viruses that cause diarrhea include:
Viral infections of the digestive system are often referred to as stomach flu . The illness has no connection to respiratory influenza, but like the "flu," it usually lasts a short period.
Other Causes of Diarrhea
In addition to germs in your food and water, you could develop diarrhea from toxins, which cause the common symptoms of food poisoning .
Parasites , or protozoal pathogens, can also cause diarrhea. In these instances, you're more likely to develop symptoms one to two weeks after exposure to the pathogen.
Dehydration is one of the most common complications related to any form of diarrhea. Multiple bowel movements that release a lot of fluid can cause you to have too little water in your body.
Severe dehydration can lead to problems such as:
- Fatigue and muscle weakness or pain
- Dizziness or lightheadedness
- Increased heart rate and breathing
- Kidney Failure
Dysentery is a serious condition that can develop from exposure to Shigella or parasites. It usually causes bloody stool, fever, and extreme dehydration. It can be fatal if it's left untreated. In addition to being picked up from contaminated food or water, the bacteria or parasites that cause dysentery can be passed from person to person in close contact, or you can get it by swimming in unclean water.
Treatment for Traveler's Diarrhea
Getting sick while far from home is more than just inconvenient. The sudden onset and severity of symptoms can be frightening. Often, symptoms will last a few days and resolve on their own, but you may need to manage the condition and take medication.
Fluid Replacement
To manage dehydration, you want to concentrate on getting enough liquids even if you feel like you don't want to put anything in your stomach.
Drinking any safe fluids can manage mild cases of traveler's diarrhea. Since tap water may be a source of infection, you need to boil non-bottled water and let it cool before you drink it. You can also drink boiled broth or prepackaged (non-citrus) fruit juice. Sports drinks like Gatorade are good, too, but not essential.
For severe dehydration, an oral rehydration solution may be needed. These are mixes or packaged beverages that contain glucose and electrolytes such as potassium and sodium. Pedialyte is an example of an oral rehydration solution for kids.
Sweating can cause dehydration as well. Try to find a cool place out of the sun to rest while you rehydrate.
Antibiotics
Antibiotics may be used for traveler's diarrhea caused by bacterial infections. A stool test should be done to identify which antibiotic might work best.
Quinolone antibiotics such as Cipro (ciprofloxacin) are most often used when antibiotics are needed.
A single dose of 750 milligrams (mg) for adults is the typical treatment. Children may be given 20 to 30 mg per kilogram of weight per day.
In some areas, bacteria are resistant to quinolones, which means the medication won't help. This is especially a problem in Southeast Asia. Another antibiotic, azithromycin , may also be used in this case, although some strains are resistant to it.
Upset Stomach Medication
Pepto-Bismol can provide short-term relief of symptoms. However, it may not be effective in small doses, and high doses put you at risk for a health condition called salicylate toxicity. Additionally, Pepto-Bismol is not recommended for people younger than 18 years because there's a risk of a condition called Reye's syndrome .
Antidiarrheal Agents
It might seem logical to reach for an anti-diarrheal product such as Imodium (loperamide) or Lomotil (diphenoxylate). However, these products should not be used if your diarrhea is related to dysentery or if you see any signs of blood in your stools.
An antidiarrheal agent should only be taken with an antibiotic. When using an antidiarrheal for traveler's diarrhea, it is especially important to keep yourself well-hydrated. Discontinue the product if your symptoms worsen or you still have diarrhea after two days.
How Long Traveler's Diarrhea Lasts
Most cases of traveler's diarrhea last from one to five days. However, symptoms may linger for several weeks.
To help prevent traveler's diarrhea:
- Wash your hands with soap and water after going to the bathroom and before eating.
- At restaurants, only eat foods that are cooked and served hot.
- Drink beverages from factory-sealed bottles or containers.
- Don't get ice in your drink since it may be made with contaminated water.
There is evidence that Pepto-Bismol may protect against traveler's diarrhea. Studies have shown a protection rate of about 60%. However, not everyone should take Pepto-Bismol, including those who are pregnant or are 18 years of age and younger.
Don't take antibiotics or antidiarrheal medicine like Pepto-Bismol as prophylaxis—that is, to prevent traveler's diarrhea— unless it's been recommended to you by your healthcare provider.
Bacteria and viruses can live in water and food. These pathogens (germs) are most common in areas where the climate is warm, refrigeration is unreliable, and there isn't proper hand washing or bathroom sanitation. Infection with these pathogens (bacterial or viral) can cause traveler's diarrhea.
Traveler's diarrhea will often resolve on its own once the bacteria or virus is out of your system. However, you may need antibiotics. You may also need to manage symptoms by staying hydrated and using over-the-counter medications. You should contact your healthcare provider if symptoms last more than a few days.
When traveling to regions that have warm climates and relaxed hygiene practices, be sure to take steps to avoid eating or drinking anything that could have pathogens. Drink pre-packed or boiled water and ensure food is handled properly.
It's important to make sure that your child gets enough fluids. Diarrhea can lead to dehydration more quickly in kids than in adults. Check with your healthcare provider if your child has signs of dehydration such as dry mouth, few or no tears when crying, irritability, reduced urination, and drowsiness.
If you're pregnant, the most important thing to do is to drink enough fluids so you don't get dehydrated. Your doctor may suggest using azithromycin if you need an antibiotic. Don't use Pepto-Bismol (bismuth subsalicylate) when pregnant because of risks to the growing fetus.
Connor BA. Preparing international travelers: Travelers’ diarrhea . In: Brunette GW, ed. CDC Yellow Book 2020: Health information for international travel . Oxford University Press; 2017.
Leung AKC, Leung AAM, Wong AHC, Hon KL. Travelers’ diarrhea: a clinical review . Recent Pat Inflamm Allergy Drug Discov . 2019;13(1):38-48. doi:10.2174/1872213X13666190514105054
Shaheen NA, Alqahtani AA, Assiri H, Alkhodair R, Hussein MA. Public knowledge of dehydration and fluid intake practices: variation by participants' characteristics . BMC Public Health . 2018;18(1):1346. doi:10.1186/s12889-018-6252-5
Strachan SR, Morris LF. Management of severe dehydration . Pediatr Crit Care Med . 2017;18(3):251-255. doi:10.1177/1751143717693859
Riddle MS, Connor BA, Beeching NJ, et al. Guidelines for the prevention and treatment of travelers’ diarrhea: a graded expert panel report . J Travel Med . 2017;24(suppl_1):S57-S74. doi:10.1093/jtm/tax026
Johns Hopkins Medicine. Traveler's Diarrhea.
Nemours Foundation. KidsHealth. Staying healthy while you travel.
Morof DF, Carroll ID. Family travel: Pregnant travelers . In: Brunette GW, ed. CDC Yellow Book 2020: Health information for international travel . Oxford University Press; 2017.
Wanke, Christine A. " Travelers' Diarrhea ." UpToDate .
By Barbara Bolen, PhD Barbara Bolen, PhD, is a licensed clinical psychologist and health coach. She has written multiple books focused on living with irritable bowel syndrome.
- Skip to content
- Accessibility help
Diarrhoea - prevention and advice for travellers
Last revised in September 2023
Travellers' diarrhoea is a clinical syndrome associated with contaminated food or water, that occurs during or shortly after travel
- Scenario: Diarrhoea - prevention and advice for travellers
Background information
- Risk factors
- Complications
Diarrhoea - prevention and advice for travellers: Summary
- Travellers' diarrhoea is defined as passing three or more unformed stools in a 24-hour period with at least one additional symptom, such as abdominal pain or cramps, nausea, vomiting, fever, or blood in the stools.
- Enteric bacteria are the most commonly documented (for example, Escherichia coli, Campylobacter spp., Salmonella spp., and Shigella spp .).
- Viruses and parasites can also cause travellers' diarrhoea.
- Low for people travelling to western European countries, the USA and Canada, Japan, Australia, and New Zealand.
- Intermediate for people travelling to southern European countries, Israel, South Africa, and some Caribbean and Pacific Islands.
- High for people travelling to Africa, Latin America, the Middle East, and most parts of Asia.
- Food hygiene and safe drinking water.
- Self-management and when to seek medical advice if diarrhoea develops during travel.
- The importance of personal hygiene, food hygiene, and safe drinking water should be emphasized.
- Advice regarding the risk of waterborne infection and avoiding contaminated recreational water should be offered.
- Antibiotic prophylaxis or 'stand-by' antibiotic treatment can be considered for certain high-risk travellers. Specialist advice should be sought.
- Most episodes are short-lived and self-limiting, lasting a few days.
- The person could consider purchasing sachets of oral rehydration salt before travelling.
- During an episode of diarrhoea, it is important to prevent dehydration — particularly for young children, pregnant women, elderly people, and those with pre-existing illnesses.
- Both loperamide and bismuth subsalicylate (for example, Pepto-Bismol ® ) may be considered in adults for the relief of mild-to-moderate diarrhoea. They should be used for a maximum of 2 days.
- When to seek medical assistance.
The content on the NICE Clinical Knowledge Summaries site (CKS) is the copyright of Clarity Informatics Limited (trading as Agilio Software Primary Care) . By using CKS, you agree to the licence set out in the CKS End User Licence Agreement .
- Current Issue
- Supplements
Gastroenterology & Hepatology
December 2018 - volume 14, issue 12, supplement 8, emerging options for the management of travelers’ diarrhea, robert steffen, md.
Emeritus Professor, Epidemiology, Biostatistics and Prevention Institute WHO Collaborating Centre for Travellers’ Health University of Zurich Zurich, Switzerland Adjunct Professor, Epidemiology, Human Genetics and Environmental Sciences Division University of Texas School of Public Health Houston, Texas
Abstract: The incidence of travelers’ diarrhea (TD) has slightly decreased due to improvements in the hygienic conditions seen in many destination countries. However, high-risk areas are still widespread. Previous classification based severity on the number of unformed stools passed in 24 hours. Now, TD is differentiated as mild, intermediate, and severe, with the new definitions focusing on the functional impact. Incapacitation is frequent and can result in the need to change travel plans, including cancellation of flights, which results in considerable expense. Guidelines published in 2017 offer detailed recommendations for the prophylaxis and management of TD. For mild TD, antibiotics are no longer recommended. However, antibiotics provide the most rapid symptom relief for patients with intermediate or severe TD, particularly when used in combination with antimotility agents. Fluoroquinolones are no longer the first-line antimicrobials against TD because of widespread resistance and evidence for the increased risk of multidrug-resistant organisms. Also, side effects are of concern. In November 2018, the US Food and Drug Administration approved a new, virtually nonabsorbed antibiotic, rifamycin, for the treatment of TD, based on data showing efficacy without evidence of an increase in multidrug resistance.
Introduction
Travelers’ diarrhea (TD) is the most frequent health problem abroad. 1 TD refers to watery or soft stools that are accompanied by symptoms such as fecal urgency, abdominal cramps, nausea, vomiting, or fever. Each year, approximately 15 to 20 million people develop TD. 2 Previous def-initions have based severity on the number of unformed stools passed in 24 hours. 3
Now, TD is differentiated as mild, intermediate, and severe, depending on the degree of incapacitation; the new definitions focus on the functional impact. 3 A mild manifestation of TD is tolerable, is not distressing, and does not interfere with planned activities. An intermediate episode is distressing and interferes with the patient’s plans and ability to function, regardless of the number of stools. The patient often has substantial accompanying symptoms. Severe TD leaves the patient completely incapacitated and unable to take part in planned activities. In general, the patient must remain close to a toilet. The presence of fever or gross blood mixed in the stool can indicate dysentery, 2 an invasive disease resulting from damage to the intestinal mucosa. Dysentery is always considered to be severe TD. The distinction between intermediate and severe TD has limited relevance; any degree of incapacitation can be troublesome. For example, if a patient must miss a business meeting, an excursion to an important tourist site, or a flight due to TD, then the episode is incapacitating, regardless of the number of unformed stools or accompanying symptoms.
Without treatment, the average episode of TD lasts approximately 4 days. 2,4,5 In approximately half of patients, symptoms resolve within 48 hours. Some people experience a spontaneous cure after a few hours, but in other cases, TD can persist for 2 weeks or even longer, particularly in patients with an underlying disease. 6
Epidemiology
The incidence of TD has slightly decreased due to improvements in the hygienic conditions seen in many destination countries in the past decades. 7 During the period just after World War II, even southern Europe had a fairly high incidence of TD. The incidence has drastically decreased in this area, 8 as well as in countries in which average income rose from low or even intermediate levels. For example, the incidence rates of TD have decreased in China and other countries in East Asia, and also in large parts of South America. 7 In these areas, the incidence rates are at an intermediate level, which means that 8% to 20% of visitors will develop TD during the first 2 weeks of their stay. Destinations with high incidence rates, in which more than 20% of visitors will develop TD, include large portions of Central America, as well as countries in South Asia, such as India, Pakistan, Bangladesh, and Nepal. Other high-risk destinations include some areas in the Middle East and nearly all of Africa (with the exception of South Africa). Tourist resorts, even in these areas, are increasingly taking great care to reduce the risk of TD among their guests, with some success.
Risk Factors
Risk factors for TD are related to the environment and the host (Table 1). 9 Environmental risk factors include the macro-epidemiology, meaning a specific country or region, as well as the micro-epidemiology, such as hotels. According to a survey on the incidence of TD at hotels in Jamaica with at least 40 clients, 10 one hotel had zero cases, a few had an incidence of less than 10%, and some exceeded 30%. The incidence of TD mirrors the hygienic conditions in the kitchen. 11 TD can affect anyone, even guests at 5-star hotels. 2
Other environmental risk factors include the characteristics of travel. Backpackers who obtain raw or improperly cooked food from street vendors are at highest risk. 7,12-14 Trips that involve multiple destinations have a higher incidence rate as compared with a visit to one location. The incidence of TD has been shown to be higher in all-inclusive trips, most likely because visitors may overindulge in unlimited alcoholic drinks. 14,15 Consumption of alcohol can result in diarrhea, even without contamination from food and beverages. Mountaineering is also associated with a considerable risk of TD. Higher rates have been seen among travelers to the Mount Everest region in Nepal and Denali (formerly known as Mount McKinley) in Alaska. 7,16-18
There are several host risk factors. In all studies, the incidence of TD is highest among young people, ages 15 to 30 years. 19-21 The reason is unknown, but it may be because younger people have a larger appetite and therefore ingest more pathogens as compared with senior travelers. 9,22 This age-related difference is maintained even among groups of travelers who stay at the same hotel with all-inclusive menus. TD is not more frequent in either sex, although women may have a stronger perception of the disorder and seek care more often. 23 Travelers originating in countries with an intermediate or high risk of TD have a far lower incidence rate compared with those from low-risk countries. 1,24,25 Also, those who stay in a developing country apparently develop some immunity, which partially protects them for a few months during subsequent travel (Table 2). 26 There may be a genetic predisposition toward developing the disorder. 9 Preexisting illnesses, such as gastrointestinal disorders and possibly immunodeficiency, may result in a high risk of TD. Lack of gastric acids has also been associated with a high risk. 27
Complications and Impact
In the acute phase of TD, the foremost complication is dehydration. Patients may collapse, particularly when in a tropical climate.
Incapacitation is frequent and can be troubling. For example, if several family members or friends are traveling together, even if only one of them develops TD, it can be disruptive for the entire group. One or more of the healthy travelers may need to stay behind to care for the person with TD. Cancellation of flights and other travel arrangements can result in considerable expense.
Longer-Term Complications
Intermediate or long-term compli-cations include irritable bowel syn-drome, 7,28 as well as acquisition of extended-spectrum beta lactamase-producing Enterobacteriaceae. 29 More rare complications include reactive arthritis, hemolytic-uremic syndrome, Guillain-Barré syndrome, and diarrhea associated with Clostridium difficile . 7,30
Etiology of TD
TD can be caused by bacteria, viruses, and parasites (Table 3). 3 Bacteria are responsible for at least 80% of cases, whereas viruses cause approximately 5% to 10%. These statistics vary according to geographic location. Up to 60% of infections have a mixed etiology.
Parasites, such as Entamoeba histolytica , Giardia lamblia , or Cryptosporidium , are a rare cause of TD, 31 accounting for less than 5%, or even less than 2%, of cases, depending on the destination. However, TD caused by parasites tends to persist after the patient returns home. In industrialized countries, the percentage of patients with TD caused by a pathogen is far higher than 5% because these cases tend to last longer and require treatment. Most cases caused by bacteria or a virus will eventually resolve without treatment. 32
Dietary Methods
The traditional rule for prevention of TD was, “Boil it, cook it, peel it, or forget it.” There is, however, no evidence that this approach substantially reduces the rates of TD. 33 There may even be higher rates of diarrhea among travelers who tried to be careful about what they ate, as compared with those who claimed to eat anything, including salads from buffets or even beef tartar and raw oysters. 4,15 These studies, however, have often been biased by a retrospective approach. There has been only one prospective study, which was performed at my clinic. 34 Unfortunately, the answer rate in this study was too low to permit any conclusions. It is therefore not known whether so-called dangerous foods should be avoided. Only a small proportion of travelers follow the advice to eat food that is boiled, cooked, or peeled. 34 Most travelers drink fruit juices, eat salads, and consume food from buffets; often, there is nothing else available. 15
Medical Prophylaxis
Drug prophylaxis for TD is more often recommended and used in the United States than elsewhere. In fact, European travelers are even reluctant to use prophylaxis against malaria. Bismuth subsalicylate is an option for any traveler for the prevention of TD. 3 Antibiotic prophylaxis is prescribed in certain circumstances, such as patients with a preexisting illness of the gastrointestinal tract or those likely to develop serious complications after TD. 3 Antimicrobial prophylaxis might also be considered for patients attending important functions, who cannot risk being incapacitated for even a few hours. Rifaximin is the recommended antibiotic for use as prophylaxis. 3 Fluoroquinolones are no longer recommended.
The treatment options for TD vary according to the severity of the case. Oral rehydration therapy as a supportive measure is the treatment of choice for infants, children, and the elderly. It is necessary to avoid dehydration and electrolyte imbalance. Such medications are available everywhere. In contrast, there are advantages to equipping future travelers with a travel kit with additional TD medication, since in many countries TD patients otherwise are at risk of counterfeit drugs, 35 obsolete medication, or even unnecessary hospitalization by some doctors practicing close to tourist resorts. 36
According to 2017 guidelines from Riddle and colleagues, mild TD should not be treated with antibiotics. 3 A mild episode can be treated with small doses of antimotility agents, such as loperamide, or bismuth subsalicylate. (In Europe, bismuth subsalicylate is not widely available and rarely used.) Activated charcoal and dimenhydrinate are not recommended for TD patients.
In some cases, antibiotics can be used to treat patients with moderate TD. 3 Antibiotics, particularly when used in combination with antimotility agents, provide the most rapid symptom relief for patients with TD. Alone, they can reduce the average duration to slightly longer than a day, or even half a day when combined with loperamide. 3 Single-dose antibiotic regimens are preferred. Options mainly include azithromycin and rifaximin. The recently approved rifamycin is also an option for moderate TD. 37
Until a few years ago, fluoroquinolones were the most commonly used class of antibiotics. There is increasing reluctance to use fluoroquinolones because of increasing resistance, acquisition of multiresistant pathogens, and risk of adverse events. 3,38,39 There is evidence for the increased risk of multi-drug-resistant organisms, particularly with ciprofloxacin. The recent guidelines cite an incrementally increasing association between travel, TD, and use of antibiotics with the acquisition of multidrug-resistant bacteria. 3 Before they leave for a trip, patients should be informed about how this risk can be balanced against the benefits associated with the use of antibiotics.
Another concern with traditional antibiotics is their side effects. Ciprofloxacin in particular has been associated with adverse events. 40,41 Caution should be exercised when using rifaximin as empirical therapy for moderate diarrhea in regions associated with a high risk of invasive pathogens. Loperamide can be used as monotherapy or adjunctive treatment for moderate TD. 3 Bismuth subsalicylate is another option.
Patients with severe TD should receive treatment with antibiotics, possibly combined with loperamide. 3 Single-dose regimens may be used. Azithromycin is the preferred antibiotic. Fluoroquinolones or nonabsorbable rifaximin may be used to treat severe, nondysenteric TD. Similar to the treatment of moderate TD, the recently approved rifamycin is an option for the treatment of severe TD. 37
Currently, the most commonly used antibiotics for TD are azithromycin and nonabsorbable rifaximin. Both of these treatments are recommended by the new guidelines. 3 A disadvantage to azithromycin is that it may cause nausea, particularly if used at a higher dosage. Rifaximin and rifamycin are nonabsorbable and therefore do not cause any such side effects. In patients with dysentery, azithromycin is the first choice because rifaximin and rifamycin are unlikely to be effective in an invasive infection. 3
The new MMX formulation of rifa-mycin (Aemcolo, Aries Pharmaceuticals, Inc. [a Cosmo Pharmaceuticals N.V. Company]) was approved for TD by the US Food and Drug Administration in November 2018. 42 Rifamycin is similar to rifaximin, an enteric antibiotic that is poorly absorbed. 43,44 Rifamycin incorporates a multimatrix (MMX) technology, which delays release of the drug after ingestion. 45 The drug is released when it encounters intestinal pH levels of 7 or higher. 46 Rifamycin is therefore active only in the colon and the lower part of the ileum. Similarly to rifaximin, as a nonabsorbable antibiotic, rifamycin is associated with a low rate of adverse events. 46 This therapy also has anti-inflammatory properties. 47
An in vitro study by Farrell and colleagues showed that rifamycin was potent against enteropathogens commonly associated with TD. 48 Rifamycin was tested for activity against 911 enteropathogens and 30 C difficile isolates gathered from global surveillance studies. Values of minimum inhibitory concentration (MIC) were measured. Against Enterobacteriaceae strains, MIC 50 values ranged from 32 µg/mL to 128 µg/mL for all but 1 strain (an enterotoxigenic Escherichia coli at >512 µg/mL). Against non-Enterobacteriaceae strains, MIC 50 values ranged from 2 µg/mL to 8 µg/mL. (The one exception was Campylobacter species, which had MIC values >512 µg/mL.)
Rifamycin was very active (MIC 50 , ≤0.03 µg/mL) against 26 of 30 strains of C difficile (including 1 hypervirulent NAP1 strain). Among the 4 remaining strains (which also included a hypervirulent NAP1 strain), MIC values ranged from 256 µg/mL to 512 µg/mL. Less activity against C difficile strains (including a hypervirulent NAP1 strain) was observed with rifaximin (MIC 50 , 0.12 µg/mL), metronidazole (MIC 50 , 0.25 µg/mL), and vancomycin (MIC 50 , 0.5 µg/mL).
In a study of healthy volunteers, rifamycin was poorly absorbed after oral treatment given in single- and multiple-dose regimens. 46 The oral bioavailability was 0.1%. The amount of systemically absorbed drug excreted in the urine was less than 0.01% of the administered dose (in both the single- and multiple-dose regimens). In the feces, the administered dose was eliminated at a rate of 87%. The absolute bioavailability was 0.0410 ±0.0617 after a single intravenous injection and after a single oral dose under fasting conditions (as calculated by the mean percent ratio between total urinary excretion amounts).
A phase 2 trial of rifamycin vs rifaximin confirmed the efficacy and safety of the new treatment among 115 patients with active infectious diarrhea. 49 Treatment was successful in 47.8% of the rifamycin arm vs 50.9% of the rifaximin arm. The median time to last unformed stool (TLUS) was 67.5 hours with rifamycin vs 65.5 hours with rifaximin. Isolates of Campylobacter jejuni , C lari , E coli , Anaerobiospirillum , Salmonella enteritidis , and Shigella flexneri identified at baseline were no longer retrievable after treatment with rifamycin.
Adverse events occurred in 29 patients treated with rifamycin vs 25 patients treated with rifaximin (Table 4). There were similar rates of constipation, worsening of diarrhea/aggravated diarrhea, and development of yeasts in feces.
A randomized, double-blind phase 3 trial by DuPont and colleagues compared rifamycin (2 × 200 mg twice daily for 3 days) vs placebo. 50 The primary endpoint was TLUS, defined as the interval between the first dose of study drug and the time the last unformed stool was passed. TLUS was 46.0 hours with rifamycin vs 68.0 hours with placebo, a significant difference ( P =.0008). A clinical cure was reported in 81.4% of the rifamycin arm vs 56.9% of the placebo arm (Figure 1). The rates of pathogen eradication were 67.0% with rifamycin vs 54.8% with placebo, but this difference did not reach statistical significance ( P =.0836). After treatment with rifamycin, in vitro studies showed resistance to rifamycin in some remaining bacteria, but this finding did not correspond to lower efficacy.
Adverse events occurred in 29.6% of the rifamycin arm vs 38.5% of the placebo arm. Diarrhea, the most common adverse event, was reported in 10.0% of the rifamycin patients and 16.9% of the placebo patients. In the rifamycin arm, the next most common adverse events were headache (occurring in 8.5%) and constipation (occurring in 3.5%). There were no reports of amebic dysentery or gastrointestinal infection among patients treated with rifamycin. Each of these adverse events occurred in 3.1% of patients receiving placebo.
My colleagues and I performed a randomized, double-blind phase 3 study comparing rifamycin (400 mg twice daily) vs ciprofloxacin (500 mg twice daily; at the time of study design, the first-line drug) for the treatment of TD. 51 The study was designed to prove noninferiority of rifamycin compared with ciprofloxacin. The primary endpoint was TLUS, which was defined as the interval between the first dose of the study drug and the last unformed stool passed before the end of the clinical cure period. This conservative definition of TLUS differs from that seen in other trials, which limits the duration to before the start of the clinical cure period.
The study randomly assigned treatment to 835 international visitors who developed acute TD. Most patients were traveling in India (n=805), and the others were in Guatemala or Ecuador (n=30). Patients had experienced at least 3 unformed, watery or soft stools within 24 hours before randomization, with the illness lasting no more than 72 hours. All patients also reported at least 1 moderate to severe sign or symptom of enteric infection (eg, gas/flatulence, nausea, vomiting, abdominal cramps or pain, rectal tenesmus, fecal urgency). Exclusion criteria included passage of gross bloody stools, known or suspected infection with a nonbacterial pathogen (eg, human immunodeficiency virus or viral hepatitis), moderate or severe dehydration, history of inflammatory bowel disease or celiac disease, and use of more than 2 doses of an antidiarrheal medication within 24 hours or use of an antibiotic within 7 days prior to randomization.
Treatment was completed by 814 patients (97.5%) in the study, and discontinuation rates were similar in both arms. In the per-protocol analysis, the median TLUS was 42.8 hours with rifamycin vs 36.8 hours with ciprofloxacin. This difference indicated that rifamycin was noninferior to ciprofloxacin ( P =.0035). The intent-to-treat analysis confirmed this noninferiority. The median TLUS was 44.3 hours with rifamycin vs 40.3 hours with ciprofloxacin ( P =.0011). There were no statistically significant differences between the treatment groups for the secondary endpoints of clinical cure rate, treatment failure rate, and requirement of rescue therapy. A subgroup analysis found that earlier initiation of either treatment corresponded to a shorter TLUS.
Adverse events occurred in 14.8% of the rifamycin arm and 14.9% of the ciprofloxacin arm. Adverse drug reactions occurred in 8.1% vs 7.5%, respectively. There were no reports of serious adverse events or deaths.
As mentioned, multidrug resistance is a concern with ciprofloxacin and other fluoroquinolones. An inter-esting finding in the trial was that rifamycin did not increase multidrug resistance. 51 Among patients in the ciprofloxacin arm, colonization rates with extended-spectrum beta lactamase– producing E coli increased by 6.9% after 3 days of treatment (Figure 2). In contrast, this rate did not increase among patients treated with rifamycin. These data were shown in a study presented at the 2017 Digestive Disease Week and the 15th Conference of the International Society of Travel Medicine. 52,53
Other Emerging Treatments
Studies of the gut microbiome in travelers with and without diarrhea may clarify the use of current and novel preventive, diagnostic, and therapeutic approaches. 54 Currently, there is insufficient evidence to recommend the use of commercially available prebiotics or probiotics to prevent or treat TD. 3,55,56 However, there is reason to expect that next-generation probiotics could bolster colonization resistance against pathogens and thus prevent TD. 57 Since there is a lack of evidence supporting the use of antisecretory agents, such as crofelemer and racecadotril, in the setting of TD, these agents are not broadly recommended. 3 Only zaldaride has been evaluated, but this agent is not marketed anywhere. 58
Currently, there is no effective vaccine against TD. In Canada, the oral cholera vaccine originally was recommended as prophylaxis against TD, but since 2015, the indication has been limited to TD associated with E coli . In the European Union, this vaccine is used only to protect against cholera, and most experts agree that it has, at best, a minimal impact on the TD incidence rate 59 —far less, for example, than the influenza vaccine. Several vaccine candidates are in the pipeline. 60
Evaluation of a Patient After a Trip
When a patient returns with TD after a trip, it is first necessary to take a history and to assess the symptoms, particularly the degree of incapacitation. The date of onset is important, to determine whether the diarrhea is acute, persistent, or chronic. Also, one needs to learn what therapy already has failed to improve the condition. Some patients may have received obsolete or inappropriate therapies during their travels. 36 For the management, it is not necessary to learn where the patient was traveling, what he or she ate, or whether the trip was for business or pleasure, but it is always nice to express a general interest and empathy for the patient’s history. The patient may urgently require rehydration and/or treatment for relief of symptoms. In general, patients should not be treated with the same medication they already received.
Traditional microbiologic testing is not needed in uncomplicated cases; hopefully, the patient will be cured before the results arrive. In contrast, testing is recommended in patients with severe TD or persistent symptoms or when antibiotic therapy was unsuccessful. 3 In patients with dysentery, there is general agreement that laboratory tests are needed. Molecular testing for clinically relevant pathogens is preferred when rapid results are needed or when nonmolecular tests did not establish a diagnosis. If the patient previously received antimicrobial agents without any improvement of the condition, then I would obtain a stool test for parasites. If the patient has received an antimicrobial agent and is very incapacitated or even has dysentery, then I may consider another antibiotic. Despite the lack of evidence, I occasionally may use probiotics when the symptoms are mild and my intention is to avoid harm while gaining time toward a spontaneous cure.
Approximately 6% of patients have diarrhea that is persistent (lasting more than 2 weeks). 6 This type of TD may be associated with undiagnosed parasitic gastrointestinal infection, which needs to be treated appropriately. The probability of a bacterial infection decreases with the duration of symptoms. In some cases, the episode of TD unmasks a previously undiagnosed gastrointestinal disease, such as idiopathic inflammatory bowel disease, celiac sprue, or lactose intolerance. Initial evaluation should include a complete blood count to check for anemia and eosinophilia, stool analysis (polymerase chain reaction is preferred), an assay for C difficile , and celiac serologies. Patients may exhibit postinfectious sequela of an enteric infection.
Management of TD continues to evolve. Guidelines offer detailed recommendations for the many different scenarios that can arise. For the many patients with mild TD, antibiotics are now contraindicated since they may be associated with adverse events and resistance. In contrast, these agents may or should be used in more severe forms of TD. While quinolones have lost their position as first-choice antimicrobials in TD for a number of reasons, the nonabsorbed agents rifaximin and the newer treatment rifamycin are attractive options for the therapy of noninvasive TD. In particular, it has been shown that patients receiving rifamycin for the treatment of TD are not at increased risk of acquiring multidrug-resistant pathogens.
Dr Steffen has been compensated as the global principal investigator in the phase 3 study sponsored by Dr. Falk Pharma comparing rifamycin to ciprofloxacin, which has been summarized in this review. He has or recently had consultancy agreements with Aries, Clasado, and Host Therabiomics, and he has been reimbursed by various vaccine producers for lectures.
This article has been prepared with the assistance of Jacquelyn Matos of Millennium Medical Publishing.
1. Angelo KM, Kozarsky PE, Ryan ET, Chen LH, Sotir MJ. What proportion of international travellers acquire a travel-related illness? A review of the literature. J Travel Med . 2017;24(5). doi:10.1093/jtm/tax046.
2. Steffen R. Epidemiology of traveler’s diarrhea. Clin Infect Dis . 2005;41(suppl 8):S536-S540.
3. Riddle MS, Connor BA, Beeching NJ, et al. Guidelines for the prevention and treatment of travelers’ diarrhea: a graded expert panel report. J Travel Med . 2017;24(suppl_1):S57-S74.
4. Steffen R, van der Linde F, Gyr K, Schär M. Epidemiology of diarrhea in travelers. JAMA . 1983; 249(9):1176-1180.
5. Kollaritsch H. Traveller’s diarrhea among Austrian tourists to warm climate countries: II. Clinical features. Eur J Epidemiol . 1989;5(3):355-362.
6. Duplessis CA, Gutierrez RL, Porter CK. Review: chronic and persistent diarrhea with a focus in the returning traveler [published online May 4, 2017]. Trop Dis Travel Med Vaccines . 2017;3:9. doi:10.1186/s40794-017-0052-2.
7. Steffen R. Epidemiology of travellers’ diarrhea. J Travel Med . 2017;24(suppl_1):S2-S5.
8. Cartwright RY. Food and waterborne infections associated with package holidays. J Appl Microbiol . 2003;94(suppl):12S-24S.
9. Steffen R, Hill DR, DuPont HL. Traveler’s diarrhea: a clinical review. JAMA . 2015;313(1):71-80.
10. Steffen R, Collard F, Tornieporth N, et al. Epidemiology, etiology, and impact of traveler’s diarrhea in Jamaica. JAMA . 1999;281(9):811-817.
11. Ashley DVM, Walters C, Dockery-Brown C, McNab A, Ashley DEC. Interventions to prevent and control food-borne diseases associated with a reduction in traveler’s diarrhea in tourists to Jamaica. J Travel Med . 2004;11(6):364-367.
12. Soonawala D, Vlot JA, Visser LG. Inconvenience due to travelers’ diarrhea: a prospective follow-up study. BMC Infect Dis . 2011;11:322.
13. Herbinger KH, Alberer M, Berens-Riha N, et al. Spectrum of imported infectious diseases: a comparative prevalence study of 16,817 German travelers and 977 immigrants from the tropics and subtropics. Am J Trop Med Hyg . 2016;94(4):757-766.
14. Piyaphanee W, Kusolsuk T, Kittitrakul C, Suttithum W, Ponam T, Wilairatana P. Incidence and impact of travelers’ diarrhea among foreign backpackers in Southeast Asia: a result from Khao San road, Bangkok. J Travel Med . 2011;18(2):109-114.
15. Steffen R, Tornieporth N, Clemens SA, et al. Epidemiology of travelers’ diarrhea: details of a global survey. J Travel Med . 2004;11(4):231-237.
16. McLaughlin JB, Gessner BD, Bailey AM. Gastroenteritis outbreak among mountaineers climbing the West Buttress route of Denali—Denali National Park, Alaska, June 2002. Wilderness Environ Med . 2005;16(2):92-96.
17. Williams KL. Exped 2010—the challenges of an expedition doctor. J R Army Med Corps . 2011;157(1):121-123.
18. Murdoch DR. Symptoms of infection and altitude illness among hikers in the Mount Everest region of Nepal. Aviat Space Environ Med . 1995;66(2):148-151.
19. Cobelens FGJ, Leentvaar-Kuijpers A, Kleijnen J, Coutinho RA. Incidence and risk factors of diarrhoea in Dutch travellers: consequences for priorities in pre-travel health advice. Trop Med Int Health . 1998;3(11):896-903.
20. Pitzinger B, Steffen R, Tschopp A. Incidence and clinical features of traveler’s diarrhea in infants and children. Pediatr Infect Dis J . 1991;10(10):719-723.
21. Hoge CW, Shlim DR, Echeverria P, Rajah R, Herrmann JE, Cross JH. Epidemiology of diarrhea among expatriate residents living in a highly endemic environment. JAMA . 1996;275(7):533-538.
22. Pitzurra R, Steffen R, Tschopp A, Mütsch M. Diarrhoea in a large prospective cohort of European travellers to resource-limited destinations. BMC Infect Dis . 2010;10:231.
23. Schlagenhauf P, Chen LH, Wilson ME, et al; GeoSentinel Surveillance Network. Sex and gender differences in travel-associated disease. Clin Infect Dis . 2010;50(6):826-832.
24. Steffen R, Sack RB. Epidemiology. In: Ericsson CD, DuPont HL, Steffen R, eds. Travelers’ Diarrhea. Hamilton, Ontario: BC Decker; 2003:112-123.
25. Dupont HL, Haynes GA, Pickering LK, Tjoa W, Sullivan P, Olarte J. Diarrhea of travelers to Mexico. Relative susceptibility of United States and Latin American students attending a Mexican University. Am J Epidemiol . 1977;105(1):37-41.
26. Kuenzli E, Juergensen D, Kling K, et al. Previous exposure in a high-risk area for travellers’ diarrhoea within the past year is associated with a significant protective effect for travellers’ diarrhoea: a prospective observational cohort study in travellers to South Asia. J Travel Med . 2017;24(5). doi:10.1093/jtm/tax056.
27. Neal KR, Scott HM, Slack RC, Logan RFA. Omeprazole as a risk factor for campylobacter gastroenteritis: case-control study. BMJ . 1996;312(7028):414-415.
28. Schwille-Kiuntke J, Mazurak N, Enck P. Systematic review with meta-analysis: post-infectious irritable bowel syndrome after travellers’ diarrhoea. Aliment Pharmacol Ther . 2015;41(11):1029-1037.
29. DuPont HL, Steffen R. Use of antimicrobial agents for treatment and prevention of travellers’ diarrhoea in the face of enhanced risk of transient fecal carriage of multi-drug resistant enterobacteriaceae: setting the stage for consensus recommendations. J Travel Med . 2017;24(suppl 1):S57-S62.
30. Michal Stevens A, Esposito DH, Stoney RJ, et al; GeoSentinel Surveillance Network. Clostridium difficile infection in returning travellers. J Travel Med . 2017;24(3). doi: 10.1093/jtm/taw099.
31. Connor BA, Rogova M, Whyte O. Use of a multiplex DNA extraction PCR in the identification of pathogens in travelers’ diarrhea. J Travel Med . 2018;25(1). doi:10.1093/jtm/tax087.
32. Jiang ZD, DuPont HL. Etiology of travellers’ diarrhea. J Travel Med . 2017;24(suppl 1):S13-S16.
33. Shlim DR. Looking for evidence that personal hygiene precautions prevent traveler’s diarrhea. Clin Infect Dis . 2005;41(suppl 8):S531-S535.
34. Kozicki M, Steffen R, Schär M. ‘Boil it, cook it, peel it or forget it’: does this rule prevent travellers’ diarrhoea? Int J Epidemiol . 1985;14(1):169-172.
35. Hajjou M, Krech L, Lane-Barlow C, et al. Monitoring the quality of medicines: results from Africa, Asia, and South America. Am J Trop Med Hyg . 2015;92(6)(suppl):68-74.
36. Wyss MN, Steffen R, Dhupdale NY, Thitiphuree S, Mutsch M. Management of travelers’ diarrhea by local physicians in tropical and subtropical countries—a questionnaire survey. J Travel Med . 2009;16(3):186-190.
37. Aemcolo [package insert]. San Diego, CA: Aries Pharmaceuticals, Inc; November 2018.
38. Taylor DN, Hamer DH, Shlim DR. Medications for the prevention and treatment of travellers’ diarrhea. J Travel Med . 2017;24(suppl 1):S17-S22.
39. Tribble DR. Resistant pathogens as causes of traveller’s diarrhea globally and impact(s) on treatment failure and recommendations. J Travel Med . 2017;24(suppl 1):S6-S12.
40. FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. Food and Drug Administration. ttps://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Posted May 12, 2016. Accessed November 19, 2018.
41. FDA updates warnings for fluoroquinolone antibiotics. Food and Drug Administration. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm513183.htm. Posted July 26, 2016. Accessed November 19, 2018.
42. FDA approves new drug to treat travelers’ diarrhea. Food and Drug Administration. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm626121.htm. Posted November 16, 2018. Accessed November 19, 2018.
43. Riddle MS, Connor P, Fraser J, et al; TrEAT TD Study Team. Trial Evaluating Ambulatory Therapy of Travelers’ Diarrhea (TrEAT TD) study: a randomized controlled trial comparing 3 single-dose antibiotic regimens with loperamide. Clin Infect Dis . 2017;65(12):2008-2017.
44. Floss HG, Yu TW. Rifamycin—mode of action, resistance, and biosynthesis. Chem Rev . 2005;105(2):621-632.
45. Nardelli S, Pisani LF, Tontini GE, Vecchi M, Pastorelli L. MMX® technology and its applications in gastrointestinal diseases. Therap Adv Gastroenterol . 2017;10(7):545-552.
46. Di Stefano AF, Rusca A, Loprete L, Dröge MJ, Moro L, Assandri A. Systemic absorption of rifamycin SV MMX administered as modified-release tablets in healthy volunteers. Antimicrob Agents Chemother . 2011;55(5):2122-2128.
47. Rosette C, Buendia-Laysa F Jr, Patkar S, et al. Anti-inflammatory and immunomodulatory activities of rifamycin SV. Int J Antimicrob Agents . 2013;42(2):182-186.
48. Farrell DJ, Putnam SD, Biedenbach DJ, et al. In vitro activity and single-step mutational analysis of rifamycin SV tested against enteropathogens associated with traveler’s diarrhea and Clostridium difficile . Antimicrob Agents Chemother . 2011;55(3):992-996.
49. Di Stefano AFD, Binelli D, Labuschagne W, et al. Efficacy of the treatment of infectious diarrhoea with non-absorbable rifamycin SV formulated as MMX modified release tablet vs. rifaximin. Antiinfect Agents . 2013;11:192-203.
50. DuPont HL, Petersen A, Zhao J, et al. Targeting of rifamycin SV to the colon for treatment of travelers’ diarrhea: a randomized, double-blind, placebo-controlled phase 3 study. J Travel Med . 2014;21(6):369-376.
51. Steffen R, Jiang Z-D, Gracias Garcia ML, et al. Rifamycin SV-MMX® for treatment of travelers’ diarrhea: equally effective as ciprofloxacin and not associated with the acquisition of multi-drug resistant bacteria [published online November 20, 2018]. J Travel Med . doi: org/10.1093/jtm/tay116.
52. DuPont HL. Rate of fecal acquisition of extended spectrum beta lactamase producing– Escherichia coli (ESBL-PE) in international travellers with diarrhea occurred more commonly in subjects treated with ciprofloxacin compared with rifamycin SV-MMX. Abstract presented at: the 15th Conference of the International Society of Travel Medicine; May 14-18, 2017; Barcelona, Spain. Abstract FC1.01.
53. Steffen R, DuPont HL, Jiang Z-D, et al. A novel multi-matrix formulation of the broad-spectrum, poorly absorbed antibiotic rifamycin SV is equally effective as systemic ciprofloxacin in the oral treatment of travelers’ diarrhea: a double-blind, double-dummy, multicentre, randomized study [AGA abstract 1]. Gastroenterology . 2017;152(5 suppl 1).
54. Rasko DA. Changes in microbiome during and after travellers’ diarrhea: what we know and what we do not. J Travel Med . 2017;24(suppl_1):S52-S56.
55. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis . 2007;5(2):97-105.
56. Sazawal S, Hiremath G, Dhingra U, Malik P, Deb S, Black RE. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis . 2006;6(6):374-382.
57. Vogt SL, Finlay BB. Gut microbiota-mediated protection against diarrheal infections. J Travel Med . 2017;24(suppl 1):S39-S43.
58. Silberschmidt G, Schick MT, Steffen R, et al. Treatment of travellers’ diarrhoea: zaldaride compared with loperamide and placebo. Eur J Gastroenterol Hepatol . 1995;7(9):871-875.
59. Steffen R, Behrens RH, Hill DR, Greenaway C, Leder K. Vaccine-preventable travel health risks: what is the evidence—what are the gaps? J Travel Med . 2015;22(1):1-12.
60. Riddle MS, Chen WH, Kirkwood CD, MacLennan CA. Update on vaccines for enteric pathogens. Clin Microbiol Infect . 2018;24(10):1039-1045.
61. Bavishi C, Dupont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther . 2011;34(11-12):1269-1281.
62. Jiang ZD, DuPont HL, Garey K, et al. A common polymorphism in the interleukin 8 gene promoter is associated with Clostridium difficile diarrhea. Am J Gastroenterol . 2006;101(5):1112-1116.
63. Jiang ZD, Okhuysen PC, Guo DC, et al. Genetic susceptibility to enteroaggregative Escherichia coli diarrhea: polymorphism in the interleukin-8 promotor region. J Infect Dis . 2003;188(4):506-511.
64. Mohamed JA, DuPont HL, Jiang ZD, et al. A novel single-nucleotide polymorphism in the lactoferrin gene is associated with susceptibility to diarrhea in North American travelers to Mexico. Clin Infect Dis . 2007;44(7):945-952.
65. Flores J, DuPont HL, Lee SA, et al. Influence of host interleukin-10 polymorphisms on development of traveler’s diarrhea due to heat-labile enterotoxin-producing Escherichia coli in travelers from the United States who are visiting Mexico. Clin Vaccine Immunol . 2008;15(8):1194-1198.
66. Mohamed JA, DuPont HL, Jiang ZD, et al. A single-nucleotide polymorphism in the gene encoding osteoprotegerin, an anti-inflammatory protein produced in response to infection with diarrheagenic Escherichia coli, is associated with an increased risk of nonsecretory bacterial diarrhea in North American travelers to Mexico. J Infect Dis . 2009;199(4):477-485.
67. Mohamed JA, DuPont HL, Flores J, et al. Single nucleotide polymorphisms in the promoter of the gene encoding the lipopolysaccharide receptor CD14 are associated with bacterial diarrhea in US and Canadian travelers to Mexico. Clin Infect Dis . 2011;52(11): 1332-1341.
68. Harris JB, Khan AI, LaRocque RC, et al; Blood group. Blood group, immunity, and risk of infection with Vibrio cholerae in an area of endemicity. Infect Immun . 2005;73(11):7422-7427.
69. Lindesmith L, Moe C, Marionneau S, et al. Human susceptibility and resistance to Norwalk virus infection. Nat Med. 2003;9(5):548-553.
70. Shah N, DuPont HL, Ramsey DJ. Global etiology of travelers’ diarrhea: systematic review from 1973 to the present. Am J Trop Med Hyg . 2009;80(4):609-614.
71. Jiang ZD, Lowe B, Verenkar MP, et al. Prevalence of enteric pathogens among international travelers with diarrhea acquired in Kenya (Mombasa), India (Goa), or Jamaica (Montego Bay). J Infect Dis . 2002;185(4):497-502.
72. Koo HL, Ajami NJ, Jiang ZD, et al. Noroviruses as a cause of diarrhea in travelers to Guatemala, India, and Mexico. J Clin Microbiol . 2010;48(5):1673-1676.
73. Pandey P, Bodhidatta L, Lewis M, et al. Travelers’ diarrhea in Nepal: an update on the pathogens and antibiotic resistance. J Travel Med . 2011;18(2):102-108.
74. Swaminathan A, Torresi J, Schlagenhauf P, et al; GeoSentinel Network. A global study of pathogens and host risk factors associated with infectious gastrointestinal disease in returned international travellers. J Infect . 2009;59(1):19-27.
75. Riddle MS, Sanders JW, Putnam SD, Tribble DR. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): a systematic review. Am J Trop Med Hyg . 2006;74(5):891-900.
- Share full article
Advertisement
Supported by
F.D.A. Approves Antibiotic for Increasingly Hard-to-Treat Urinary Tract Infections
Pivmecillinam, which has been used in Europe for decades, will become available next year to women 18 and older.

By Andrew Jacobs
The Food and Drug Administration on Wednesday approved the sale of an antibiotic for the treatment of urinary tract infections in women, giving U.S. health providers a powerful new tool to combat a common infection that is increasingly unresponsive to the existing suite of antimicrobial drugs.
The drug, pivmecillinam, has been used in Europe for more than 40 years, where it is often a first-line therapy for women with uncomplicated U.T.I.’s, meaning the infection is confined to the bladder and has not reached the kidneys. The drug will be marketed in the U.S. as Pivya and will be made available by prescription to women 18 and older.
It is the first time in two decades that the F.D.A. has approved a new antibiotic for U.T.I.s, which annually affect 30 million Americans. U.T.I.s are responsible for the single-greatest use of antibiotics outside a hospital setting.
“Uncomplicated U.T.I.s are a very common condition impacting women and one of the most frequent reasons for antibiotic use,” Dr. Peter Kim, director of the Division of Anti-Infectives at the F.D.A.’s Center for Drug Evaluation and Research, said in a statement . “The F.D.A. is committed to fostering new antibiotic availability when they prove to be safe and effective.”
Utility Therapeutics , the U.S. company that acquired the rights to pivmecillinam, said it would be available in 2025. The company is also seeking F.D.A. approval for an intravenous version of the drug that is used for more serious infections and is usually administered in a hospital setting.
Health practitioners said they were elated to have another tool in their arsenal given the growing challenge of antimicrobial resistance, which makes existing medications less effective as pathogens mutate in ways that allow them to survive a course of antibiotics.
The problem, largely an outgrowth of antibiotic overuse around the world, is associated with five million deaths, according to the World Health Organization .
“This is an exciting new possibility for treatment of lower urinary tract infections,” said Dr. Shruti Gohil, a professor of infectious diseases at the University of California, Irvine School of Medicine, and an author of a recent study in JAMA that focused on ways to reduce antibiotic overuse in hospitals. “But I would also say that it is going to be important that we use the drug responsibly in this country so that we don’t breed resistance against it.”
Most U.T.I.s occur when bacteria like E. coli travel from the rectum, genital area or vagina into the urethra and enter the bladder. As they multiply, the pathogens can cause abdominal cramping, burning and bloody urination.
More than half of all women in the United States will acquire a U.T.I. in their lifetime, compared with 14 percent of men. That is in large part because of the differing architecture of the urinary tract in the sexes: Women have shorter urethras than men, which makes it easier for bacteria to reach the urinary tract.
The majority of U.T.I.s are now resistant to one or more antibiotics; ampicillin, once a common treatment, has been largely abandoned. Infections that travel to the kidneys or that enter the bloodstream are more difficult to treat and more dangerous.
People with weakened immune systems or chronic medical conditions are usually the most vulnerable to drug-resistant infections. But U.T.I.s have a dubious distinction: They are the single biggest risk to healthy people from drug-resistant germs.
In the four decades since it was first approved for use in Europe, Pivmecillinam has been prescribed more than 30 million times, mostly in Nordic countries, with few reported complications.
The F.D.A. said that nausea and diarrhea were the most common side effects in the clinical trials that paved the way for pivmecillinam’s approval in the United States.
Tom Hadley, the president and chief operating officer of Utility Therapeutics, said his company moved to acquire the U.S. rights to pivmecillinam after Congress, in 2012, granted an additional five years of exclusivity to manufacturers of new antimicrobial drugs.
Henry Skinner, the chief executive at the AMR Action Fund , a venture capital fund that invested in Utility Therapeutics’ bid to bring pivmecillinam to the U.S., said he was gratified by the F.D.A.’s approval but said the long-term prognosis for new antimicrobial drugs remained grim. The $1 billion fund, financed by the pharmaceutical industry, invests in biotech start-ups working on promising antimicrobials.
Most of the nation’s biggest drug makers, unable to turn a profit on antibiotics, have long since abandoned the field, he said, and the dearth of investment has prompted an exodus of talented researchers.
A federal initiative that would create a subscription-based model for antibiotic development has been languishing in Congress. The $6 billion measure, the Pasteur Act , would provide pharmaceutical companies an upfront payment in exchange for unlimited access to a drug once it is approved by the F.D.A.
Mr. Skinner said he was haunted by one recent estimate suggesting that drug-resistant infections could claim 10 million lives by 2050.
“There are definitely bright spots,” he said. “But more people are dying today than ought to be because we are moving backward, and not delivering the physicians, drugs and diagnostics needed to address the crisis of antimicrobial resistance.”
Andrew Jacobs is a Times reporter focused on how healthcare policy, politics and corporate interests affect people’s lives. More about Andrew Jacobs
- Tour Account ›
- Travel Forum ›
- Travel Forum
- Independent Trave...
Independent Travel to Moscow and St. Petersburg
My wife and I have traveled independently to many European countries and, after reading many of the comments in this forum, feel like we may be able to do so in Moscow and St. Petersburg as well, although we feel somewhat less comfortable than the other countries where we have traveled in Europe.
My biggest question is how much we will miss by visiting the main sites without a tour guide. In the other European countries we have visited on our own we have been comfortable and satisfied with the level of knowledge we have gained by studying and visiting on our own, although we believe a tour guide or tour company in any country would normally be able to provide greater insight than visiting a site on our own, but about in Moscow and St. Petersburg?
Any thoughts would be appreciated.
Unless you speak at least some Russian and read the alphabet it would be difficult without a guide. Very few signs in English especially in Moscow. Also not too many people speak English there.
I visited St Petersburg last Autumn for a protracted period , and did not find it anymore daunting than any other European city . As Ilja says , learning the Cyrillic alphabet is a big plus . I also found that it was fairly easy to grasp . It will , among other things , enable you to read signs with relative ease . I would answer the main part or your question ( about tour guides ) thus - I am not enamored of tour guides or tours. While I only use a tour under duress ( A sites rules prohibiting an independent visit - ie Glasgow School of Art , Municipal House in Prague , etc ) . Doing your own preparation and homework is the best way , in my opinion . My wife and I ventured through St Petersburg with nary a concern ,and made two day trips outside of the central district ( one was twenty five miles away , and no English speakers in sight ) Figuring out the logistical details , did not present any difficulties . While I never use tours , I am an inveterate eavesdropper , have done so many times , and find , that on balance , the information that they impart is fairly elementary . If you want greater detail , they are unlikely to supply it . I also prefer to go at my own pace , not being rushed and being able to dwell on things that most other visitors barely give a passing glance . If you are interested , let me know . I would be happy to provide you with information that will enable you to travel independently
Thank you, Steven. I am encouraged by your comments and feel the same way you do about tour groups. We are very much willing to prepare in advance and learn the basics of the Cyrillic alphabet to help us have a better experience.
I would be very interested in learning more about your experience in Russia and receiving any additional information you can provide on independent travel there, starting perhaps with obtaining the visa.
I am not opposed to hiring a private or small group guide for a specific site or for a day trip outside St Petersburg and Moscow, but, like you said, I much prefer the freedom of staying at places as long or short as I want and seeing the sites that interest me most. For example, neither my wife nor I are big into shopping, yet most tour itineraries leave afternoon time for just that. Not interested!
Please provide whatever you may feel would be helpful for us.
Russ , I see you are quite near San Francisco - you can start by looking at the VISA procedure , it's fairly straightforward . Since there is a consulate in San Francisco - look here http://www.consulrussia.org/eng/visa-sub1.html
And here - http://ils-usa.com/main.php
Having always traveled independently, we thought we could do a better job than any tour. How misguided (pun intended) we were. Now that we have had the experience of having someone native to the area walk us around and explain things through a personal perspective we realize how much we missed. For St. Petersburg I highly recommend http://www.peterswalk.com/tours.html . This is not a traditional guided tour, but an opportunity to receive some orientation to the area as well as insight into the "Russian soul". I think if you did this upon arrival the rest of your time would be much more meaningful. We really liked the http://www.pushka-inn.com . The location is superb (just around the corner from the Hermitage square), the rooms lovely, the included breakfast at the restaurant next door ample and overall an excellent value. We used this company to get our visa: https://www.passportvisasexpress.com/site/san_francisco_customer_service Note that it costs about the same for a 3-year visa as a one-year, and you never know if you might want to return within that more extended window of time. It is not cheap, so factor that into your planning.
If you like traveling without a guide in other countries and find this satisfying, the same will be true in Moscow and St. Petersburg. Of course it's not either/or - you can certainly take a guided walk or boat tour, for instance.
I cannot emphasize enough the importance of learning Cyrillic if you're going on your own. The book I used was Teach Yourself Beginner's Russian Script, which was great. It breaks down the alphabet into letters that are the same as English, letters that look the same but are pronounced differently, etc. It's out of print, but you can get used copies on Amazon: http://www.amazon.com/Teach-Yourself-Beginners-Russian-Script/dp/0071419861/ref=sr_1_2?ie=UTF8&qid=1459701143&sr=8-2&keywords=teach+yourself+russian+script
Russia is indeed a bit more "foreign" than say, Italy. However, in Moscow and St. Petersburg, I found enough English to be able to get by. Many restaurants had English menus and/or English speaking staff, for instance. This was most emphatically not true in Vladimir and Suzdal (two cities in the Golden Ring outside Moscow). I went with my sister, a Russian speaker, and if she hadn't been there, I would have been in big trouble. So, if you want to see places outside these two big cities, use a guided tour (even if just for that part). Also, Moscow and St. Petersburg are huge cities. Coming from New York, I wasn't intimidated, but those not used to a megacity may not be so cavalier (even I found them overwhelming at times, especially Moscow).
I found both Lonely Planet and Rough Guide to be helpful, and both to have various errors. Look at both, buy whichever one has a more recent edition, and then be prepared to have to discard some of the advice therein. Also, these places change more quickly than places in Western Europe. Be very careful of outdated advice. For instance, I was there in 2001 and 2010, so I won't give you any specifics on getting a visa - that changes constantly.
Just as a teaser, two things I saw and loved that I doubt would be included in any escorted tour are the Gorky House in Moscow (an Art Nouveau wonder) and the Sheremyetov Palace in St. Petersburg (it's now a museum of musical instruments, and the decor is amazing, particularly in the Etruscan Room).
Thanks for all the good advice. Any additional thoughts are welcomed.
One of history's seminal works pertaining to Russian history and culture and a MUST for anyone contemplating a visit or simply interested , is this fine work from 1980 - http://www.amazon.com/Land-Firebird-The-Beauty-Russia/dp/096441841X
This is about you and not about Petersburg. Do you like guided tours? We don't and didn't find that a guide added to our experience in China where we did hire private guides mostly for the logistics; it was easy to have someone drive us places. But once at a site, we didn't need the guide. I felt the same way about our 9 nights in Petersburg. We did hire a guide for the trip to the Catherine Palace again for the ease of logistics for us Olds. Here is our visit: https://janettravels.wordpress.com/2016/01/23/an-easy-trip-to-the-catherine-palace/ There are also snapshots of the Church on Spilled Blood in this photo journal. Having someone pick us up at the apartment and get us in without line ups and shepherd us through the palace steering clear of the tour groups was lovely. But we didn't need commentary because we can read and prepare.
You certainly don't need a guide for the Hermitage (we spent 4 days there), the Russian Museum, the Kazan Cathedral or Church on Spilled Blood or the Faberge Museum. We enjoyed a number of self guided walking tours including a couple from Rick Steves guidebooks. We took the canal cruise suggested by RS that had an English commentator. I would not take one without that as you will be totally clueless. The commentator was not all that good but at least we had some idea what we were seeing. So for people like us who like to do our own thing and can read a guidebook and don't particularly like to be led about, a tour is not needed. If you enjoy tour groups, then go for it. Petersburg is easy to negotiate. It helps if you can read the cyrillic alphabet and it is also useful to have the google translate ap on your phone. We found ourselves translating packages in grocery stores with it and the occasional museum sign or menu. I have one food I need to avoid and so it was handy to have the translator to talk with waiters (I could either show them the sentence, or play it for them or play it to myself and then repeat it to the waiter -- that all worked well)
Dear Russ, I cannot help you with Moscow, but about four years ago, my husband and I went to St Petersburg on our own. But, we did use a private guide for 4 half days. We both feel that our guide absolutely made our trip (we stayed 6 or 7 nights). We used a company owned by Tatyana Chiurikova, www.tour-stpetersburg.com I cannot say enough good things about her and our experience. I emailed her and we worked out a schedule/ sights that was tailored to our interests. She also offered some recommendations, which we took. The guide will meet you at your hotel. And frequently, at certain places, with the guide, we were able to skip the long entrance lines. We had an half day driving tour of the city (car, driver, & guide). You are taken to & go in places such as Peter & Paul Fortress, some of the cathedrals, etc. We had a half day with the guide at the Hermitage which ensured that we would see the major sights there. And, of course, you can stay after your guide leaves or return another day. Also, we had the guide for Peterhof (a must & go by boat) and Catherine's Palace. I hope that you will go to the website. As I said, our guide made our trip. I am positive that we would have missed quite a bit on our own everyday. And I'm sure we would have wasted a lot of time trying to get to various place.This was the best of both worlds, a guide where needed and plenty of time on our own. Whatever you do, I'm sure that you will love St Petersburg! Ashley
I am curious about the lines as we encountered no lines on our trip -- but it was in September. We got tickets for the Hermitage at machines and skipped those lines and our guide for the Catherine Palace which was our only guided experience (as noted before, chosen for the logistics of getting there) had arranged tickets and we didn't have a line, but then we also didn't see lines. We did not find lines at any other site.
Both Moscow and St Petersburg I've done on my own, that is together with the Dear Partner. I can't remember any problem getting where we wanted to go. The Metro systems are well signed, and with a little exercise and patience you can recognize the station names. With a good map and a good guide - we had the Rough Guides - that part of the logistics is solved. The language is a major problem, but the usual tricks of pointing, looking helpless, and making a joke of it all do wonders. I would hate to be led by a guide, but for others it is a comfortable thought.
We also did both cities on our own. I found the DK Eyewitness guide for Moscow has the best map. I used the one from our library (kept the book at home). Took the smaller RS book for St. Petersburg ( his book on northern cities). His map and restaurant ideas were all good. We also downloaded the Google maps in our Android Samsung tablets/phones for both cities and then could get directions to any place we typed in. The blue ball guided us everywhere. I'm sure we missed somethings by not having a guide, but we just enjoy walking around and getting a sense of a place. If you like art, The Hermitage is great. We went 3 times and still missed alot. In St. Peterburg we stayed at the 3 Mosta which we loved (quite and not far from the Church of Spilled Blood.) We also loved the Georgian food in both cities. There's a great Georgian restaurant near the 3 Mosta hotel. We're now in Belarus- very scenic. Enjoy your trip!!
This topic has been automatically closed due to a period of inactivity.

COMMENTS
Treatment. Travelers' diarrhea (TD) is the most predictable travel-related illness. Attack rates range from 30%-70% of travelers during a 2-week period, depending on the destination and season of travel. Traditionally, TD was thought to be prevented by following simple dietary recommendations (e.g., "boil it, cook it, peel it, or forget ...
Empiric treatment of traveler's diarrhea with antibiotics and loperamide is effective and often limits symptoms to one day. ... with an antibiotic. 29, 34, 38 A conservative approach would be to ...
Travelers' diarrhea (TD) is the most common travel-related illness, ... Pharmacists can draw from the guidelines to ensure patients are counselled on safe and appropriate antibiotic therapy during international travel and can direct patients to important nonprescription products supported by the guidelines and provide advice on their safe and ...
Episodes of travelers' diarrhea are nearly always benign and self-limited, but symptoms may disrupt planned activities and result in health care visits for some travelers . There is a growing recognition that travelers' diarrhea and its self-treatment abroad are associated with the acquisition of organisms harboring antibiotic resistance .
Lifestyle and home remedies. If you do get traveler's diarrhea, avoid caffeine, alcohol and dairy products, which may worsen symptoms or increase fluid loss. But keep drinking fluids. Drink canned fruit juices, weak tea, clear soup, decaffeinated soda or sports drinks to replace lost fluids and minerals.
Benefit. For the past 30 years, randomized controlled trials have consistently and clearly demonstrated that antibiotics shorten the duration of illness and alleviate the disability associated with travelers' diarrhea (TD). Treatment with an effective antibiotic shortens the average duration of a TD episode by 1-2 days, and if the traveler ...
Travellers' diarrhoea (TD) affects a large proportion of international travellers. These people will often present to general prac-tice for advice before they travel. ... Avoidance, immunisation, non-antibiotic interventions and antibiotic prophylaxis are all methods for preventing TD. However, advice regarding self-management through ...
Traveler's diarrhea affects travelers and others who consume contaminated food or water. It's a brief but unpleasant gastrointestinal infection that typically causes loose stools and abdominal cramps. Most of the time, it's caused by bacteria, but sometimes viruses or parasites are to blame. ... Metronidazole (Flagyl). Nitazoxanide (Alinia).
What you need to know. Enterotoxic Escherichia coli (ETEC) is the most common cause of acute travellers' diarrhoea globally. Chronic (>14 days) diarrhoea is less likely to be caused by bacterial pathogens. Prophylactic antibiotic use is only recommended for patients vulnerable to severe sequelae after a short period of diarrhoea, such as those with ileostomies or immune suppression
Traveler's diarrhea is a digestive tract disorder that commonly causes loose stools and stomach cramps. It's caused by eating contaminated food or drinking contaminated water. Fortunately, traveler's diarrhea usually isn't serious in most people — it's just unpleasant. When you visit a place where the climate or sanitary practices are ...
Treatment. Mild traveler's diarrhea can usually be managed with the judicious use of antimotility agents such as loperamide (Imodium A-D), in a dosage of two 2-mg tablets initially, then one ...
Traveller's diarrhoea. Traveller's diarrhoea is diarrhoea that develops during, or shortly after, travel abroad. It is caused by consuming food and water, contaminated by germs (microbes) including bacteria, viruses and parasites. Other symptoms can include high temperature (fever), being sick (vomiting) and tummy (abdominal) pain.
The typical symptoms of traveler's diarrhea include: Abrupt onset of diarrhea. Fever. Nausea and vomiting. Bloating. Urgent need to have a bowel movement. Malaise (weakness or discomfort ...
Traveler's diarrhea may be caused by any of several bacteria, viruses, or, less commonly, parasites. The most common cause of traveler's diarrhea is . Enterotoxigenic Escherichia coli (E. coli). E. coli is common in the water supplies of areas that lack adequate purification. Infection is common among people traveling to low-resource countries.
Antibiotics may be used for traveler's diarrhea caused by bacterial infections. A stool test should be done to identify which antibiotic might work best. Quinolone antibiotics such as Cipro (ciprofloxacin) are most often used when antibiotics are needed. A single dose of 750 milligrams (mg) for adults is the typical treatment.
Travellers' diarrhoea is defined as passing three or more unformed stools in a 24-hour period with at least one additional symptom, such as abdominal pain or cramps, nausea, vomiting, fever, or blood in the stools. ... Antibiotic prophylaxis or 'stand-by' antibiotic treatment can be considered for certain high-risk travellers. Specialist advice ...
Alone, they can reduce the average duration to slightly longer than a day, or even half a day when combined with loperamide. 3 Single-dose antibiotic regimens are preferred. Options mainly include azithromycin and rifaximin. The recently approved rifamycin is also an option for moderate TD. 37.
The Food and Drug Administration on Wednesday approved the sale of an antibiotic for ... Most U.T.I.s occur when bacteria like E. coli travel from the rectum, genital area or vagina into the ...
2487 posts. Both Moscow and St Petersburg I've done on my own, that is together with the Dear Partner. I can't remember any problem getting where we wanted to go. The Metro systems are well signed, and with a little exercise and patience you can recognize the station names.
Richard and Greg Davies clash with army tanks and head into space in the Russian capital. To watch the full episode click here http://www.channel4.com/progra...
Travelling around the city by tram is a great way to explore Moscow and is ideal for urban adventurers and amateur geographers.
Richard and Greg Davies attempt to extract the essence of Moscow in two days, as they clash with army tanks, head into space and visit one of the strangest c...