- Quick Links
- Make An Appointment
- Our Services
- Price Estimate
- Price Transparency
- Pay Your Bill
- Patient Experience
- Careers at UH

Schedule an appointment today

Hepatitis A Vaccine: Where to Get It and Why You Need It
What is hepatitis a.
Hepatitis A is a serious disease caused by the Hepatitis A virus (HAV). The disease primarily affects the liver and can, in rare cases, lead to liver failure and/or death. Severe complications are most common in persons over 50 years of age or in those with other liver diseases.
How Does Hepatitis A Spread?
Hepatitis A is most often transmitted via the consumption of contaminated food or water . It can also be contracted through intimate contact with an infected person.
Symptoms of Hepatitis A
Symptoms of Hepatitis A usually appear two to six weeks after exposure and can persist for up to two months. In severe cases, the illness can last for up to six months. Symptoms may include:
- Fever, fatigue and/or joint pain
- Loss of appetite, nausea or vomiting
- Abdominal pain and diarrhea
- Yellowing of the skin or eyes (jaundice)
Hepatitis A Vaccine for Travel
The vaccine contains inactivated virus and can prevent the disease. It is often recommended for those who are traveling to a country where the virus is common.
Your doctor may recommend a Hepatitis A vaccine before travel to the following countries:
- South Africa
This is not a comprehensive list. If your destination country is not listed, talk to the travel medicine specialists at the UH Roe Green Center for Travel Medicine & Global Health for more information about recommended vaccines.

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
Vaccines for Travelers
Vaccines protect travelers from serious diseases. Depending on where you travel, you may come into contact with diseases that are rare in the United States, like yellow fever. Some vaccines may also be required for you to travel to certain places.
Getting vaccinated will help keep you safe and healthy while you’re traveling. It will also help make sure that you don’t bring any serious diseases home to your family, friends, and community.
On this page, you'll find answers to common questions about vaccines for travelers.
Which vaccines do I need before traveling?
The vaccines you need to get before traveling will depend on few things, including:
- Where you plan to travel . Some countries require proof of vaccination for certain diseases, like yellow fever or polio. And traveling in developing countries and rural areas may bring you into contact with more diseases, which means you might need more vaccines before you visit.
- Your health . If you’re pregnant or have an ongoing illness or weakened immune system, you may need additional vaccines.
- The vaccinations you’ve already had . It’s important to be up to date on your routine vaccinations. While diseases like measles are rare in the United States, they are more common in other countries. Learn more about routine vaccines for specific age groups .
How far in advance should I get vaccinated before traveling?
It’s important to get vaccinated at least 4 to 6 weeks before you travel. This will give the vaccines time to start working, so you’re protected while you’re traveling. It will also usually make sure there’s enough time for you to get vaccines that require more than 1 dose.
Where can I go to get travel vaccines?
Start by finding a:
- Travel clinic
- Health department
- Yellow fever vaccination clinic
Learn more about where you can get vaccines .
What resources can I use to prepare for my trip?
Here are some resources that may come in handy as you’re planning your trip:
- Visit CDC’s travel website to find out which vaccines you may need based on where you plan to travel, what you’ll be doing, and any health conditions you have.
- Download CDC's TravWell app to get recommended vaccines, a checklist to help prepare for travel, and a personalized packing list. You can also use it to store travel documents and keep a record of your medicines and vaccinations.
- Read the current travel notices to learn about any new disease outbreaks in or vaccine recommendations for the areas where you plan to travel.
- Visit the State Department’s website to learn about vaccinations, insurance, and medical emergencies while traveling.
Traveling with a child? Make sure they get the measles vaccine.
Measles is still common in some countries. Getting your child vaccinated will protect them from getting measles — and from bringing it back to the United States where it can spread to others. Learn more about the measles vaccine.
Find out which vaccines you need
CDC’s Adult Vaccine Quiz helps you create a list of vaccines you may need based on your age, health conditions, and more.
Take the quiz now !
Get Immunized
Getting immunized is easy. Vaccines and preventive antibodies are available at the doctor’s office or pharmacies — and are usually covered by insurance.
Find out how to get protected .
- GP practice services
- Health advice
- Health research
- Medical professionals
- Health topics
Advice and clinical information on a wide variety of healthcare topics.
All health topics
Latest features
Allergies, blood & immune system
Bones, joints and muscles
Brain and nerves
Chest and lungs
Children's health
Cosmetic surgery
Digestive health
Ear, nose and throat
General health & lifestyle
Heart health and blood vessels
Kidney & urinary tract
Men's health
Mental health
Oral and dental care
Senior health
Sexual health
Signs and symptoms
Skin, nail and hair health
Travel and vaccinations
Treatment and medication
Women's health
Healthy living
Expert insight and opinion on nutrition, physical and mental health.
Exercise and physical activity
Healthy eating
Healthy relationships
Managing harmful habits
Mental wellbeing
Relaxation and sleep
Managing conditions
From ACE inhibitors for high blood pressure, to steroids for eczema, find out what options are available, how they work and the possible side effects.
Featured conditions
ADHD in children
Crohn's disease
Endometriosis
Fibromyalgia
Gastroenteritis
Irritable bowel syndrome
Polycystic ovary syndrome
Scarlet fever
Tonsillitis
Vaginal thrush
Health conditions A-Z
Medicine information
Information and fact sheets for patients and professionals. Find out side effects, medicine names, dosages and uses.
All medicines A-Z
Allergy medicines
Analgesics and pain medication
Anti-inflammatory medicines
Breathing treatment and respiratory care
Cancer treatment and drugs
Contraceptive medicines
Diabetes medicines
ENT and mouth care
Eye care medicine
Gastrointestinal treatment
Genitourinary medicine
Heart disease treatment and prevention
Hormonal imbalance treatment
Hormone deficiency treatment
Immunosuppressive drugs
Infection treatment medicine
Kidney conditions treatments
Muscle, bone and joint pain treatment
Nausea medicine and vomiting treatment
Nervous system drugs
Reproductive health
Skin conditions treatments
Substance abuse treatment
Vaccines and immunisation
Vitamin and mineral supplements
Tests & investigations
Information and guidance about tests and an easy, fast and accurate symptom checker.
About tests & investigations
Symptom checker
Blood tests
BMI calculator
Pregnancy due date calculator
General signs and symptoms
Patient health questionnaire
Generalised anxiety disorder assessment
Medical professional hub
Information and tools written by clinicians for medical professionals, and training resources provided by FourteenFish.
Content for medical professionals
FourteenFish training
Professional articles
Evidence-based professional reference pages authored by our clinical team for the use of medical professionals.
View all professional articles A-Z
Actinic keratosis
Bronchiolitis
Molluscum contagiosum
Obesity in adults
Osmolality, osmolarity, and fluid homeostasis
Recurrent abdominal pain in children
Medical tools and resources
Clinical tools for medical professional use.
All medical tools and resources
Hepatitis A vaccine
Peer reviewed by Dr Colin Tidy, MRCGP Last updated by Dr Hayley Willacy, FRCGP Last updated 15 Feb 2023
Meets Patient’s editorial guidelines
In this series: Travel vaccinations Hepatitis B vaccine Rabies vaccine Tick-borne encephalitis vaccine Typhoid vaccine Yellow fever vaccine
You should consider vaccination against hepatitis A before you travel to certain countries, such as the Indian subcontinent.
In this article :
What is hepatitis a, who should be immunised against hepatitis a, are there any side-effects from the hepatitis a vaccine, who should not receive hepatitis a vaccine, other points.
Check with your practice nurse at least two weeks before you travel to see if you should have this vaccination.
Continue reading below
Hepatitis A is an illness caused by the hepatitis A virus. The virus mainly causes inflammation of the liver. Symptoms include:
Generally feeling unwell.
Yellowing of your skin or the whites of your eyes ( jaundice ).
Sometimes, being sick (vomiting).
A raised temperature (fever).
However, some people who are infected do not develop any symptoms (a subclinical illness). The illness is not usually serious and full recovery is usual but the symptoms can be quite unpleasant for a while. The hepatitis A virus is passed out in the stools (faeces) of infected people and infection is usually spread by eating dirty (contaminated) food or drink.
Hepatitis A infection can occur in the UK but it is more common in countries where there is poor sanitation or where disposal of sewage is poor. In the UK, most cases of hepatitis A are seen in people who have recently returned after travelling to such countries. If you catch hepatitis A, the illness is not usually serious but it may ruin a holiday or business trip. See the separate leaflet called Hepatitis A for more details .
This leaflet is just about vaccination to help prevent hepatitis A infection.
Travellers to countries outside Western Europe, North America and Australasia should consider being immunised. The highest-risk areas include the Indian subcontinent (in particular India, Pakistan, Bangladesh and Nepal), Africa, parts of the Far East (except Japan), South and Central America and the Middle East. Vaccination is generally recommended for anyone over the age of 1 year. Your doctor or practice nurse can advise if you should be immunised against hepatitis A for your travel destination.
You can find out if immunisation against hepatitis A is recommended for any countries you are planning to visit from the NHS website Fitfortravel.
Close contacts of someone with hepatitis A
Occasional outbreaks of hepatitis A occur in the UK within families or in institutions. Close contacts of someone found to have hepatitis A infection (for example, family members or other members of the institution) may be offered vaccination. This only happens rarely. The most important measure for anybody with hepatitis A is good personal hygiene. In particular, washing hands after going to the toilet or before eating.
People with chronic liver disease
If you have a persistent (chronic) liver disease (for example, cirrhosis ) it is suggested that you have the hepatitis A vaccine. Hepatitis A infection is not more common in those with chronic liver disease but, if infection does occur, it can cause a more serious illness.
People exposed to hepatitis A at work
For example, laboratory workers who are exposed to hepatitis A during their work and sewage workers are advised to be immunised against hepatitis A.
Staff of some large residential institutions
Outbreaks of hepatitis A have been associated with large residential institutions for people with learning difficulties, where standards of personal hygiene among clients or patients may be poor. Therefore, vaccination of staff and residents of some institutions may be recommended.
Injecting drug users
Drug users who share drug injecting equipment are also thought to have an increased risk of hepatitis A infection and so should consider vaccination.
People with blood clotting problems
If someone has certain blood clotting problems (such as haemophilia) and needs to receive blood clotting factors, they may have an increased risk of hepatitis A infection. This is because the hepatitis A virus may not be completely destroyed during the preparation of these blood products. Vaccination is therefore suggested for these people.
Oral-anal contact
Men who have sex with men, and other people whose sexual practices involve oral-anal contact, may also like to consider vaccination against hepatitis A.
Note : if you have been infected with hepatitis A in the past, you should be immune to further infection and therefore not need vaccination. A blood test can detect antibodies to check if you are already immune. This may be worthwhile doing if you have had a history of yellowing of your skin or the whites of your eyes (jaundice) or come from an area where hepatitis A is common.
There are a number of different hepatitis A vaccines available. Some just protect against hepatitis A, but there are also some combined vaccines for both hepatitis A and hepatitis B or hepatitis A and typhoid fever . A combined vaccine may be useful if you require protection against both diseases.
The hepatitis A single vaccine is given as two doses. The first dose of the vaccine protects against hepatitis A for about one year. The vaccine causes your body to make antibodies against the virus. These antibodies protect you from illness should you become infected with this virus. Ideally, you should have an injection at least two weeks before travel to allow immunity to develop. However, the vaccine may still be advised even if there is less than two weeks before you travel.
How long does a hepatitis A vaccine last?
A second dose of the vaccine 6-12 months after the first gives protection for about 20 years. If you are late with this second dose, you should have it as soon as possible but you don't need to start with the first dose again. Another booster dose of hepatitis A vaccine after 20 years can be given to those people still at risk of infection.
The doses of the combined vaccines against both hepatitis A and hepatitis B or hepatitis A and typhoid may need to be given at slightly different time intervals. Your doctor or practice nurse will be able to advise you in detail.
Some people develop a temporary soreness and redness at the injection site. Much less common are:
A mild raised temperature (fever).
Feeling sick (nauseated).
Feeling off your food for a few days.
Severe reactions are extremely rare.
There are a very few situations where the hepatitis A vaccine is not recommended. They include:
If you have an illness causing a high temperature. In this situation, it is best to postpone vaccination until after you have fully recovered from the illness.
If you have had an allergic reaction to the vaccine or to any of its components in the past.
One type of vaccine (Epaxal®) should not be given to anyone who is known to be allergic to eggs.
Children under the age of 1 year. However, the risk of hepatitis A in children under the age of 1 year is very low. The hepatitis A vaccine is not licensed for this age group.
The vaccine may be given if you are pregnant or breastfeeding and vaccination against hepatitis A is thought to be necessary.
Remember - vaccination for travellers is only one aspect of preventing illness. No vaccination is 100% effective. So when travelling to at-risk areas, you should have very good personal hygiene and also be careful about what you eat and drink.
You should avoid eating and drinking the following when travelling to areas where the risk of hepatitis A is higher:
Raw or inadequately cooked shellfish .
Raw salads and vegetables that may have been washed in unclean (contaminated) water. (Wash fruit and vegetables in safe water and peel them yourself.)
Other foods that may have been grown close to the ground , such as strawberries.
Untreated drinking water, including ice cubes made from untreated water. (Remember also to use only treated or bottled water when brushing your teeth.)
Unpasteurised milk, cheese, ice cream and other dairy products.
Also, be careful when buying food from street traders. Make sure that food has been recently prepared and that it is served hot and on clean serving plates. Food that has been left out at room temperature (for example, for a buffet) or food that may have been exposed to flies could also pose a risk.
Further reading and references
- Travel Health Pro ; National Travel Health Network and Centre (NaTHNaC)
- Immunisation against infectious disease - the Green Book (latest edition) ; UK Health Security Agency.
- Travellers' Health ; US Centers for Disease Control and Prevention
- Travel and Diabetes ; Diabetes UK
Article History
The information on this page is written and peer reviewed by qualified clinicians.
Next review due: 14 Feb 2028
15 feb 2023 | latest version.
Last updated by
Peer reviewed by

Feeling unwell?
Assess your symptoms online for free
Thanks for visiting! GoodRx is not available outside of the United States. If you are trying to access this site from the United States and believe you have received this message in error, please reach out to [email protected] and let us know.
- Português Br
- Journalist Pass
9 common questions about vaccines and travel
Joel Streed
Share this:

Travel does more than just transport you to a different place. It can broaden your perspective, increase your happiness, give you a chance to try new things, boost your creativity and help you recharge. Even planning a trip can be an exciting task. The anticipation of mapping an itinerary and scheduling your must-see attractions can bring a lot of joy and happiness.
One of the most important tasks before taking a trip is to make an appointment with a travel medicine specialist. These health care professionals help keep travelers safe and happy before and after their journeys.
Here are answers to common questions about travel medicine:
1. who should make an appointment with a travel medicine specialist.
Anyone planning a trip overseas can benefit from seeing a travel medicine specialist. However, a travel clinic appointment is critical if you are traveling to underdeveloped or developing countries where there's a higher risk of contracting severe communicable illnesses while abroad. It is also important for patients with certain medical conditions that make their immune systems weaker and more vulnerable to infectious diseases.
2. What vaccinations do I need to travel overseas?
All travelers should be vaccinated against the flu and current with COVID-19 vaccines and boosters.
In addition, it's important to complete the adult vaccination schedule that includes vaccinations for:
- Chickenpox (varicella)
- Diphtheria, tetanus and pertussis (DTP)
- Pneumococcal
- Measles, mumps and rubella (MMR)
Additional vaccines may be recommended depending on your travel itinerary. For example, hepatitis A vaccination is recommended if you are traveling to Southeast Asia. During your appointment, we can discuss which vaccines are appropriate for your itinerary.
3. Are there travel destinations that have different vaccination recommendations?
Yes. Infectious diseases thrive in different climates. If you travel to a new climate, you may be exposed to diseases to which you don't have any immunity.
Some infections are more prevalent in tropical settings compared to temperate climates. For example, typhoid and hepatitis A are more common in Southeast Asia because these communicable diseases can be spread through contaminated water. Some areas of Africa and South America have a higher prevalence of yellow fever and malaria, which are mosquito-borne infections.
The Centers for Disease Control and Prevention (CDC) has good information online for travelers for each travel destination.
Recommended vaccines may include:
- Hepatitis A
- Hepatitis B
- Japanese encephalitis
- Yellow fever
4. Can my primary care provider give me travel vaccinations?
It depends on your travel destinations and vaccine recommendations. I recommend starting the conversation with your primary care provider and reviewing the CDC recommendations .
If you have a complex itinerary with multiple countries or are traveling to Southeast Asia or Africa, it's better to make an appointment at the travel clinic. I also would recommend patients with organ transplants and immunocompromising conditions seek travel medicine consultation to reduce the risk of illness during travel. During that appointment, we will review your itinerary, provide necessary vaccinations and discuss ways to prevent mosquito-borne or tick-borne diseases.
5. How long before my trip should I go to the travel clinic?
Plan to have an appointment at least four weeks before you travel. Some vaccines require several weeks for immunity to develop, while others require more than one dose of vaccine for full protection.
If your trip is to an underdeveloped or developing country, you may need to schedule an appointment up to two months in advance to receive a complete set of immunizations. This gives your body time to produce the protective antibodies, so you are well protected when you land at your destination.
6. Can I only go to the travel clinic before I travel?
No. The Travel and Tropical Medicine Clinic is available before or after travel. The team can provide consultative services and treatment if you get sick after you return home.
7. I'm going to an all-inclusive resort. Will I have a lower risk of getting sick?
Maybe, but no traveler should take safety for granted. Even in an all-inclusive resort, knowing how food is prepared or the water supply quality is not possible. Mosquitos and other insects could still be a concern. It's important to take all necessary precautions and follow vaccination recommendations when you travel, regardless of your accommodations.
8. How do I lower my risk of malaria when traveling?
Malaria is a disease caused by a parasite. It's spread to humans through the bites of infected mosquitoes. Prophylactic malaria medications are available and are started before the travel, continued during the stay and for a certain duration after returning home. A travel medicine specialist can review the risks and benefits of all prevention and treatment options.
9. How do I stay healthy while traveling?
Nothing can ruin a trip like illness. Make sure all your vaccinations and boosters are up to date, and get any new vaccinations recommended for your destinations.
Food and water safety is important while traveling. Only eat well-cooked food. Avoid eating from roadside stands and uncooked foods, like salad and raw vegetables. Drink bottled beverages only, including bottled water. This is especially important if you travel in resource-limited regions, such as Southeast Asia or Africa.
Hand hygiene is important at home and overseas. Wash your hands often using soap and hot water. Avoid crowded places, follow respiratory etiquette and consider optional masking. Mosquitos and bugs can transmit parasites and diseases, like yellow fever and malaria. Use mosquito repellents. Mosquito nets may be appropriate in some parts of the world, as well.
As you make travel plans, schedule an appointment with a travel medicine specialist to get the vaccinations and information you need to be healthy and safe on your journey.
Raj Palraj, M.D. , is a physician in Infectious Diseases and Travel and Tropical Medicine in La Crosse , Wisconsin.
- Mayo Clinic Q and A: Thyroid nodules, cancer and treatment Mayo Clinic Minute: Dangers of late cervical cancer diagnosis in women of color
Related Articles

- Extra 15% off $35+ sitewide* with code SPRING15
- Up to 60% off clearance
- BOGO FREE & BOGO 50% off select vitamins + extra 10% off
- Your Account
- Walgreens Cash Rewards
- Prescription Refills & Status
- Vaccination Records
- Order Status & History
- Buy It Again
Select a store
Schedule hepatitis a vaccine | walgreens.
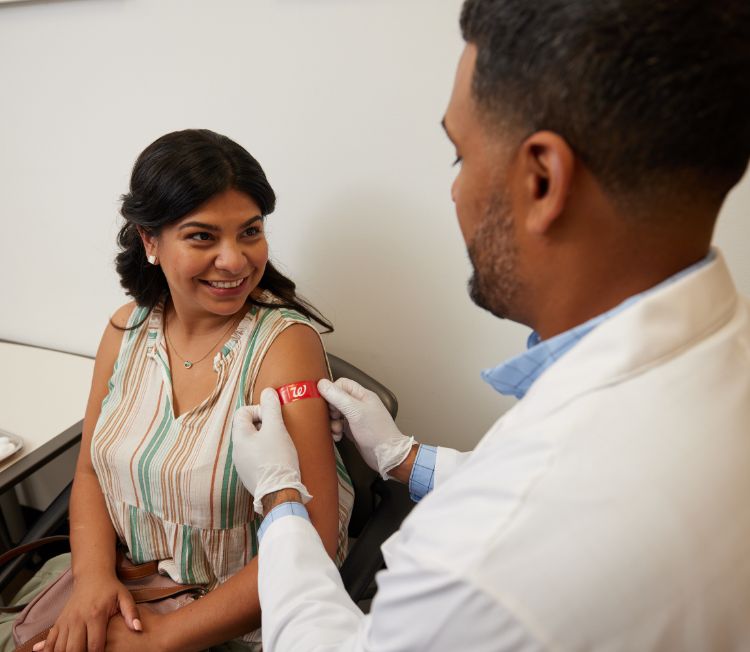
Hepatitis A vaccine
Schedule your vaccine today.
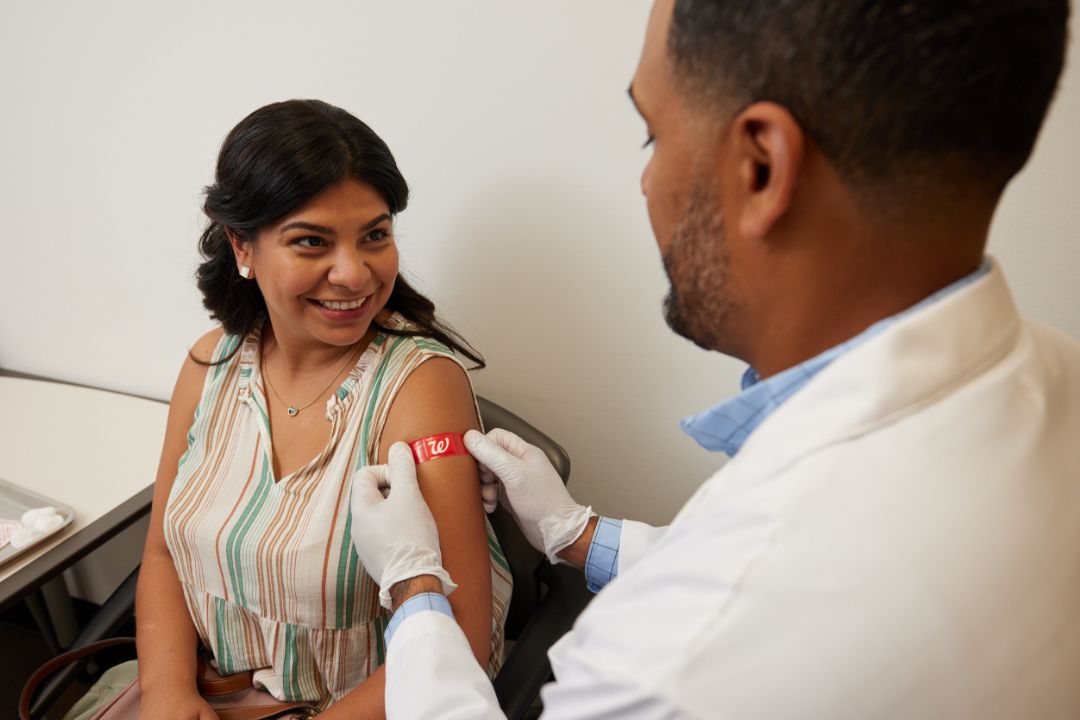
What is hepatitis A?
Hepatitis A is one of several types of hepatitis viruses that cause inflammation affecting the liver’s ability to function. It’s usually spread through ingesting contaminated food or water or close contact (including sexual contact) with an infected person.
Symptoms of hepatitis A include flu-like illness, jaundice, stomach cramping and diarrhea. Frequent handwashing with soap and warm water after using the bathroom, changing a diaper or before preparing food can help prevent the spread of hepatitis A.
Frequently Asked Questions
The hepatitis A vaccine is the best way to prevent infection and is safe and effective. The vaccine, given in 2 doses 6 months apart, is injected into the arm or thigh muscle. Both shots are needed for long-term protection.
To learn more about the hepatitis A vaccine from the CDC, download this PDF or visit the CDC website .
All children ages 12–23 months should get vaccinated against hepatitis A. Children and adolescents ages 2–18 years who have never received a hepatitis A vaccine may also get vaccinated.
People at an increased risk from hepatitis A include:
- Travelers to countries that have high rates of hepatitis A
- Families planning to adopt a child or care for an adopted child from a country with high rates of hepatitis A
- Men who have sex with men
- Users of injection and non-injection drugs
- People with chronic liver diseases, such as hepatitis B or hepatitis C
- People with HIV
- People experiencing homelessness
- People who work with hepatitis A infected animals or in a hepatitis A research laboratory
- Any unvaccinated person who has been exposed to the hepatitis A virus
The safety of the hepatitis A vaccine for pregnant people hasn't been determined. However, there's no evidence of harm or risk to either pregnant people or their unborn babies. People who are pregnant or plan to become pregnant should ask their doctor if they should receive the vaccine.
- Anyone with a life-threatening allergy to any vaccine component, such as aluminum and neomycin sulfate
- Anyone with moderate or severe illness should wait until they recover to be vaccinated
Mild-to-moderate side effects:
- Soreness, redness or swelling at the injection site
Severe side effects, although rare, may include serious allergic reactions. Symptoms include:
- Difficulty breathing
- Fast heartbeat
Over-the-counter pain relievers such as acetaminophen or ibuprofen can help ease pain and reduce fever. Contact your doctor or pharmacist if you have any unexpected or worsening reactions after receiving a vaccine.
If you believe you have a medical emergency, please call 911 .
References:
Hamborsky J, Kroger A, Wolfe S, eds. Centers for Disease Control and Prevention (CDC).Epidemiology and Prevention of Vaccine-Preventable Diseases. 14th ed. Washington, DC: Public Health Foundation, 2021. Vaccine Information Statement: Hepatitis A Vaccine (What You Need to Know) October 25, 2020. https://www.cdc.gov/vaccines/hcp/vis/vis-statements/hep-a.pdf . Accessed September 2021. This publication should be used for general educational purposes only and is not intended to be a substitute for professional medical advice. Although it is intended to be accurate, neither Walgreen Co., its subsidiaries or affiliates, nor any other party assumes liability for loss or damage due to reliance on this publication.

- About the Handbook
Hepatitis A
Information about hepatitis A disease, vaccines and recommendations for vaccination from the Australian Immunisation Handbook.
Recently added
This page was added on 06 June 2018 .
Updates made
This page was updated on 03 June 2020 . View history of updates
Vaccination for certain groups of people is funded under the National Immunisation Program and by states and territories .
Hepatitis A is an acute viral infection of the liver, which can cause mild to severe illness. The illness is usually self-limiting and needs no treatment. It is transmitted primarily by the faecal–oral route by ingesting contaminated food and water, or by direct contact with an infectious person. Hepatitis A is highly contagious.
Hepatitis A vaccination is recommended for:
- Aboriginal and Torres Strait Islander children living in the Northern Territory, Queensland, South Australia and Western Australia
- people with medical risk factors, including chronic liver disease and developmental disabilities
- people who live or work in rural and remote Aboriginal and Torres Strait Islander communities in the Northern Territory, Queensland, South Australia and Western Australia
- people who regularly provide care for Aboriginal and Torres Strait Islander children in the Northern Territory, Queensland, South Australia and Western Australia
- early childhood educators and carers
- carers of people with developmental disabilities
- plumbers and sewage workers
- people aged ≥1 year who travel to hepatitis A– endemic areas
- people who have anal intercourse (including men who have sex with men, and sex industry workers)
- people who inject drugs
- inmates of correctional facilities
Hepatitis A vaccination is recommended for Aboriginal and Torres Strait Islander children living in hepatitis A– endemic areas in a 2-dose schedule at 18 months and 4 years of age.
Hepatitis A vaccination is recommended for all other risk groups (medical, occupational, travel, lifestyle) in a 2-dose schedule, with a minimum interval of 6 months between doses.
Hepatitis A virus survives well in the environment outside its human host. Hepatitis A occurs worldwide. In Australia, before the introduction of the NIP funded Hepatitis A vaccination program in children, it was particularly prevalent in Aboriginal and Torres Strait Islander communities. The prevalence of Hepatitis A disease has decreased significantly since the implementation of the Hepatitis A vaccination program.
Recommendations
Aboriginal and torres strait islander people.
Aboriginal and Torres Strait Islander children living in these states and territories are recommended to receive 2 doses of monovalent hepatitis A vaccine:
- the Northern Territory
- South Australia
- Western Australia
This is due to the increased risk for hepatitis A in this population. 1 See Epidemiology .
These children should receive:
- 1st dose at 18 months of age
- 2nd dose at 4 years of age
Aboriginal and Torres Strait Islander children <10 years of age who have not received hepatitis A vaccine at the recommended schedule points may need extra doses of vaccine and/or an alternative schedule.
Hepatitis A vaccine is funded through the NIP for all Aboriginal and Torres Strait Islander children living in the Northern Territory, Queensland, South Australia and Western Australia. For details see the National Immunisation Program schedule .
See Catch-up vaccination for more details, including minimum intervals between doses.
People with medical risk factors
Hepatitis A vaccination is recommended for people with chronic liver disease of any aetiology, if they are not immune to hepatitis A. 2,3 This includes:
- people with chronic liver disease
- people who have received a liver solid organ transplant
- people with chronic hepatitis B
- people with chronic hepatitis C
2 doses are required, with a recommended minimum interval between doses of 6 months.
See Table. Recommended doses and schedules for monovalent hepatitis A vaccines .
People with chronic liver disease should receive the vaccine as early in the course of the disease as possible. Immune responses to vaccination in these people can vary — for example:
- people with chronic liver disease of mild to moderate severity usually mount a good immune response
- people with end-stage liver disease do not respond as well
- liver transplant recipients may not respond at all 4,5
People with chronic hepatitis C or hepatitis B are recommended to receive hepatitis A vaccine because of the high case-fatality rate in this group if they acquire hepatitis A. 2
If the person is also at risk of acquiring hepatitis B, they can receive a combination hepatitis A/hepatitis B vaccine. This is usually given in 3 doses. People aged 1 to <16 years can receive a 2-dose schedule using Twinrix 720/20.
See Table. Recommended doses and schedules for combination hepatitis A and hepatitis B vaccines .
For more details, see:
- Hepatitis B
- Groups with special vaccination requirements
People with developmental disabilities are recommended to receive hepatitis A vaccine.
2 doses are required, with a recommended interval between doses of 6 months.
See Table. Recommended doses and schedules for monovalent hepatitis A vaccines .
If the person is also at risk of acquiring hepatitis B, they can receive a combination hepatitis A/hepatitis B vaccine. This is usually given in 3 doses. People aged 1 to <16 years can receive a 2-dose schedule using Twinrix 720/20 (minimum 6 months interval doses).
For more details, see Hepatitis B .
People whose occupation increases their risk of acquiring hepatitis A
People who live or work in rural and remote Aboriginal and Torres Strait Islander communities in the Northern Territory, Queensland, South Australia or Western Australia are recommended to receive hepatitis A vaccine.
See also Vaccination for people at occupational risk .
If the person is also at risk of acquiring hepatitis B, they can receive a combination hepatitis A/hepatitis B vaccine. This is usually given in 3 doses.
See Table. Recommended doses and schedules for combination hepatitis A and hepatitis B vaccines .
People who regularly provide care for Aboriginal and Torres Strait Islander children in the Northern Territory, Queensland, South Australia and Western Australia are recommended to receive hepatitis A vaccine.
See Table. Recommended doses and schedules for monovalent hepatitis A vaccines .
See also Vaccination for people at occupational risk .
Early childhood educators and carers are recommended to receive hepatitis A vaccine.
See Table. Recommended doses and schedules for monovalent hepatitis A vaccines .
Carers of people with developmental disabilities are recommended to receive hepatitis A vaccine.
Plumbers and sewage workers are recommended to receive hepatitis A vaccine.
People aged ≥1 year are recommended to receive hepatitis A vaccine if they travel to moderately to highly endemic areas for hepatitis A. 6 This includes expatriates, and people who are visiting friends and relatives.
1 dose of a monovalent hepatitis A vaccine provides protective levels of antibodies against hepatitis A virus. 7 A 2nd dose is recommended 6–12 months after the 1st dose, to increase the duration of protection.
If the person travelling is also at risk of acquiring hepatitis B, they can receive a combination hepatitis A/hepatitis B vaccine. This is usually given in 3 doses. Travellers aged 1 to <16 years can receive a 2-dose schedule using Twinrix 720/20 (minimum 6 months interval between doses).
Travellers aged ≥16 years can receive hepatitis A/hepatitis B vaccine in a ‘rapid’ schedule if there is limited time before departure. 8 This consists of a single dose on each of:
The 4th dose is important to ensure longer-term protection.
The combination hepatitis A/typhoid vaccine is an option for people ≥16 years of age who are travelling to countries where there is an increased risk of both these diseases. This combination is particularly useful for people who are already immunised against hepatitis B.
See Table. Recommended doses and schedules for combination hepatitis A and typhoid vaccine .
To provide longer-term protection against hepatitis A in travellers who are receiving a combination hepatitis A/typhoid vaccine:
- give 1 dose of combination hepatitis A/typhoid vaccine
- at a minimum of 6 months later, give 1 dose of a monovalent adult formulation hepatitis A vaccine
A person who received a previous dose of a monovalent adult formulation hepatitis A vaccine can receive a combination hepatitis A/typhoid vaccine as a booster dose. They should receive the combination vaccine at least 6 months after the 1st dose of hepatitis A vaccine.
For more details, see Typhoid fever .
People whose lifestyle increases their risk of acquiring hepatitis A
People who have anal intercourse (including men who have sex with men, and sex industry workers) are recommended to receive hepatitis A vaccine.
See Epidemiology .
If the person’s lifestyle also increases their risk of acquiring hepatitis B, they can receive a combination hepatitis A/hepatitis B vaccine. This is usually given in 3 doses.
- Vaccination for other groups
People who inject drugs are recommended to receive hepatitis A vaccine.
See Epidemiology .
People who are inmates of correctional facilities are recommended to receive hepatitis A vaccine. See Epidemiology .
Serological testing for hepatitis A immunity
Serological testing for immunity to hepatitis A is not recommended before receiving hepatitis A vaccine.
It is not appropriate to test people who cannot remember whether they have ever had a hepatitis A vaccine. If a person is recommended for vaccination and has no records of previous vaccination, they should receive a vaccine. It is not harmful to vaccinate a person who is already immune to hepatitis A.
However, serological testing before hepatitis A immunity may be helpful for certain groups of people to avoid unnecessary vaccination in individuals with natural immunity:
- people who were born before 1950
- people who spent their early childhood in hepatitis A–endemic areas
- people with an unexplained previous episode of hepatitis or jaundice
People with unexplained jaundice should also be tested for other causes of hepatitis, including hepatitis B.
These people may need to be tested for total hepatitis A antibodies or IgG antibodies against hepatitis A virus. A positive test indicates immunity to hepatitis A. People who are immune do not need hepatitis A vaccination.
To better interpret serological testing results, discuss them with the laboratory that performed the test. Ensure that the laboratory receives the relevant clinical information.
Serological testing to assess immunity after vaccination against hepatitis A is neither necessary nor appropriate. This is because, even in persons who are likely immune, antibody titres are often below the detection limits of the routinely available commercial tests for antibodies against hepatitis A virus. 7
Vaccines, dosage and administration
Hepatitis a vaccines available in australia.
The Therapeutic Goods Administration website provides product information for each vaccine .
See also Vaccine information and Variations from product information for more details.
Monovalent vaccines
Registered for use in people aged ≥2 years.
Each 0.5 mL monodose pre-filled syringe contains:
- 160 antigen units of inactivated hepatitis A virus (GBM strain)
- 0.3 mg aluminium as aluminium hydroxide
- 2.5 µL phenoxyethanol
- 12.5 µg formaldehyde
- ≤2.5 µg neomycin
- <50 ng bovine serum albumin
- traces of polysorbate 80
For Product Information and Consumer Medicine Information about Avaxim visit the Therapeutic Goods Administration website .
Registered for use in people aged ≥16 years.
Each 1.0 mL monodose vial or pre-filled syringe contains:
- 1440 ELISA units of inactivated hepatitis A virus (HM175 strain)
- 0.5 mg aluminium as aluminium hydroxide hydrate
Also contains traces of:
- formaldehyde
- polysorbate 20
For Product Information and Consumer Medicine Information about Havrix 1440 visit the Therapeutic Goods Administration website .
Registered for use in people aged 2–15 years.
Each 0.5 mL monodose vial or pre-filled syringe contains:
- 720 ELISA units of inactivated hepatitis A virus (HM175 strain)
- 0.25 mg aluminium as aluminium hydroxide hydrate
For Product Information and Consumer Medicine Information about Havrix Junior visit the Therapeutic Goods Administration website .
Registered for use in people aged ≥12 months.
Monovalent hepatitis A vaccine
Adult formulation
- approximately 50 units of hepatitis A virus protein
- 0.45 mg aluminium as aluminium hydroxide
- 70 µg borax
Paediatric/adolescent formulation
- approximately 25 units of hepatitis A virus protein
- 0.225 mg aluminium as aluminium hydroxide
- 35 µg borax
Both formulations contain traces of:
- bovine serum albumin
For Product Information and Consumer Medicine Information about Vaqta visit the Therapeutic Goods Administration website .
Combination vaccines
Registered for use in people aged ≥1 year.
Hepatitis A and hepatitis B combination vaccine
- 720 ELISA units of inactivated hepatitis A virus (HM175 strain)
- 20 µg recombinant hepatitis B surface antigen protein
- 0.45 mg aluminium as aluminium phosphate and aluminium hydroxide
May contain yeast proteins.
For Product Information and Consumer Medicine Information about Twinrix (720/20) visit the Therapeutic Goods Administration website .
Registered for use in children aged 1–15 years.
Hepatitis A and hepatitis B vaccine combination vaccine.
- 360 ELISA units of inactivated hepatitis A virus (HM175 strain)
- 10 µg recombinant hepatitis B surface antigen protein
- 0.225 mg aluminium as aluminium phosphate and aluminium hydroxide
For Product Information and Consumer Medicine Information about Twinrix (360/10) visit the Therapeutic Goods Administration website .
Combination hepatitis A and typhoid vaccine
Supplied in a dual-chamber syringe that enables the 2 vaccines to be mixed just before administration.
Each 1.0 mL dose of mixed vaccine contains:
- 25 µg purified Vi capsular polysaccharide of Salmonella typhi strain Ty2
- ≤5 µg neomycin
- <10 ng bovine serum albumin
For Product Information and Consumer Medicine Information about Vivaxim visit the Therapeutic Goods Administration website .
Dose and route
Inactivated hepatitis A vaccines are given by intramuscular injection .
Recommended doses and schedules are shown in:
- Table. Recommended doses and schedules for monovalent hepatitis A vaccines
- Table. Recommended doses and schedules for combination hepatitis A and hepatitis B vaccines
- Table. Recommended doses and schedules for combination hepatitis A and typhoid vaccine
For more details on combination hepatitis A/hepatitis B vaccines and schedules, see Hepatitis B .
Co-administration with other vaccines
Hepatitis A vaccines are inactivated vaccines.
Travellers can receive hepatitis A vaccines in Australia at any time before or after, or with, all other vaccines relevant to international travel 9 and vaccines on the National Immunisation Program schedule.
If a combination hepatitis A/hepatitis B vaccine is not available, the person can receive both monovalent vaccines at the same time. Use separate syringes and administer at separate sites. See Interchangeability of hepatitis A vaccines and review the recommended minimum intervals.
Interchangeability of hepatitis A vaccines
Vaccine manufacturers use slightly different methods to produce the vaccines and quantify the hepatitis A virus antigen content. All monovalent hepatitis A vaccines that are given as a 2-dose course are interchangeable. See Table. Recommended doses and schedules for monovalent hepatitis A vaccines .
Schedules that mix combination hepatitis A/hepatitis B vaccines with monovalent vaccines are not routinely recommended.
An adult dose of Twinrix 720/20 (1.0 ml) contains half the hepatitis A antigen content of an adult dose (1.0 ml) of Havrix adult vaccine. These vaccines are therefore not interchangeable.
Contraindications and precautions
Contraindications.
The only absolute contraindications to hepatitis A vaccines are:
- anaphylaxis after a previous dose of any hepatitis A vaccine
- anaphylaxis after any component of a hepatitis A vaccine
Combination hepatitis A/hepatitis B vaccines are contraindicated in people with a history of anaphylaxis to yeast.
Precautions
Women who are pregnant or breastfeeding.
Hepatitis A vaccines are not routinely recommended for pregnant or breastfeeding women. However, these women can receive these vaccines if necessary.
See Vaccination for women who are planning pregnancy, pregnant or breastfeeding and Table. Vaccines that are not routinely recommended in pregnancy: inactivated viral vaccines for more details.
People with latex allergy
The product information for both Vaqta paediatric/adolescent formulation and adult formulation states that the vial stopper, syringe plunger stopper and tip cap of the syringe contain latex. Consider the use of an alternative product in people with an allergy or sensitivity to latex.
Adverse events
The most common adverse events after receiving a hepatitis A vaccine are mild injection site reactions of short duration.
In both adults and children, systemic adverse events such as headache and fever are much less common than local adverse events. 3
Injection site pain is reported in up to 60% of adults. About 15% of adults report headache, and about 5% report malaise or fatigue after vaccination. 3 Up to 20% of children who receive hepatitis A vaccine report injection site pain.
Hepatitis A vaccines do not affect liver enzyme levels.
People with HIV can safely receive hepatitis A vaccines. The vaccines do not adversely affect the HIV viral load or CD4 + cell count. 10

Nature of the disease
Hepatitis A is an acute infection of the liver. It is caused by hepatitis A virus ( HAV ). HAV is a picornavirus (a small single-stranded RNA virus ). 7
Pathogenesis
The incubation period is 15–50 days, with a mean of about 28 days. 2 Infected people excrete HAV in faeces for:
- up to 2 weeks before the onset of illness
- at least 1 week after the illness 7
HAV does not cause chronic infection . Immunity after infection is lifelong. 2
Transmission
Hepatitis A is a human infection . There is no animal reservoir. 7 HAV is mainly transmitted by the faecal–oral route, primarily by ingesting contaminated food or water. The infecting dose is unknown, but is probably low.
HAV survives well in the environment outside the human host. It persists on hands for several hours and in food kept at room temperature for much longer. It is also relatively resistant to heat and freezing.
Laboratory diagnosis
Hepatitis A is diagnosed by detecting IgM antibodies against HAV in serum during the acute illness.
Anti- HAV IgM is always present by the time the person presents with symptoms. IgM persists for 3–6 months after the acute illness. 7
Serum anti- HAV IgG alone indicates past infection (or possibly immunisation) and therefore immunity. 7
Clinical features
Symptoms of hepatitis a.
In young children, hepatitis A virus usually causes either:
- asymptomatic infection , or
- very mild illness without jaundice
In adults, more than 70% of infected people have symptomatic infection . 2
Patients with symptomatic illness typically have a 4–10-day prodrome of systemic and gastrointestinal symptoms, including:
- abdominal pain
- joint aches and pains
- yellow skin and eyes.
Dark urine is usually the 1st specific manifestation of acute hepatitis A infection . After 1–2 more days, the symptoms include jaundice and pale faeces. 2 Prodromal symptoms tend to wane when jaundice starts, but anorexia and malaise may persist. Pruritus and localised hepatic discomfort or pain may follow. 7
The duration of illness varies. Most people feel better and have normal, or near normal, liver function tests within 1–2 months from the onset of illness. 2
Complications of hepatitis A
Complications of hepatitis A are uncommon. Rarely, it may develop into fulminant hepatitis, for which mortality can be as high as 60%. 2,11 The case-fatality rate of hepatitis A increases with age and varies according to the population. 2
Hepatitis A does not cause chronic liver disease. Relapse occurs in up to 10% of cases, but all relapsed cases recover.
Epidemiology
Hepatitis a in australia.
In recent years, hepatitis A notifications and hospitalisations have been low and trending down. 1 An increasing proportion of cases relate to travel to countries where hepatitis A is endemic . 12-14
Hepatitis A in Aboriginal and Torres Strait Islander children
The hepatitis A vaccination program was initially established in north Queensland in 1999 for Aboriginal and Torres Strait Islander children aged 18 months. 15 In 2005, it expanded to include all Aboriginal and Torres Strait Islander children aged ≤2 years in:
Before the vaccination program, rates of hepatitis A in Aboriginal and Torres Strait Islander communities were very high. Factors associated with high rates were poor living conditions, overcrowding and poor sanitation. 16 The hepatitis A vaccination program for Aboriginal and Torres Strait Islander children in endemic areas substantially reduced hospitalisations and notifications for this population. 17
Many Aboriginal and Torres Strait Islander children >2 years of age in states and territories targeted by the hepatitis A vaccination program have received hep A vaccine. However, Aboriginal and Torres Strait Islander children remain at greater risk than non-Indigenous children of acquiring hepatitis A. 17
See also Vaccination for Aboriginal and Torres Strait Islander people .
History of hepatitis A in Australia
Hepatitis A was a considerable public health problem in Australia in the 1990s. During this time, numerous outbreaks occurred in:
- childcare centres and preschools 18
- Aboriginal and Torres Strait Islander communities 15
- communities of men who have sex with men 19
- schools and residential facilities for people with disability 20
- communities of people who inject drugs 19
More recently, Hepatitis A outbreaks have been associated with a common food source. 17,21,22
Hepatitis A in other countries
Hepatitis A occurs worldwide. Developing countries with poor hygiene measures are at higher risk of hepatitis A infection and transmission.
In areas of high endemicity, such as parts of Africa, Asia, Central America and South America, up to 90% of children have been infected with hepatitis A. 2
Hepatitis A is commonly reported in foodborne outbreaks.
Vaccine information
Inactivated hepatitis A vaccines are prepared from hepatitis A virus ( HAV ) harvested from human diploid cell cultures.
Different strains of HAV are in different vaccines, but there is only 1 known serotype. Immunity induced by a particular strain probably protects against all strains. 7
Immunogenicity of hepatitis A vaccines
Hepatitis A vaccines are highly immunogenic in both children and adults. Seroconversion is nearly universal 4 weeks after vaccination. 7,23,24
Several studies have shown no significant difference in immunogenicity when comparing standard (6-12 months) and extended (20-31 months) dose intervals. 25-27
Efficacy of hepatitis A vaccines
Randomised controlled trials show that the vaccines have protective efficacy of nearly 100%. 28,29 This is supported by the apparent eradication of hepatitis A from Aboriginal and Torres Strait Islander communities in north Queensland and the Northern Territory since the vaccination program started in these regions. 15,17,30
A single dose of Hepatitis A vaccine can confer protection for several years. There is evidence to suggest that a single dose of HAV hepatitis A vaccine can be 100% efficacious in preventing hepatitis A infection in seronegative young children in the study period from 6 weeks to 15 months post vaccination. 31 Other studies have demonstrated effectiveness of a single dose in preventing hepatitis A infection up to 7 years after vaccination. 25,32
Duration of immunity
The duration of immunity after vaccination is uncertain. However, vaccine-induced antibodies against HAV probably persist for many years. Booster doses are not required. 33
Transporting, storing and handling vaccines
Transport according to National Vaccine Storage Guidelines: Strive for 5 . 34 Store at +2°C to +8°C. Do not freeze.
Public health management
Hepatitis A is a notifiable disease in all states and territories in Australia. Hepatitis A National Guidelines for Public Health Units has details about managing hepatitis A cases and contacts. 35
State and territory public health authorities can provide further advice about hepatitis A case management.
Post-exposure prophylaxis using hepatitis A vaccine or normal human immunoglobulin ( NHIG ) can prevent secondary cases in close contacts of hepatitis A cases. Refer to the most recent version of the Hepatitis A National Guidelines for Public Health Units for recommendations regarding the use of hepatitis A vaccine or NHIG for post-exposure prophylaxis for these close contacts, which vary according to the age or presence of medical conditions of the contact.
Normal human immunoglobulin
NHIG is a sterile solution of immunoglobulin , mainly IgG. It contains antibodies that are commonly present in adult human blood.
In Australia, the Australian Red Cross Blood Service supplies NHIG as a 16% solution.
Normal human immunoglobulin-VF (human)
160 mg/mL immunoglobulin (mainly IgG) prepared from Australian blood donations.
Supplied in 2 mL and 5 mL vials.
Also contains glycine.
Administration
Give NHIG by deep intramuscular injection , using an appropriately sized needle. Introduce NHIG slowly into the muscle, to reduce pain.
Never administer NHIG intravenously because of possible severe adverse events. To ensure that the needle is not in a small vessel, try to draw back on the syringe after intramuscular insertion.
A special product for intravenous use — called NHIG (intravenous) — is for people who need large doses of immunoglobulin .
For more details about the use of intravenous immunoglobulins, see Criteria for the Clinical Use of Intravenous Immunoglobulin in Australia . 36
Recommendations for using NHIG following possible Hepatitis A exposure
Use NHIG in close contacts of hepatitis A cases: 35
- when hepatitis A vaccine is contraindicated
- in infants <12 months of age
- in people who are immunocompromised and who might not mount a sufficient immune response after vaccination
- as an alternative option to hepatitis A vaccine for close contacts of hepatitis A cases who are aged >40 years – refer to the Hepatitis A National Guidelines for Public Health Units for further information and guidance on selection
NHIG in Australia provided by the Australian Red Cross Blood Service (ARCBS) contains enough antibody against hepatitis A virus to prevent infection or reduce disease severity if received within 2 weeks of exposure. 35
Variations from product information
Routine vaccination in children
Havrix Junior
The product information for Havrix Junior vaccine states that when given in a two dose schedule, the second dose of the vaccine should be given 6 to 12 months after the first dose.
The Australian Technical Advisory Group on Immunisation ( ATAGI ) recommends that the second dose of Havrix Junior may be given anytime between 6 to 36 months after the first dose.
Vaqta Paediatric/Adolescent
The product information for the Vaqta Paediatric/Adolescent vaccine states that when given in a two dose schedule, the second dose of the vaccine should be given 6 to 18 months after the first dose.
The Australian Technical Advisory Group on Immunisation ( ATAGI ) recommends that the second dose of Vaqta Paediatric/Adolescent may be given anytime between 6 to 36 months after the first dose.
- Naidu L, Chiu C, Habig A, et al. Vaccine preventable diseases and vaccination coverage in Aboriginal and Torres Strait Islander people, Australia 2006–2010. Communicable Diseases Intelligence 2013;37 Suppl:S1-95.
- Averhoff FM, Khudyakov Y, Nelson NP. Hepatitis A vaccines. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, eds. Plotkin's vaccines. 7th ed. Philadelphia, PA: Elsevier; 2018.
- Centers for Disease Control and Prevention (CDC), Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recommendations and Reports 2006;55(RR-7):1-23.
- Keeffe EB, Iwarson S, McMahon BJ, et al. Safety and immunogenicity of hepatitis A vaccine in patients with chronic liver disease. Hepatology 1998;27:881-6.
- Dumot JA, Barnes DS, Younossi Z, et al. Immunogenicity of hepatitis A vaccine in decompensated liver disease. American Journal of Gastroenterology 1999;94:1601-4.
- Nelson NP. Infectious diseases related to travel: hepatitis A . In: CDC yellow book 2018: health information for international travel. New York: Oxford University Press; 2017.
- Koff RS. Hepatitis A. The Lancet 1998;351:1643-9.
- Nothdurft HD, Dietrich M, Zuckerman JN, et al. A new accelerated vaccination schedule for rapid protection against hepatitis A and B. Vaccine 2002;20:1157-62.
- Dumas R, Forrat R, Lang J, Farinelli T, Loutan L. Safety and immunogenicity of a new inactivated hepatitis A vaccine in concurrent administration with a typhoid fever vaccine or a typhoid fever + yellow fever vaccine. Advances in Therapy 1997;14:160-7.
- Wallace MR, Brandt CJ, Earhart KC, et al. Safety and immunogenicity of an inactivated hepatitis A vaccine among HIV-infected subjects. Clinical Infectious Diseases 2004;39:1207-13.
- Hanna JN, Warnock TH, Shepherd RW, Selvey LA. Fulminant hepatitis A in Indigenous children in north Queensland. Medical Journal of Australia 2000;172:19-21.
- Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). Update: Prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Morbidity and Mortality Weekly Report 2007;56:1080-4.
- Hendrickx G, Van Herck K, Vorsters A, et al. Has the time come to control hepatitis A globally? Matching prevention to the changing epidemiology. Journal of Viral Hepatitis 2008;15 Suppl 2:1-15.
- Ward K, McAnulty J. Hepatitis A: who in NSW is most at risk of infection ? New South Wales Public Health Bulletin 2008;19:32-5.
- Hanna JN, Hills SL, Humphreys JL. Impact of hepatitis A vaccination of Indigenous children on notifications of hepatitis A in north Queensland. Medical Journal of Australia 2004;181:482-5.
- Menzies R, Turnour C, Chiu C, McIntyre P. Vaccine preventable diseases and vaccination coverage in Aboriginal and Torres Strait Islander people, Australia, 2003 to 2006. Communicable Diseases Intelligence 2008;32 Suppl:S2-67.
- Thompson C, Dey A, Fearnley E, Polkinghorne B, Beard F. Impact of the national targeted hepatitis A immunisation program in Australia: 2000–2014. Vaccine 2017;35:170-6.
- Hanna JN, Humphreys JL, Hills SL, Richards AR, Brookes DL. Recognising and responding to outbreaks of hepatitis A associated with child day-care centres. Australian and New Zealand Journal of Public Health 2001;25:525-8.
- Ferson MJ, Young LC, Stokes ML. Changing epidemiology of hepatitis A in the 1990s in Sydney, Australia. Epidemiology and Infection 1998;121:631-6.
- Bell JC, Crewe EB, Capon AG. Seroprevalence of hepatitis A antibodies among residents of a centre for people with developmental disabilities. Australian and New Zealand Journal of Medicine 1994;24:365-7.
- Franklin N, Camphor H, Wright R, et al. Outbreak of hepatitis A genotype IB in Australia associated with imported frozen pomegranate arils. Epidemiology and Infection 2019;147:e74.
- NSW Health. Hepatitis A control guideline. 2020. (Accessed 27 May 2020). https://www.health.nsw.gov.au/Infectious/controlguideline/Pages/hepa.as…
- MacIntyre CR, Burgess M, Isaacs D, et al. Epidemiology of severe hepatitis A in Indigenous Australian children. Journal of Paediatrics and Child Health 2007;43:383-7.
- World Health Organization (WHO). WHO position paper on hepatitis A vaccines – June 2012. Weekly Epidemiological Record 2012;87:261-76.
- Mayorga O, Bühler S, Jaeger VK, et al. Single-Dose Hepatitis A Immunization: 7.5-Year Observational Pilot Study in Nicaraguan Children to Assess Protective Effectiveness and Humoral Immune Memory Response. Journal of Infectious Diseases 2016;214:1498-506.
- Williams JL, Bruden DA, Cagle HH, et al. Hepatitis A vaccine: immunogenicity following administration of a delayed immunization schedule in infants, children and adults. Vaccine 2003;21:3208-11.
- Lolekha S, Pratuangtham S, Punpanich W, et al. Immunogenicity and safety of two doses of a paediatric hepatitis A vaccine in thai children: comparison of three vaccination schedules. Journal of Tropical Pediatrics 2003;49:333-9.
- Werzberger A, Mensch B, Kuter B, et al. A controlled trial of a formalin-inactivated hepatitis A vaccine in healthy children. New England Journal of Medicine 1992;327:453-7.
- Innis BL, Snitbhan R, Kunasol P, et al. Protection against hepatitis A by an inactivated vaccine. JAMA 1994;271:1328-34.
- Markey P. Nearing elimination of hepatitis A in the Northern Territory following immunisation of Indigenous infants. The Northern Territory Disease Control Bulletin 2010;17(3):1-6.
- Mayorga Pérez O, Herzog C, Zellmeyer M, et al. Efficacy of virosome hepatitis A vaccine in young children in Nicaragua: randomized placebo-controlled trial. Journal of Infectious Diseases 2003;188:671-7.
- Zhang Z, Zhu X, Hu Y, et al. Five-year antibody persistence in children after one dose of inactivated or live attenuated hepatitis A vaccine. Human Vaccines and Immunotherapeutics 2017;13:1-6.
- Van Damme P, Banatvala J, Fay O, et al. Hepatitis A booster vaccination: is there a need? The Lancet 2003;362:1065-71.
- National Vaccine Storage Guidelines: Strive for 5 . 3rd ed. Canberra: Australian Government Department of Health and Ageing; 2019. https://www.health.gov.au/resources/publications/national-vaccine-storage-guidelines-strive-for-5
- Communicable Diseases Network Australia ( CDNA ). Hepatitis A: national guidelines for public health units . Canberra: Australian Government Department of Health and Ageing; 2009.
- Ig Governance. Criteria for the clinical use of intravenous immunoglobulin in Australia . Version 2.1. Canberra: National Blood Authority Australia; 2016.
Page history
Changes to the vaccination schedule for Aboriginal and Torres Strait Islander children in Northern Territory, Queensland, South Australia and Western Australia.
Recommendations for Aboriginal and Torres Strait Islander children in Northern Territory, Queensland, South Australia and Western Australia have changed. Aboriginal and Torres Strait Islander children in these states and territories are now recommended to receive Hepatitis A vaccine in a 2-dose schedule at 18 months and 4 years of age.
Changes to 4.4.4 Vaccines and 4.4.6 Dosage and administration
4.4.4 Vaccines
Amendment of text to align with new product information.
4.4.6 Dosage and administration
Correction of incorrect text, replacing ELISA units with antigen units and changing 12 months to 36 months.
Help us improve the Australian Immunisation Handbook
Printed content may be out of date. For up to date information, always refer to the digital version:
All vaccine preventable diseases
Subscribe to receive notifications regarding updates to the Australian Immunisation Handbook and changes to immunisation policy.
Help us improve
We are always looking for ways to improve our website, the NICC and mobile app.
Provide feedback
Acknowledgement
The Department of Health and Aged Care acknowledges First Nations peoples as the Traditional Owners of Country throughout Australia, and their continuing connection to land, sea and community. We pay our respects to them and their cultures, and to all Elders both past and present.
© Commonwealth of Australia | Department of Health and Aged Care
Link , share or bookmark directly to this section of the page.
Immunisation Handbook - 2024, version 1
On this page, pdf download, summary of changes.
This publication, which has been prepared for, and is published by, Health New Zealand | Te Whatu Ora, is for the assistance of those involved in providing immunisation services in New Zealand.
While the information and advice included in this publication are believed to be correct, no liability is accepted for any incorrect statement or advice. No person proposing to administer a vaccine to any other person should rely on the advice given in this publication without first exercising his or her professional judgement as to the appropriateness of administering that vaccine to another person.
The Health New Zealand | Te Whatu Ora website hosts the most current version of the Immunisation Handbook. Check the website for the updates that will occur as and when required.
- Main sources
- Commonly used abbreviations
- Glossary of vaccine brand names and abbreviations
- Introduction
- 1. General immunisation principles
- 2. Processes for safe immunisation
- 3. Vaccination questions and addressing concerns
- 4. Immunisation of special groups
- 5. Coronavirus disease (COVID-19)
- 6. Diphtheria
- 7. Haemophilus influenzae type b (Hib) disease
- 8. Hepatitis A
- 9. Hepatitis B
- 10. Human papillomavirus
- 11. Influenza
- 12. Measles
- 13. Meningococcal disease
- 15. Pertussis (whooping cough)
- 16. Pneumococcal disease
- 17. Poliomyelitis
- 18. Rotavirus
- 19. Rubella
- 20. Tetanus
- 21. Tuberculosis
- 22. Varicella (chickenpox)
- 23. Zoster (herpes zoster/shingles)
- Appendix 1: The history of immunisation in New Zealand
- Appendix 2: Planning immunisation catch-ups
- Appendix 3: Immunisation standards for vaccinators and guidelines for organisations offering immunisation services
- Appendix 4: Authorisation of vaccinators and criteria of vaccinators
- Appendix 5: Immunisation certificate
- Appendix 6: Passive immunisation
- Appendix 7: Vaccine presentation, preparation, disposal, and needle-stick recommendations
- Appendix 8: Websites and other online resources
- Funded vaccines for special groups
- Anaphylaxis response/management
- National Immunisation Schedule
Download: Immunisation Handbook 2024 version 1 PDF download - PDF, 4.8 MB
This is version 1 of the Immunisation Handbook 2024, released 7 March 2024.
Summary of changes from previously available Immunisation Handbook 2020 Version 23 and follow-up notice of changes published on the on the Ministry of Health website in December 2023.
See summary of changes
- Chapter 4, Immunisation of Special Groups: a. Updated relevant COVID-19 sections in each high-risk group.
- Chapter 5, COVID-19: a. Extensive update to replace original and bivalent mRNA-CV (30 µg) with XBB.1.5 mRNA-CV (30µg) b. Removed reference to booster doses and replaced with ‘additional’ doses. c. XBB formulation is a one dose primary course. d. Updated recommendations for additional doses and additional doses in pregnancy. e. Updated public health measures.
- Chapter 6, Diphtheria: a. Updated antimicrobial prophylaxis and exclusion of contacts, to match CD manual. b. Corrected vaccine details in the vaccination of contacts.
- Chapter 9, Hepatitis B: a. Table 9.6 – clarified that 40µg means two doses of 1.0 ml. b. Figure 9.4 – updated non-responder protocol.
- Chapter 11, Influenza: a. Updated for the 2024 influenza programme.
- Chapter 12, Measles: a. Improved clarity in wording about when children received MMR at 11 months and when 2nd dose is given. b. In an outbreak scenario, recommend referring to clinical guidance provided in the CD Manual.
- Chapter 13, Meningococcal: a. Removed mention of MenB catch up for 13–25-year-olds. b. Added note in Table 13.2 to check NZ Formulary for antibiotic dosages. c. Corrected footnote in table 13.6 – MenB doesn’t have an upper age limit on licensure.
- Chapter 15, Pertussis: a. Updated wording around unfunded (10-yearly) recommendations. b. Table 15.3, added note to check NZ Formulary for current dosing details.
- Chapter 15, Pneumococcal: a. Tables 16.3 and 16.4, added footnote about giving 23PPV to children born prematurely with ongoing lung disease.
- Appendix 6: a. A6.4.1, Interaction with other drugs: updated re MMR and VV after receipt of blood products.
- Travel Insurance
The journalists on the editorial team at Forbes Advisor Australia base their research and opinions on objective, independent information-gathering.
When covering investment and personal finance stories, we aim to inform our readers rather than recommend specific financial product or asset classes. While we may highlight certain positives of a financial product or asset class, there is no guarantee that readers will benefit from the product or investment approach and may, in fact, make a loss if they acquire the product or adopt the approach.
To the extent any recommendations or statements of opinion or fact made in a story may constitute financial advice, they constitute general information and not personal financial advice in any form. As such, any recommendations or statements do not take into account the financial circumstances, investment objectives, tax implications, or any specific requirements of readers.
Readers of our stories should not act on any recommendation without first taking appropriate steps to verify the information in the stories consulting their independent financial adviser in order to ascertain whether the recommendation (if any) is appropriate, having regard to their investment objectives, financial situation and particular needs. Providing access to our stories should not be construed as investment advice or a solicitation to buy or sell any security or product, or to engage in or refrain from engaging in any transaction by Forbes Advisor Australia. In comparing various financial products and services, we are unable to compare every provider in the market so our rankings do not constitute a comprehensive review of a particular sector. While we do go to great lengths to ensure our ranking criteria matches the concerns of consumers, we cannot guarantee that every relevant feature of a financial product will be reviewed. We make every effort to provide accurate and up-to-date information. However, Forbes Advisor Australia cannot guarantee the accuracy, completeness or timeliness of this website. Forbes Advisor Australia accepts no responsibility to update any person regarding any inaccuracy, omission or change in information in our stories or any other information made available to a person, nor any obligation to furnish the person with any further information.
Travel Insurance For South Africa: Everything You Need To Know
Updated: Apr 30, 2024, 1:13pm
Table of Contents
Featured Partners
Do I Need Travel Insurance for South Africa?
What does travel insurance for south africa cover, frequently asked questions (faqs).
Tourism is on the rise in South Africa according to the local government , with more than four million tourists visiting the nation in the first half of 2023 alone. Australians form a sizable chunk of those numbers, with an estimated 125,000 Australians touring the country each year pre-pandemic.
With travel still high on the agenda for many Australians, that figure is expected to rise in the coming years.
If you’re considering a trip to South Africa, you’ll want to purchase travel insurance. Our guide explains everything you need to know.
Fast Cover Travel Insurance
On Fast Cover’s Secure Website
Medical cover
Unlimited, 24/7 Emergency Assistance
Cancellations
Unlimited, (Trip Disruption $50,000)
Key Features
25-Day Cooling Off Period, Australian Based Call Centre, 4.6 Star Product Review Rating
Cover-More Travel Insurance

On Cover-more’s secure website
Unlimited, with a $2000 limit to dental
Yes, amount chosen by customer
Southern Cross Travel Insurance

Medical Cover
Including medical treatment, doctors’ visits, prescribed medication, specialist treatment & medical transport costs
$2,500 with option to increase to unlimited
Yes, Australians should purchase travel insurance for South Africa. While not a legal requirement to enter the country, it is highly recommended from the Australian government—especially for medical care.
The standard of medical facilities in South Africa can vary by region, but medical facilities are generally of a much lower standard than Australia. In fact, many regional hospitals only provide basic facilities, meaning you may have to be relocated in order to receive the right medical attention.
There is no shared healthcare agreement between Australia and South Africa, which makes travel insurance even more essential. If you need to be transferred by air evacuation to a major city in order to receive treatment, and you don’t have travel insurance, you’ll likely face a hefty bill out of your own pocket.
As Smartraveller advises all Australians, if you can’t afford travel insurance, you can’t afford to travel.
Vaccinations to Consider for Your Trip to South Africa
There is a high risk of certain diseases in South Africa, so it is worth making sure your vaccinations are up to date before you travel and taking any preventative measures with you, such as medications.
This can help reduce your chances of needing to seek medical attention.
There is a risk of Hepatitis A and B throughout South Africa, so vaccinations for Australian travellers are recommended. There is also a moderate risk for most travellers of typhoid, so a vaccination is also recommended if you are travelling to smaller cities, villages and rural areas.
Malaria is present throughout the country, so it could be a good idea to equip yourself with malaria tablets before you travel.
It is essential that you consult a medical practitioner regarding your need for vaccinations before you travel to South Africa, especially as some medical conditions can predispose travellers to certain infections.
When purchasing a travel insurance policy for South Africa, you will have the option to choose a basic policy or a comprehensive policy. A basic policy is cheaper , but may turn out to be more expensive in the long run if you aren’t covered for the things you need.
While a basic policy will usually cover medical needs, it may not provide cover for things such as lost luggage and cancellations (or, if it does, will provide it at a much lower claim level).
That’s why a comprehensive policy is highly recommended for travel to South Africa, as you will receive cover for stolen items, lost luggage, delays and more, in addition to medical and emergency dental care.
Smartraveller asks Australians to exercise a high degree of caution due to the threat of violent crime in South Africa, which includes robbery and carjacking.
The government website warns that opportunistic criminals will target travellers at the approaches to tourist-hotspot Kruger National Park, at well-known resorts, and on public transport.
Additionally, as ATM and credit card fraud are common crimes in South Africa, a comprehensive policy can be the more financially sound choice to give you peace of mind.
Going on a Safari?
South Africa is a popular tourist region for many reasons, including wildlife safaris. If you wish to partake in a safari or a game walk—walking with wild animals and a professional guide—you will need to ensure that these activities are covered in your policy’s list of included sports and activities.
If they are not, you will not receive cover for anything that occurs during the safari.
However, your policy may offer the option for you to choose an ‘adventure pack’ at an additional cost, which can include many activities that aren’t covered in the standard offering.
This can also include hiking or trekking to certain altitudes.
It’s important to consider which activities you may be participating in during your trip to South Africa in order to ensure you have the appropriate coverage, and purchase an additional add-on if necessary.
What Travel Insurance Won’t Cover
Your travel insurance policy won’t cover anything that is set out in its exclusions, as per the product disclosure statement (PDS). This could include certain sports and activities (such as a safari), or travel to certain regions in South Africa due to safety.
While each travel insurance policy differs on the fine-print, it is standard for most policies not to cover:
- Cancellations due to ‘disinclination to travel’, being if you change your mind about your holiday;
- Accidents or injuries that occur when not following the appropriate safety guidance or official guidelines;
- Intoxicated behaviour, including recreational drugs;
- Any illegal activity.
Be sure to carefully read the PDS of your policy so you know exactly what you can and cannot claim on your trip to South Africa.
Is it safe to travel to South Africa?
Smartraveller recommends that Australians exercise a high degree of caution when travelling to South Africa, due to the threat of violent crime. This includes armed robbery, mugging, carjacking, credit card theft, and more.
There is a higher risk of violent crime in major cities after dark, or during “rolling blackout” periods.
For these reasons (and more), Smartraveller urges Australians to take out a travel insurance policy before travelling to South Africa.
Do Australians need a visa for South Africa?
No, Australians do not need a visa for South Africa if they are visiting for tourism for stays of up to 90 days.
Where can I buy travel insurance for South Africa?
Most Australian travel insurance providers will cover Aussies wanting to head abroad to South Africa. When shopping around for a policy, you will be able to choose your destination when you request a quote. If there is no option to choose South Africa, this would be a clear indicator that the insurance provider does not provide policies to this region.
At the time of writing, a few of our top picks for comprehensive travel insurance cover South Africa, including Allianz and Cover-More .
Travel insurance providers can revoke the issuing of new policies to certain destinations at any time, especially if Smartraveller changes the alert warning for a country to ‘Do Not Travel’.
- Best Comprehensive Travel Insurance
- Best Seniors Travel Insurance
- Best Domestic Travel Insurance
- Best Cruise Travel Insurance
- Best Family Travel Insurance
- Travel Insurance Cost
- Pregnancy Travel Insurance Guide
- Travel Insurance Cancellation Cover
- Travel Insurance For Bali
- Travel Insurance For Fiji
- Travel Insurance For The USA
- Travel Insurance For Thailand
- Travel Insurance For New Zealand
- Travel Insurance For Japan
- Travel Insurance For Europe
- Travel Insurance For Singapore
- Travel Insurance For Indonesia
- Travel Insurance For Vietnam
- Travel Insurance For Canada
- Cover-More Travel Insurance Review
- Fast Cover Travel Insurance Review
- Travel Insurance Saver Review
- Allianz Comprehensive Travel Insurance Review
- 1Cover Comprehensive Travel Insurance Review
- Australia Post Comprehensive Travel Insurance Review
- Tick Travel Insurance Review
More from
Top travel insurance tips for australians, our pick of the best comprehensive travel insurance providers in australia, do frequent flyer points expire, travel insurance for canada: what you need to know before you go, travel insurance for vietnam: everything you need to know, tick travel insurance top cover review: features, pros and cons.
Sophie Venz is an experienced editor and features reporter, and has previously worked in the small business and start-up reporting space. Previously the Associate Editor of SmartCompany, Sophie has worked closely with finance experts and columnists around Australia and internationally.
- Share full article
Advertisement
Supported by
U.S. Lags Behind Other Countries in Hepatitis-C Treatment
Despite an arsenal of drugs, many Americans are still unaware of their infections until it’s too late. A Biden initiative languishes without Congressional approval.

By Ted Alcorn
In the 10 years since the drugmaker Gilead debuted a revolutionary treatment for hepatitis C, a wave of new therapies have been used to cure millions of people around the world of the blood-borne virus.
Today, 15 countries, including Egypt, Canada and Australia, are on track to eliminate hepatitis C during this decade, according to the Center for Disease Analysis Foundation, a nonprofit. Each has pursued a dogged national screening and treatment campaign.
But the arsenal of drugs, which have generated tens of billions of dollars for pharmaceutical companies, has not brought the United States any closer to eradicating the disease.
Spread through the blood including IV drug use, hepatitis C causes liver inflammation, though people may not display symptoms for years. Only a fraction of Americans with the virus are aware of the infection, even as many develop the fatal disease.
A course of medications lasting eight to 12 weeks is straightforward. But the most at-risk, including those who are incarcerated, uninsured or homeless, have difficulty navigating the American health system to get treatment.
Of those diagnosed in the United States since 2013, just 34 percent have been cured, according to a recent analysis by the Centers for Disease Control and Prevention.
“We’re not making progress,” said Dr. Carolyn Wester, who heads the agency’s division of viral hepatitis. “We have models of care that are working, but it is a patchwork.”
Dr. Francis Collins, who headed the National Institutes of Health for decades until retiring in 2021, has been spearheading a White House initiative aimed at eliminating the disease.
In an interview, he said he was motivated by memories of his brother-in-law, Rick Boterf, who died of hepatitis C just before the introduction of the new cures. An outdoorsman, Mr. Boterf endured five years of liver failure waiting for a transplant, and even that procedure wasn’t enough to save him from the destructive virus.
“The more I looked at this, the more it just seemed impossible to walk away,” Dr. Collins said.
The initiative, which was included in President Biden’s latest budget proposal , calls for about $5 billion to establish a five-year “subscription” contract. The federal government would pay a flat fee and, in return, receive drugs for every patient it enrolled for treatment.
Several states already use similar subscription contracts, with limited success. Louisiana was the first to deploy such a scheme, in 2019, and reported a significant increase in people treated through Medicaid and in correctional facilities. But the state’s treatment numbers dwindled during the pandemic, and have not rebounded. Now, nearing the end of its five-year contract, Louisiana has treated barely half the people it had proposed to reach.
Dr. Collins acknowledged that on its own, a national drug-purchasing agreement like Louisiana’s would not be sufficient to turn the tide.
“Anybody who tries to say, ‘Oh, it’s just the cost of the drug, that’s the only thing that’s gotten in the way,’ hasn’t looked at those lessons carefully,” he said. To that end the proposal also calls for a $4.3 billion campaign to raise awareness, train clinicians and promote treatment at health centers, prisons and drug treatment programs.
Carl Schmid, who directs the H.I.V. and Hepatitis Policy Institute, a nonprofit, said he worried that the White House proposal was overly focused on drug prices. “The real problem is you have to get money for the outreach, the testing and the providers,” he said.
Advocates say some states have cobbled together robust efforts, like New Mexico, which has been connecting hard-to-reach populations with treatment, largely without federal support.
“New Mexico is one of our superstars,” said Boatemaa Ntiri-Reid, a health policy expert with the National Alliance of State and Territorial AIDS Directors.
Andrew Gans, who manages the state’s hepatitis C program, said an estimated 25,800 residents needed treatment , and that multiple strategies would be required to eradicate the disease by the end of this decade. “You can’t do that through just one door.”
In the village of Ruidoso, in southeastern New Mexico, Christie Haase, a nurse practitioner, had been working at a small private clinic for just two weeks when a patient with abnormal liver enzymes tested positive for hepatitis C.
Like many primary care providers, Ms. Haase had not been trained to treat hepatitis C and offered to refer the patient to a gastroenterologist. But none practiced in the town, and the patient balked at traveling to Albuquerque, three hours away.
“I didn’t know where to go from there,” Ms. Haase said.
One of the biggest hurdles to eliminating hepatitis C is the specialists most qualified to treat the disease are often the least accessible to patients, especially those who lack insurance or stable shelter , both risk factors for infection.
Even when referrals are possible, they require follow-up visits that patients may miss and co-payments they may be unable to afford.
So instead of handing off the patient, Ms. Haase joined a video conference with other rural providers, where she presented the case, and more experienced clinicians recommended further tests and medications. The meeting was part of a program called ECHO (Extension for Community Healthcare Outcomes), which Dr. Sanjeev Arora, a gastroenterologist, developed in the early 2000s to connect primary care doctors in sparsely populated areas with specialists.
Dr. Arora, who later founded the nonprofit Project ECHO to promote the model around the world, estimated that the New Mexico program had provided hepatitis C treatment for more than 10,000 patients. “It really changed the game,” he said.
Care behind bars
Few people are at higher risk of hepatitis C infection than those who are incarcerated. A recent study estimated that over 90,000 people in U.S. state prisons are infected, 8.7 times the prevalence of people outside the correctional system.
For many years, New Mexico’s prisons did a good job of screening for hepatitis C and a terrible job treating it. More than 40 percent of prisoners were infected , the highest prevalence of any state correctional system, but no funds were available for the needed treatment. Prisons then rationed the drugs, including by denying medication to inmates accused of disciplinary infractions. In 2018, of some 3,000 infected inmates, just 46 received treatment .
That changed in 2020 when state lawmakers appropriated $22 million specifically for treating prisoners with hepatitis C. New Mexico’s corrections department also arranged to buy the medications at a steep discount through the 340 B federal drug pricing program.
But some prisoners continued to decline treatment, so the state enlisted incarcerated people to win them over. Since 2009, the Peer Education Project , a collaboration between Project ECHO and the corrections department, has trained more than 800 people to counsel others about preventing infections and getting treated.
Last May, incarcerated peer educators around the state tuned into a videoconference to discuss the reasons their fellow inmates were reluctant to seek treatment and to share their approaches for assuaging those concerns.
Daniel Rowan, who now manages the Prison Education Program, had himself formerly been incarcerated. He said the program had gone a long way toward improving the relationship between inmates and their medical providers, although it remains “a gantlet of challenges, to say the least.”
Between 2020 and 2022, the number of imprisoned people receiving treatment for hepatitis C quadrupled, to more than 600. Last year, the New Mexico State Legislature appropriated another $27 million to sustain the effort.
Another group it is crucial to reach are people with a history of IV drug use: Two-thirds of newly infected people had previously injected drugs, according to the C.D.C.
In New Mexico, where opiate addiction is a generational scourge, harm reduction programs are deeply integrated into the state’s public health department. The state legalized needle exchanges more than 25 years ago, and was the first to allow the distribution of naloxone.
Early last year, a county public health clinic in Las Cruces paired treatment for hepatitis C with existing services including needle-exchange and prescriptions for buprenorphine, an opioid addiction treatment. Over the next year, a lower-than-expected share of patients in the buprenorphine program tested positive for hepatitis C, which health officer Dr. Michael Bell attributed, in part, to changes in drug use. People who once injected heroin now smoke fentanyl instead, limiting their exposure to unsanitary needles that could transmit the virus. The C.D.C. believes this shift also contributed to a slight decline in new hepatitis C infections nationwide, which fell 3.5 percent in 2022 .
Still not enough
Despite statewide efforts, no tracking system exists to accurately measure the number of people cured. A little more than 2,200 people were treated in 2022 by the largest providers. The state estimated it needed to treat 4,000 people that year to stay on track.
As in other states, clinicians in New Mexico also struggle to persuade patients to return and begin treatment. Some countries have approved a rapid test that makes it possible to diagnose and start treatment in one visit. The test is under accelerated review at the National Institutes of Health in the United States, with data expected to be ready this summer, an agency spokesperson said.
The president’s initiative was also in last year’s budget, but lawmakers have not yet introduced legislation to fund it, and there may be few opportunities to pass it before the election in November.
The Congressional Budget Office is evaluating a draft bill for its impact on the budget. Dr. Collins acknowledged that lawmakers in Congress might balk at the price tag, but contended that it would eventually save not just lives, but money.
In a paper published by the National Bureau of Economic Research , a group of scientists calculated that the initiative would prevent 24,000 deaths in the next decade and save $18.1 billion in medical costs for people with untreated hepatitis C.
“This is a deficit reduction program in the long term,” Dr. Collins said. “Just don’t expect it to be deficit reduction this year.”
By continuing to browse the site you are agreeing to our use of cookies and similar tracking technologies described in our privacy policy .
Resource Library
The AAEP develops numerous resources to assist veterinarians and the broader equine industry with issues affecting the horse and connection to those with common goals.
Search & Filter
Resource Type
Audience Type
Snake Bite Vaccination Guidelines
Tetanus Vaccination Guidelines
Tetanus toxoid is a core equine vaccine and should be included in equine immunization programs for every horse annually.
Venezuelan Equine Encephalomyelitis Vaccination Guidelines
Equine Viral Arteritis (EVA) Vaccination Guidelines
Equine Influenza Vaccination Guidelines
West Nile Virus Vaccination Guidelines
- Next »
Join Us Today!
Our community of horse doctors connects you to more than 9,000 veterinarians and veterinary students who make a difference every day in horse health, just like you!

Vaccine (Shot) for Hepatitis A

How to pronounce: “HEP” + “uh” + “TY” + “tis”
Two doses of the hepatitis A vaccine are recommended for children by doctors as the best way to protect against hepatitis A.
Why should my child get the hepatitis A shot?
- Protects your child from hepatitis A, a potentially serious disease.
- Protects other people from the disease because children under 6 years old with hepatitis A usually don’t have symptoms, but they often pass the disease to others without anyone knowing they were infected.
- Keeps your child from missing school or childcare and you from missing work.
When should my child get the hepatitis A shot?
Your child will need two doses of the Hepatitis A shot for best protection. One dose at each of the following ages:

The hepatitis A shot is safe.
The hepatitis A vaccine is very safe, and it is effective at preventing the hepatitis A disease. Vaccines, like any medicine, can have side effects. These are usually mild and go away on their own.
What are the side effects?
The most common side effects are usually mild and last 1 or 2 days. They include:
- Sore arm from the shot
- Loss of appetite (not wanting to eat)
Prepare for your child's vaccine visit and learn about how you can:
- Research vaccines and ready your child before the visit
- Comfort your child during the appointment
- Care for your child after the shot
What is hepatitis A?
Hepatitis A is a serious liver disease caused by the hepatitis A virus. Children with the virus often don’t have symptoms, but they often pass the disease to others, including their unvaccinated parents or caregivers. These individuals can get very sick.
What are the symptoms of hepatitis A disease?
Children under 6 years old often have no symptoms. Older children and adults feel very sick and weak. Symptoms usually appear 2 to 6 weeks after a person gets the virus. The symptoms may include
- Stomach pain
- Yellow skin and eyes
Is it serious?
Older children, adolescents and adults often feel sick and symptoms can last for up to 6 months. There is no specific treatment for hepatitis A.
Hepatitis A is a serious disease that used to be more common in the United States. In the 1980s, the United States used to see as many as 30,000 cases a year. Thanks to the vaccine, the number of hepatitis A cases in the United States has dropped by 95%.

How does hepatitis A spread?
Hepatitis A virus is found in the stool (poop) of a person who has the virus. It spreads when a person puts something in his or her mouth that has the hepatitis A virus on it. Even if the item looks clean, it can still have virus on it that can spread to others. The amount of stool can be so tiny that it cannot be seen with the naked eye. You can get it by touching objects such as doorknobs or diapers or eating food that has the virus on it.
Follow the vaccine schedule
The Centers for Disease Control and Prevention, American Academy of Family Physicians, and American Academy of Pediatrics strongly recommend children receive all vaccines according to the recommended vaccine schedule .
- Get a list of vaccines that your child may need based on age, health conditions, and other factors.
- Learn the reasons you should follow the vaccine schedule .
Birth - 6 years schedule
WARNING: Some of these photos might be unsuitable for children. Viewing discretion is advised.
- Vaccines & Immunizations
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
You are using an outdated browser. Upgrade your browser today or install Google Chrome Frame to better experience this site.
- Section 5 - Rubella
- Section 5 - Smallpox & Other Orthopoxvirus-Associated Infections
Rubeola / Measles
Cdc yellow book 2024.
Author(s): Paul Gastañaduy, James Goodson
Infectious Agent
Transmission, epidemiology, clinical presentation.
INFECTIOUS AGENT: Measles virus
TRAVELER CATEGORIES AT GREATEST RISK FOR EXPOSURE & INFECTION
PREVENTION METHODS
Rubeola is a vaccine-preventable disease
DIAGNOSTIC SUPPORT
Measles virus is a member of the genus Morbillivirus of the family Paramyxoviridae .
Measles is transmitted from person to person via respiratory droplets and by the airborne route as aerosolized droplet nuclei. Infected people are usually contagious from 4 days before until 4 days after rash onset. Measles is among the most contagious viral diseases known; secondary attack rates are ≥90% among susceptible household and institutional contacts. Humans are the only natural host for sustaining measles virus transmission, which makes global eradication of measles feasible.
Measles was declared eliminated (defined as the absence of endemic measles virus transmission in a defined geographic area for ≥12 months in the presence of a well-performing surveillance system) from the United States in 2000. Measles virus continues to be imported into the country from other parts of the world, however, and recent prolonged outbreaks in the United States resulting from measles virus importations highlight the challenges faced in maintaining measles elimination.
Given the large global measles burden and high communicability of the disease, travelers could be exposed to the virus in any country they visit where measles remains endemic or where large outbreaks are occurring. Most measles cases imported into the United States occur in unvaccinated US residents who become infected while traveling abroad, often to the World Health Organization (WHO)–defined Western Pacific and European regions. These travelers become symptomatic after returning to the United States and sometimes infect others in their communities, causing outbreaks.
Nearly 90% of imported measles cases are considered preventable by vaccination (i.e., the travelers lacked recommended age- and travel-appropriate vaccination). Furthermore, observational studies in travel clinics in the United States have shown that 59% of pediatric and 53% of adult travelers eligible for measles-mumps-rubella (MMR) vaccine at the time of pretravel consultation were not vaccinated at the visit, highlighting a missed opportunity to reduce the likelihood of measles introductions and subsequent spread. Encourage all eligible travelers to receive appropriate MMR vaccination. Outbreak investigations are costly and resource intensive, and infected people—in addition to productivity losses—can incur direct costs for the management of their illness, including treatment, quarantine, and caregiving.
The incubation period averages 11–12 days from exposure to onset of prodrome; rash usually appears ≈14 days after exposure. Symptoms include fever, with temperature ≤105°F (≤40.6°C); conjunctivitis; coryza (runny nose); cough; and small spots with white or bluish-white centers on an erythematous base appearing on the buccal mucosa (Koplik spots). A characteristic red, blotchy (maculopapular) rash appears 3–7 days after onset of prodromal symptoms. The rash begins on the face, becomes generalized, and lasts 4–7 days.
Common measles complications include diarrhea (8%), middle ear infection (7%–9%), and pneumonia (1%–6%). Encephalitis, which can result in permanent brain damage, occurs in ≈1 per 1,000–2,000 cases of measles. The risk for serious complications or death is highest for children aged ≤5 years, adults aged ≥20 years, and in populations with poor nutritional status or that lack access to health care.
Subacute sclerosing panencephalitis (SSPE) is a progressive neurologic disorder caused by measles virus that usually presents 5–10 years after recovery from the initial primary measles virus infection. SSPE manifests as mental and motor deterioration, which can progress to coma and death. SSPE occurs in ≈1 of every 5,000 reported measles cases; rates are higher among children <5 years of age.
Measles is a nationally notifiable disease. Laboratory criteria for diagnosis include a positive serologic test for measles-specific IgM, IgG seroconversion, or a significant rise in measles IgG level by any standard serologic assay; isolation of measles virus; or detection of measles virus RNA by reverse transcription PCR (RT-PCR) testing. The Centers for Disease Control and Prevention’s Measles Virus Laboratory is the national reference laboratory; it provides serologic and molecular testing for measles and technical assistance to state public health laboratories for the collection and shipment of clinical samples for molecular diagnostics and genetic analysis. See detailed information on diagnostic support .
A clinical case of measles illness is characterized by generalized maculopapular rash lasting ≥3 days; temperature ≥101°F (38.3°C); and cough, coryza, or conjunctivitis. A confirmed case is one with an acute febrile rash illness with laboratory confirmation or direct epidemiologic linkage to a laboratory-confirmed case. In a laboratory-confirmed or epidemiologically linked case, the patient’s temperature does not need to reach ≥101°F (38.3°C) and the rash does not need to last ≥3 days.
Treatment is supportive. The WHO recommends vitamin A for all children with acute measles, regardless of their country of residence, to reduce the risk for complications. Administer vitamin A as follows: for infants <6 months old, give 50,000 IU, once a day for 2 days; for infants 6 months old and older, but younger than 12 months, give 100,000 IU once a day for 2 days; for children ≥12 months old give 200,000 IU once a day for 2 days. For children with clinical signs and symptoms of vitamin A deficiency, administer an additional (i.e., a third) age-specific dose of vitamin A 2–4 weeks following the first round of dosing.
Measles has been preventable through vaccination since a vaccine was licensed in 1963. People who do not have evidence of measles immunity should be considered at risk for measles, particularly during international travel. Acceptable presumptive evidence of immunity to measles includes birth before 1957; laboratory confirmation of disease; laboratory evidence of immunity; or written documentation of age-appropriate vaccination with a licensed, live attenuated measles-containing vaccine 1 , namely, MMR or measles-mumps-rubella-varicella (MMRV). For infants 6 months old and older, but younger than 12 months, this includes documented administration of 1 dose of MMR; for people aged ≥12 months, documentation should include 2 doses of MMR or MMRV (the first dose administered at age ≥12 months and the second dose administered no earlier than 28 days after the first dose). Verbal or self-reported history of vaccination is not considered valid presumptive evidence of immunity.
1 From 1963–1967, a formalin-inactivated measles vaccine was available in the United States and was administered to ≈600,000–900,000 people. It was discontinued when it became apparent that the immunity it produced was short-lived. Consider people who received this vaccine unvaccinated.
Vaccination
Measles vaccine contains live, attenuated measles virus, which in the United States is available only in combination formulations (e.g., MMR and MMRV vaccines). MMRV vaccine is licensed for children aged 12 months–12 years and can be used in place of MMR vaccine if vaccination for measles, mumps, rubella, and varicella is needed.
International travelers, including people traveling to high-income countries, who do not have presumptive evidence of measles immunity and who have no contraindications to MMR or MMRV, should receive MMR or MMRV before travel per the following schedule.
Infants (6 months old and older, but younger than 12 months): 1 MMR dose. Infants vaccinated before age 12 months must be revaccinated on or after the first birthday with 2 doses of MMR or MMRV separated by ≥28 days. MMRV is not licensed for children aged <12 months.
Children (aged ≥12 months): 2 doses of MMR or MMRV separated by ≥28 days.
Adults born in or after 1957: 2 doses of MMR separated by ≥28 days.
One dose of MMR is ≈85% effective when administered at age 9 months; MMR and MMRV are 93% effective when administered at age ≥1 year. Vaccine effectiveness of 2 doses is 97%.
Adverse Reactions
In rare circumstances, MMR vaccination has been associated with anaphylaxis (≈2–14 occurrences per million doses administered); febrile seizures (≈1 occurrence per 3,000–4,000 doses administered, but overall, the rate of febrile seizures after measles-containing vaccine is much lower than the rate with measles disease); thrombocytopenia (≈1 occurrence per 40,000 doses during the 6 weeks after immunization); or joint symptoms (arthralgia develops among ≈25% of nonimmune postpubertal females from the rubella component of the MMR vaccination, and ≈10% have acute arthritis-like signs and symptoms that generally persist for 1–21 days and rarely recur; chronic joint symptoms are rare, if they occur at all). No evidence supports a causal link between MMR vaccination and autism, type 1 diabetes mellitus, or inflammatory bowel disease.
Contraindications
People who experienced a severe allergic reaction (difficulty breathing, hives, hypotension, shock, swelling of the mouth or throat) following a prior dose of MMR or MMRV vaccine, or who had an anaphylactic reaction to topically or systemically administered neomycin, should not be vaccinated or revaccinated. People who are allergic to eggs can receive MMR or MMRV vaccine without prior routine skin testing or the use of special protocols.
Immunosuppression
Enhanced replication of live vaccine viruses can occur in people who have immune deficiency disorders. Death related to vaccine-associated measles virus infection has been reported among severely immunocompromised people; thus, severely immunosuppressed people should not be vaccinated with MMR or MMRV vaccine. For a thorough discussion of recommendations for immunocompromised travelers, see Sec. 3, Ch. 1, Immunocompromised Travelers .
MMR vaccination is recommended for all people with HIV infection aged ≥12 months who do not have evidence of measles, mumps, and rubella immunity, and who do not have evidence of severe immunosuppression. The assessment of severe immunosuppression can be based on CD4 values (count or percentage); absence of severe immunosuppression is defined as CD4 ≥15% for ≥6 months for children aged ≤5 years, or CD4 ≥15% and CD4 count ≥200 cells/mL for ≥6 months for people aged >5 years.
People with leukemia in remission and off chemotherapy, who were not immune to measles when diagnosed with leukemia, may receive MMR vaccine. At least 3 months should elapse after termination of chemotherapy before administering the first dose of vaccine.
Steroids & Other Immunosuppressive Therapies
Avoid vaccinating people who have received high-dose corticosteroid therapy (in general, considered to be ≥20 mg or 2 mg/kg body weight of prednisone, or its equivalent, daily for ≥14 days) with MMR or MMRV for ≥1 month after cessation of steroid therapy. Corticosteroid therapy usually is not a contraindication when administration is short-term (<14 days) or a low to moderate dose (<20 mg of prednisone or equivalent per day).
In general, withhold MMR or MMRV vaccine for ≥3 months after cessation of other immunosuppressive therapies and remission of the underlying disease. See Sec. 3, Ch. 1, Immunocompromised Travelers , for more details.
MMR vaccines should not be administered to pregnant people or people attempting to become pregnant. Because of the theoretical risk to the fetus, people should be counseled to avoid becoming pregnant for 28 days after receiving a live-virus (e.g., MMR) vaccine.
Precautions
Personal or family history of seizures of any etiology.
Compared with administration of separate MMR and varicella vaccines at the same visit, use of MMRV vaccine is associated with a higher risk for fever and febrile seizures 5–12 days after the first dose among children aged 12–23 months. Approximately 1 additional febrile seizure occurs for every 2,300–2,600 MMRV vaccine doses administered. Use of separate MMR and varicella vaccines avoids this increased risk for fever and febrile seizures.
Thrombocytopenia
The benefits of primary immunization are usually greater than the potential risks for vaccine- associated thrombocytopenia. Avoid giving subsequent doses of MMR or MMRV vaccine, however, if an episode of thrombocytopenia occurred ≤6 weeks after a previous dose of vaccine.
Postexposure Prophylaxis
Measles-containing vaccine or immune globulin (IG) can be effective as postexposure prophylaxis. MMR or MMRV administered ≤72 hours after initial exposure to measles virus might provide some protection. If the exposure does not result in infection, the vaccine should induce protection against subsequent measles virus infection.
When administered ≤6 days of exposure, IG can be used to confer temporary immunity in a susceptible person. If the exposure does not result in modified or typical measles, vaccination with MMR or MMRV is still necessary to provide long-lasting protection. Six months after receiving intramuscularly administered IG, or 8 months after receiving intravenously administered IG, administer MMR or MMRV vaccine, provided the patient is aged ≥12 months and the vaccine is not otherwise contraindicated.
CDC website: Measles
The following authors contributed to the previous version of this chapter: Paul A. Gastañaduy, James L. Goodson
Bibliography
Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR-04):1–34.
Gastañaduy P, Redd S, Clemmons N, Lee AD, Hickman CJ, Rota PA, et al. Measles. In: Roush SW, Baldy LM, Kirkconnell Hall MA, editors. Manual for the surveillance of vaccine-preventable diseases. Atlanta: Centers for Disease Control and Prevention; 2019. Available from: www.cdc.gov/vaccines/pubs/surv-manual/chpt07-measles.html .
Hyle EP, Fields NF, Fiebelkorn AP, Taylor Walker A, Gastañaduy P, Rao SR, et al. The clinical impact and cost-effectiveness of measles-mumps-rubella vaccination to prevent measles importations among US international travelers. Clin Infect Dis. 2019;69(2):306–15.
Hyle EP, Rao SR, Bangs AC, Gastañaduy P, Parker Fiebelkorn A, Hagmann SHF, et al. Clinical practices for measles-mumps-rubella vaccination among US pediatric international travelers. JAMA Pediatr. 2020;174(2):e194515.
Hyle EP, Rao SR, Jentes ES, Parker Fiebelkorn A, Hagmann SHF, Taylor Walker A, et al. Missed opportunities for measles, mumps, rubella vaccination among departing U.S. adult travelers receiving pretravel health consultations. Ann Intern Med. 2017;167(2):77–84.
Lee AD, Clemmons NS, Patel M, Gastañaduy PA. International importations of measles virus into the United States during the post-elimination era, 2001–2016. J Infect Dis. 2019;219(10):1616–23.
National Notifiable Diseases Surveillance System. Measles (rubeola): 2013 case definition. Atlanta: CDC; 2013. Available from: https://ndc.services.cdc.gov/conditions/measles/ .
Patel MK, Goodson JL, Alexander JP Jr., Kretsinger K, Sodha SV, Steulet C, et al. Progress toward regional measles elimination—Worldwide, 2000–2019. MMWR Morb Mortal Wkly Rep. 2020;69(45):1700–5.
Pike J, Leidner AJ, Gastañaduy PA. A review of measles outbreak cost estimates from the US in the post-elimination era (2004–2017): Estimates by perspective and cost type. Clin Infect Dis. 2020;1(6):1568–76.
World Health Organization. Measles vaccines: WHO position paper—April 2017. Wkly Epidemiol Rec. 2017;92(17):205–27.
File Formats Help:
- Adobe PDF file
- Microsoft PowerPoint file
- Microsoft Word file
- Microsoft Excel file
- Audio/Video file
- Apple Quicktime file
- RealPlayer file
- Zip Archive file
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.

IMAGES
VIDEO
COMMENTS
The hepatitis A vaccine is very effective and has been a routine childhood vaccine since 1996. The vaccine is recommended for international travelers 6 months of age or older going to countries where hepatitis A infection is common. Check if hepatitis A vaccine is recommended for your destination. Hepatitis A vaccine is given in two doses.
Prevention modalities: vaccination, medication, consultation. Hepatitis A. Contaminated food & water. Vaccination (2-dose vaccine): Recommended for most travelers. --Administer 2 doses, at least 6 months apart. --At least 1 dose should be given before travel. Consultation: Advise patient to wash hands frequently and avoid unsafe food and water.
Children need 2 doses of hepatitis A vaccine:. First dose: 12 through 23 months of age; Second dose: at least 6 months after the first dose; Infants 6 through 11 months old traveling outside the United States when protection against hepatitis A is recommended should receive 1 dose of hepatitis A vaccine. These children should still get 2 additional doses at the recommended ages for long ...
Examples of Vaccines. Here is a list of possible vaccines that you may need to get for the first time or boosters before you travel. COVID-19. Chickenpox. Cholera. Flu (Influenza) Hepatitis A. Hepatitis B.
Non-routine vaccinations required for travel to some countries Staying up to date on typical vaccines like those for COVID-19, flu, tetanus and hepatitis A and B is a smart choice for everyone.
Because hepatitis A vaccine consists of inactivated virus, and hepatitis B vaccine consists of a recombinant protein, no special precautions are needed for vaccination of immunocompromised travelers with single-antigen vaccines or Twinrix. Check precautions and contraindications before administering IG. Pregnancy
The hepatitis A vaccine series should be completed according to the routine schedule. Information on immune globulin dosing and additional information on hepatitis A vaccine and travel is available. What should be done to protect international travelers <6 months of age and other travelers unable to receive hepatitis A vaccine?
Hepatitis A Vaccination. Pronounced (hep-ah-TY-tiss) Hepatitis A is a liver disease caused by the hepatitis A virus (HAV). Hepatitis A can affect anyone. Vaccines are available for long-term prevention of HAV infection in persons 1 year of age and older. Good personal hygiene and proper sanitation can also help prevent the spread of hepatitis A.
Hepatitis A Vaccine for Travel. The vaccine contains inactivated virus and can prevent the disease. It is often recommended for those who are traveling to a country where the virus is common. Your doctor may recommend a Hepatitis A vaccine before travel to the following countries: Brazil; Caribbean; China; Ghana; India; Kenya; Peru; South ...
Hepatitis A Vaccine and International Travel. Who should get the hepatitis A vaccine before traveling internationally? All unvaccinated people, along with those who have never had hepatitis A, should be vaccinated before traveling to countries where hepatitis A is common. Travelers to urban areas, resorts, and luxury hotels in countries where ...
Vaccines for Travelers. Vaccines protect travelers from serious diseases. Depending on where you travel, you may come into contact with diseases that are rare in the United States, like yellow fever. Some vaccines may also be required for you to travel to certain places. Getting vaccinated will help keep you safe and healthy while you're ...
In this series: Travel vaccinations Hepatitis B vaccine Rabies vaccine Tick-borne encephalitis vaccine Typhoid vaccine Yellow fever vaccine. You should consider vaccination against hepatitis A before you travel to certain countries, such as the Indian subcontinent. In this article:
The three most commonly recommended travel vaccines are: Hepatitis A vaccine for travel to most other countries in the world. Hepatitis A is a common viral infection that you can get from contaminated water or food. Yellow fever vaccine for travel to some parts of Africa and South America. Yellow fever is a virus that spreads through mosquito ...
For instance, the yellow fever vaccine offers lifelong protection for most people. But typhoid vaccine boosters are recommended every 2 to 5 years. The typical yellow fever vaccine cost is around $170 — but this can vary by clinic and location. GoodRx can help make your travel vaccines more affordable.
Pneumococcal. Measles, mumps and rubella (MMR) Polio. Shingles. Additional vaccines may be recommended depending on your travel itinerary. For example, hepatitis A vaccination is recommended if you are traveling to Southeast Asia. During your appointment, we can discuss which vaccines are appropriate for your itinerary. 3.
People at an increased risk from hepatitis A include: Travelers to countries that have high rates of hepatitis A; Families planning to adopt a child or care for an adopted child from a country with high rates of hepatitis A ... The safety of the hepatitis A vaccine for pregnant people hasn't been determined. However, there's no evidence of harm ...
But some multiple-dose vaccines (like hepatitis A) can still give you partial protection after just one dose. Some can also be given on an "accelerated schedule," meaning doses are given in a shorter period of time. Before you travel, find out which vaccines you and your family will need. Plan ahead to get the shots required for all ...
The best way to prevent hepatitis A infection is through vaccination. In March 2024, 64% of cases were not up-to-date on hepatitis A vaccinations and 18% of cases had unknown hepatitis A vaccination status. Since 2006, hepatitis A vaccine has been recommended for all children at age 1 year. Hepatitis A vaccine is also
If the person is departing for travel soon, consider an accelerated schedule, if appropriate, such as for hepatitis B vaccine or the combination hepatitis A-hepatitis B vaccine (see Hepatitis A and Hepatitis B). Although immunity may be established sooner with the accelerated schedule, people who receive an accelerated schedule need another ...
Hepatitis A is an acute viral infection of the liver, which can cause mild to severe illness. The illness is usually self-limiting and needs no treatment. It is transmitted primarily by the faecal-oral route by ingesting contaminated food and water, or by direct contact with an infectious person. Hepatitis A is highly contagious.
Hepatitis A Hepatitis B Meningococcal Hib Mpox At least 1 dose of an updated COVID-19 vaccine Every Year Tdap every pregnancy. Td/Tdap every 10 years for all adults. 27-45 years If aged 66 years or younger If U.S. born and aged 43 years or younger If pregnant during RSV season If aged 60 years or older Through 59 years You need vaccines ...
Routine hepatitis A vaccination for children aged ≥12 months consists of 2 doses, separated by ≥6 months. Ideally, the first dose should be administered ≥2 weeks before travel. When protection against hepatitis A is recommended, infants aged 6-11 months should receive 1 dose of hepatitis A vaccine before travel outside the United States.
Immunisation Handbook. 2024, version 1. The Immunisation Handbook provides clinical guidelines for health professionals on the safest and most effective use of vaccines in their practice. These guidelines are based on the best scientific evidence available at the time of publication, from published and unpublished literature.
There is a risk of Hepatitis A and B throughout South Africa, so vaccinations for Australian travellers are recommended. ... It is essential that you consult a medical practitioner regarding your ...
Hepatitis A vaccine info for healthcare professionals: vaccine recommendations, about hepatitis A vaccine, storage and handling, administering vaccine, references and resources Skip directly to site content Skip directly to search. ... Preventive measures for travelers Top of Page. Surveillance. Surveillance manual's chapter on Hepatitis A
Even with limited time before departure, research supports the use of certain single-dose vaccines, if indicated, to initiate protection in LMTs. These include cholera for selected travelers, hepatitis A (monovalent), meningococcal (quadrivalent, ACWY), polio booster (inactivated), and typhoid (injectable) vaccines.
Adria Malcolm for The New York Times. By Ted Alcorn. April 28, 2024. In the 10 years since the drugmaker Gilead debuted a revolutionary treatment for hepatitis C, a wave of new therapies have been ...
Tetanus toxoid is a core equine vaccine and should be included in equine immunization programs for every horse annually. Learn More. Venezuelan Equine Encephalomyelitis Vaccination Guidelines. Venezuelan Equine Encephalomyelitis Vaccination Guidelines.
Follow the vaccine schedule. The Centers for Disease Control and Prevention, American Academy of Family Physicians, and American Academy of Pediatrics strongly recommend children receive all vaccines according to the recommended vaccine schedule. Get a list of vaccines that your child may need based on age, health conditions, and other factors.
Nearly 90% of imported measles cases are considered preventable by vaccination (i.e., the travelers lacked recommended age- and travel-appropriate vaccination). Furthermore, observational studies in travel clinics in the United States have shown that 59% of pediatric and 53% of adult travelers eligible for measles-mumps-rubella (MMR) vaccine at ...