We have a new app!
Take the Access library with you wherever you go—easy access to books, videos, images, podcasts, personalized features, and more.
Download the Access App here: iOS and Android . Learn more here!
- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs


Wandering Atrial Pacemaker
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
Key features, clinical presentation, diagnostic evaluation, ongoing management.
- Full Chapter
- Supplementary Content
ESSENTIALS OF DIAGNOSIS
Progressive cyclic variation in P-wave morphology
Heart rate 60–100 bpm
Variation of P-wave morphology, P-P interval, and P-R interval
GENERAL CONSIDERATIONS
This rhythm is benign
This rhythm and multifocal atrial tachycardia are similar except for heart rate
The other possible explanation is that there is significant respiratory sinus arrhythmia, with uncovering of latent foci of pacemaker activity
Usually, it is associated with underlying lung disease
In the elderly, it may be a manifestation of sick sinus syndrome
In the young and athletic heart, it may represent enhanced vagal tone
SYMPTOMS AND SIGNS
Usually causes no symptoms and is incidentally discovered
Occasional patient may feel skipped beats
PHYSICAL EXAM FINDINGS
Variable S 1
DIFFERENTIAL DIAGNOSIS
Multifocal atrial tachycardia (heart rate > 100 bpm)
Frequent premature atrial complexes and atrial bigeminy
LABORATORY TESTS
None specific
ELECTROCARDIOGRAPHY
ECG to document rhythm
CARDIOLOGY REFERRAL
Not required
MEDICATIONS
No specific treatment
Monitor and treat the underlying cause, such as sick sinus syndrome or lung disease
DIET AND ACTIVITY
No restrictions
General healthy lifestyle
Once a year if sinus node abnormality is suspected; otherwise when symptoms arise
COMPLICATIONS
May progress to sick sinus syndrome
This condition by itself is benign
PRACTICE GUIDELINES
Indications for pacemaker:
– If part of sick sinus syndrome
– If associated with documented symptomatic bradycardia
Sign in or create a free Access profile below to access even more exclusive content.
With an Access profile, you can save and manage favorites from your personal dashboard, complete case quizzes, review Q&A, and take these feature on the go with our Access app.
Pop-up div Successfully Displayed
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
Sick sinus syndrome
On this page, preparing for your appointment.
To diagnose sick sinus syndrome, a health care provider performs a physical exam and asks about symptoms and medical history.
Symptoms of sick sinus syndrome — such as dizziness, shortness of breath and fainting — only occur when the heart is beating irregularly. You may not have symptoms at the time of the appointment.
To determine whether symptoms are related to problems with the sinus node and heart function, a health care provider may use the following tests:
- Electrocardiogram (ECG or EKG). This simple test measures the electrical activity of the heart. Sensors (electrodes) are attached to the chest and legs. Wires attach the sensors to a computer, which displays or prints results. An electrocardiogram (ECG) can tell how fast or slow the heart is beating. A health care provider can look for signal patterns to determine if sick sinus syndrome is present.
- Holter monitor. This portable device can be worn for a day or more during daily activities. It automatically records the heart's activity for 24 to 72 hours. A person wearing a monitor might also keep a diary of symptoms.
- Event recorder. This portable device is intended to be worn for up to 30 days or until you have an irregular heartbeat or symptoms. You typically press a button when symptoms occur.
- Other monitors. Some personal devices, such as smart watches, offer electrocardiogram monitoring. Ask your health care provider if this is an option for you.
- Implantable loop recorder. This small device is implanted just under the skin of the chest. It's used for continuous, long-term monitoring of the heart's electrical activity, particularly for people who have infrequent symptoms.
.jpg)
- Electrocardiogram (ECG or EKG)
An electrocardiogram (ECG or EKG) is a simple test to determine how the heart is beating. Sensors (electrodes) placed on the chest record the heart's electrical signals. The signals are shown as waves on an attached computer monitor or printer.

- Holter monitor
A Holter monitor uses electrodes and a recording device to track the heart's rhythm for 24 to 72 hours. A health care provider can print an electrocardiogram strip using the data on the recording device to see the heart's rhythm during the period the monitor was worn.
Electrophysiologic (EP) testing
This test, also called an EP study, is rarely used to screen for sick sinus syndrome. However, it may be done to check the function of the sinus node and to evaluate other electrical properties of the heart.
During an electrophysiologic (EP) study, thin, flexible wires tipped with electrodes are threaded through blood vessels to different areas within the heart. Once in place, the electrodes can map the spread of electrical signals through the heart.
More Information
The goals of sick sinus treatment are to reduce or eliminate symptoms and to manage any other contributing health conditions.
Treatment of sick sinus syndrome may include:
- Regular checkups
Medications
- Catheter procedures
- Surgery to implant a device to maintain a regular heartbeat (pacemaker)
If you don't have symptoms, your health care provider may simply recommend regular health checkups to monitor your condition. Most people with symptoms need to have a procedure to implant a device to maintain a regular heartbeat (pacemaker).
Some medications, including those used to treat high blood pressure or heart disease, may interfere with sinus node function. Your health care provider will likely review the medications you take and may adjust them or prescribe different ones.
Medications may be needed to prevent or to slow down fast heartbeats.
Blood-thinners (anticoagulants), such as warfarin (Jantoven), dabigatran (Pradaxa) or others, may be prescribed if sick sinus syndrome is associated with atrial fibrillation or other irregular heart rhythms linked to stroke.
Surgeries or other procedures
Most people with sick sinus syndrome eventually need a permanent device to control the heart rhythm (pacemaker). A pacemaker is a small, battery-powered device that's implanted under the skin near the collarbone during a minor surgical procedure. The pacemaker stimulates (paces) the heart as needed to keep it beating regularly.
If sick sinus syndrome symptoms are mild or infrequent, the decision to use a pacemaker will depend on the results of electrocardiograms (ECGs), your overall health and the risk of more-serious problems.
The type of pacemaker you need depends on the type of irregular heart rhythm you have. Types of pacemakers include:
- Single chamber pacemaker. This type usually carries electrical signals to the right lower heart chamber (ventricle) of the heart.
- Dual chamber pacemaker. This type paces the right lower heart chamber (ventricle) and the right upper heart chamber (atrium) separately. Most people with sick sinus syndrome benefit from dual-chamber pacemakers.
- Biventricular pacemaker. Biventricular pacing, also called cardiac resynchronization therapy, is for people who have heart failure and heartbeat problems. This type of pacemaker stimulates both lower heart chambers (the right and left ventricles) to make the heart beat more efficiently.
If your heart rate is still irregular after getting a pacemaker, you may need medications or a catheter-based procedure called cardiac ablation to correct or control it. Cardiac ablation uses heat or cold energy to create tiny scars in the heart to block faulty signals and restore a regular heartbeat. It's most often done using thin, flexible tubes called catheters inserted through the veins or arteries. Less commonly, ablation is performed during cardiac surgery. A type of cardiac ablation called atrioventricular (AV) node ablation is often used to control fast heart rhythms in people with pacemakers.

AV node ablation
In atrioventricular (AV) node ablation, a heart doctor uses radiofrequency energy to destroy the electrical connection between the upper and lower heart chambers ( node), blocking the heart's electrical impulses. Once the node is destroyed, the heart doctor then implants a small medical device to maintain a heart rhythm (pacemaker).

- Cardiac ablation
Cardiac ablation uses heat or cold energy to create tiny scars in the heart to block irregular electrical signals and restore the heart rhythm. One or more thin, flexible tubes (catheters) are inserted through an artery, usually in the groin, and guided to the heart. Sensors on the tip of the catheters apply the heat or cold energy. This illustration shows ablation catheters being applied near the pulmonary veins in a type of cardiac ablation called pulmonary vein isolation.
It's important to take steps to lower the risk of heart disease. Try these heart-healthy strategies:
- Eat a healthy diet. Choose generous portions of nonstarchy vegetables, fruits and whole grains and modest portions of fish, lean meats, poultry and dairy.
- Exercise and maintain a healthy weight. Being overweight increases the risk of developing heart disease. Unless your provider tells you otherwise, aim for at least 30 minutes of moderate physical activity every day. Ask your health care provider what your goal weight should be.
- Keep blood pressure and cholesterol under control. Make lifestyle changes and take medications as prescribed to manage high blood pressure or high cholesterol.
- Don't smoke. If you smoke and can't quit on your own, talk to your health care provider about ways or programs to help break a smoking habit.
- If you drink, do so in moderation. For some conditions it's recommended that you completely avoid alcohol. Ask your health care provider for advice specific to your condition. If you can't control your alcohol use, talk to your provider about a program to quit drinking and manage other behaviors related to alcohol use.
- Don't use illegal drugs. Talk to your provider about a program if you need help quitting.
- Control stress. Getting more exercise, practicing mindfulness and connecting with others in support groups are some ways to reduce stress.
- Go to scheduled checkups. Have regular physical exams and report any signs or symptoms to your health care provider.
Call your health care provider if you have symptoms of sick sinus syndrome. You might be referred to a doctor trained in diagnosing and treating heart conditions (cardiologist).
Be prepared to answer questions about your medical history and symptoms. Write down your answers to help you remember details.
Questions your provider may ask about symptoms include:
- Do your symptoms include feeling lightheaded or dizzy?
- Have you ever fainted?
- Do you have rapid, fluttering or pounding heartbeats?
- Do you feel pressure, heaviness, tightness or pain in your chest?
- Does exercise or activity worsen your symptoms?
- Does anything improve your symptoms?
- How often have you had symptoms?
- How long have the symptoms lasted?
Other questions may include the following:
- Have you been diagnosed with high blood pressure, high cholesterol, diabetes or a heart condition?
- What medications do you take and what dosage? Who is the prescribing doctor?
- Why were the prescription drugs prescribed?
- Have you been taking the medication as prescribed?
- Have you recently stopped, started or changed medications?
- What over-the-counter medications, herbal remedies or supplements do you take?
Write down any questions you have for your provider. You might bring a friend or relative to write down information during the appointment.
What you can do in the meantime
If exercise makes your symptoms worse, avoid exercise until you see your provider.
Apr 30, 2022
- Issa ZF. Sinus node dysfunction. In: Clinical Arrhythmology and Electrophysiology: A Companion to Braunwald's Heart Disease. 3rd ed. Elsevier; 2019. https://www.clinicalkey.com. Accessed Feb. 15, 2022.
- Homoud MK. Sinus node dysfunction: Epidemiology, etiology, and natural history. https://www.uptodate.com/contents/search. Accessed Feb. 15, 2022.
- Homoud MK. Sinus node dysfunction: Treatment. https://www.uptodate.com/contents/search. Accessed Feb. 15, 2022.
- Homoud MK. Sinus node dysfunction: Clinical manifestations, diagnosis, and evaluation. https://www.uptodate.com/contents/search. Accessed Feb. 15, 2022.
- Libby P, et al., eds. Genetics of cardiac arrhythmias. In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 12th ed. Elsevier; 2022. https://www.clinicalkey.com. Accessed Feb. 15, 2022.
- Kusumoto FM, et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology. 2018; doi: 10.1016/j.jacc.2018.10.044.
- Hayes DL. Permanent cardiac pacing: Overiew of devices and indications. https://www.uptodate.com/contents/search. Accessed Feb. 15, 2022.
- Pacemakers. National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health-topics/pacemakers. Accessed Feb. 15, 2022.
- Ablation for arrhythmias. American Heart Association. https://www.heart.org/en/health-topics/arrhythmia/prevention--treatment-of-arrhythmia/ablation-for-arrhythmias. Accessed Feb. 15, 2022.
- Heart-healthy lifestyle changes. National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health-topics/heart-healthy-living. Accessed Feb. 15, 2022.
- Noseworthy PA (expert opinion). Mayo Clinic. Feb. 15, 2022.
- How the heart works. National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health-topics/how-heart-works. Accessed Feb. 15, 2022.
- Symptoms & causes
- Doctors & departments
- Diseases & Conditions
- Sick sinus syndrome diagnosis & treatment
Associated Procedures
CON-XXXXXXXX
Make twice the impact
Your gift can go twice as far to advance cancer research and care!

MATTHEW KENDALL HAWKS, MD, MADISON L.B. PAUL, MD, AND OMOJO ODIHI MALU, MD, MSc
Am Fam Physician. 2021;104(2):179-185
Author disclosure: No relevant financial affiliations.
Sinus node dysfunction, previously known as sick sinus syndrome, describes disorders related to abnormal conduction and propagation of electrical impulses at the sinoatrial node. An abnormal atrial rate may result in the inability to meet physiologic demands, especially during periods of stress or physical activity. Sinus node dysfunction may occur at any age, but is usually more common in older persons. The causes of sinus node dysfunction are intrinsic (e.g., degenerative idiopathic fibrosis, cardiac remodeling) or extrinsic (e.g., medications, metabolic abnormalities) to the sinoatrial node. Many extrinsic causes are reversible. Electrocardiography findings include sinus bradycardia, sinus pauses or arrest, sinoatrial exit block, chronotropic incompetence, or alternating bradycardia and tachycardia (i.e., bradycardia-tachycardia syndrome). Clinical symptoms result from the hypoperfusion of end organs. About 50% of patients present with cerebral hypoperfusion (e.g., syncope, presyncope, lightheadedness, cerebrovascular accident). Other symptoms include palpitations, decreased physical activity tolerance, angina, muscular fatigue, or oliguria. A diagnosis is made by directly correlating symptoms with a bradyarrhythmia and eliminating potentially reversible extrinsic causes. Heart rate monitoring using electrocardiography or ambulatory cardiac event monitoring is performed based on the frequency of symptoms. An exercise stress test should be performed when symptoms are associated with exertion. The patient's inability to reach a heart rate of at least 80% of their predicted maximum (220 beats per minute – age) may indicate chronotropic incompetence, which is present in 50% of patients with sinus node dysfunction. First-line treatment for patients with confirmed sinus node dysfunction is permanent pacemaker placement with atrial-based pacing and limited ventricular pacing when necessary.
Sinus node dysfunction, previously known as sick sinus syndrome, is characterized by abnormal initiation and propagation of electrical impulses from the sinoatrial node (SAN). The resulting abnormalities include bradycardia (less than 50 beats per minute [bpm]), sinus pause (more than three seconds), sinus arrest, and sinoatrial exit blocks, which are sometimes associated with supraventricular tachyarrhythmias in bradycardia-tachycardia syndrome 1 – 4 ( Table 1 5 – 11 ) . Bradycardia-tachycardia syndrome occurs in approximately 50% of patients with sinus node dysfunction and increases the risk of stroke and death. 5 , 12 Symptoms manifest as end-organ hypoperfusion, including palpitations, decreased physical activity tolerance, easy fatigability, dizziness, and syncope. 2 , 5 , 6 , 13 To diagnose sinus node dysfunction, a combination of symptoms and documented electrical abnormalities must be present. 5 , 7
Epidemiology
Sinus node dysfunction may occur at any age 7 , 14 ; however, increasing age is the most significant risk factor with the highest disease prevalence in patients 70 to 89 years of age. 2 , 7 , 8 , 14 The incidence of sinus node dysfunction is 0.8 per 1,000 person-years and is expected to double by 2060 due to the aging population. 15 Conditions associated with advanced age such as hypertension, chronic kidney disease, diabetes mellitus, and coronary heart disease are overlapping risk factors and potential causes of sinus node dysfunction. 2 , 15 Brugada syndrome, a rare inherited ion channel disorder that results in ventricular tachyarrhythmias and sudden cardiac death, is also associated with sinus node dysfunction. 5 , 16 , 17
Causes of sinus node dysfunction are generally categorized as intrinsic or extrinsic based on their effect on the SAN ( Table 2 2 , 5 – 8 , 18 ) . It is important to note that sinus node dysfunction is usually a progressive condition and most causes are chronic and irreversible. 5
INTRINSIC CAUSES
Intrinsic causes originate from structural or functional changes within the SAN. These changes can occur because of fibrosis, ischemia, cardiac remodeling, infiltrative disease, or ion channel dysfunction. 8 , 18 , 19 Degenerative idiopathic fibrosis of the SAN is the most common cause of sinus node dysfunction. 4 , 5 , 7 , 8 , 15 Elastic fiber and fatty and fibrous tissue buildup at the SAN and surrounding myocardial tissue increases with age and may lead to prolonged SAN refractory time and, therefore, a decreased intrinsic heart rate. 2 , 4 , 8 Ischemic heart disease and embolization of the sinus node artery may cause ischemic necrosis of the node, resulting in sinus node dysfunction. 8 Acute myocardial infarction may induce a transient sinus node dysfunction caused by autonomic disturbance and increased vagal tone. 2 , 12 Cardiac remodeling following myocardial infarction, congestive heart failure, or advanced age can result in structural changes that decrease cardiac tissue voltage transmission and ultimately delay or block the SAN and result in sinus node dysfunction. 3 , 8 , 14
Another result of this remodeling is the formation of bradycardia-tachycardia syndrome. It is unclear if a supraventricular tachycardia or sinus node dysfunction is the primary disorder in bradycardia-tachycardia syndrome. The etiology is further complicated by current contradictory evidence about the role of supraventricular tachycardia and atrial fibrillation as a cause of sinus node dysfunction. 3 At a minimum, it is clear that these diagnoses are associated even if the causal pathway is unclear.
Infiltrative diseases such as sarcoidosis, amyloidosis, hemochromatosis, and connective tissue diseases can disrupt the cardiac tissue and result in abnormal SAN function. 2 , 5 Similarly, sinus node dysfunction has been associated with cardiomyopathy from infection with Chagas disease, with which the arrhythmia may be permanent. 6 , 8 Rhythm abnormalities associated with myocarditis from infections such as diphtheria and typhoid and immune-mediated disorders such as rheumatic fever may cause sinus node dysfunction temporarily. 6 , 8
About 80% of patients younger than 21 years with sinus node dysfunction have a history of congenital heart malformations (e.g., atrial septal defect, transposition of the great arteries) that required surgical intervention. 18 , 20 Genetic mutations in genes responsible for coding ion channels, such as HCN4 and SCN5A , have also been identified as a cause of intrinsic sinus node dysfunction. 18
EXTRINSIC CAUSES
Extrinsic causes are related to external factors causing abnormal conduction at the SAN. These causes include medications, metabolic abnormalities, autonomic imbalances, toxins, and endocrine disorders ( Table 2 2 , 5 – 8 , 18 ) . Extrinsic causes may be reversible, such as electrolyte abnormalities, hypothyroidism, metabolic abnormalities, and certain medications. 2 , 8 Anesthesia (e.g., sympatholytic drugs) has been shown to induce autonomic imbalances that may mimic sinus node dysfunction or may reveal the underlying dysfunction in previously asymptomatic patients. 8 , 21 Other pharmacotherapies known to cause sinus node dysfunction include beta blockers, nondihydropyridine calcium channel blockers, digoxin, lithium, and antiarrhythmics. 5 , 7 , 8 Toxins such as nicotine and marijuana have also been implicated in sinus node dysfunction. 7 , 8 , 22
Patients with sinus node dysfunction typically present with end-organ hypoperfusion symptoms from decreased cardiac output caused by the underlying arrhythmia ( Table 1 5 – 11 ) . The most common symptoms of cerebral hypoperfusion are syncope, presyncope, lightheadedness, and cerebrovascular accidents, with syncope occurring in 50% of patients with sinus node dysfunction. 2 , 5 , 7 , 23 Cardiovascular hypoperfusion can present with palpitations, decreased physical activity tolerance, angina, or, less commonly, heart failure. Musculoskeletal hypoperfusion can present with muscle fatigue. Renal hypoperfusion can present as oliguria. 2 , 5 , 6 , 13 , 23 Correlation between symptoms and arrhythmias is considered the diagnostic standard (on electrocardiography [ECG] or other cardiac monitoring). 2 , 5 , 6
A definitive diagnosis of sinus node dysfunction is established when symptoms are directly associated with cardiac monitoring that demonstrates a bradyarrhythmia 2 , 6 ( Table 1 5 – 11 ) . The initial assessment should begin with a history and physical examination ( Figure 1 2 – 4 , 8 , 14 , 17 , 21 , 24 ) . Clinicians should focus their history by investigating the intrinsic and extrinsic causes of sinus node dysfunction ( Table 2 2 , 5 – 8 , 18 ) . It should include a medication review to assess for a potential extrinsic cause. Initial diagnostic evaluation should include 12-lead ECG, a basic chemical panel to assess for metabolic abnormalities, and any additional laboratory tests needed to rule out other extrinsic causes that were not excluded by the history or physical examination (i.e., thyroid-stimulating hormone to rule out hypothyroidism or A1C to rule out diabetic atrial myopathy). 5 , 6
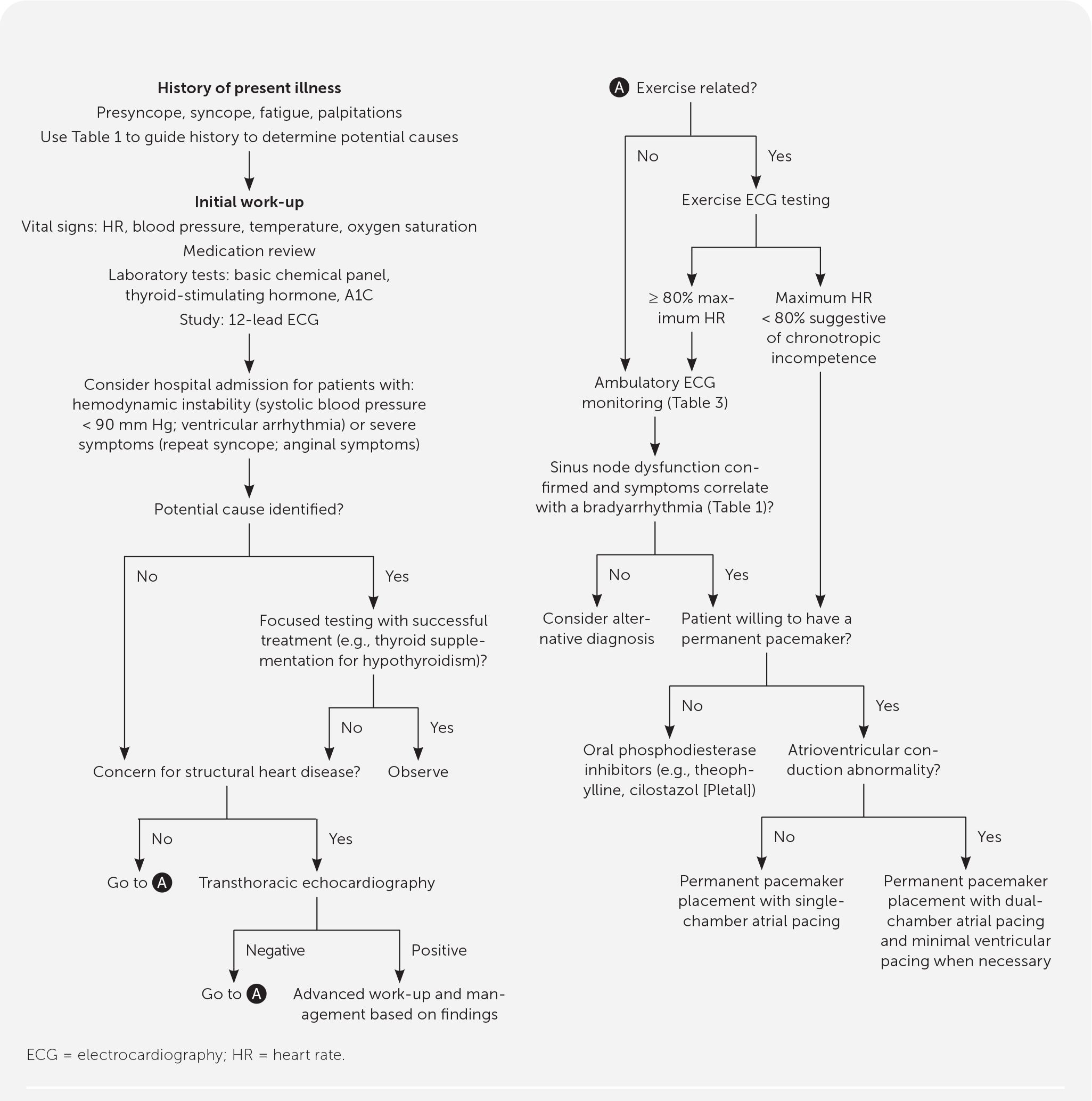
Initial evaluation of sinus node dysfunction can be performed in an outpatient setting; patients with hemodynamic instability (i.e., systolic blood pressure less than 90 mm Hg, ventricular arrhythmias) or severe symptoms (i.e., recurrent syncope, anginal symptoms) should be hospitalized because these patients need urgent evaluation and may require temporary transcutaneous pacing for stabilization. 2 When a potential extrinsic factor is identified during the workup, further evaluation should focus on confirming the diagnosis followed by a trial of therapy. With successful treatment of the extrinsic factor (e.g., continuous positive airway pressure for confirmed sleep apnea or thyroid supplementation for hypothyroidism) and subsequent resolution of sinus node dysfunction, no further workup is indicated.
Patients with history or physical examination findings for underlying structural defects such as a history of valvular disease, new cardiac murmur, bibasilar crackles, lower extremity edema, or concerning ECG findings (e.g., left bundle branch block, second-degree Mobitz type II block, third-degree atrioventricular block) should have transthoracic echocardiography. 2 If the results suggest a specific pathology, further workup and treatment should be initiated based on the suspected etiology. Abnormal results may be because of complications from sinus node dysfunction or an alternative diagnosis, and will require further workup and potential specialty referral for evaluation and treatment.
Chronotropic incompetence is associated with sinus node dysfunction, with 50% of patients diagnosed with sinus node dysfunction also meeting chronotropic incompetence criteria. 25 , 26 It is a separate diagnosis associated with an array of diseases, including sinus node dysfunction. Chronotropic incompetence is defined as the sinus node's inability to mount a heart rate high enough to meet physiologic demands during exertion, resulting in symptoms of central nervous system hypoperfusion similar to those found in sinus node dysfunction. 5 , 25 When symptoms are associated with exertion, the patient should have an exercise ECG test to assess chronotropic incompetence. A diagnosis of chronotropic incompetence is made if the patient is unable to meet 80% of the maximum heart rate (220 bpm – age) during exertion. 2 , 25 It is important to distinguish that chronotropic incompetence is a separate disease process and alone is not enough to diagnose sinus node dysfunction. Sinus node dysfunction and chronotropic incompetence follow the same treatment algorithm ( Figure 1 2 – 4 , 8 , 14 , 17 , 21 , 24 ) .
When initial 12-lead ECG is unable to confirm sinus node dysfunction by correlating symptoms with a definitive bradyarrhythmia ( Table 1 5 – 11 ) , further electrical monitoring is indicated. 2 , 5 , 9 There are multiple types of ambulatory monitoring available. These devices include continuous monitoring (e.g., Holter monitor, external patch recorder, ambulatory telemetry) and patient- or event-activated devices (e.g., event monitor, external loop recorder, implantable loop recorder). First-line devices include the original Holter monitor or the external patch recorder. 8 , 9 , 27 – 32 The external patch recorder is a smaller second-generation monitor that is water-resistant and can be worn for seven to 14 days. The external patch recorder detected more arrhythmias and was better tolerated by patients compared with the Holter monitor. 31 , 33 The frequency of symptoms, the patient's ability to use the device, and the need for continuous vs. intermittent monitoring should be considered when deciding the best form of monitoring for a patient. 9 Table 3 lists cardiac monitoring options with associated indications and pros and cons of use. 9 , 27 – 31 If sinus node dysfunction cannot be definitively established after ambulatory monitoring is completed, further evaluation for an alternative diagnosis and expert consultation should be considered.
Permanent pacemaker placement is the first-line treatment for patients with confirmed sinus node dysfunction , 2 , 5 , 34 – 36 accounting for 50% of pacemakers implanted in the United States. 5 , 7 , 12 Pacemaker therapy has been found to provide symptom relief and improve quality of life, but it is unclear if it provides a mortality benefit. 5 , 36 This treatment includes patients with chronotropic incompetence and patients with pharmacologically induced sinus node dysfunction where continued treatment is clinically necessary. Atrial-based pacing has been established as superior because right ventricular pacing has been associated with an increased risk of arrhythmias and decreased cardiac function. 2 , 37 , 38 A well-powered randomized controlled trial demonstrated no difference in mortality, stroke, heart failure, or atrial fibrillation hospitalizations when comparing single-chamber atrial pacing with dual-chamber atrial pacing. 21 However, over five years of follow-up, 3% to 35% of patients will transition from a single-chamber atrial device to a dual-chamber device with minimized ventricular pacing. 2 , 6 To minimize the risk of these additional procedures, patients with evidence of atrioventricular nodal or bundle branch conduction dysfunction should be considered for initial dual-chamber device placement. 2 It is common in the United States for patients to receive a dual-chamber device with right atrial pacing unless otherwise indicated. Overall, permanent pacemaker placement is relatively safe, with complications estimated at less than 1% to 6%. Most common complications include lead dislodgment (5.7% in left ventricular leads), hematomas (3.5%), venous thrombus/obstruction (2%), infections (1%), and pneumothorax (1%). 34
Medication control of sinus node dysfunction is a secondary option for patients who decline permanent pacemaker placement. Phosphodiesterase inhibitors (e.g., theophylline, cilostazol [Pletal]) have a positive chronotropic effect, resulting in symptom control for patients with sinus node dysfunction. However, the long-term impact of medication control on disease progression and mortality is unclear. 2 , 35 Other pharmaceuticals (e.g., atropine, dopamine, epinephrine, glucagon) used in advanced cardiac life support protocols for acutely unstable bradycardic patients are effective for short-term control of unstable patients but are not appropriate for long-term management of sinus node dysfunction because of significant adverse effect profiles. 2 , 35
The role of oral anticoagulation in patients with sinus node dysfunction is unclear. There is limited evidence to support its use in patients with sinus node dysfunction who do not have another indication for anticoagulation therapy. 2 , 37 , 39 Anticoagulation is currently not routinely recommended for the treatment of sinus node dysfunction.
This article updates previous articles on this topic by Semelka, et al. , 5 and by Adán and Crown . 7
Data Sources: We searched Essential Evidence, PubMed, and Google Scholar. Key words included sinus node dysfunction, sick sinus syndrome, causes, bradyarrhythmia, permanent pacemaker indications, chronotropic incompetence, loop recorders, ambulator cardiac monitoring. The search included practice guidelines, randomized controlled trials, a retrospective case control study, and review articles. Search dates: December 2019 to October 2020.
The opinions and assertions contained herein are those of the authors and are not to be construed as official or as reflecting the views of the Uniformed Services University, the U.S. Air Force Medical Department, the Air Force at large, the U.S. Army Medical Department, the Army at large, or the U.S. Department of Defense.
Ferrer MI. The sick sinus syndrome in atrial disease. JAMA. 1968;206(3):645-646.
Kusumoto FM, Schoenfeld MH, Barrett C, et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society [published correction appears in J Am Coll Cardiol . 2019;74(7):1016–1018]. J Am Coll Cardiol. 2019;74(7):e51-e156.
Sanders P, Kistler PM, Morton JB, et al. Remodeling of sinus node function in patients with congestive heart failure: reduction in sinus node reserve. Circulation. 2004;110(8):897-903.
Csepe TA, Kalyanasundaram A, Hansen BJ, et al. Fibrosis: a structural modulator of sinoatrial node physiology and dysfunction. Front Physiol. 2015;6:37.
Semelka M, Gera J, Usman S. Sick sinus syndrome: a review. Am Fam Physician. 2013;87(10):691-696. Accessed September 28, 2020. https://www.aafp.org/afp/2013/0515/p691.html
De Ponti R, Marazzato J, Bagliani G, et al. Sick sinus syndrome. Card Electrophysiol Clin. 2018;10(2):183-195.
Adán V, Crown LA. Diagnosis and treatment of sick sinus syndrome. Am Fam Physician. 2003;67(8):1725-1732. Accessed September 28, 2020. https://www.aafp.org/afp/2003/0415/p1725.html
Kistler PM, Sanders P, Fynn SP, et al. Electrophysiologic and electro-anatomic changes in the human atrium associated with age. J Am Coll Cardiol. 2004;44(1):109-116.
Zimetbaum P, Goldman A. Ambulatory arrhythmia monitoring: choosing the right device. Circulation. 2010;122(16):1629-1636.
Bagliani G, Leonelli F, Padeletti L. P wave and the substrates of arrhythmias originating in the atria. Card Electrophysiol Clin. 2017;9(3):365-382.
Park DS, Fishman GI. The cardiac conduction system. Circulation. 2011;123(8):904-915.
Go AS, Mozaffarian D, Roger VL, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127(1):143-152.
Alonso A, Jensen PN, Lopez FL, et al. Association of sick sinus syndrome with incident cardiovascular disease and mortality: the Atherosclerosis Risk in Communities study and Cardiovascular Health Study. PLoS One. 2014;9(10):e109662.
Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation. 2007;115(14):1921-1932.
Jensen PN, Gronroos NN, Chen LY, et al. Incidence of and risk factors for sick sinus syndrome in the general population. J Am Coll Cardiol. 2014;64(6):531-538.
Sarquella-Brugada G, Campuzano O, Arbelo E, et al. Brugada syndrome: clinical and genetic findings. Genet Med. 2016;18(1):3-12.
Mizusawa Y, Wilde AAM. Brugada syndrome. Circ Arrhythm Electrophysiol. 2012;5(3):606-616.
Benson DW, Wang DW, Dyment M, et al. Congenital sick sinus syndrome caused by recessive mutations in the cardiac sodium channel gene (SCN5A). J Clin Invest. 2003;112(7):1019-1028.
Bashour TT. Classification of sinus node dysfunction. Am Heart J. 1985;110(6):1251-1256.
Yabek SM, Swensson RE, Jarmakani JM. Electrocardiographic recognition of sinus node dysfunction in children and young adults. Circulation. 1977;56(2):235-239.
Khanna S, Sreedharan R, Trombetta C, et al. Sick sinus syndrome: sinus node dysfunction in the elderly. Anesthesiology. 2020;132(2):377-378.
Iqbal AM, Mubarik A, Cheetirala VG, et al. Marijuana induced sick sinus syndrome: a case report. Am J Case Rep. 2019;20:882-885.
Nielsen JC, Thomsen PEB, Højberg S, et al.; DANPACE Investigators. A comparison of single-lead atrial pacing with dual-chamber pacing in sick sinus syndrome. Eur Heart J. 2011;32(6):686-696.
John RM, Kumar S. Sinus node and atrial arrhythmias. Circulation. 2016;133(19):1892-1900.
Melzer C, Witte J, Reibis R, et al. Predictors of chronotropic incompetence in the pacemaker patient population. Europace. 2006;8(1):70-75.
Lukl J, Doupal V, Sovová E, et al. Incidence and significance of chronotropic incompetence in patients with indications for primary pacemaker implantation or pacemaker replacement. Pacing Clin Electrophysiol. 1999;22(9):1284-1291.
Sivakumaran S, Krahn AD, Klein GJ, et al. A prospective randomized comparison of loop recorders versus Holter monitors in patients with syncope or presyncope. Am J Med. 2003;115(1):1-5.
Gula LJ, Krahn AD, Massel D, et al. External loop recorders: determinants of diagnostic yield in patients with syncope. Am Heart J. 2004;147(4):644-648.
Olson JA, Fouts AM, Padanilam BJ, et al. Utility of mobile cardiac outpatient telemetry for the diagnosis of palpitations, presyncope, syncope, and the assessment of therapy efficacy. J Cardiovasc Electrophysiol. 2007;18(5):473-477.
Krahn AD, Klein GJ, Skanes AC, et al. Insertable loop recorder use for detection of intermittent arrhythmias. Pacing Clin Electrophysiol. 2004;27(5):657-664.
Steinberg JS, Varma N, Cygankiewicz I, et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry [published corrections appear in Heart Rhythm . 2018; 15(5):789, and Heart Rhythm . 2018;15(8):1276]. Heart Rhythm. 2017;14(7):e55-e96.
Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS Guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society [published correction appears in Circulation . 2017;136(16):e271–e272]. Circulation. 2017;136(5):e60-e122.
Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med. 2014;127(1):95.e11-95.e17.
Mulpuru SK, Madhavan M, McLeod CJ, et al. Cardiac pacemakers: function, troubleshooting, and management: part 1 of a 2-part series. J Am Coll Cardiol. 2017;69(2):189-210.
Sonoura T, Kodera S, Shakya S, et al. Efficacy of cilostazol for sick sinus syndrome to avoid permanent pacemaker implantation: a retrospective case-control study. J Cardiol. 2019;74(4):328-332.
Vardas PE, Auricchio A, Blanc JJ, et al.; European Society of Cardiology; European Heart Rhythm Association. Guidelines for cardiac pacing and cardiac resynchronization therapy. Europace. 2007;9(10):959-998.
Andersen HR, Thuesen L, Bagger JP, et al. Prospective randomised trial of atrial versus ventricular pacing in sick-sinus syndrome. Lancet. 1994;344(8936):1523-1528.
Andersen HR, Nielsen JC, Thomsen PE, et al. Long-term follow-up of patients from a randomised trial of atrial versus ventricular pacing for sick-sinus syndrome. Lancet. 1997;350(9086):1210-1216.
Svendsen JH, Nielsen JC, Darkner S, et al.; DANPACE Investigators. CHADS 2 and CHA2DS 2 -VASc score to assess risk of stroke and death in patients paced for sick sinus syndrome. Heart. 2013;99(12):843-848.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2021 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
Clinical review of sick sinus syndrome and atrial fibrillation
Klinischer Überblick über das Syndrom des kranken Sinusknotens und Vorhofflimmern
- Review articles
- Published: 22 June 2021
- Volume 47 , pages 244–250, ( 2022 )
Cite this article
- Wenxing Chang MD 1 &
- Guangsen Li PhD ORCID: orcid.org/0000-0002-5693-4481 1
1492 Accesses
7 Citations
Explore all metrics
Sick sinus syndrome (SSS) is a set of diseases with abnormal cardiac pacing, which manifests as diverse cardiac arrhythmias, especially bradycardia. The clinical presentation is inconspicuous in the early stage, but with the progression of this disease, patients may present with symptoms and signs of end-organ hypoperfusion. As a common result in the natural history of the disease, SSS coexisting with atrial fibrillation (AF) forms the basis of bradycardia–tachycardia syndrome. Age-related interstitial fibrosis is considered to be the common pathophysiological mechanism between SSS and AF. The combination of these diseases will adversely affect the condition of patients and the efficiency of subsequent treatment. Although the exact mechanism is not clear to date, the extensive structural and electrical remodeling of the atrium are considered to be the important mechanism for the occurrence of AF in patients with SSS. Pacemaker implantation is the first-line treatment for symptomatic patients with SSS and documented bradycardia history. In view of the adverse effects of AF on the treatment of SSS, researchers have focused on evaluating different pacing modes and algorithms to reduce the risk of AF during pacing. Catheter ablation may also be used as an alternative second-line therapy for some patients with SSS and AF.
Zusammenfassung
Das Syndrom des kranken Sinusknotens („sick sinus syndrome“ [SSS]) setzt sich aus einer Reihe von Erkrankungen mit anomaler Schrittmacherfunktion des Herzens zusammen, was sich in Form verschiedener kardialer Arrhythmien zeigt, insbesondere als Bradykardie. Im Frühstadium sind die klinischen Anzeichen unauffällig, aber mit Fortschreiten der Erkrankung können sich bei den Patienten Symptome einer Endorganhypoperfusion einstellen. Als eine häufige Folge des natürlichen Krankheitsverlaufs bildet das SSS bei gleichzeitigem Vorhofflimmern (VF) die Basis für ein Bradykardie-Tachykardie-Syndrom. Eine altersbezogene interstitielle Fibrose gilt als der gemeinsame pathophysiologische Mechanismus zwischen SSS und VF. Die Kombination dieser Erkrankungen hat negative Auswirkungen auf den Zustand der Patienten und auf die Wirksamkeit der nachfolgenden Behandlung. Zwar ist der genaue Mechanismus bisher noch nicht bekannt, aber das ausgedehnte strukturelle und elektrische Remodeling des Vorhofs werden als die entscheidenden Mechanismen für das Auftreten von VF bei Patienten mit SSS betrachtet. Die Schrittmacherimplantation stellt die Therapie der ersten Wahl bei symptomatischen Patienten mit SSS und dokumentierter Bradykardie in der Vorgeschichte dar. Angesichts der günstigen Auswirkungen von VF auf die Behandlung des SSS lag der Schwerpunkt von wissenschaftlichen Untersuchungen auf der Beurteilung verschiedener Schrittmachermodi und –algorithmen, um das Risiko eines VF während der Schrittmacheraktivität zu vermindern. Als alternative Zweitlinientherapie für manche Patienten mit SSS und VF kann auch eine Katheterablation infrage kommen.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Right versus left atrial pacing in patients with sick sinus syndrome and paroxysmal atrial fibrillation (Riverleft study): study protocol for randomized controlled trial
Tanwier TTK Ramdjan, Lisette JME van der Does, … Natasja MS de Groot

Catheter ablation of paroxysmal atrial fibrillation in patients with sick sinus syndrome
Masahiro Hada, Shinsuke Miyazaki, … Yoshito Iesaka
Prevalence and predictors of atrial arrhythmias in patients with sinus node dysfunction and atrial pacing
Abdallah Bukari, Eisha Wali, … Cevher Ozcan
Rogińska N, Bieganowska K (2014) Sick sinus syndrome: a family study. Cardiol Young 24(1):136–139. https://doi.org/10.1017/s1047951113000991
Article PubMed Google Scholar
Kristensen L, Nielsen J, Mortensen P, Pedersen O, Pedersen A et al (2004) Incidence of atrial fibrillation and thromboembolism in a randomised trial of atrial versus dual chamber pacing in 177 patients with sick sinus syndrome. Heart 90(6):661–666
Article CAS Google Scholar
Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ et al (2017) 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice Guid. Circulation. https://doi.org/10.1161/CIR.0000000000000548
Article PubMed PubMed Central Google Scholar
Adán V, Crown LA (2003) Diagnosis and treatment of sick sinus syndrome. Am Fam Physician 67(8):1725–1732
PubMed Google Scholar
Shiferaw Y, Aistrup G, Wasserstrom J (2017) Mechanism for triggered waves in atrial myocytes. Biophys J 113(3):656–670. https://doi.org/10.1016/j.bpj.2017.06.026
Article CAS PubMed PubMed Central Google Scholar
Yang PS, Kim D, Jang E, Yu HT, Joung B (2021) Risk of sick sinus syndrome in patients diagnosed with atrial fibrillation: a population-based cohort. Authorea. https://doi.org/10.22541/au.161555510.06165605/v1
Article Google Scholar
Dobrzynski H, Boyett M, Anderson R (2007) New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation 115(14):1921–1932. https://doi.org/10.1161/circulationaha.106.616011
Kronborg M, Nielsen J (2012) Pacing in sinus node disease to prevent atrial fibrillation. Expert Rev Cardiovasc Ther 10(7):851–858. https://doi.org/10.1586/erc.12.79
Article CAS PubMed Google Scholar
Alonso A, Jensen P, Lopez F, Chen L, Psaty B et al (2014) Association of sick sinus syndrome with incident cardiovascular disease and mortality: the Atherosclerosis Risk in Communities study and Cardiovascular Health Study. Plos One 9(10):e109662. https://doi.org/10.1371/journal.pone.0109662
Lamas GA, Lee KL, Sweeney MO (2002) Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. ACC Curr J Rev. https://doi.org/10.1016/S1062-1458(02)00963-7
Nielsen JC, Thomsen PEB, Hojberg S, Moller M, Vesterlund T et al (2011) A comparison of single-lead atrial pacing with dual-chamber pacing in sick sinus syndrome. Eur Heart J 32(6):686–696
Epstein A, Dimarco J, Ellenbogen K, Estes N, Freedman R et al (2008) ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: executive summary. Heart Rhythm 5(6):934–955. https://doi.org/10.1016/j.hrthm.2008.04.015
Ciszewski JB, Chwyczko T, Kowalik I, Majda W, Farkowski MM et al (2018) Clinical, echocardiographic, and pacing parameters affecting atrial fibrillation burden in patients with tachycardia-bradycardia syndrome. Kardiol Pol 76(2):338–346. https://doi.org/10.5603/KP.a2017.0207
Kim DH, Choi JI, Lee KN, Ahn J, Roh SY et al (2018) Long-term clinical outcomes of catheter ablation in patients with atrial fibrillation predisposing to tachycardia-bradycardia syndrome: a long pause predicts implantation of a permanent pacemaker. BMC Cardiovasc Disord 18(1):106. https://doi.org/10.1186/s12872-018-0834-0
Mangrum JM, DiMarco JP (2000) The evaluation and management of bradycardia. N Engl J Med. https://doi.org/10.1056/NEJM200003093421006
Sanchez-Quintana D, Cabrera JA, Farre J, Climent V, Anderson RH et al (2005) Sinus node revisited in the era of electroanatomical mapping and catheter ablation. Heart 91(2):189–194
Giuseppe BF, Leonelli L et al (2017) P wave and the substrates of arrhythmias originating in the atria. Cardiac Electrophysiology Clinics. https://doi.org/10.1016/j.ccep.2017.05.001
Lin YS, Guo BF, Chen YL, Tsai TH, Chen M‑C (2010) Atrial size independently correlates with the development of paroxysmal atrial fibrillation in patients with sick sinus syndrome. Biomed J 33(6):659–667
Google Scholar
Kistler PM, Sanders P, Fynn SP, Stevenson IH, Spence SJ et al (2004) Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol 44(1):109–116
Anyukhovsky EP, Sosunov EA, Alexei P, Gainullin RZ, Jhang JS et al (2002) Cellular electrophysiologic properties of old canine atria provide a substrate for arrhythmogenesis. Cardiovasc Res. https://doi.org/10.1016/S0008-6363(02)00271-7
John RM, Kumar S (2016) Sinus node and atrial arrhythmias. Circulation 133(19):1892–1900
Sparks PB, Jayaprakash S, Vohra JK, Kalman JM (2000) Electrical remodeling of the atria associated with paroxysmal and chronic atrial flutter. Circulation 102(15):1807–1813
Ishikawa T, Ohno S, Murakami T, Yoshida K, Mishima H et al (2017) Sick sinus syndrome with HCN4 mutations shows early onset and frequent association with atrial fibrillation and left ventricular noncompaction. Heart Rhythm 14(5):717–724. https://doi.org/10.1016/j.hrthm.2017.01.020
Jenewein T, Beckmann BM, Rose S, Osterhues HH, Schmidt U et al (2017) Genotype-phenotype dilemma in a case of sudden cardiac death with the E1053K mutation and a deletion in the SCN5A gene. Forensic Sci Int 275:187–194. https://doi.org/10.1016/j.forsciint.2017.02.038
Holm H, Gudbjartsson D, Sulem P, Masson G, Helgadottir H et al (2011) A rare variant in MYH6 is associated with high risk of sick sinus syndrome. Nat Genet 43(4):316–320. https://doi.org/10.1038/ng.781
Monfredi O, Dobrzynski H, Mondal T, Boyett M, Morris G (2010) The anatomy and physiology of the sinoatrial node—a contemporary review. Pacing Clin Electrophysiol 33(11):1392–1406. https://doi.org/10.1111/j.1540-8159.2010.02838.x
Gunes H, Sonmez F, Canga H, Saritas A (2017) An unexpected presentation of sick sinus syndrome: Isolated ventricular asystole. Am J Emerg Med 35(8):1212.e1215–1212.e1216. https://doi.org/10.1016/j.ajem.2017.04.069
Ewy G (2014) Sick sinus syndrome: synopsis. J Am Coll Cardiol 64(6):539–540. https://doi.org/10.1016/j.jacc.2014.05.029
Healey JS, Connolly SJ, Gold MR (2016) Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 374(10):998–998
Amasyali B, Kilic A, Kilit C (2014) Sinus node dysfunction and atrial fibrillation: which one dominates? Int J Cardiol 175(2):379–380
Iwasaki YK, Nishida K, Kato T, Nattel S (2011) Atrial fibrillation pathophysiology: implications for management. Circulation 124(20):2264–2274
Jackson L, Rathakrishnan B, Campbell K, Thomas K, Piccini J et al (2017) Sinus node dysfunction and atrial fibrillation: a reversible phenomenon? Pacing Clin Electrophysiol 40(4):442–450. https://doi.org/10.1111/pace.13030
Elvan A (2001) Sinoatrial remodeling caused by persistent atrial fibrillation: what is the relationship between postcardioversion sinus node dysfunction and increased atrial vulnerability? J Cardiovasc Electrophysiol 12(7):807–808. https://doi.org/10.1046/j.1540-8167.2001.00807.x
Roberts-Thomson KC, Sanders P, Kalman JM (2007) Sinus node disease: an idiopathic right atrial myopathy. Trends Cardiovasc Med 17(6):211–214. https://doi.org/10.1016/j.tcm.2007.06.002
Zhong H, Wang T, Lian G, Xu C, Wang H et al (2018) TRPM7 regulates angiotensin II-induced sinoatrial node fibrosis in sick sinus syndrome rats by mediating Smad signaling. Heart Vessels 33(9):1094–1105. https://doi.org/10.1007/s00380-018-1146-0
Prashanthan S, Joseph BM, Peter MK, Steven JS, Jonathan MK (2004) Electrophysiological and electroanatomic characterization of the atria in sinus node disease evidence of diffuse atrial remodeling. Circulation 109(12):1514–1522. https://doi.org/10.1161/01.CIR.0000121734.47409.AA
Jang SW (2021) Left atrial enlargement and sick sinus syndrome for pacemaker indication were associated with atrial high rate episodes. Korean Circ J 51(3):248–250. https://doi.org/10.4070/kcj.2020.0515
Ramdjan T, van der Does L, Knops P, Res J, de Groot N (2014) Right versus left atrial pacing in patients with sick sinus syndrome and paroxysmal atrial fibrillation (Riverleft study): study protocol for randomized controlled trial. Trials 15:445. https://doi.org/10.1186/1745-6215-15-445
Kochhäuser S, Verma A, Dalvi R, Suszko A, Alipour P et al (2017) Spatial relationships of complex fractionated atrial electrograms and continuous electrical activity to focal electrical sources: implications for substrate ablation in human atrial fibrillation. JACC Clin Electrophysiol 3(11):1220–1228. https://doi.org/10.1016/j.jacep.2017.05.013
Monfredi O, Boyett MR (2015) Sick sinus syndrome and atrial fibrillation in older persons—A view from the sinoatrial nodal myocyte. J Mol Cell Cardiol 83:88–100
Li G, Liu E, Liu T, Wang J, Dai J et al (2011) Atrial electrical remodeling in a canine model of sinus node dysfunction. Int J Cardiol 146(1):32–36. https://doi.org/10.1016/j.ijcard.2009.06.002
Luck J, Engel T (1979) Dispersion of atrial refractoriness in patients with sinus node dysfunction. Circulation 60(2):404–412. https://doi.org/10.1161/01.cir.60.2.404
Guray U, Guray Y, YAlmaz M, Mecit B, Sasmaz H et al (2003) Evaluation of P wave duration and P wave dispersion in adult patients with secundum atrial septal defect during normal sinus rhythm. Int J Cardiol 91(1):75–79. https://doi.org/10.1016/s0167-5273(02)00598-3
Kojodjojo P, Kanagaratnam P, Markides V, Davies D, Peters N (2006) Age-related changes in human left and right atrial conduction. J Cardiovasc Electrophysiol 17(2):120–127. https://doi.org/10.1111/j.1540-8167.2005.00293.x
Letsas KP, Korantzopoulos P, Efremidis M, Weber R, Lioni L et al (2013) Sinus node disease in subjects with type 1 ECG pattern of Brugada syndrome. J Cardiol 61(3):227–231. https://doi.org/10.1016/j.jjcc.2012.12.006
Bocchi F, Marques-Vidal P, Pruvot E, Waeber G, Vollenweider P et al (2020) Clinical and biological determinants of P‑wave duration: cross-sectional data from the population-based CoLaus|PsyCoLaus study. BMJ Open 10(11):e38828. https://doi.org/10.1136/bmjopen-2020-038828
Dinov B, Knopp H, Lobe S, Nedios S, Bode K et al (2016) Patterns of left atrial activation and evaluation of atrial dyssynchrony in patients with atrial fibrillation and normal controls: Factors beyond the left atrial dimensions. Heart Rhythm 13(9):1829–1836. https://doi.org/10.1016/j.hrthm.2016.06.003
Wang M, Tse H, Lee K, Zhang X, Siu C et al (2007) Differential in inter-atrial dyssynchrony and atrial mechanical function in sick sinus syndrome with or without paroxysmal atrial fibrillation. Circulation 116(suppl_16):II_687–II_688. https://doi.org/10.1161/circ.116.suppl_16.II_687-c
Wang M, Lau C, Zhang X, Siu C, Lee K et al (2009) Interatrial mechanical dyssynchrony worsened atrial mechanical function in sinus node disease with or without paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 20(11):1237–1243. https://doi.org/10.1111/j.1540-8167.2009.01547.x
Ponti RD, Marazzato J, Bagliani G, Leonelli FM, Padeletti L (2018) Sick sinus syndrome. Card Electrophysiol Clin 10(2):183–195. https://doi.org/10.1016/j.ccep.2018.02.002
Andersen H, Thuesen L, Bagger J, Vesterlund T, Thomsen P (1994) Prospective randomised trial of atrial versus ventricular pacing in sick-sinus syndrome. Lancet 344(8936):1523–1528. https://doi.org/10.1016/s0140-6736(94)90347-6
Mattioli A, Vivoli D, Mattioli G (1998) Influence of pacing modalities on the incidence of atrial fibrillation in patients without prior atrial fibrillation. A prospective study. Eur Heart J 19(2):282–286. https://doi.org/10.1053/euhj.1997.0616
Lamas GA, Orav EJ, Stambler BS, Ellenbogen KA, Sgarbossa EB et al (1999) Quality of life and clinical outcomes in elderly patients treated with ventricular pacing as compared with dual-chamber pacing. Surv Anesthesiol 43(1):14
Kim W, Joung B, Shim J, Park J, Hwang E et al (2010) Long-term outcome of single-chamber atrial pacing compared with dual-chamber pacing in patients with sinus-node dysfunction and intact atrioventricular node conduction. Yonsei Med J 51(6):832–837. https://doi.org/10.3349/ymj.2010.51.6.832
Hiroshi M, Yuichi U, Rinya K, Akihiko U, Takashi M et al (2004) Long-term clinical performance of AAI pacing in patients with sick sinus syndrome: a comparison with dual-chamber pacing. Europace 2004(5):5. https://doi.org/10.1016/j.eupc.2004.05.003
Tripp I, Armstrong G, Stewart J, Hood M, Smith W (2005) Atrial pacing should be used more frequently in sinus node disease. Pacing Clin Electrophysiol 28(4):291–294. https://doi.org/10.1111/j.1540-8159.2005.08672.x
Nielsen JC, Kristensen L, Andersen HR, Mortensen PT, Pedersen OL et al (2003) A randomized comparison ofatrial and dual-chamber pacing in177 consecutive patients with sick sinus syndrome: echocardiographic and clinical outcome. J Am Coll Cardiol 42(4):614–623
Pastore G, Marcantoni L, Lanza D, Maines M, Noventa F et al (2021) Occurrence of persistent atrial fibrillation during pacing for sinus node disease: The influence of His bundle pacing versus managed ventricular pacing. J Cardiovasc Electrophysiol 32(1):110–116. https://doi.org/10.1111/jce.14810
Edwards SJ, Karner C, Trevor N, Wakefield V, Salih F (2015) Dual-chamber pacemakers for treating symptomatic bradycardia due to sick sinus syndrome without atrioventricular block: a systematic review and economic evaluation. Health Technol Assess 19(65):1–210. https://doi.org/10.3310/hta19650
DeSilvey D (2008) Minimizing ventricular pacing to reduce atrial fibrillation in sinus node disease. Amer J Geriatric Cardiol 17(1):57–58. https://doi.org/10.1111/j.1076-7460.2007.07671.x
Martin S, Serge B, Javier M, Da CA, Robert H et al (2015) Long-term clinical effects of ventricular pacing reduction with a changeover mode to minimize ventricular pacing in a general pacemaker population. Eur Heart J. https://doi.org/10.1093/eurheartj/ehu336
Botto G, Ricci R, Banzet J, Nielsen J, De Roy L et al (2014) Managed ventricular pacing compared with conventional dual-chamber pacing for elective replacement in chronically paced patients: results of the Prefer for Elective Replacement Managed Ventricular Pacing randomized study. Heart Rhythm 11(6):992–1000. https://doi.org/10.1016/j.hrthm.2014.01.011
Chutani S, Shah A, Kantharia B (2017) Pacing to prevent atrial fibrillation. Curr Opin Cardiol 32(1):22–26. https://doi.org/10.1097/hco.0000000000000355
Boriani G, Tukkie R, Manolis A, Mont L, Santini M et al (2014) Atrial antitachycardia pacing and managed ventricular pacing in bradycardia patients with paroxysmal or persistent atrial tachyarrhythmias: the MINERVA randomized multicentre international trial. Eur Heart J 35(35):2352–2362. https://doi.org/10.1093/eurheartj/ehu165
Calkins H, Hindricks G, Cappato R, Kim Y, Saad E et al (2017) 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. Heart Rhythm 14(10):e445–e494. https://doi.org/10.1016/j.hrthm.2017.07.009
Download references
Acknowledgements
The authors would like to acknowledge Prof. Li for support with this review.
Author information
Authors and affiliations.
Department of Ultrasound, the Second Affiliated Hospital of Dalian Medical University, 116027, Dalian, China
Wenxing Chang MD & Guangsen Li PhD
You can also search for this author in PubMed Google Scholar

Corresponding author
Correspondence to Guangsen Li PhD .
Ethics declarations
Conflict of interest.
W. Chang and G. Li declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the ethical standards indicated in each case.
Rights and permissions
Reprints and permissions
About this article
Chang, W., Li, G. Clinical review of sick sinus syndrome and atrial fibrillation. Herz 47 , 244–250 (2022). https://doi.org/10.1007/s00059-021-05046-x
Download citation
Received : 23 July 2020
Revised : 29 April 2021
Accepted : 06 May 2021
Published : 22 June 2021
Issue Date : June 2022
DOI : https://doi.org/10.1007/s00059-021-05046-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Sick sinus syndrome
- Bradycardia–tachycardia syndrome
- Pacemaker implantation
- Sinus node dysfunction
- Atrial fibrillation
Schlüsselwörter
- Syndrom des kranken Sinusknotens
- Bradykardie-Tachykardie-Syndrom
- Schrittmacherimplantation
- Sinusknoten-Dysfunktion
- Vorhofflimmern
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Cardiovasc Dev Dis

Cellular and Molecular Mechanisms of Functional Hierarchy of Pacemaker Clusters in the Sinoatrial Node: New Insights into Sick Sinus Syndrome
The sinoatrial node (SAN), the primary pacemaker of the heart, consists of a heterogeneous population of specialized cardiac myocytes that can spontaneously produce action potentials, generating the rhythm of the heart and coordinating heart contractions. Spontaneous beating can be observed from very early embryonic stage and under a series of genetic programing, the complex heterogeneous SAN cells are formed with specific biomarker proteins and generate robust automaticity. The SAN is capable to adjust its pacemaking rate in response to environmental and autonomic changes to regulate the heart’s performance and maintain physiological needs of the body. Importantly, the origin of the action potential in the SAN is not static, but rather dynamically changes according to the prevailing conditions. Changes in the heart rate are associated with a shift of the leading pacemaker location within the SAN and accompanied by alterations in P wave morphology and PQ interval on ECG. Pacemaker shift occurs in response to different interventions: neurohormonal modulation, cardiac glycosides, pharmacological agents, mechanical stretch, a change in temperature, and a change in extracellular electrolyte concentrations. It was linked with the presence of distinct anatomically and functionally defined intranodal pacemaker clusters that are responsible for the generation of the heart rhythm at different rates. Recent studies indicate that on the cellular level, different pacemaker clusters rely on a complex interplay between the calcium (referred to local subsarcolemmal Ca 2+ releases generated by the sarcoplasmic reticulum via ryanodine receptors) and voltage (referred to sarcolemmal electrogenic proteins) components of so-called “coupled clock pacemaker system” that is used to describe a complex mechanism of SAN pacemaking. In this review, we examine the structural, functional, and molecular evidence for hierarchical pacemaker clustering within the SAN. We also demonstrate the unique molecular signatures of intranodal pacemaker clusters, highlighting their importance for physiological rhythm regulation as well as their role in the development of SAN dysfunction, also known as sick sinus syndrome.
1. Introduction
The sinoatrial node (SAN) is the primary pacemaker of the heart, which can spontaneously produce electrical impulses coordinating heart contractions. Anatomical SAN is located at the junction where the superior vena cava enters the right atrium [ 1 ]. Despite the species difference in size, the SAN covers a relatively large area rather than a small group of cells (SAN cells, or SANCs) where the electrical impulses originate. SAN is bordered from the crista terminalis and may extend from the superior to inferior vena cava. Across this area, the SAN consists of highly heterogeneous populations of cells that significantly vary in size, ionic current and gap junction repertoire, and expression profiles of other biomarkers [ 2 ]. At the center of the SAN, a group of small spindle-shaped cells, that can spontaneously generate electrical impulses, is extensively studied and traditionally recognized as “typical nodal cells” or leading pacemaker cells. However, emerging evidence have suggested that they are not the only group of SANCs with automaticity. It was recognized that the origin of electrical impulses is not static or limited exclusively to center SAN, rather, it has been shown that pacemaker location is dynamic and changes according to the prevailing conditions [ 3 ], including neurohormonal modulation, pharmacological interventions, mechanical stretch, and a change in temperature among others. Importantly, such pacemaker shift is not arbitrary but rather associated with distinct anatomical regions within the SAN, also known as pacemaker clusters [ 3 , 4 , 5 , 6 ]. These intranodal pacemaker clusters can be activated and dominate the heart beating under certain conditions [ 6 , 7 , 8 ]. It was proposed that any substantial changes in heart rate are associated with the pacemaker shift within the SAN, and larger changes in heart rate were linked with greater distance of pacemaker shift [ 6 ]. Thereby, a complex and dynamic system of intranodal pacemaker clusters, which, individually, are responsible for narrow ranges of beating rates, can represent a hierarchical rhythmic system tightly adjusted to regulate the heart’s performance and meet the physiological needs of the body. When activated during physiological stimulations, pacemaker clusters follow a hierarchical way to take turns in determining the heart beating at the exact rate to meet body needs and assures the robustness of heart rhythm maintenance. Importantly, multiple studies have shown that center and peripheral SAN pacemaker clusters possess diverse functional and molecular signatures and presumably can be activated via distinct cellular mechanisms [ 7 , 9 , 10 ]. In this review, we summarize the evidence supporting the hierarchy of SAN pacemaking system and examine the structural, functional, and molecular evidence for the pacemaker clustering within the SAN. We also demonstrate the unique molecular signatures of intranodal pacemaker clusters highlighting their importance for physiological rhythm regulation as well as for the development of SAN dysfunction, also known as sick sinus syndrome.
2. Development of the SAN
Extensive studies have been performed to explore the embryonic origin of the pacemaker tissue [ 11 , 12 , 13 ]. The clear elucidation of the mechanisms underlying SAN development would extend our understanding of SAN physiology and regulation, and thereby provide foundation for therapeutic intervention of pacemaker diseases. Moreover, the studies of genetic programing that controls SAN development have been critical in the regenerative medicine where nodal-like pluripotent stem cells are preferentially generated [ 14 ] and selected [ 15 ], which were then used to develop the concept of “biological pacemakers” [ 16 ]. In reviewing the development of SAN, we also observe evidence that may support the hierarchy of pacemaker clusters.
2.1. Structural Changes in the SAN Development
During SAN development, spontaneous contractions can be detected at early stages [ 11 , 12 , 13 ]. Caudal pacemaker activity conducts slowly in the early heart tube resulting in a sinusoidal morphology ECG [ 13 ] and the cells of the early embryonic heart tube present similar properties to mature SAN before the tube elongates and develops into ventricular and atrial chambers. SAN primordium was observed in histological sections as early as embryonic day E10.5 in mice [ 17 ], and the nodal structure then becomes obvious in the right sinus horn at the junction with the atrium. A transient development of a small SAN follows [ 18 ], which then develop to a full SAN in case of atrial right isomerism [ 19 ].
2.2. Genetic Programing Controlling SAN Development
A series of specific biomarkers has been reported in identification and localization of sinoatrial precursors in its development. One widely accepted specific biomarker of SAN is the hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, which mediate the I f current known to gradually depolarize membrane potential during diastolic phase and is essential to SAN automaticity. Among all the isoforms, HCN4 is reported to be the highest expressed one in the adult SAN in different species including human [ 20 , 21 , 22 ]. HCN4 expression was reported in cells as early as the gastrulation stage (E6.0) in mice [ 23 ] and mRNA expression of HCN4 is also detected in the cardiac crescent at E7.5 in mice [ 24 ], suggesting that HCN4 is a specific marker that can be used to characterize pacemaker cells at very early stage of the development. However, HCN4 does not seem to be functioning until a later stage as evidenced by the observations that HCN4 knockout mice die between E9.5 to E11.5, but not as early as it was observed in cells [ 25 ].
Besides HCN4, there are also other markers that facilitate researchers to study and identify SAN during its development despite less specificity. In the adult rabbit heart, neurofilament-M (NF-M) transcript is found to be localized in all the cardiac condition system, including the SAN, but not the working cardiomyocytes. NF-M mRNA can be detected at E9.5 which also helps to locate SAN origin during the development [ 26 ]. Moreover, gap junctions, though not sufficiently expressed during early embryonic stages, are also markers to differentiate the SANCs from surrounding atrial cells. Gap junction protein, connexin 43 (Cx43) which form high conductance junctions between atrial and ventricular cells, is negligibly expressed in the SAN [ 27 , 28 ]. Similarly, the gene for atrial natriuretic peptide, natriuretic peptides A ( Nppa ), shows low expression in the SAN unlike in atrial cells.
During the development of the SAN, the genetic program for pacemaker properties is promoted while the genetic program that promotes chamber specification is inhibited. There are a series of transcription factors that were reported to be fundamental in such genetic program [ 28 ]. For example, T-box transcription factors, are known to be critical in maintaining the pacemaker features during SAN development. Tbx3 is found to be specifically expressed in the regions that eventually form the mature conduction system of the heart [ 29 ]. The lack of Tbx3 can induce SANCs to express specific genes of the working myocardium including Cx43 and nppa [ 30 ]. Similar to Tbx3, Shox2 (short-stature homeobox protein 2) is also reported to facilitate the maintenance of SAN properties during development. Shox2 is a transcriptional repressor with its expression restricted to the right side of the sinus venosus, where it forms the SAN later in development. Knockdown of Shox2 causes the ectopic expressions atrial genes like Cx43 and Nppa and lack of of Tbx3 and HCN4 [ 31 ]. Tbx18 was also identified to be an important factor in the formation of SAN head during development [ 32 ]. Mice lacking Tbx18 are found to have smaller SAN, which is attributed to delayed recruitment of SAN progenitors [ 32 ]. ISL1 (Islet-1) is also found to be critical in the functioning of pacemaker cells during development. In an SAN-specific Isl1 deletion model, it is revealed that a number of critical genes for SAN function including L-type Ca 2+ channel, Tbx3 and Ank2 are downstream of ISL1 activity [ 33 ].
Other transcription factors, including Tbx5 and Nkx2.5, on the other hand, promote the expression of these specific genes in working myocytes and therefore are considered as negative markers of the SAN. Studies also showed that deficiency of Nkx2.5 promotes the atrial myocytes to express SAN phenotype proteins including HCN4 and Tbx3 [ 18 ]. Overexpression of Nkx2.5 in the atrium on the other hand, causes the expression of atrial genes. Shox2, which promotes SAN gene expression, is reported to repress the Nkx2.5 gene [ 31 ]. The genetic programing is very complex, where tiny interventions in this cascade may result in cells not with SAN phenotypes, but with both atrial and SAN characteristics [ 18 , 30 ].
It is established that a conserved gene regulatory network of these transcription factors controls the development and function of sinus node cells. A recent study which employs the an epigenetic assay for transposase-accessible chromatin with sequencing further investigated the precise mechanism that connect the expression of these regulators with their gene targets and found that sinus node cells have distinct regions of accessible chromatin that correlate with their gene expression profile and contain novel SAN enhancers during development [ 34 ].
3. Concept of Hierarchical Pacemaker Clustering in the SAN
3.1. pacemaker shift.
In early histological studies, the SAN was described as a small condensed area of specialized tissue located near the crista terminalis where the superior vena cava enters the right atrium [ 35 ]. Recent studies in canine [ 36 ], rabbit [ 1 ] and human [ 37 ] hearts, however, suggested that SAN is a more diffusive and extensive structure than it was appreciated previously. Both functional and structural assessments have shown that the SAN region covers a relatively large region extending from the superior to inferior vena cava, with a indistinct and irregular margin to its surrounding atrial myocytes [ 38 ]. Within this region, highly heterogeneous populations of cells with varying morphologies and properties (see Section 4 below) are observed. In 1985, Kreitner isolated the inter-caval region, including the SAN and peripheral atrium, from the rabbit atria and cut it into several small strips. He found that multiple (but not all) strips, including those from the SAN center, near the SAN periphery and even from adjacent atria, possess spontaneous automatic activity [ 39 ]. The author concluded that, in addition to typical nodal cells, there are other pacemaker cells that occupy a relatively large area around the SAN and can generate spontaneous electrical impulses when they are physically (or electrically) uncoupled from the center of the SAN.
Later studies confirmed that indeed such “non-typical nodal cells” with intrinsic automaticity belong to the SAN and can serve as a leading pacemaker under certain conditions. In a series of studies, the location of the leading pacemaker was found to be highly variable and not limited to a certain anatomical area (i.e., the central SAN) [ 7 , 40 , 41 , 42 , 43 , 44 , 45 ]. It was shown that the leading pacemaker can change its location within the anatomically and structurally defined SAN region which was described as a “pacemaker shift” [ 46 ]. The leading pacemaker can shift both superiorly, inferiorly, or laterally in response to various physiological stimulations. Sympathetic stimulation shifts the leading pacemaker superiorly as labeled by red dots, and parasympathetic stimulation shifts the leading pacemaker inferiorly as labeled by whit dots in mouse, rabbit ( Figure 1 ), and canine ( Figure 2 ). Importantly, pacemaker shift is accompanied by changes in SAN beating rate. Pacemaker shift also alters the activation sequence of the atria and thereby could be observed from the changes of ECG P wave morphology and polarity [ 47 ].

Pacemaker shift in mouse and rabbit SANs. Figures are modified from Glukhov [ 48 , 49 ] for mouse, and Lang [ 7 ], for rabbit. ( A ) Pacemaker shift in the mouse SAN under sympathetic (isoproterenol, or Iso; red dots) and parasympathetic (acetylcholine, or ACh; white dots) stimulations. Leading pacemakers under different conditions were visualized by optical mapping in isolated mouse right atrial preparations. SAN area was identified by connexin 43 (Cx43)-negative and HCN4-positive immunofluorescent staining. Iso shifted the leading pacemakers superiorly and ACh shifted them inferiorly within the anatomically and functionally defined SAN. ( B ) Pacemaker shift in rabbit SAN under sympathetic (Iso; red dots) and parasympathetic (ACh; white dots) stimulations. Leading pacemakers distributed within three distinct areas of the SAN, which is identified as HCN4-positive area. Similar to mouse, Iso and ACh shifted the leading pacemakers from the center of the rabbit SAN to superior and inferior intranodal pacemaker clusters, respectively.

Evidence of several pacemaker clusters in canine and human SANs. Figures are modified from Lou [ 50 ] and Li [ 4 ]. ( A ) Evidence supporting existence of pacemaker clusters in the canine SAN. Three pacemaker clusters located at head, center and tail SAN were identified during autonomic stimulation. Similar to mouse and rabbit SANs, Iso and ACh shifted the leading pacemaker from the center cluster (blue dots in a light blue area) into superior (red dots in a light red area, Iso) and inferior (white dots in a gray area, ACh) clusters. The figure summarizes four studies that optically located the leading pacemaker in the canine SAN [ 42 , 43 , 44 , 51 ]. Immunofluorescent detection showed various Cx43 expression patterns in distinct intranodal clusters. Further evidence from electrophysiological characterization also supported the existence of pacemaker clusters located at head, center and tail SAN, which is consistent with the findings from the pacemaker shift. Three clusters showed different action potential duration (APD) and dominate frequency distribution measured during atrial pacing. ( B ) Evidence supporting existence of multiple pacemakers in the human SAN. Similar to the canine SAN, three pacemaker clusters were identified in the human SAN evidenced from pacemaker shift and dominant frequency distribution during atrial pacing. Adenosine (Ado) shifted the leading pacemaker outside the central SAN majorly into the superior cluster and rarely to the inferior cluster. Green *: sinus exit pathway.
Importantly, the anatomical sites that the leading pacemaker shifts to is consistent between multiple species under various conditions [ 7 , 42 , 52 ]. Figure 1 and Figure 2 summarize the location of the leading pacemaker observed under baseline conditions and during sympathetic and parasympathetic stimulations in a series of studies in isolated mouse [ 48 ], rabbit [ 7 ], canine [ 42 , 43 , 44 , 45 , 50 , 51 ], and human [ 4 ] SANs. In those studies, location of the leading pacemaker was accurately captured by a high-resolution optical mapping [ 53 , 54 ] based on the earliest impulse origination [ 55 , 56 ]. Sympathetic stimulation was found to shift the leading pacemaker location superiorly in every individual animal to confined locations shown by red circles. Similar, parasympathetic simulation resulted in an inferior shift as shown by white circles. Importantly, all the leading pacemakers, identified both at baseline and under autonomic stimulation, were located within the Cx43 negative and HCN4 positive region that confirms their localization within the SAN. Furthermore, under specific conditions, the leading pacemaker locations are concentrated within distinct “clusters”. Such clusters could be attributed to certain populations of pacemaker cells that dominate under certain physiological conditions and generate a faster pacemaking rate comparing to baseline condition resulting in a concomitant pacemaker shift. In addition to the pacemaker shift, evidence from studies from canine and human have further supported the existence of multi intranodal pacemaker clusters. Several groups have independently reported the superior and inferior pacemaker shift during anatomical stimulation in canine ( Figure 2 A, middle panel) [ 42 , 43 , 44 , 45 ]. Immunofluorescent studies showed different Cx43 expression within these superior and inferior pacemaker clusters ( Figure 2 A, top panel) [ 42 ]. Moreover, distinct electrophysiological parameters could also differentiate the multi intranodal pacemaker cluster as shown in Figure 2 A, bottom panel. Specifically, three pacemaker clusters located at SAN head, center and tail have different action potential durations [ 42 ]. These clusters also present different dominant frequencies during fast atrial pacing. Similarly, in the human heart, adenosine was found to shift the leading pacemaker to both the SAN head and tail ( Figure 2 B, middle panel), and, as reported in the canine SAN, the dominant frequencies in these three pacemaker clusters during atrial pacing were also different ( Figure 2 B, bottom panel) [ 4 ].
Pacemaker shift could be also observed in various physiological conditions. Besides autonomic stimulation, cardiac glycosides [ 57 ], L-type Ca 2+ channel blocker nifedipine [ 58 ], ryanodine treatment [ 43 ], and changes in temperature [ 41 ] were all observed to shift the leading pacemaker to clusters located at different anatomical regions within the SAN, as summarized in Figure 3 .

Summary of the pacemaker shifts among multiple SAN clusters. Figure is modified based on the data from Boyett et al. [ 5 ] with adding data from Lang et al. (point 3) [ 7 ], Li et al. (point 4) [ 4 ] and Shinohara et al. (point 13) [ 43 ]. All points are from rabbit SAN experiments except otherwise indicated. Diagram summarizes pacemaker shift into different clusters under the following conditions. *—pacemaker at baseline condition. Shift to the superior pacemaker cluster: 1—low Cl - concentration; 2—treatment with E-4031 (class III antiarrhythmic drug that blocks hERG K + channels); 3—isoproterenol (non-selective β-adrenergic agonist); 4—adenosine (human data), and 5—4-aminopyridine (4-AP, selective blocker of I to current). Shift within the center pacemaker cluster: 6—low Na + concentration; 7—low Ca 2+ concentration, low temperature, or high K + concentration. Shift to the inferior pacemaker cluster: 8,9—same condition as for 7, but to different extend. Shift to the inferior pacemaker cluster: 10—acetylcholine (ACh, muscarinic receptor agonist); 11—nifedipine (Ca 2+ channel blocker); 12—ryanodine with high extracellular K + concentration (canine data); and 13—adrenaline (α- and β-adrenergic receptor agonist).
3.2. Dynamic Hierarchy of Cluster Pacemaking
Pacemaker shift is always accompanied by a change in beating rate, which seems to be closely correlated with the location the leading pacemaker moves to, or in other words, the beating rate seems to be closely correlated with distinct pacemaker clusters [ 7 , 9 ]. Shibata et al. found that vagal stimulation results in the shift of the leading pacemaker to a “lower” (inferior) position in accordance with a slowing of the beating rate. Importantly, the authors noticed that the extent of rate slowing is proportional to the distance of the pacemaker shift. They found the greater the slowing, the larger distance of the pacemaker shift [ 6 ]. In the pacemaker shift path, multiple pacemaker clusters were dominating at different beating rates, with each cluster working within a narrow frequency window [ 6 , 59 ]. The pacemaker shifts appear to follow a dynamic hierarchical pattern, in which another pacemaker is activated and dominate at rhythms that exceed the current pacemaker’s working range. This dynamic hierarchical pacemaker shift is supposed to be mediated by a combination of complex factors discussed in Section 4 .
The intrinsic automaticity of each pacemaker cluster is not static but rather dynamic and regulatable in response to prevailing conditions. In a recent study, Lang et al. used optical mapping of the rabbit SAN to identify and isolate intranodal pacemaker clusters that dominated at baseline and sympathetic stimulation. The cluster that dominated under sympathetic tone showed no automaticity at baseline condition; whereas, after application of 100 nM isoproterenol, the cluster’s pacemaking activity dramatically increased. Under sympathetic stimulation, the pacemaker cluster which dominated at baseline condition have also showed an increase in pacemaking rate which, however, was not as fast as that in other clusters. The baseline pacemaker then lost its dominating role which resulted in a pacemaker shift to another cluster activated by sympathetic stimulation [ 10 ]. This study suggests that each intranodal pacemaker cluster can be specifically activated to start providing the dominant rhythm.
Besides adjusting the heart rates by shifting to distinct pacemaker clusters, the hierarchical pacemaking system could also provide specific mechanisms preventing rhythm failure. Li and colleagues found that in humans, distinct intranodal pacemaker clusters work in a hierarchical and complementary pattern that ensures a “fail-safe” mechanism for robust maintenance of sinus rhythm [ 4 ]. During adenosine stimulation, when the primary central SAN pacemaker cluster is suppressed, other clusters (superior and inferior), which were previously inactive, take over and maintain a robust heart rhythm. This cluster system works synergistically with the sinoatrial conduction pathways to rescue sinus rhythm when needed [ 4 , 60 , 61 ]. When preferential sinoatrial conduction pathways were shown to be suppressed together with the inactivation of certain pacemaker clusters under conditions like adenosine stimulation, another sinoatrial conduction pathway could be activated along with change of pacemaker clusters.
3.3. Synchronization of the Hierarchical Pacemaker Clusters
With distinct intrinsic automaticity of each pacemaker cluster, the SAN synchronizes all of them and generates an “integral” rhythm. This rhythm is mainly determined by the cluster that displays the fastest firing rate [ 62 ]. Pacemaker clusters are connected through low resistance gap junctions, and affect each other by local circuit currents, which is known as mutual entrainment [ 63 , 64 ]. The mutual entrainment is associated with the respective intrinsic frequencies of the pacemaker clusters, their phase relations and the electrical coupling degrees among them, and eventually synchronizes all the pacemaker cells [ 63 ]. In a recent study by Fenske and colleagues, a nonfiring mode of pacemaker cells was introduced [ 62 ]. Pacemaker cells can be hyperpolarized to a degree that prevents its own spontaneous firing, i.e., nonfiring mode. This mode is specifically regulated by the cyclic adenosine monophosphate (cAMP)-dependent regulation of the HCN4 channels [ 62 ]. The interactions between nonfiring and firing pacemaker cells form the tonic adjustment of heart rate via synchronization of these two types of pacemaker cells. Specifically, pacemaker cells, that hyperpolarize themselves to enter nonfiring mode, will activate tonic flows of cations from the neighbor firing cells via gap junctions. The current then depolarizes the nonfiring cells and results in a relatively slow firing rate in these cells instead of nonfiring mode. Meanwhile, the source firing cells would be slightly hyperpolarized during this process and fire more slowly, therefore adjusting the SAN to fire at a slower heart rate.
4. Functional and Molecular Signatures of SAN Pacemaker Clusters
4.1. fine architecture.
Substantial heterogeneity of pacemaker cells within the SAN has been demonstrated in various studies [ 9 , 10 , 65 , 66 ]. It was shown that SANCs significantly vary in their spontaneous beating rate [ 9 , 65 ], cell size [ 1 , 66 , 67 , 68 ], cellular microarchitecture [ 5 , 69 , 70 ], ionic current densities [ 9 , 65 , 70 ], ratios between depolarizing and repolarizing ionic current [ 5 , 9 , 65 , 71 , 72 ], and cell response to autonomic modulation [ 9 ]. SANCs show a distinct cell morphology as reviewed in detail by Boyett et al. [ 5 ]. In general, SANCs are smaller and lack of organized myofilaments and mitochondria (cells look “empty” under the light microscope) as compared to the surrounding atrial cells, with an approximately twice higher density of nuclei than in the atrial muscle. James et al. described human and canine SANCs as P. cells as they appear pale in contrast to working myocardium, many of their features resemble those of embryonal primitive myocardial cells, and they also have many similarities to Purkinje cells [ 68 ]. Interestingly, the authors noticed that most of the central portion of the SAN is composed of clusters of P. cells, but the homogeneity of the node varies in different areas. The cells in the center of the SAN are reported to be 5–10 μm in diameter in the human and dog [ 68 ] as well as in other species [ 5 ], compared to 15–25 μm atrial myocytes [ 73 ] and even larger ventricular myocytes [ 74 ]. Furthermore, several authors observed an increase in cell size and density with more myofilament organization and mitochondria in the SAN periphery [ 1 , 59 , 66 , 67 ].
4.2. Electrophysiological Heterogeneity
Several groups tried to correlate the SANC size with electrophysiological characteristics, including electrical activity and ion channel composition. Honjo et al. reported in the rabbit SAN that the action potential amplitude, maximum diastolic potential, take-off potential, and action potential upstroke velocity were greater in larger SANC which correlated with a faster spontaneous beating rate [ 65 ]. The authors linked this to a higher density of I f current and the presence of tetrodotoxin-sensitive I Na current found in larger SANCs which are believed to be located in the periphery of the SAN (see above). In contrast, Monfredi et al. recently used 150 spontaneously beating rabbit SANCs (versus 61 cells in Honjo et. study) to study correlations between cell size, spontaneous beating rate and ion current densities, and found no relationship between beating rate and cell size, but also observed a positive correlation between the density of I f current (105 cells versus 12 cells in Honjo et. study) and spontaneous beating rate [ 9 ]. The authors also observed a strong negative correlation between the spontaneous beating rate and the density of I Ca,L current, i.e., those cells that beat the quickest at baseline tend to have the lowest density of I Ca,L , in contrast to Honjo et. study where no correlation between I Ca,L and beating rate was found. Importantly, either study did not attempt geographical categorization of the studies cells or intra-SAN localization of these cells. Therefore, while significant morphological and electrophysiological heterogeneity of SANCs could be appreciate from these studies, it is quite difficult to locate different populations of SANCs to certain intra-nodal pacemaker clusters. However, this could be approximated based on functional behaviors of those SANC populations and their response to various pharmacological interventions.
4.3. Pacemaking Mechanisms: Voltage Versus Calcium Clocks
In the study by Monfredi et al., the authors used cyclopiazonic acid (CPA), a moderate disruptor of Ca 2+ cycling and action potential firing rate in SANCs [ 75 ], to determine the effect of Ca 2+ cycling inhibition on beating rate and the relationship with cell size and ionic current density [ 9 ]. It was found that cells with lower I f current densities demonstrated a significantly greater susceptibility to the effect of CPA in terms of beating rate slowing. The authors also noted that a relatively large cell population (21 of 90 cells) stopped beating when the sarcoplasmic reticulum pumping rate decreased in the presence of CPA, despite a relatively high I f density. It should be noted that in spontaneously beating SANCs, I f current density varied dramatically from 0 to ~50 pA/pF, i.e., some of the spontaneously beating SANCs had little to zero I f . The authors then suggested that those cells may represent a divergent population of SANCs compared with those that simply slow their beating rather than stopping, and thus may rely on pacemaker mechanisms other than I f , e.g., Ca 2+ clock. By using computational simulations, Monfredi et al. confirmed that indeed a higher sarcoplasmic reticulum Ca 2+ pump rate in cells with low I f can accelerate diastolic depolarization via increase in I NCX .
Subsequently, two recent reports from Lakatta’s group demonstrated in guinea pig [ 76 ] and human SANCs [ 77 ] several populations of cells isolated from the SAN anatomically and functionally defined area which show rhythmic pacemaking activity, dysrhythmic firing, and no spontaneous activity (i.e., “dormant” cells). Dysrhythmic and dormant SANCs have smaller and desynchronized LCR activity than rhythmic SANCs. However, in response to sympathetic stimulation, all dysrhythmic cells and a third of dormant SANCs increased their LCR activity and developed automaticity resulting in spontaneous electrical beating [ 76 ]. Importantly, under sympathetic stimulation, dysrhythmic and dormant cells can generate spontaneous activity at the same rate as rhythmic cells at baseline or even faster. This may further support their ability to lead the SAN rhythm under sympathetic stimulation.
Whether or not these different types of SANCs are associated with different pacemaker clusters are responsible for certain heart rates, remains open to question. However, it may provide a mechanistic basis for dynamic pacemaker location shifts within the SAN as it was observed experimentally in intact optically mapped mouse, rabbit, canine, and human SAN preparations ( Figure 1 and Figure 2 ) [ 4 , 7 , 42 , 48 , 49 , 50 , 52 ]. As summarized in Figure 1 , sympathetic stimulation by isoproterenol in isolated mouse and rabbit SAN preparations results in a shift of the leading pacemaker location superiorly within the anatomically and structurally (identified by connexin 43-negative and HCN4-positive expression patterns) defined SAN area ( Figure 1 ). Therefore, it might be possible to associate SANCs identified by Monfredi et al. as those with lower I f current densities which pacemaking greatly relies on the Ca 2+ clock, with the superior SAN cluster. Indeed, optical mapping of electric activity from isolated superfused rabbit SAN preparation showed that selective inhibition of I f current by ivabradine (1–10 μM) resulted in the inferior shift of the leading pacemaker location within the SAN region [ 78 ]. These results further highlight a region-specific expression of I f current, its contribution to SANC pacemaker activity, and a potentially Ca 2+ clock driven superior SAN pacemaker cluster.
Concurrently, Li and colleagues performed molecular mapping of HCN isoform expression in human SAN and observed no difference in the expression levels of HCN1, HCN2 and HCN4 between superior, center and inferior SAN pacemaker clusters [ 79 ]. However, as different isoforms of the HCN channels exhibit varied sensitivity to cAMP [ 80 ], which may differ between intranodal pacemaker clusters, cAMP may have a larger role in regulating SAN pacemaking. In general, SAN is characterized by a significantly higher level of basal cAMP than that found in atrial or ventricular myocytes [ 81 ] and appears to be critical for SAN pacemaking. High cAMP concentration is the SAN has been linked to both activation of I f current and stimulation of the Ca 2+ cycling via protein kinase A (PKA)- and Ca 2+ /calmodulin-dependent protein kinase II (CaMKII)-dependent phosphorylation of Ca 2+ handling proteins, including RyRs, SERCA and phospholamban [ 62 , 82 , 83 ]. Overexpression of a Ca 2+ -activated adenylyl cyclase, that drives the phosphorylation-driven automaticity, has been shown to be sufficient, in the absence of I f current activation, to produce biological pacemaking for 7 days in experimental dogs [ 84 ]. Therefore, it is possible that a highly dynamic cAMP cycling profile, rather than HCN expression, determines the shift of the leading pacemaker upon I f block.
Recent studies have proposed the structural foundation for cAMP-dependent so-called Ca 2+ “super-hubs” which have been identified in mouse, rat, rabbit and human atrial myocytes [ 85 , 86 ]. These Ca 2+ “super-hubs” where linked to specialized axial tubule junctions which are highly enriched by cholesterol-rich caveolae-shaped nanodomains visualized by the fluorescent cholesterol analog dye Chol-PEG-KK114 in live cells. This is different from ventricular myocytes, where transversal (T)-tubules rarely contain caveolae-shaped membrane structures. It was shown that axial tubule junctions form contact junctions with the sarcoplasmic reticulum and are associated with cAMP-dependent highly phosphorylated RyRs [ 85 ] which may play an important role in SANC pacemaking in cells located in the periphery of the SAN. Indeed, transmission electron microscopy studies by Ayettey and Navaratnam demonstrated that transversal-axial tubule system is either absent or significantly less developed in the center of the rat SAN [ 87 ] but appears in transitional cells within the SAN region. These transitional myocytes resemble nodal cells in diminutiveness of size and lack of atrial granules but also possess a sparse and disorganized transversal-axial tubule system [ 87 ]. Overall, these studies highlight the importance of cAMP signaling and Ca 2+ clock in peripheral SAN clusters.
Indeed, parasympathetic stimulation by acetylcholine perfusion [ 4 , 48 , 49 , 52 ] or vagal nerve stimulation [ 45 ], which inhibits both voltage and Ca 2+ clocks, suppresses both center and superior SAN pacemaker clusters and shifts the leading pacemaker location site inferiorly within the SAN area, as summarized in Figure 1 and Figure 2 . This may indicate that the inferior SAN pacemaker cluster depends less on Ca 2+ clock, in general, and cAMP level, in particular. It also may indicate the presence of tetrodotoxin-sensitive I Na current as it was identified by Honjo et al. in the periphery of the SAN [ 65 ]. More intensive parasympathetic stimulations result in local SAN inexcitability while cells from subsidiary pacemaker areas as well as atrium remain excitable [ 88 , 89 ]. Interestingly, cholinergically induced suppression of action potential amplitude was shown to be predictable based on the maximal rate of action potential upstroke (dV/dt). Vinogradova and colleagues showed in the rabbit SAN that the probability of amplitude suppression was the highest among pacemaker cells (dV/dt, < 3 V/s), in which acetylcholine suppressed amplitude in 93% of cells, and vagal stimulation did so in 81% of cells. With increasing upstroke velocity, the probability of amplitude suppression is significantly decreased and inexcitability did not occur in cells whose dV/dt was >15 V/s. The observed effects were completely reversible and were abolished by atropine, a muscarinic receptor antagonist. These results further support the presence of I Na in the peripheral SAN clusters. Indeed, Lipsius and Vassalle [ 90 ] and Kreitner [ 39 ] observed that in the intact SAN of the guinea-pig and rabbit, tetrodotoxin (a selective I Na blocker) reduced the upstroke velocity in the periphery but not in the center. The presence of I Na may facilitate spontaneous activity in peripheral SAN cells which primarily rely on Ca 2+ clock spontaneous local releases from the sarcoplasmic reticulum.
A higher contribution of the Ca 2+ clock into pacemaking in the periphery of the SAN was supported by immunocytochemical labeling of Ca 2+ handling proteins, including Ca v 1.2 isoform of the L-type Ca 2+ channel as well as NCX1, RyR2, and SERCA2 proteins. Musa and colleagues showed that, in all cases, there was significantly denser labeling of cells from the periphery of the SAN than of cells from the center [ 70 ]. The authors demonstrated that a difference in cell size exists between the center and periphery of the SAN, where large cells (C m > 30 pF) tend to be from the periphery and small cells (C m < 30 pF) tend to be from the center of the SAN [ 70 ]. In contrast, in the rabbit SAN study by Lyashkov et al., the density of Ca 2+ machinery proteins were found to be independent of cell size. It should be, however, noted that the authors did not differentiate cells from center or periphery SAN area [ 91 ]. Moreover, the studies by Musa et al. and Lyashkov et al. have used different criteria to classify SANC populations. In the study by Musa and colleagues, the authors used two groups of cells. The size of the center SANCs was averagely around 500 µm 2 and the size of the periphery cells was averagely around 800 µm 2 . In contrast, Lyashkov et al. separated cells into three groups based on their size: 156–262 µm 2 , 324–779 µm 2 and 806–1187 µm 2 . Thus, the difference between center (~500 µm 2 ) and periphery (~800 µm 2 ) SAN cells found by Musa et al. might be hidden in the middle group (324–779 µm 2 ) of Lyashkov et al. study. The conclusions from two separate studies may not be opposite, but rather the data analysis and interpretations are different.
Intranodal heterogeneity in the expression of repolarizing potassium currents could be also appreciated from the distribution of action potential duration within the SAN. The changes in the action potential are complex and occur in two dimensions. The action potential duration is greatest at or near the leading pacemaker site and it decreases in all directions as demonstrated by Boyett et al. in the rabbit SAN [ 71 ]. Similar action potential duration distribution patterns have been shown in the canine SAN as well [ 50 ]. Such heterogeneity could be associated with a cluster-specific expression of transient outward K + current I to and delayed rectifier K + currents I K,r and I K,s . Boyett et al. showed that inhibition of I to by 4-aminopyridine increased the duration of the action potential and the increase was greater in peripheral tissue than in central tissue [ 71 ], indicating a greater impact of I to in the SAN periphery. The authors also demonstrated a higher impact of 4-aminopyridine-sensitive currents in the inferior region of the SAN than the superior region. In contrast, an opposite pattern was observed for I K,r . Kodama et al. examined the effect of I K,r antagonist E-4031 in the superior and inferior direction within the rabbit SAN and found that the center and inferior SAN clusters were more sensitive to I K,r inhibition than the superior cluster [ 72 ]. As a result, E-4031 suppressed the pacemaker activity in the center SAN and shifted the leading pacemaker superiorly ( Figure 3 ).
4.4. Autonomic Innervation
The rich innervation of the SAN is well documented [ 92 ]. Anderson showed that the SAN is highly supplied with plexuses of both acetylcholinesterase- and catecholamine-containing nerves [ 93 ]. SAN autonomic control is achieved by a complex interplay between the extra-cardiac neural circuits that interface with specific ganglionated plexus found in epicardial fat pad within the intercaval region at the dorsal aspect of the right atrium [ 94 , 95 ]. As discussed in Section 4.3 above, autonomic stimulation of the SAN is associated with a pacemaker shift within or outside of the SAN. This was attributed to the varying sensitivity of the SAN pacemaker clusters to autonomic stimulation, both sympathetic and parasympathetic. Different sensitivity of the SAN pacemaker clusters to autonomic stimulation, in principle, could be linked to specific ion channel repertoire in distinct clusters, heterogeneous autonomic innervation, or both.
It is believed that a negative chronotropic effect of acetylcholine, released from parasympathetic vagal nerves, on the SAN is mediated by activation of acetylcholine-activated inwardly-rectifying potassium current I K,ACh , hyperpolarizing shift in the I f activation curve, and inhibition of I Ca,L . While I K,ACh activation is achieved through a direct interaction of M 2 muscarinic receptors with GIRK4 channels, I f and I Ca,L currents are regulated by changes in cAMP concentration induced via acetylcholine-dependent inhibition of adenylyl cyclase activity. In contrast, a positive chronotropic effect of noradrenalin, released from the sympathetic nerves, on the SAN purely depends on changes in cAMP concentration, associated with β-adrenergic receptor dependent stimulation of adenylyl cyclase, and a subsequent depolarizing shift in the I f activation curve and a potentiation of I Ca,L and I K activities. In addition, studies indicate that cAMP-mediated PKA-dependent modulation of the Ca 2+ component of the SAN pacemaker system can significantly contribute to autonomic modulation of heart rate [ 96 ]. Therefore, different sensitivity of the SAN pacemaker clusters to both parasympathetic and sympathetic stimulations, that was observed experimentally and clinically, could be associated with heterogeneity in the expression levels of both voltage (including I f , I Ca,L , and I K ) and Ca 2+ clock proteins as discussed above. It, however, could be also linked to heterogeneous autonomic innervation of the SAN.
Indeed, different densities of muscarinic and adrenergic receptors as well as different innervation has been proposed in the SAN [ 97 , 98 ]. Beau et al. [ 98 ] applied quantitative light-microscopic autoradiography of radioligand binding sites to characterize the spatial distribution of muscarinic cholinergic and β-adrenergic receptor subtypes in the canine SAN. The authors have found that the region of dominant pacemaker activity, localized with in vitro electrical mapping, consistently exhibited greater densities of muscarinic and β 1 -adrenergic receptors than other SAN regions. Muscarinic receptor density in the dominant pacemaker region was 18 ± 2% and 29 ± 7% higher than in adjacent superior and inferior SAN clusters, respectively. Density of β 1 -adrenergic receptors in the dominant site was 53 ± 5% and 26 ± 4% higher than in adjacent superior and inferior SAN regions, respectively. Later study by Kurogouchi et al. in dogs [ 99 ] confirmed a higher density of muscarinic receptors in the central SAN pacemaker cluster compared with superior and inferior clusters, but did not find any difference for β-adrenergic receptor subtypes. In contrast, in the rat SAN, Sutyagin and colleagues [ 100 ] have shown that the relative density of binding sites for β-adrenergic and muscarinic cholinergic receptors was minimum in the central SAN cluster and asymmetrically increased to maximum values to cranial (sharply) and caudal (smoothly) directions.
These data indicate that pacemaker shift can result from a complex interaction between both intrinsic properties of SAN clusters (including expression levels of voltage and Ca 2+ clock proteins as well as muscarinic and adrenergic receptors) and heterogeneous innervation of intranodal clusters. Importantly, these changes can be species-dependent and rely on the dynamic balance (i.e., “accentuated antagonism” [ 101 , 102 ]) between the sympathetic and parasympathetic branches of the autonomic nervous system.
Overall, these findings demonstrate substantial heterogeneity of cells within the SAN that could be associated with distinct functional intranodal pacemaker clusters. Importantly, these clusters are characterized by different ion channel and Ca 2+ cycling protein compositions and, likely, varying contribution of voltage and Ca 2+ clock components of the coupled-clock system into their pacemaker mechanisms. The latter may be critical in pathological conditions associated with mutations/impairment of certain pacemaker proteins which would affect SAN pacemaking in a cluster-specific manner and thus be manifested differently and upon specific circumstances as discussed in the next section.
5. Hierarchical Pacemaker Clusters in Sinus Node Dysfunction
Pacemaker clusters have diverse functional and molecular signatures and thereby can be activated during physiological stimulations and hierarchically take turns to dominate the heart beating with rhythm of exact rates that meets body needs. To generate rhythm at different rates, pacemaker clusters may have distinct pacemaker mechanisms that probably involve a dynamic balance between the components of the coupled clock system. As mentioned earlier, this system integrates molecular cues on the cell membrane surface (i.e., voltage clock) and the intracellular Ca 2+ machinery proteins (Ca 2+ clock) to synergistically depolarize membrane potential during diastole and eventually boost it to the threshold for I Ca,L activation and initiate spontaneous action potentials.
The diverse molecular foundation of each pacemaker cluster may suggest unequal contribution of pacemaker components from the coupled clock system in the pacemaking, or, some clusters may depend more on membrane clock in its pacemaking, whereas pacemaking in other clusters may be mediated more by the rhythmic Ca 2+ oscillations. The remodeling of certain pacemaking components then is anticipated to disrupt pacemaker clusters to different extents leading to SAN dysfunctions. SAN dysfunction is a major public health problem that refers to abnormalities in SAN impulse formation and propagation, which has been associated with a series of gene mutations [ 69 ]. Since the contribution of each pacemaking component varies between the intranodal clusters, genetic mutations will affect the clusters differently depending on the encoded mutant gene. Therefore, SAN dysfunction could occur only when the affected cluster is supposed to be activated. For example, in patients with catecholaminergic polymorphic ventricular tachycardia (CPVT), a genetic disorder associated with mutations in Ca 2+ handling proteins, 5–20% are documented with SAN dysfunction [ 103 ]. Though the majority of CPVT patients show normal SAN function at rest, researches have shown that the effect of sympathetic and parasympathetic stimulations on heart rate regulation are dramatically attenuated [ 49 , 104 ]. It is likely that the pacemaker clusters that dominate at sympathetic and parasympathetic tone are mediated majorly by the Ca 2+ clock which is disrupted in CPVT patients by mutations in Ca 2+ handling proteins.
It has been known for over a century that pacemaker cells are widely distributed throughout the entire intercaval region located between the superior and inferior vena cava and between the crista terminalis and intra-atrial septum [ 105 , 106 , 107 ]. Canine and human studies [ 61 , 108 , 109 , 110 , 111 , 112 ] in which potentials have been recorded from multiple electrodes simultaneously have revealed an extensive distributed system of atrial pacemakers (the atrial pacemaker complex), which includes but extends well beyond an anatomically defined SAN. In some circumstances, when the SAN is sick (i.e., sinus node dysfunction or sick sinus syndrome) or temporally suppressed (for instance, during extensive parasympathetic stimulation), automaticity could be observed from other clusters of subsidiary atrial pacemakers located within the distributed atrial pacemaker complex [ 49 , 113 ]. These cells have been characterized to express both pacemaker marker HCN4 and non-pacemaker marker Cx43 [ 20 , 113 , 114 ]. Though these subsidiary pacemaker clusters can provide a relatively regular rhythm, they are characterized by a slower resting heart, slower exertional heart rates, a prolonged post-pacing recovery time (a parameter similar to SAN recovery time but for non-SAN pacemakers), and an increased beat-to-beat heart rate variability [ 48 , 49 , 114 , 115 , 116 ]. Furthermore, the electrical activity of this subsidiary pacemakers is more akin to that of the SAN than to the surrounding atrial muscle; the subsidiary pacemaker action potential exhibits prominent diastolic depolarization and a significantly lower maximum diastolic potential, take-off potential, overshoot, rate of rise, and amplitude than typical atrial muscle [ 117 ]. Finally, while being bradycardic in general, subsidiary atrial pacemakers can contribute to the development of atrial tachycardia [ 118 , 119 , 120 ].
6. Conclusions
Here, we summarize the experimental evidence supporting the presence of hierarchical pacemaker clustering within the SAN. The studies discussed highlight the importance of these intranodal pacemaker clusters in a complex and dynamic regulation of the heart rhythm in response to various interventions. Importantly, we show that SAN clusters may differentially rely on the components of the coupled clock pacemaker system which, in turn, determines their sensitivity to both physiological and pathophysiological perturbations as well as genetic mutations. The latter may critically contribute to the development of the sick sinus syndrome and its manifestation under certain conditions that affect certain pacemaker clusters. These findings further highlight a complex nature of the SAN pacemaker complex and can introduce novel targets that could be used for the treatment of various SAN pathologies.
Acknowledgments
We thank Daniel Turner (University of Wisconsin-Madison) for critical reading, editing and valuable comments on the manuscript.
Author Contributions
D.L. and A.V.G. wrote and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
This work was supported by NIH/NHLBI 1R01HL141214-01, American Heart Association Scientist Development Award 16SDG29120011, and the Wisconsin Partnership Program 4140 grant (A.V.G.). American Heart Association Fellowship 17POST33370089 (to D.L.).
Conflicts of Interest
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- Search Menu
- Advance Articles
- Editor's Choice
- Supplements
- Europace Image Library
- ESC Journals App
- Review Series
- ESC Content Collections
- Author Guidelines
- Submission Site
- Why publish with Europace?
- Open Access Options
- Read & Publish
- Author Resources
- Self-Archiving Policy
- Advertising and Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- Journals Career Network
- About Europace
- Editorial Board
- About the European Heart Rhythm Association
- About the European Society of Cardiology
- ESC Publications
- Developing Countries Initiative
- Dispatch Dates
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Introduction, limitations.
- < Previous
Does atrial pacing lead to atrial fibrillation in patients with sick sinus syndrome? Insights from the DANPACE trial
Participants in The Danish Multicenter Randomized Trial on Single Lead Atrial Pacing vs. Dual Chamber Pacing in Sick Sinus Syndrome (DANPACE) are listed in the Appendix.
- Article contents
- Figures & tables
- Supplementary Data
Søren Hjortshøj, Sam Riahi, Jens Cosedis Nielsen, Flemming Skjøth, Søren Lundbye-Christensen, Henning R. Andersen, on behalf of the DANPACE Investigators, Does atrial pacing lead to atrial fibrillation in patients with sick sinus syndrome? Insights from the DANPACE trial, EP Europace , Volume 16, Issue 2, February 2014, Pages 241–245, https://doi.org/10.1093/europace/eut306
- Permissions Icon Permissions
Paroxysmal atrial fibrillation (AF) is common in patients with sick-sinus syndrome (SSS) and pacemakers leading to morbidity and an increased risk of stroke or death. Previous studies indicate that atrial pacing may precipitate AF. We investigated the relation between atrial pacing and the occurrence of AF during long-term follow-up among patients with SSS, no prior AF, and dual-chamber pacemakers (DDDRs).
We analysed data from 396 patients who received DDDR pacemakers in the DANPACE trial. The percentage of atrial pacing (%AP) was compared with the number of mode-switch (MS) episodes collected by the pacemaker at each follow-up as an indicator of AF. Mean follow-up was 4.2 ± 2.4 years. The mean proportion of atrial and ventricular pacing was 59 ± 31 and 65 ± 33%, respectively. Approximately 72% developed AF as indicated by MS episodes at some point during follow-up. Unadjusted regression analysis indicated a relation between %AP and AF ( P = 0.04), but after adjustment for possible confounders (sex, age, hypertension, diabetes, myocardial infarction, PQ interval, and left atrial diameter) there was no significant relationship ( P = 0.37).
Atrial fibrillation is very common among patients with SSS. No association between %AP and development of AF was found in patients with SSS. Future trials may randomize patients to different levels of AP exposure.
Atrial fibrillation is very common among patients with sick-sinus syndrome.
No association between increasing atrial pacing and development of atrial fibrillation was found.
Future randomized trials should expose patients to different degrees of atrial pacing to investigate any causal relationship between atrial pacing and atrial fibrillation.
Paroxysmal atrial fibrillation (AF) is common in patients with sick-sinus syndrome (SSS) and pacemakers leading to morbidity and an increased risk of stroke or death. 1 Patients with SSS and bradycardia can be treated by single lead atrial (AAIR) or dual-chamber pacemakers (DDDR) 2–4 and require various degrees of atrial pacing to maintain adequate functional capacity. Although both AAIR and DDDR pacing preserve the normal sequential activation of the atria and ventricles, small studies as well as a meta-analysis have previously indicated that atrial pacing may precipitate AF. 5 , 6
The mode-switch (MS) feature in DDDRs detects atrial tachyarrhythmias and is a sensitive and specific indicator of AF. 7 , 8 In the present study, we examined patients with no previous history of AF who received DDDR pacemakers in the DANPACE trial, 4 with the aim of determining the effect of atrial pacing on the occurrence of AF among patients with SSS.
Study design
The DANPACE trial has been described previously. 4 The current study included a cohort of all patients who during the DANPACE trial were randomly assigned to DDDR, and treated with DDDR pacing due to SSS and bradycardia. Furthermore, all patients who were randomized to AAIR pacing, and who received a DDDR pacemaker at the time of first pacemaker implantation (typically due to a low Wenckebach point) ( N = 46), were included in the analysis. Patients who on randomization had electrocardiogram documented AF (tachycardia–bradycardia) or had a previous history of paroxysmal AF were excluded from the analysis, as were patients who died before their first follow-up visit.
The criteria for inclusion in the DANPACE trial were: symptomatic bradycardia; documented sino-atrial block or sinus arrest with pauses >2 s or sinus bradycardia <40 b.p.m. for >1 min while awake; PR interval ≤0.22 s if aged 18–70 years or PR interval ≤0.26 s if aged ≥70 years; and QRS width ≤0.12 s.
The main exclusion criteria were: atrioventricular block; bundle branch block; long-standing persistent AF (>12 months); AF with ventricular rate <40 b.p.m. for ≥1 min or pauses >3 s; a positive test for carotid sinus hypersensitivity.
Enrolment began in March 1999 and was terminated in June 2008. Patients were followed until September 2009.
The trial was conducted in accordance with the Helsinki Declaration and approved by the regional Ethics Committee and the Danish Data Protection Agency. All patients gave written informed consent before inclusion.
Implantation and programming of pacemakers
Bipolar leads were implanted in the right atrium and ventricle. Contemporary DDDR pacemakers from Boston Scientific, Medtronic, and St Jude Medical were used. The rate adaptive function was activated in all pacemakers and programmed with a lower rate of 60 b.p.m. and an upper rate of 130 b.p.m. In patients with DDDR pacemakers, the paced atrioventricular interval was programmed to 140–220 ms according to a pre-specified algorithm. 4 The maximum tracking rate was individualized and the MS function was activated. Mode switch occurred, when the atrial rate exceeded 170–180 b.p.m. for a given number of beats or period of time according to the default settings of the manufacturer of the pacemaker. Atrial sensitivity was programmed to 0.5 mV.
Patient follow-up
Follow-up took place after 3 months and again every year after implantation up to 10 years. Mean follow-up was 5.4 ± 2.6 years. At each planned follow-up visit, a printout of the pacemaker memory data accumulated since the previous resetting of the memory was obtained. The percentage of atrial pacing (%AP) at each follow-up was calculated using the number of paced and the number of sensed beats. The occurrence and duration of MS events were recorded.
Definition of atrial fibrillation
Episodes of MS detected by the pacemaker at yearly follow-up visits were used as a surrogate marker of AF. The total duration of MS episodes were rounded to the nearest number of whole hours. Thus, a total MS duration of 30 min was recorded as 0 h, whereas 31 min were recorded as 1 h. Analyses for MS durations of 6 and 24 h as the criterion for AF were also carried out.
Statistical analysis
A crude analysis is reported for the occurrence of MS since last interrogation/follow-up.
The potentially non-linear relationship between development of %AP and AF was analysed by fitting splines 9 in a logistic regression with %AP being a time-varying covariate. Serial correlation was accounted for by using a population averaged generalized estimation equation. 10 Adjustments for pre-specified confounders known to be associated with AF were performed [sex, age, hypertension, diabetes, previous myocardial infarction (MI), PQ interval, and left atrial diameter].
Statistical tests were two-tailed, and P < 0.05 was considered significant. Stata version 11.2 (StataCorp. 2009. Stata: Release 11. Statistical Software. Stata Corp. LP) was used.
A total of 746 patients received DDDR pacemakers in the trial and were eligible for evaluation at 3 months follow-up. Twenty-five patients were excluded from the analysis due to crossover to other programming modes, e.g. VVI mode at the time of first follow-up. A further 325 patients had a previous history of AF (tachycardia–bradycardia) and were also excluded. Therefore, the data analysis is based on a total of 2058 follow-up visits in 396 patients spanning over a period of 4.2 ± 2.4 years. Table 1 displays the baseline characteristics of our patient cohort with DDDR pacemakers without a previous history of AF. The mean proportion of atrial and ventricular pacing was 59 ± 31 and 65 ± 33%, respectively.
Baseline characteristics of study cohort ( N = 396) with DDDR without known AF at study inclusion
Atrial pacing and atrial fibrillation
Figure 1 displays a crude analysis of the occurrence of one or more MS episode at different follow-up visits collected since the latest earlier follow-up interrogation. Approximately 72% developed AF as indicated by at least one MS episode (≥31 min) during follow-up. During the first year after implantation, the proportion of patients with MS episodes increased from ∼50% but levelled off during the remaining observation period at 60–70%. The figure displays large standard deviations at 8–10 years of follow-up reflecting a small number of participating patients at these time points.
Mean proportion of patients with mode-switch (MS) episodes at different follow-up visits with 1 SD. Number of patients at each follow-up below figure.
To describe a possible effect of atrial pacing, splines were fitted with MS at individual follow-ups and %AP being a time-varying covariate. Figure 2 displays a crude analysis indicating the association between the probability of observing MS (≥31 min) since last follow-up in relation to the proportion of time in atrial pacing (%AP). The curve shape is essentially the same at all time points ( P = 0.93) and exhibits a clear relationship with %AP ( P = 0.04). For most curves, there is a two-phased course with a tendency to a decrease in the proportion of MS episodes with higher %AP, although a peak in MS is seen with ∼70% AP.
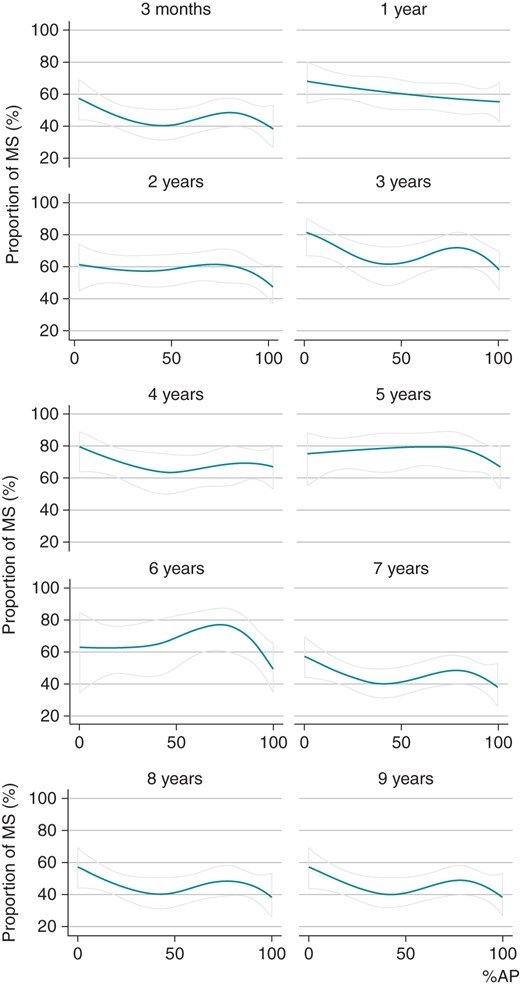
Spline analysis with indication of the association between the probability of observing MS since last follow-up in relation to the proportion of time in atrial pacing (%AP). The curve shape is the same at all follow-up visits ( P = 0.93).
However, when adjusting for possible confounders (sex, age, hypertension, diabetes, MI, PQ interval, and left atrial diameter), the curve shape remains the same at all time points ( P = 0.20), but there is no significant association with %AP ( P = 0.37).
In these analyses, the criterion for AF detection was defined by MS ≥ 1 h (rounded to 1 h if ≥31 min). Further analyses with MS durations of 6 and 24 h as the criterion for AF were also carried out, but the above-mentioned curves remained the same for both crude and adjusted analysis and with essentially similar P values.
A simulation study was designed to evaluate the statistical power of our analysis. Based on the final crude analysis, a number of datasets of binary MS indicators were simulated with the same population characteristics as the present data, but with varying effect sizes. The effect size, b , is the ratio between the highest and the lowest proportion and varied relative to the present study from b = 0.0, 0.1, 0.2,…, 2.0. Hence, the effect size in the present study corresponds to b = 1, no effect to b = 0, and twice the effect to b = 2 etc.
For each effect size, 1000 datasets were simulated. Each set of data were analysed using the same model as in the crude analysis. The statistical power is defined as the proportion of datasets with significant test results.
The observed power in the current analysis ( b = 1) was 90.4%, and a power of 80% was reached when the simulated effect size was approximately b = 0.9 corresponding to a slightly flattened curve shape.
In this study, we found no significant association between increasing %AP and subsequent occurrence of AF in patients with SSS, although unadjusted results suggested an inversely proportional relationship.
Previously, Adelstein and Saba 5 found that in patients with heart failure undergoing cardiac resynchronization therapy (CRT), increasing %AP was a significant predictor of AF development, and in a recent meta-analysis, Elkayam et al . 6 showed that atrial pacing was a predisposing factor for development of AF. The reason for the apparent association between atrial pacing and AF is not clear and may be multifactorial. First, patients needing atrial pacing may have more diseased atria in which AF is more likely to develop, e.g. in heart failure patients. Secondly, atrial pacing may itself cause AF by different mechanisms: pacing the right atrium may induce atrial dyssynchrony and subsequent AF. 11 Further, right atrial pacing may lead to prolonged atrioventricular conduction times, which in turn can promote AF. 11–13 Also, increases in conduction times can lead to more ventricular pacing, which, in some studies, has been established to promote AF in patients with SSS. 13–15 Pacing from the low interatrial septum has been suggested to be superior to right atrial appendage pacing in terms of AF prevention in patients with SSS, but the subject remains controversial. 16
A relatively low number of patients in the DANPACE cohort had pre-existing cardiovascular disease, e.g. ischaemic heart disease and hypertension, and only a modest number of patients developed heart failure during the trial indicating the relatively benign course of SSS apart from the development of AF. 17 Thus, patients with SSS may respond differently or not at all to atrial pacing compared with other patient groups, e.g. CRT patients.
While atrial pacing by some researchers is considered potentially pro-arrhythmogenic, others have focused on atrial overdrive pacing strategies for the prevention of AF. Results from previous trials with overdrive pacing have not shown a convincing benefit in terms of AF prevention, and these trials included only modest sample sizes. 18–20 Recently, the ASSERT trial with 2580 patients was not able to demonstrate any benefit of continuous atrial overdrive pacing. 21
In the current study, we also show that a very high number of patients (∼72%) developed AF during follow-up. Notably, during the first year after implantation the proportion of patients with MS episodes since last follow-up was >50%, underscoring the importance of regular follow-up procedures of SSS patients to detect patients with AF requiring anticoagulation treatment.
A recent study by Svendsen et al . 22 has shown that the rate of stroke was similar in the single-chamber and dual-chamber pacemaker arms in the DANPACE trial. For the whole DANPACE trial, the authors did not find an association between AF and stroke, and they speculate that patients with AF (due to taking part in a randomized trial) were adequately anticoagulated and therefore less likely to develop stroke. Interestingly, the authors also report a higher ‘MS burden’ among patients with DDDRs who developed stroke during follow-up, thus supporting an association between AF and stroke after all.
Episodes of MS were used as a surrogate marker of AF, and sensitivities and specificities of the used MS algorithms are thus crucial. Pacemakers from five different manufacturers were used in the DANPACE trial, thus introducing different MS algorithms. However, the manufacturer of individual pacemakers was not recorded in DANPACE, and it is thus not possible to discriminate between the various algorithms. However, it is widely accepted that today's MS algorithms display exhibit a very high degree of sensitivity and specificity for the reliable detection of AF. 7 , 8 , 23 The occurrence of AF may sometimes lead to undersensing in the atrium and thus inappropriate atrial pacing. However, given the efficiency of modern MS algorithms, we consider it unlikely that this would not result in MS episodes which would be detected at the next scheduled follow-up. Although patients with known AF prior to pacemaker implantation were excluded from the study cohort, some patients may have had episodes of undiagnosed AF. It may be argued that by using MS durations of 1 h (rounded to 1 h if ≥31 min) as the criterion for AF, we may detect clinically irrelevant episodes of atrial tachycardia and possibly far-field R-wave oversensing, thus overestimating the true incidence of AF. Today, it is not possible to make adjudications of electrogram tracings from the pacemaker memory to exclude significant overdiagnosis of AF. However, we performed the same analyses with MS durations of 6 and 24 h as the criterion for AF leading to similar results.
The use of beta-blockers may inhibit the sinus node and thus cause an increase in %AP. It was, however, impossible to satisfactorily adjust for beta-blocker use because of the heterogeneity of drugs and doses used and their wide variation in patient effects. It is possible that various atrial lead positions, e.g. septal vs. lateral/appendage, may determine propagation of pacing impulses in the atria thus contributing differently to the development of AF. In the present study, we did not have information on the exact position of the atrial lead, yet most leads were implanted in the right atrial appendage or on the lateral wall of the right atrium as is practice in most clinics today.
The present study did not find an association between increasing atrial pacing and development of AF. Whether any causal relationship exists between atrial pacing and AF should be the focus of future randomized trials, exposing patients to different degrees of atrial pacing.
Conflict of interest: J.C.N. received speakers fees from Biotronik and research grant for the MANTRA-PAF trial from Biosense-Webster. All the other authors declare no conflicts of interest.
The DANPACE trial was funded by unrestricted grants from Medtronic, St Jude Medical, Boston Scientific, Ela Medical, Pfizer, and the Danish Heart Foundation (10-04-R78-A2954-22779).
Google Scholar
Google Preview
Author notes
- artificial cardiac pacemaker
- atrial fibrillation
- myocardial infarction
- hypertension
- diabetes mellitus
- left atrium
- sick sinus syndrome
- diabetes mellitus, type 2
- ventricular pacing
- atrial-based pacing
Supplementary data
Email alerts, more on this topic, related articles in pubmed, citing articles via.
- Recommend to Your Librarian
Affiliations
- Online ISSN 1532-2092
- Print ISSN 1099-5129
- Copyright © 2024 European Society of Cardiology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

- Heart Failure
- Cardiovascular Clinical Consult
- Adult Immunization
- Hepatic Disease
- Rare Disorders
- Pediatric Immunization
- Implementing The Topcon Ocular Telehealth Platform
- Weight Management
- Men's Health
- Women's Health
- Substance Use
- Kidney Disease
- Complimentary & Alternative Medicine
- Dermatology
- Endocrinology
- Oral Medicine
- Otorhinolaryngologic Diseases
- Gastrointestinal Disorders
- Musculoskeletal Disorders
- Rheumatology
- Pulmonology
Atrial Fibrillation and Sick Sinus Syndrome: A Clinical Catch-22
Concurrence of AF and SSS is common in older patients and presents a treatment conundrum. Can you balance safety and efficacy in this patient?
Both atrial fibrillation and sick sinus syndrome (a subset of which is also known as “tachy-brady” syndrome) are more common in elderly patients. The problem comes when they co-exist and as a result, make management of each disease more challenging. Let’s illustrate with a case.
Case. Mrs. Robertson is an 85-year-old patient with a history of long-standing paroxysmal atrial fibrillation (AF). She has been taking dabigatran and metoprolol 50 mg twice daily. She also takes lisinopril 10 mg and metformin 500 mg BID. Recently, she has started complaining of episodes of palpitations with feelings of heart racing followed by episodes of lightheadedness. The episodes occur 1-2 times a day and usually last for a few minutes. You are concerned about whether these represent poorly rate-controlled AF.
1. What type of monitoring do you recommend?
A. Weekly ECG
B. 48h Holter monitor
C. 7d event monitor
D. Implantable loop recorder
Answer, discussion, next question>>>
Answer: B. 48h Holter monitoring
Weekly ECG monitoring in a patient with chronic AF is likely to provide only a “snapshot” and may not capture the acute episode. Although Mrs. Robertson needs a baseline ECG, weekly monitoring is not indicated. A 48h Holter monitor will not only capture the episodes but will also provide histograms of heart rate trends as well as average heart rate so that overall rate control can be assessed. The 48h monitoring will also autocapture any tachy- or bradyarrhythmias.
A 7d event monitor (prolonged monitoring) would be indicated if the episodes were less frequent than 72 hours (longest duration of a Holter recording). An event monitor only autocaptures notable arrhythmias or patient-triggered events and does not provide the more comprehensive information about AF rate control recoreded by the Holter monitor. Finally, extended monitoring with an implantable loop recorder is only indicated when an occult arrhythmia is suspected, such as in cryptogenic stroke or unexplained recurrent syncope.
Case. You order a 48h holter monitor and it returns, showing multiple bursts of AF with rapid ventricular response (RVR) followed by episodes of conversion back to sinus rhythm with post-conversion pauses (longest 3.2 seconds). There are also some AF episodes with slow ventricular response (heart rate in the 40s) and pauses in AF of as long as 5.1 seconds.
2. What is the underlying pathophysiology?
A. Sinus node dysfunction
B. AV node dysfunction
C. His-Purkinje disease
D. Iatrogenic from medications
Answer, discussion, next question> >>
Answer: A. Sinus node dysfunction
Tachy-brady syndrome is a variant of sick sinus syndrome (SSS), where there is intrinsic sinus node dysfunction that leads to alternating slow and fast heart rates. In this case, the rapid discharge of AF can result in sinus node “fatigue” and exacerbation of bradyarrhythmias or post-conversion (to sinus rhythm) pauses. Risk factors for SSS are similar to those for AF (age, CAD, HTN, DM, valvular disease) and therefore, the two often coexist. Underlying pathology often involves microfibrosis of the SA node. SSS can also be aggravated by many of the medications used to manage AF (rate control agents such as metoprolol, diltiazem, digoxin).
Case. You stop Mrs. Robertson’s metoprolol in the hopes that it will improve her symptoms. However, her episodes are now occurring more frequently with more episodes of palpitations.
3. What is the next best step in management?
A. Restart metoprolol
B . Start a calcium channel blocker
C. Start amiodarone
D. Refer to electrophysiology for a pacemaker
E. None of the above
Answer, discussion>>>
Answer: D. Refer to electrophysiology for a pacemaker
The patient has multiple indications for a pacemaker. First, she has symptoms of lightheadedness with her pauses (Class I). Second, the nodal agents she is taking to manage AF with RVR are contraindicated in patients with bradyarrhythmias and pauses (Class II). Additional indications include sinus pause/arrest of >3 sec and pause of >5 sec while in AF. Restarting nodal agents (metoprolol or diltiazem) or choosing an antiarrhythmic would help the RVR but could worsen the sinus node dysfunction and pauses.
Often, the concurrence of AF and SSS in elderly patients is a contraindication for traditional therapies such as beta-blockers and calcium channel blockers. In these instances, the goal of therapy is to establish the diagnosis with monitoring and pursue a pacemaker only if there are prolonged pauses, symptoms of bradycardia, or if rate control agents are needed.
Continue to Part II of this case where you will identify the appropriate pacemaker for Mrs. Robertson and continue to manager her progress.
Please leave any comments you may have on Part I, below.
Individuals with CVD Consume More Daily Sodium than Recommended, According to New Data
ACC.24: Adults with a history of CVD consumed more than double the recommended daily sodium amount, reported researchers.

Navigating Cardiovascular Complications of Obesity: Expert Insights for Primary Care
Listen to our latest podcast episode for details on top CVD risk factors to screen patients with obesity for, medications to help prevent CVD, and more.

Bempedoic Acid Reduces CV Risk in Latinx Population with Statin-Intolerance: Daily Dose
Your daily dose of the clinical news you may have missed.

Primary Viewpoints Episode 4: Lp(a): The Next CVD Target?
Patrick Moriarty, MD, from the University of Kansas Medical Center, discusses the role of Lp(a) as a risk factor for cardiovascular disease and the phase 3 trials of a potential treatment.

Icosapent Ethyl Reduces MACE Across Range of Baseline Lp(a): REDUCE-IT Analysis
ACC.24: Icosapent ethyl was associated with reduced rate of MACE at all baseline levels of Lp(a) in participants with elevated TG and at high risk for CVD.
Remission from Type 2 Diabetes via Lifestyle Intervention Reduces Risk for CVD, CKD
Longer duration of remission was associated with a greater reduction in CKD and CVD risk, according to a post hoc analysis of the Look AHEAD study.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

Patient with sick sinus syndrome and implanted dual-chamber pacemaker with reduced P-wave duration following low interatrial septal pacing: Case report
Affiliations.
- 1 Department of Emergency Medicine, Wroclaw Medical University, Wroclaw, Poland.
- 2 Klodzko County Hospital, Klodzko, Poland.
- 3 Department of Anesthesia, Critical Care and Emergency Medicine, Collegium Medicum of University in Zielona Góra, Zielona Góra, Poland.
- 4 Department of Internal and Occupational Diseases, Hypertension and Clinical Oncology, Wroclaw Medical University, Wroclaw, Poland.
- 5 Department of Emergency Medical Service, Wroclaw Medical University, Wroclaw, Poland.
- PMID: 34477142
- PMCID: PMC8415954
- DOI: 10.1097/MD.0000000000027076
Introduction: A dual-chamber pacemaker (DDD/R) for a sinus node disease is sometimes referred to as a physiological pacemaker as it maintains atrioventricular synchrony, however several clinical trials have proved its inferiority to a nonphysiological single-chamber ventricular back-up pacing.
Patient concerns: A subject of the study is a 74-year-old woman with a sick sinus syndrome (SSS) and a previously implanted physiological DDD/R pacemaker. The SSS was diagnosed because of patient's very slow sinus rhythm of about 36 bpm, and due to several episodes of dizziness. After the DDD/R implantation the percentage of atrial pacing approached 100%, with almost none ventricular pacing.
Diagnoses: Sick sinus syndrome, complete Bachmann's bundle block, atrial fibrillation, atrial flutter.
Interventions: The patient was previously implanted with a physiological DDD/R pacemaker. Several years after the implantation, the atrial fibrillation was diagnosed and the pulmonary vein isolation was then performed by cryoablation. During the follow-up after pulmonary vein isolation, the improvement of mitral filling parameters was assessed using echocardiography. Shortly thereafter the patient developed the persistent paroxysm of a typical atrial flutter which was successfully terminated using a radiofrequency ablation. No recurrence thereof has been observed ever since (24 months).
Outcomes: The atrial electrode of the pacing system was implanted within the low interatrial septal region that resulted in a reduced P-wave duration compared to native sinus rhythm P-waves. The said morphology was deformed because of the complete Bachmann bundle block. That approach, despite a nonphysiological direction of an atrial activation, yielded relatively short P-waves (paced P-wave: 179 ms vs intrinsic sinus P-wave: 237 ms). It also contributed to a significantly shorter PR interval (paced PR: 204 ms vs sinus rhythm PR: 254 ms).
Conclusions: The authors took into consideration different aspects of alternative right atrial pacing sites. This report has shown that in some patients with a sinus node disease, low interatrial septal pacing can reduce the P-wave duration but does not prevent from the development of atrial arrhythmias.
Copyright © 2021 the Author(s). Published by Wolters Kluwer Health, Inc.
Publication types
- Case Reports
- Arrhythmias, Cardiac / etiology*
- Arrhythmias, Cardiac / therapy
- Cardiac Pacing, Artificial / adverse effects*
- Cardiac Pacing, Artificial / methods
- Cardiac Pacing, Artificial / statistics & numerical data
- Cardiac Resynchronization Therapy Devices / standards*
- Cardiac Resynchronization Therapy Devices / statistics & numerical data
- Sick Sinus Syndrome / physiopathology
- Sick Sinus Syndrome / therapy*

IMAGES
VIDEO
COMMENTS
Sick sinus syndrome, also known as sinus node dysfunction (SND), is a disorder of the sinoatrial (SA) node caused by impaired pacemaker function and impulse transmission producing a constellation of abnormal rhythms. These include atrial bradyarrhythmias, atrial tachyarrhythmias and, sometimes, bradycardia alternating with tachycardia often referred to as "tachy-brady syndrome." These ...
This rhythm and multifocal atrial tachycardia are similar except for heart rate. The other possible explanation is that there is significant respiratory sinus arrhythmia, with uncovering of latent foci of pacemaker activity. Usually, it is associated with underlying lung disease. In the elderly, it may be a manifestation of sick sinus syndrome.
as sick sinus syndrome, is characterized by ... Permanent pacemaker place - ment with atrial-based pacing is ... Wandering pacemaker: at least three distinct P waves with HR < 50 bpm
Wandering atrial pacemaker (WAP) is an atrial rhythm where the pacemaking activity of the heart originates from different locations within the atria. This is different from normal pacemaking activity, where the sinoatrial node (SA node) is responsible for each heartbeat and keeps a steady rate and rhythm. Causes of wandering atrial pacemaker are unclear, but there may be factors leading to its ...
Sick Sinus Syndrome. Sick sinus syndrome can give you a heartbeat that's too slow, too fast or a combination of both. Your sinoatrial (SA) node, where your heartbeat begins, is to blame. Some people don't have symptoms with sick sinus syndrome, but others need a pacemaker to get a normal heart rhythm. This affects mostly older people.
A wandering atrial pacemaker is a rare form of a condition called arrhythmia. That's a problem with your heartbeat. It can happen anytime, even when you're sleeping. It's usually nothing to ...
With wandering atrial pacemaker, the ECG shows variable P-wave morphology and PR intervals. ... Atrial adaptive rate pacing in sick sinus syndrome: effects on exercise capacity and arrhythmias. Br ...
Most people with sick sinus syndrome eventually need a permanent device to control the heart rhythm (pacemaker). A pacemaker is a small, battery-powered device that's implanted under the skin near the collarbone during a minor surgical procedure. The pacemaker stimulates (paces) the heart as needed to keep it beating regularly.
Symptomatic sinus node dysfunction (SND), also known as sick sinus syndrome, is usually due to age-related degeneration of the sinus node. SND can manifest on the ECG as a variety of ECG abnormalities, including sinus bradycardia, sinus arrest, sino-atrial block, chronotropic incompetence and the tachy- brady syndrome. 1 The most common symptoms of SND include syncope, dizzy spells, fatigue ...
This recommendation was based on the fact that though there was no statistically significant difference in death between the 2 groups, the AAIR group was associated with a higher incidence of atrial fibrillation (28.4% vs 23.0%) and a 2-fold increased risk of pacemaker reoperation during follow-up (22.1% vs 11.9%).
To the Editor: I read with interest the article on the treatment of patients who have sick sinus syndrome (SSS) with single-chamber atrial pacing by Anderson et al. 1 However, their finding of the annual risk of second- or third-degree atrioventricular (AV) block of only 0.6% per year contrasts somewhat with the reported findings that abnormal AV conduction was demonstrated in 57% to 67% of ...
Sick sinus syndrome (SSS) and atrial fibrillation (AF) frequently coexist and show a bidirectional relationship. This systematic review and meta-analysis aimed to decipher the precise relationship between SSS and AF, further exploring and comparing different therapy strategies on the occurrence or progression of AF in patients with SSS.
Sinus node dysfunction may occur at any age 7, 14; however, increasing age is the most significant risk factor with the highest disease prevalence in patients 70 to 89 years of age. 2, 7, 8, 14 ...
A subject of the study is a 74-year-old woman with a sick sinus syndrome (SSS) and a previously implanted physiological DDD/R pacemaker. The SSS was diagnosed because of patient's very slow sinus rhythm of about 36 bpm, and due to several episodes of dizziness. After the DDD/R implantation the percentage of atrial pacing approached 100%, with ...
Sick sinus syndrome (SSS) is a set of diseases with abnormal cardiac pacing, which manifests as diverse cardiac arrhythmias, especially bradycardia. The clinical presentation is inconspicuous in the early stage, but with the progression of this disease, patients may present with symptoms and signs of end-organ hypoperfusion. As a common result in the natural history of the disease, SSS ...
In some circumstances, when the SAN is sick (i.e., sinus node dysfunction or sick sinus syndrome) or temporally suppressed (for instance, during extensive parasympathetic stimulation), automaticity could be observed from other clusters of subsidiary atrial pacemakers located within the distributed atrial pacemaker complex [49,113].
Introduction. Paroxysmal atrial fibrillation (AF) is common in patients with sick-sinus syndrome (SSS) and pacemakers leading to morbidity and an increased risk of stroke or death. 1 Patients with SSS and bradycardia can be treated by single lead atrial (AAIR) or dual-chamber pacemakers (DDDR) 2-4 and require various degrees of atrial pacing to maintain adequate functional capacity.
The wandering atrial pacemaker has nothing to do with extrinsic cardiac hardware. The sino-atrial node is the natural pacemaker of the heart. Remember also that if P waves all appear similar and they're arriving at a rate of 60 - 100 beats per minute we assume them to be sinus.
Aims: In patients with sick sinus syndrome, bradycardia can be treated with a single-lead pacemaker or a dual-chamber pacemaker. Previous trials have revealed that pacing modes preserving atrio-ventricular synchrony are superior to single-lead ventricular pacing, but it remains unclear if there is any difference between single-lead atrial pacing (AAIR) and dual-chamber pacing (DDDR).
Tachy-brady syndrome is a variant of sick sinus syndrome (SSS), where there is intrinsic sinus node dysfunction that leads to alternating slow and fast heart rates. In this case, the rapid discharge of AF can result in sinus node "fatigue" and exacerbation of bradyarrhythmias or post-conversion (to sinus rhythm) pauses.
Introduction: A dual-chamber pacemaker (DDD/R) for a sinus node disease is sometimes referred to as a physiological pacemaker as it maintains atrioventricular synchrony, however several clinical trials have proved its inferiority to a nonphysiological single-chamber ventricular back-up pacing. Patient concerns: A subject of the study is a 74-year-old woman with a sick sinus syndrome (SSS) and ...