- Find in topic
INTRODUCTION
The treatment and prevention of travelers' diarrhea are discussed here. The epidemiology, microbiology, clinical manifestations, and diagnosis of travelers' diarrhea are discussed separately. (See "Travelers' diarrhea: Epidemiology, microbiology, clinical manifestations, and diagnosis" .)
Clinical approach — Management of travelers’ diarrhea depends on the severity of illness. Fluid replacement is an essential component of treatment for all cases of travelers’ diarrhea. Most cases are self-limited and resolve on their own within three to five days of treatment with fluid replacement only. Antimotility agents can provide symptomatic relief but should not be used when bloody diarrhea is present. Antimicrobial therapy shortens the disease duration, but the benefit of antibiotics must be weighed against potential risks, including adverse effects and selection for resistant bacteria. These issues are discussed in the sections that follow.
When to seek care — Travelers from resource-rich settings who develop diarrhea while traveling to resource-limited settings generally can treat themselves rather than seek medical advice while traveling. However, medical evaluation may be warranted in patients who develop high fever, abdominal pain, bloody diarrhea, or vomiting. Otherwise, for most patients while traveling or after returning home, medical consultation is generally not warranted unless symptoms persist for 10 to 14 days.
Fluid replacement — The primary and most important treatment of travelers' (or any other) diarrhea is fluid replacement, since the most significant complication of diarrhea is volume depletion [ 11,12 ]. The approach to fluid replacement depends on the severity of the diarrhea and volume depletion. Travelers can use the amount of urine passed as a general guide to their level of volume depletion. If they are urinating regularly, even if the color is dark yellow, the diarrhea and volume depletion are likely mild. If there is a paucity of urine and that small amount is dark yellow, the diarrhea and volume depletion are likely more severe.
To continue reading this article, you must sign in . For more information or to purchase a personal subscription, click below on the option that best describes you:
- Medical Professional
- Resident, Fellow or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver

Print Options
You are using an outdated browser. Upgrade your browser today or install Google Chrome Frame to better experience this site.
- Section 2 - Travelers’ Diarrhea
- Section 2 - Food & Water Precautions
Perspectives : Antibiotics in Travelers' Diarrhea - Balancing Benefit & Risk
Cdc yellow book 2024.
Author(s): Mark Riddle, Bradley Connor
For the past 30 years, randomized controlled trials have consistently and clearly demonstrated that antibiotics shorten the duration of illness and alleviate the disability associated with travelers’ diarrhea (TD). Treatment with an effective antibiotic shortens the average duration of a TD episode by 1–2 days, and if the traveler combines an antibiotic with an antimotility agent (e.g., loperamide), duration of illness is shortened even further. Emerging data on the potential long-term health consequences of TD (e.g., chronic constipation, dyspepsia, irritable bowel syndrome) might suggest a benefit of early antibiotic therapy given the association between more severe and longer disease and risk for postinfectious consequences.
Antibiotics commonly used to treat TD have side effects, some of which are severe but rare. Perhaps of greater concern is the recent understanding that antibiotics used by travelers can contribute to changes in the host microbiome and to the acquisition of multidrug-resistant bacteria. Multiple observational studies have found that travelers (in particular, travelers to South and Southeast Asia) who develop TD and take antibiotics are at risk for colonization with extended-spectrum β-lactamase–producing Enterobacteriaceae (ESBL-PE).
The direct effect of colonization on the average traveler appears limited; carriage is most often transient, but it does persist in a small percentage of colonized persons. Elderly travelers (because of the serious consequences of bloodstream infections in this population) and those with a history of recurrent urinary tract infections (because Escherichia coli is a common cause) might be at an increased risk for health consequences from ESBL-PE colonization. At a minimum, clinicians should make these travelers aware of the risk and counsel them to convey their travel exposure history to their treating providers if they become ill after travel. Of broader importance, international travel has been associated with subsequent ESBL-PE colonization among close-living contacts, suggesting potentially wider public health consequences from ESBL-PE acquisition during travel.
The Challenge
The challenge providers and travelers face is how to balance the health benefit of short-course antibiotic treatment of TD with the risk for colonization and global spread of resistance. The role played by travelers in the translocation of infectious disease and resistance cannot be ignored, but the ecology of ESBL-PE infections is complex and includes diet, environment, immigration, and local nosocomial transmission dynamics. ESBL-PE infections are an emerging health threat, and addressing this complex problem will require multiple strategies, including antibiotic stewardship.
An Approach
Health care providers need to have conversations with travelers about the multilevel (individual, community, global) and multifactorial risks of developing TD: travel, individual behaviors (e.g., hand hygiene), diet (e.g., safe selection of foods and beverages), and other risk avoidance measures. But then, knowing it is often difficult to prevent or even reduce the risk for TD through behaviors and diet alone, what is the most reasonable way to prepare travelers for empiric TD self-treatment before a trip? Clinicians can strongly emphasize reserving antibiotics for moderate to severe TD and using antimotility agents for self-treatment of mild TD.
When it comes to managing TD, we expect the traveler to be both diagnostician and health care provider. For even the most astute traveler, making an appropriately informed decision about their own health can be challenged by the anxiety-provoking onset of that first abdominal cramp in sometimes austere and inconvenient settings. Given that TD counseling is competing with numerous other pretravel health topics that need to be covered, travel medicine providers might want to develop and implement simple messaging, handouts, or easy-to-access electronic health guidance. Providing travelers with clear written guidance about TD prevention and step-by-step instructions about how and when to use medications for TD is crucial.
Though further studies are needed (and many are under way), a rational approach involves using a single-dose regimen of an antibiotic that minimizes microbiome disruption and risk for colonization. Additionally, as travel and untreated TD independently increase the risk for ESBL-PE colonization, nonantibiotic chemoprophylactic strategies (e.g., self-treatment with bismuth subsalicylate), can decrease both the acute and posttravel risk concerns. Strengthening the resilience of the host microbiota to prevent infection and unwanted colonization, as with the use of prebiotics or probiotics, are promising potential strategies but need further investigation.
The following authors contributed to the previous version of this chapter: Mark S. Riddle, Bradley A. Connor
Bibliography
Arcilla MS, van Hattem JM, Haverkate MR, Bootsma MCJ, van Genderen PJJ, Goorhuis A, et al. Import and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): a prospective, multicentre cohort study. Lancet Infect Dis. 2017;17(1):78–85.
Riddle MS, Connor BA, Beeching NJ, DuPont HL, Hamer DH, Kozarsky P, et al. Guidelines for the prevention and treatment of travelers’ diarrhea: a graded expert panel report. J Travel Med. 2017;24 (Suppl 1):S57–74.
. . . perspectives chapters supplement the clinical guidance in this book with additional content, context, and expert opinion. The views expressed do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
File Formats Help:
- Adobe PDF file
- Microsoft PowerPoint file
- Microsoft Word file
- Microsoft Excel file
- Audio/Video file
- Apple Quicktime file
- RealPlayer file
- Zip Archive file
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Enter search terms to find related medical topics, multimedia and more.
Advanced Search:
- Use “ “ for exact phrases.
- For example: “pediatric abdominal pain”
- Use – to remove results with certain keywords.
- For example: abdominal pain -pediatric
- Use OR to account for alternate keywords.
- For example: teenager OR adolescent
Traveler’s Diarrhea
, MD, Lewis Katz School of Medicine at Temple University
More Information
Traveler’s diarrhea is an infection characterized by diarrhea, nausea, and vomiting that commonly occur in travelers to areas of the world with poor water purification.
Traveler's diarrhea can be caused by bacteria, parasites, or viruses.
Organisms that cause the disorder are usually acquired from food or water, especially in countries where the water supply may be inadequately treated.
Nausea, vomiting, abdominal cramping, and diarrhea can occur with any degree of severity.
The diagnosis is usually based on the doctor's evaluation, but sometimes stool is tested for organisms.
Treatment involves drinking plenty of safe fluids and sometimes taking antidiarrheal medications or antibiotics.
Preventive measures include drinking only bottled carbonated beverages, avoiding uncooked vegetables or fruits, not using ice cubes, and using bottled water to brush teeth.

Traveler’s diarrhea occurs when people are exposed to bacteria, viruses, or, less commonly, parasites to which they have had little exposure and thus no immunity. The organisms are usually acquired from food or water (including water used to wash foods).
Traveler’s diarrhea occurs mostly in countries where the water supply is inadequately treated.

Travelers who avoid drinking local water may still become infected by brushing their teeth with an improperly rinsed toothbrush, drinking bottled drinks with ice made from local water, or eating food that is improperly handled or washed with local water. People who take medications that decrease stomach acid (such as antacids, H2 blockers, and proton pump inhibitors) are at risk of developing a more severe illness.
Symptoms of Traveler’s Diarrhea
The following symptoms of traveler's diarrhea can occur in any combination and with any degree of severity:
Intestinal rumbling
Abdominal cramping
These symptoms begin 12 to 72 hours after ingesting contaminated food or water. Vomiting, headache, and muscle pain are particularly common in infections caused by norovirus. Rarely, diarrhea is bloody.
Most cases are mild and disappear without treatment within 3 to 5 days.
Diagnosis of Traveler’s Diarrhea
A doctor's evaluation
Rarely stool tests
Diagnostic tests are rarely needed, but sometimes stool samples are tested for bacteria, viruses, or parasites, typically in people who have fever, severe abdominal pain, and bloody diarrhea.
Treatment of Traveler’s Diarrhea
Medications that stop diarrhea (antidiarrheal medications)
Sometimes antibiotics or antiparasitic medications
When symptoms occur, treatment includes drinking plenty of fluids and taking antidiarrheal medications such as loperamide .
These medications are not given to children under 18 years of age with acute diarrhea. Antidiarrheal medications are also not given to people who have recently used antibiotics, who have bloody diarrhea, who have small amounts of blood in the stool that are too small to be seen, or who have diarrhea and fever.
Antibiotics are not necessary for mild traveler's diarrhea.
However, if diarrhea is more severe (3 or more loose stools over 8 hours), antibiotics are often given. Adults may be given ciprofloxacin , levofloxacin , azithromycin , or rifaximin . Children may be given azithromycin . Antibiotics are not given if a virus is the cause.
Antiparasitic medications are given if a parasite is identified in the stool.
Travelers are encouraged to seek medical care if they develop fever or blood in the stool.
Prevention of Traveler’s Diarrhea
Safe consumption of food and water
Travelers should eat only in restaurants with a reputation for safety and should not consume any food or beverages from street vendors. Cooked foods that are still hot when served are generally safe. Salads containing uncooked vegetables or fruit and salsa left on the table in open containers should be avoided. Any fruit should be peeled by the traveler.
Travelers should drink only bottled carbonated beverages or beverages made with water that has been boiled. Even ice cubes should be made with water that has been boiled.
Buffets and fast food restaurants pose an increased risk of infection.
Preventive antibiotics are recommended only for people who are particularly susceptible to the consequences of traveler’s diarrhea, such as those whose immune system is impaired, those who have inflammatory bowel disease, those who have HIV, those who have received an organ transplant, and those who have severe heart or kidney disease. The antibiotic most commonly given is rifaximin . Some travelers instead take bismuth subsalicylate rather than an antibiotic for prevention.
The following English-language resource may be useful. Please note that THE MANUAL is not responsible for the content of this resource.
Centers for Disease Control and Prevention (CDC): Choose Safe Food and Drinks When Traveling
Drugs Mentioned In This Article

Was This Page Helpful?

Test your knowledge
Brought to you by Merck & Co, Inc., Rahway, NJ, USA (known as MSD outside the US and Canada)—dedicated to using leading-edge science to save and improve lives around the world. Learn more about the Merck Manuals and our commitment to Global Medical Knowledge .
- Permissions
- Cookie Settings
- Terms of use
- Veterinary Edition
- IN THIS TOPIC
- Search Menu
- Advance articles
- Collections
- Editor's Choice
- Supplements
- Author Guidelines
- Submission Site
- Open Access
- About Journal of Travel Medicine
- About the International Society of Travel Medicine
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction.
- < Previous
Medications for the prevention and treatment of travellers’ diarrhea
- Article contents
- Figures & tables
- Supplementary Data
David N. Taylor, Davidson H. Hamer, David R. Shlim, Medications for the prevention and treatment of travellers’ diarrhea, Journal of Travel Medicine , Volume 24, Issue suppl_1, April 2017, Pages S17–S22, https://doi.org/10.1093/jtm/taw097
- Permissions Icon Permissions
Background . Travellers’ diarrhea (TD) remains one of the most common illnesses encountered by travellers to less developed areas of the world. Because bacterial pathogens such as enterotoxigenic Escherichia coli (ETEC), enteroaggregative E. coli , Campylobacter spp. and Shigella spp. are the most frequent causes, antibiotics have been useful in both prevention and treatment of TD.
Methods. Results of trials that assessed the use of medications for the prevention and treatment of TD were identified through PubMed and MEDLINE searches using search terms ‘travellers’ diarrhea’, ‘prevention’ and ‘treatment’. References of articles were also screened for additional relevant studies.
Results. Prevention of TD with antibiotics has been recommended only under special circumstances. Doxycycline, trimethoprim–sulfamethoxazole, fluoroquinolones and rifaximin have been used for prevention, but at present the first three antibiotics may have limited use secondary to increasing resistance, leaving rifaximin as the only current option. Bismuth subsalicylate (BSS) (Pepto-Bismol tablets) is also an option for prophylaxis. Treatment with antibiotics has been recommended for moderate to severe TD. Azithromycin is the drug of choice, especially in Asia where Campylobacter is common. Fluoroquinolone antibiotics continue to be effectively used in Latin America and Africa where ETEC is predominant. BSS and loperamide (LOP) also are effective as standalone treatments. LOP may be used alone for treatment of mild TD or in conjunction with antibiotics for treatment of TD.
Conclusions . Historically, antibiotic prophylaxis has not been routinely recommended and has been reserved for special circumstances such as when a traveller with an underlying illness cannot tolerate TD. Antibiotics with or without LOP have been useful in shortening the duration and severity of TD. Emerging antibiotic resistance, limited new antibiotic alternatives and faecal carriage of antibiotic-resistant bacteria by travellers may prompt a re-evaluation of classic recommendations for treatment and prevention of TD with antibiotics.
Preventing and treating travellers’ diarrhea (TD) during and after a journey continue to be important clinical challenges. Bacterial pathogens such as enterotoxigenic Escherichia coli (ETEC), enteroaggregative E. coli , Campylobacter species and Shigella spp. still predominate as causes; however the list of etiologic agents causing diarrheal disease includes a broad spectrum of bacteria, viruses and parasites. 1–3 As diagnostic methods improve, multiple pathogens are detected in a higher proportion of TD illnesses. 4 , 5 Antimicrobial resistance of enteric bacterial pathogens continues to increase and requires surveillance to monitor current trends. 6 Emerging research has further highlighted the acute and chronic health consequences of TD. 7 There is now increasing awareness of alterations to gut microflora caused by even a short course of antibiotics. 8 Some of these changes to the gut microflora appear to be associated with chronic gastrointestinal symptoms and enteropathy, and there is growing recognition of colonization with multi‐drug resistant bacteria in returning travellers. 9 This review outlines the classical recommendations for use of antimicrobial and other agents in the treatment and prevention of TD and sets the stage for the consensus statements that follow on how recommendations might need to change in view of emerging concerns for the use of antimicrobial agents.
Results of trials that assessed the use of antibiotics for the prevention and treatment of TD were identified through PubMed and MEDLINE searches using search terms ‘travellers’ diarrhea’, ‘prevention’ and ‘treatment’. References of articles were also screened for additional relevant studies.
Historical Definitions of Diarrhea
Diarrhea is one of the most common illnesses acquired during travel. TD caused by ETEC, the most common aetiology, is usually a watery diarrhea associated with nausea, vomiting, and abdominal cramping or pain. Shigella and Campylobacter may also be associated with bloody diarrhea instead of watery diarrhea and a longer and more severe course of illness, associated with invasion of the gut mucosa. ETEC is confined to the lumen of the gut and disease is mediated by toxin. Invasion of the gut mucosa or spread to blood or other tissues does not occur. The spectrum of illness has been categorized as mild, moderate or severe. Severe diarrhea is characterized by more than 10 loose, watery stools in a single day, moderate diarrhea is more than a few diarrhea stools but less than 10 diarrhea stools in a day, and mild diarrhea is a few diarrhea stools in a day. In clinical and epidemiologic studies, TD has been defined as three or more unformed stools or two unformed stools with at least one accompanying symptom (nausea, vomiting, abdominal pain, fever, blood in stool) within 24 h. 10
History of Antibiotic Use for TD
When Dr Benjamin Kean first started investigating diarrhea in travellers in Mexico in the 1950s, the aetiology was elusive. The known bacterial pathogens of the time were not present in these patients. 11 An exhaustive search for protozoan pathogens proved fruitless. 12 An interesting observation, at the time, was that non-absorbable antibiotics (phthalylsulfathiazole and neomycin) prevented diarrhea in travellers. 13 , 14 Eventually, the discovery of ETEC confirmed the predominantly bacterial aetiology of TD in Mexico. 15 , 16 Studies in the mid-East, Asia and South America provided additional proof that bacteria were the main cause of TD. 2 , 17 , 18 By 1970s, the threat of TD was such a concern that studies were done to see if prophylactic antibiotics would prevent TD. Doxycycline was proven to prevent TD in two studies in the late 1970s. 19 , 20 At the same time, clinicians were convinced that travellers who were able to make good decisions about what to avoid eating and drinking could also avoid TD. This feeling continues to persist, despite the absence of any reliable studies demonstrating that travellers can avoid TD by watching what they eat. 21 Since TD was not fully preventable by taking precautions with food and beverages, the discussion naturally focused on antimicrobial prophylaxis or treatment, both of which had been shown to be effective. 22 However, at the same time, many clinicians still considered TD to be a minor, self-limited illness for which antibiotic treatment was not indicated. There was also an ongoing feeling among clinicians that empiric treatment was not justified, and that a definitive diagnosis should be made before treatment could be offered.
Whatever the concerns were about treatment, the impact of TD was and still remains significant for travellers. The incidence of TD in resource-poor countries is never less than 20–30% of travellers, and can be as much as 88%. That means the chance that a traveller will have their trip impacted by an episode of uncomfortable diarrhea ranges from 1 in 5 to over 4 in 5.
The concept that TD is a self-limited illness that does not require treatment is belied by the magnitude of the symptoms present in a majority of cases. Vomiting, fever, severe cramps and blood in the stool are present in a substantial subset of travellers with acute gastroenteritis. A description of the natural history of TD acquired among Finnish travellers in Morocco found that 23% of patients with an identifiable invasive pathogen were still ill at day 7. Among patients with no pathogen identified, or a non-invasive pathogen, 20% were still ill after 4 days. 23 Significantly, 37% of patients with an identified bacterial pathogen had fever.
Empiric Treatment of Diarrhea—Historical Perspective
Once it was confirmed that pathogenic bacteria were the main cause of acute TD, this paved the way for empiric treatment without obtaining stool exams or cultures. 18 , 24 Since TD often occurred in a setting far from medical care, the next step was to provide travellers with empiric antibiotics to carry with them, to minimize the impact of TD when it occurred. This approach was recommended as early as 1985. The initial antibiotic that was used was trimethoprim–sulfamethoxazole (TMP–SMX), twice a day for 5 days. 25 . Clinical experience later demonstrated that many patients were better within 24 h of taking their first one or two doses, and they did not always finish the remaining course. Subsequently, treatment was shortened to three days, and even less. In the latter half of the 1980s, resistance to TMP–SMX began to be noted, with resulting treatment failures. 26 Fluoroquinolones were then employed. 27–29 The length of treatment with ciprofloxacin was twice a day for three days, which later was gradually shortened to one day, as studies confirmed that even single dose treatment could be effective. 30 Early randomized, placebo-controlled trial of ciprofloxacin in 1987 demonstrated that the mean duration of diarrhea in the treatment group was 1.5 vs. 2.9 days in the placebo group. 29 In addition, the mean duration of fever was 1.3 vs. 3.1 days. In this study, two patients in the placebo group subsequently received ciprofloxacin: one patient with fever after 4 days, and one patient with 5 days of diarrhea. Both patients improved after one day of treatment with ciprofloxacin 500 mg BID.
Starting in the mid-1990s in Thailand, fluoroquinolone-resistant Campylobacter jejuni began to be noted. 31 This increased rapidly, and since C. jejuni was found to account for 20–25% of the etiology of diarrhea in Thailand (and Nepal), this had an impact on treatment failure. Based on the observation that Campylobacter was sensitive to erythromycin, azithromycin (AZM) began to be used for fluoroquinolone treatment failures, and later as empiric treatment. 30 , 32
In 1985, an influential TD consensus statement concluded that it was difficult to prevent TD with eating precautions, although they conjectured that it was possible. 21 , 33 Prophylactic antibiotics were discouraged, due to the risk of side effects from the mass administration of antibiotics, and the possibility of creating resistant organisms. Empiric treatment was the recommended approach to TD. 24 , 30 In the late 1990s, a non-absorbable antibiotic, rifaximin, was tested for both the prophylaxis and treatment of TD. 34–36 However, rifaximin could not be used to treat invasive organisms, such as Campylobacter or Shigella , so its usefulness for empiric self-treatment of TD was limited in many parts of the world.
Non-Antibiotic Therapy for Empiric Treatment of diarrhea—Historical Perspective
In the mid-1970s, bismuth subsalicylate (BSS) as a liquid formulation was first evaluated for the treatment of TD. 37 US subjects with TD acquired in Mexico were treated with 4.2 g of BSS over a 3.5-h period, which resulted in a decrease in the number of unformed stools compared to placebo. In a study of TD prevention, US students in Mexico received BSS or placebo for 21 days. TD occurred in 23% of 62 students taking BSS compared to 61% of 66 students taking placebo. 38 A second prevention study was performed using BSS tablets. US students visiting Mexico received two tablets (high dose) or one tablet (low dose) of BSS four times daily or a placebo during a three-week period. TD occurred in 7 (14%) of 51 receiving the high-dose regimen compared with 15 (24%) of 63 receiving the low-dose regimen and 23 (40%) of 58 in the placebo group. BSS in both dosages caused blackening of tongues and stools and infrequently was associated with tinnitus. 39 BSS at doses of 150 mg/kg was also found to be safe and effective in treating infantile diarrhea even when the cause of illness was viral. 40 , 41
BSS was compared to loperamide (LOP) for the treatment of non-dysenteric TD in US students in Mexico. 39 , 42 LOP was more effective than BSS in reducing the number of unformed stools at all time points and was more often associated with constipation than BSS. BSS was also shown to be effective in Africa 43 and treatment with BSS or LOP became an other option for the treatment of TD. 44 The utility of an anti-motility drug such as LOP to lessen diarrhea, particularly in the first 24 h of illness, has historically been offset by the concern that it could worsen an illness caused by an invasive pathogen such as Shigella . This led to evaluation of the safety of LOP when taken along with an antibiotic. One of the first studies of this kind, was the evaluation of TMP–SMX with LOP versus LOP alone. 45 The combination of antibiotic plus LOP gave the shortest duration of diarrhea compared with the placebo group (1 vs 59 h). The authors concluded that the combination of TMP–SMX plus LOP can be highly recommended for the treatment of most patients with traveller's diarrhea. Other studies found that the combination of ciprofloxacin plus LOP was also effective and safe to use even in the presence of dysentery caused by Shigella . 27 , 46
Hill evaluated the choices made by US travellers for TD. 47 Overall, 80% of persons with TD chose to take some medication. BSS or LOP were taken by 48%, while 30% took an antibiotic with an antimotility drug and 15% took antibiotic alone. About 80% of persons said that their symptoms improved regardless of treatment category. In a recent placebo-controlled study, BSS was evaluated to reduce the use of antibiotics among Pakistani adults who presented to outpatient clinics with diarrhea. 48 In this study 9.1% of 220 patients were eventually treated with antibiotics in the BSS group and 15.5% in the placebo group. There was a 41% reduction in antibiotic use among those who received BSS, but the study did not report on the length of time that the patients remained ill. These studies suggest that BSS may be tried first and will often allow patients to avoid antibiotics. LOP could also be tried as a first medication but appears to be more reliably used in combination with an antibiotic.
Current Recommendations on Medications for the Prevention and Treatment of TD
Medications for td prophylaxis.
Agents available for the management of TD
Adapted from Hill et al. 49 .
Should be considered mainly for trips <2 weeks in duration.
The recommended over-the-counter dose is 8 mg daily.
Effectiveness of rifaximin and fluoroquinolones in preventing TD: systematic review and meta-analysis
Alajbegovic et al. 51 .
In the meta-analysis performed by Alajbegovic et al. , the fluoroquinolone antibiotics, such as ciprofloxacin and norfloxacin, given for prevention showed a high degree of protection ( Table 2 ). 51 However, many of the studies were performed in the 1980s and 1990s before fluoroquinolone resistance was detected. It is likely that if the studies were repeated today in Asia, fluoroquinolone antibiotics would be less effective due to the increasing prevalence of fluoroquinolone resistance documented in South and South East Asia. 52–55 Prophylactic fluoroquinolone use can also be discouraged because fluoroquinolones are one of the mainstays of treatment. In addition, in May 2016, the US Food and Drug Administration (FDA) released a new Black Box warning for fluoroquinolones ( http://www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf ), advising that the potentially serious side effects (tendon, joint and muscle pain; paresthesia; confusion and hallucination) associated with this class of antibiotic generally outweigh the benefits for patients with acute sinusitis, acute bronchitis and uncomplicated urinary tract infections who have other treatment options. While these recommendations do not apply to short treatment courses of fluoroquinolones and the FDA did not specifically comment on serious adverse events associated with the use of fluoroquinolones for TD, the risk of longer term treatment that is likely to result during prophylactic use might be another reason for avoiding this class of agents for the prevention of TD.
If diarrhea occurs while on fluoroquinolone prophylaxis, the only remaining option is AZM. Additionally, fluoroquinolone prophylaxis, and antibiotic use in general, may have unintended consequences such as selection for antibiotic resistant gut flora 9 , 56 or disruption of the gut microbiome that could lead to colonization with pathogenic bacteria such as Clostridium difficile .
Risk factors associated with TD
Adapted from Ericsson CD. Prevention of TD in Travel Medicine 2 nd edition. 59
Medications for Empiric Self-Treatment
The alternative to prophylaxis is self-treatment. Studies have shown that up to half of all TD cases are mild and self-limited after a day or two of illness. The use of empiric self-treatment of antibiotics for bacterial diarrhea has been a very successful method of keeping travellers from getting stranded at destinations, on treks, and facing unnecessary hospitalization in a developing country. Given that it is difficult to anticipate which traveller to which destination might become severely ill, current recommendations are to provide antibiotics to travellers for use in an emergency, even in lower risk destinations. Many recommendations encourage the use of antibiotics only for more severe illness. A step wise approach to treatment has been espoused in recommendations; first try BSS or LOP to see if they provide enough relief for mild illness and reserve antibiotic self-treatment for moderate or severe TD.
Treatment of travellers’ diarrhea with rifaximin and/or LOP
DuPont et al. 36
TLUS, time to last unformed stool.
Comparative efficacy and tolerability of single doses of AZM and the combination of LOP and single dose AZM in the treatment of acute TD
Ericsson et al. 58 .
The routine use of empiric antibiotics for TD has recently been questioned again, this time due to the specific concern about travellers acquiring highly resistant E. coli during travel, along with general concerns of change in the intestinal microbiome, and increasing antibiotic resistant of intestinal pathogens. Already, there are signs of bacterial resistance to AZM, with no obvious replacement antibiotic on the horizon. As a result, the ongoing history of antibiotic treatment of TD remains unsettled. This is a disturbing prospect for those clinicians old enough to remember the pre-antibiotic era of TD.
Conflict of interest : None declared.
Jiang ZD , Lowe B , Verenkar MP et al. Prevalence of enteric pathogens among international travelers with diarrhea acquired in Kenya (Mombasa), India (Goa), or Jamaica (Montego Bay) . J Infect Dis 2002 ; 185 : 497 – 502 .
Google Scholar
Shah N , Ldupont H , Ramsey DJ. Global etiology of travelers’ diarrhea: systematic review from 1973 to the present . Am J Trop Med Hyg 2009 ; 80 : 609 – 14 .
Swaminathan A , Torresi J , Schlagenhauf P et al. A global study of pathogens and host risk factors associated with infectious gastrointestinal disease in returned international travellers . J Infect 2009 ; 59 : 19 – 27 .
Koo HL , Ajami NJ , Jiang ZD et al. Noroviruses as a cause of diarrhea in travelers to Guatemala, India, and Mexico . J Clin Microbiol 2010 ; 48 : 1673 – 6 .
Mattila L , Siitonen A , Kyrönseppä H et al. Seasonal variation in etiology of travelers’ diarrhea. Finnish-Moroccan Study Group . J Infect Dis 1992 ; 165 : 385 – 8 .
Ouyang-Latimer J , Jafri S , VanTassel A et al. In vitro antimicrobial susceptibility of bacterial enteropathogens isolated from international travelers to Mexico, Guatemala, and India from 2006 to 2008 . Antimicrob Agents Chemother 2011 ; 55 : 874 – 8 .
Youmans BP , Ajami NJ , Jiang ZD et al. Characterization of the human gut microbiome during travelers’ diarrhea . Gut Microbes 2015 ; 6 : 110 – 9 .
Dethlefsen L , Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation . Proc Natl Acad Sci USA 2011 ; 108 ( Suppl 1 ): 4554 – 61 .
Kantele A , Lääveri T , Mero S et al. Antimicrobials increase travelers’ risk of colonization by extended-spectrum betalactamase-producing enterobacteriaceae . Clin Infect Dis 2015 ; 60 : 837 – 46 .
DuPont HL , Cooperstock M , Corrado ML , Fekety R. Evaluation of new anti-infective drugs for the treatment of acute infectious diarrhea. Infectious Diseases Society of America and the Food and Drug Administration . Clin Infect Dis 1992 ; 15 ( Suppl 1 ): S228 – 35 .
Varela G , Kean BH , Barrett EL , Keegan CJ. The diarrhea of travelers. II. Bacteriologic studies of U. S. students in Mexico . Am J Trop Med Hyg 1959 ; 8 : 353 – 7 .
Rosenbluth MA , Schaffner W , Kean BH. The diarrhea of travelers. IV. Viral studies of visiting students in Mexico with further bacteriologic and parasitologic observations . Am J Trop Med Hyg 1963 ; 12 : 239 – 45 .
Kean BH , Waters SR. The diarrhea of travelers . N Engl J Med 1959 ; 261 : 71 – 4 .
Kean B , Schaffner W , Brennan RW. The diarrhea of travelers V. prophylaxis with phthalylsulfathiazole and neomycin sulphate . JAMA 1962 ; 180 : 367 – 71 .
Gorbach SL , Kean BH , Evans DG , Evans DJ , Bessudo D. Travelers’ diarrhea and toxigenic Escherichia coli . N Engl J Med 1975 ; 292 : 933 – 6 .
Merson MH , Morris GK , Sack DA et al. Travelers’ diarrhea in Mexico . N Engl J Med 1976 ; 294 : 1299 – 305 .
Rowe B , Taylor J , Bettelheim KA. An investigation of traveller’s diarrhoea . Lancet 1970 ; 1 : 1 – 5 .
Taylor DN , Houston R , Shlim DR , Bhaibulaya M , Ungar BL , Echeverria P. Etiology of diarrhea among travelers and foreign residents in Nepal . JAMA 1988 ; 260 : 1245 – 8 .
Sack RB , Froehlich JL , Zulich AW et al. Prophylactic doxycycline for travelers’ diarrhea: results of a prospective double-blind study of Peace Corps volunteers in Morocco . Gastroenterology 1979 ; 76 : 1368 – 73 .
Sack DA , Kaminsky DC , Sack RB et al. Prophylactic doxycycline for travelers’ diarrhea . N Engl J Med 1978 ; 298 : 758 – 63 .
Shlim DR. Looking for evidence that personal hygiene precautions prevent traveler’s diarrhea . Clin Infect Dis 2005 ; 41 (Suppl 8) : S531 – 5 .
Gorbach S. How to hit the runs for fifty million travelers at risk . Ann Intern Med 2005 ; 142 : 861 – 2 .
Mattila L. Clinical features and duration of traveler’s diarrhea in relation to its etiology . Clin Infect Dis 1994 ; 19 : 728 – 34 .
Hoge CW , Shlim DR , Echeverria P , Rajah R , Herrmann JE , Cross JH. Epidemiology of diarrhea among expatriate residents living in a highly endemic environment . JAMA 1996 ; 275 : 533 – 8 .
DuPont HL , Reves RR , Galindo E , Sullivan PS , Wood LV , Mendiola JG. Treatment of travelers’ diarrhea with trimethoprim/sulfamethoxazole and with trimethoprim alone . N Engl J Med 1982 ; 307 : 841 – 4 .
Rudy RP , Murray BE. Evidence for an epidemic trimethoprim-resistance plasmid in fecal isolates of Escherichia coli from citizens of the united states studying in Mexico . J Infect Dis 1984 ; 150 : 25 – 9 .
Taylor DN , Sanchez JL , Candler W , Thornton S , McQueen C , Echeverria P. Treatment of travelers diarrhea: Ciprofloxacin plus loperamide compared with ciprofloxacin alone: a placebo-controlled, randomized trial . Ann Intern Med 1991 ; 114 : 731 .
Mattila L , Peltola H , Siitonen A , Kyrönseppä H , Simula I , Kataja M. Short-term treatment of traveler’s diarrhea with norfloxacin: a double-blind, placebo-controlled study during two seasons . Clin Infect Dis 1993 ; 17 : 779 – 82 .
Pichler HE , Diridl G , Stickler K , Wolf D. Clinical efficacy of ciprofloxacin compared with placebo in bacterial diarrhea . Am J Med 1987 ; 82 : 329 – 32 .
Tribble DR , Sanders JW , Pang LW et al. Traveler’s diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen . Clin Infect Dis 2007 ; 44 : 338 – 46 .
Kuschner RA , Trofa AF , Thomas RJ et al. Use of azithromycin for the treatment of campylobacter enteritis in travelers to Thailand, an area where ciprofloxacin resistance is prevalent . Clin Infect Dis 1995 ; 21 : 536 – 41 .
Sanders JW , Frenck RW , Putnam SD et al. Azithromycin and loperamide are comparable to levofloxacin and loperamide for the treatment of traveler’s diarrhea in United States military personnel in Turkey . Clin Infect Dis 2007 ; 45 : 294 – 301 .
Travelers’ diarrhea. NIH Consensus Development Conference . JAMA 1985 ; 253 : 2700 – 4 .
DuPont HL , Jiang Z , Ericsson CD et al. Rifaximin versus ciprofloxacin for the treatment of traveler’s diarrhea: a randomized, double‐blind clinical trial . Clin Infect Dis 2001 ; 33 : 1807 – 15 .
Taylor DN , Bourgeois AL , Ericsson CD et al. A randomized, double-blind, multicenter study of rifaximin compared with placebo and with ciprofloxacin in the treatment of travelers’ diarrhea . Am J Trop Med Hyg 2006 ; 74 : 1060 – 6 .
Dupont HL , Jiang ZD , Belkind-Gerson J et al. Treatment of travelers’ diarrhea: randomized trial comparing rifaximin, rifaximin plus loperamide, and loperamide alone . Clin Gastroenterol Hepatol 2007 ; 5 : 451 – 6 .
DuPont HL , Sullivan P , Pickering LK , Haynes G , Ackerman PB. Symptomatic treatment of diarrhea with bismuth subsalicylate among students attending a Mexican university . Gastroenterology 1977 ; 73 : 715 – 8 .
DuPont HL , Sullivan P , Evans DG et al. Prevention of traveler’s diarrhea (emporiatric enteritis). Prophylactic administration of subsalicylate bismuth) . JAMA 1980 ; 243 : 237 – 41 .
DuPont HL , Ericsson CD , Johnson PC , Bitsura JA , DuPont MW , de la Cabada FJ. Prevention of travelers’ diarrhea by the tablet formulation of bismuth subsalicylate . JAMA 1987 ; 257 : 1347 – 50 .
Soriano-Brücher H , Avendaño P , O’ryan M et al. Bismuth subsalicylate in the treatment of acute diarrhea in children: a clinical study . Pediatrics 1991 ; 87 : 18 – 27 .
Figueroa-Quintanilla D , Salazar-Lindo E , Sack RB et al. A controlled trial of bismuth subsalicylate in infants with acute watery diarrheal disease . N Engl J Med 1993 ; 328 : 1653 – 8 .
DuPont HL , Flores Sanchez J , Ericsson CD et al. Comparative efficacy of loperamide hydrochloride and bismuth subsalicylate in the management of acute diarrhea . Am J Med 1990 ; 88 : 15S – 9S .
Steffen R , Mathewson JJ , Ericsson CD et al. Travelers’ diarrhea in West Africa and Mexico: fecal transport systems and liquid bismuth subsalicylate for self-therapy . J Infect Dis 1988 ; 157 : 1008 – 13 .
Steffen R. Worldwide efficacy of bismuth subsalicylate in the treatment of travelers’ diarrhea . Rev Infect Dis 1990 ; 12(Suppl 1) : S80 – 6 .
Ericsson CD , DuPont HL , Mathewson JJ , West MS , Johnson PC , Bitsura JA. Treatment of traveler’s diarrhea with sulfamethoxazole and trimethoprim and loperamide . JAMA 1990 ; 263 : 257 – 61 .
Murphy GS1 , Bodhidatta L , Echeverria P et al. Ciprofloxacin and loperamide in the treatment of bacillary dysentery . Ann Intern Med 1993 ; 118 : 582 – 6 .
Hill DR. Occurrence and self-treatment of diarrhea in a large cohort of Americans traveling to developing countries . Am J Trop Med Hyg 2000 ; 62 : 585 – 9 .
Bowen A , Agboatwalla M , Pitz A , Brum J , Plikaytis BD. Bismuth subsalicylate reduces antimicrobial use among adult diarrhea patients in Pakistan: a randomized, placebo-controlled, triple-masked clinical trial. In: 65th Annual Meeting of the ASTMH. Atlanta, GA; 2016 . p. Abstract 1202.
Hill DR , Ericsson CD , Pearson RD et al. The practice of travel medicine: guidelines by the Infectious Diseases Society of America . Clin Infect Dis 2006 ; 43 : 1499 – 539 .
Ponziani FR , Scaldaferri F , Petito V et al. The role of antibiotics in gut microbiota modulation: the eubiotic effects of rifaximin . Dig Dis 2016 ; 34 : 269 – 78 .
Alajbegovic S , Sanders JW , Atherly DE , Riddle MS. Effectiveness of rifaximin and fluoroquinolones in preventing travelers’ diarrhea (TD): a systematic review and meta-analysis . Syst Rev 2012 ; 1 : 39.
Hoge CW , Gambel JM , Srijan A , Pitarangsi C , Echeverria P. Trends in antibiotic resistance among diarrheal pathogens isolated in Thailand over 15 years . Clin Infect Dis 1998 ; 26 : 341 – 5 .
Barlow RS , Debess EE , Winthrop KL , Lapidus JA , Vega R , Cieslak PR. Travel-associated antimicrobial drug-resistant nontyphoidal Salmonellae , 2004-2009 . Emerg Infect Dis 2014 ; 20 : 603 – 11 .
De Lappe N , O’connor J , Garvey P , McKeown P , Cormican M. Ciprofloxacin-resistant Shigella sonnei associated with travel to India . Emerg Infect Dis 2015 May ; 21 : 894 – 6 .
Vila J , Vargas M , Ruiz J , Corachan M , De Anta MTJ , Gascon J. Quinolone resistance in enterotoxigenic Escherichia coli causing diarrhea in travelers to India in comparison with other geographical areas . Antimicrob Agents Chemother 2000 ; 44 : 1731 – 3 .
Connor BA , Keystone JS. Antibiotic self-treatment of travelers’ diarrhea: helpful or harmful? . Clin Infect Dis 2015 ; 60 : 847 – 8 .
DuPont HL , Jiang ZD , Okhuysen PC et al. A randomized, double-blind, placebo-controlled trial of rifaximin to prevent travelers’ diarrhea . Ann Intern Med 2005 ; 142 : 805 – 12 .
DuPont HL , Jiang ZD , Okhuysen PC et al. Loperamide plus azithromycin more effectively treats travelers’ diarrhea in Mexico than azithromycin alone . J Travel Med 2007 ; 14 : 312 – 9 .
Ericsson C. D. Prevention of Travelers Diarrhea. 2nd ed. In: Keystone JS , Kozarsky PE , Freedman DO , Nothdurft HD , Connor B (ed). Travel Medicine . Elsevier Ltd ., 2008 . pp. 191 – 196
Google Preview
- antibiotics
- bismuth subsalicylate
- fluoroquinolones
- trimethoprim-sulfamethoxazole combination
- pathogenic organism
- enterotoxigenic escherichia coli
- campylobacter
Email alerts
More on this topic, related articles in pubmed, citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1708-8305
- Copyright © 2024 International Society of Travel Medicine
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Patient Care & Health Information
- Diseases & Conditions
- Traveler's diarrhea
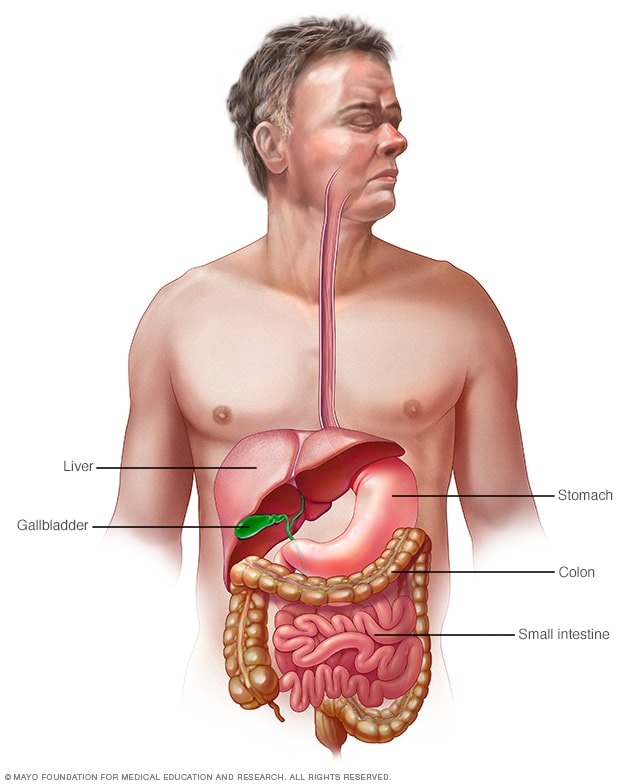
Gastrointestinal tract
Your digestive tract stretches from your mouth to your anus. It includes the organs necessary to digest food, absorb nutrients and process waste.
Traveler's diarrhea is a digestive tract disorder that commonly causes loose stools and stomach cramps. It's caused by eating contaminated food or drinking contaminated water. Fortunately, traveler's diarrhea usually isn't serious in most people — it's just unpleasant.
When you visit a place where the climate or sanitary practices are different from yours at home, you have an increased risk of developing traveler's diarrhea.
To reduce your risk of traveler's diarrhea, be careful about what you eat and drink while traveling. If you do develop traveler's diarrhea, chances are it will go away without treatment. However, it's a good idea to have doctor-approved medicines with you when you travel to high-risk areas. This way, you'll be prepared in case diarrhea gets severe or won't go away.
Products & Services
- A Book: Mayo Clinic Book of Home Remedies
- A Book: Mayo Clinic on Digestive Health
Traveler's diarrhea may begin suddenly during your trip or shortly after you return home. Most people improve within 1 to 2 days without treatment and recover completely within a week. However, you can have multiple episodes of traveler's diarrhea during one trip.
The most common symptoms of traveler's diarrhea are:
- Suddenly passing three or more looser watery stools a day.
- An urgent need to pass stool.
- Stomach cramps.
Sometimes, people experience moderate to severe dehydration, ongoing vomiting, a high fever, bloody stools, or severe pain in the belly or rectum. If you or your child experiences any of these symptoms or if the diarrhea lasts longer than a few days, it's time to see a health care professional.
When to see a doctor
Traveler's diarrhea usually goes away on its own within several days. Symptoms may last longer and be more severe if it's caused by certain bacteria or parasites. In such cases, you may need prescription medicines to help you get better.
If you're an adult, see your doctor if:
- Your diarrhea lasts beyond two days.
- You become dehydrated.
- You have severe stomach or rectal pain.
- You have bloody or black stools.
- You have a fever above 102 F (39 C).
While traveling internationally, a local embassy or consulate may be able to help you find a well-regarded medical professional who speaks your language.
Be especially cautious with children because traveler's diarrhea can cause severe dehydration in a short time. Call a doctor if your child is sick and has any of the following symptoms:
- Ongoing vomiting.
- A fever of 102 F (39 C) or more.
- Bloody stools or severe diarrhea.
- Dry mouth or crying without tears.
- Signs of being unusually sleepy, drowsy or unresponsive.
- Decreased volume of urine, including fewer wet diapers in infants.
It's possible that traveler's diarrhea may stem from the stress of traveling or a change in diet. But usually infectious agents — such as bacteria, viruses or parasites — are to blame. You typically develop traveler's diarrhea after ingesting food or water contaminated with organisms from feces.
So why aren't natives of high-risk countries affected in the same way? Often their bodies have become used to the bacteria and have developed immunity to them.
Risk factors
Each year millions of international travelers experience traveler's diarrhea. High-risk destinations for traveler's diarrhea include areas of:
- Central America.
- South America.
- South Asia and Southeast Asia.
Traveling to Eastern Europe, South Africa, Central and East Asia, the Middle East, and a few Caribbean islands also poses some risk. However, your risk of traveler's diarrhea is generally low in Northern and Western Europe, Japan, Canada, Singapore, Australia, New Zealand, and the United States.
Your chances of getting traveler's diarrhea are mostly determined by your destination. But certain groups of people have a greater risk of developing the condition. These include:
- Young adults. The condition is slightly more common in young adult tourists. Though the reasons why aren't clear, it's possible that young adults lack acquired immunity. They may also be more adventurous than older people in their travels and dietary choices, or they may be less careful about avoiding contaminated foods.
- People with weakened immune systems. A weakened immune system due to an underlying illness or immune-suppressing medicines such as corticosteroids increases risk of infections.
- People with diabetes, inflammatory bowel disease, or severe kidney, liver or heart disease. These conditions can leave you more prone to infection or increase your risk of a more-severe infection.
- People who take acid blockers or antacids. Acid in the stomach tends to destroy organisms, so a reduction in stomach acid may leave more opportunity for bacterial survival.
- People who travel during certain seasons. The risk of traveler's diarrhea varies by season in certain parts of the world. For example, risk is highest in South Asia during the hot months just before the monsoons.
Complications
Because you lose vital fluids, salts and minerals during a bout with traveler's diarrhea, you may become dehydrated, especially during the summer months. Dehydration is especially dangerous for children, older adults and people with weakened immune systems.
Dehydration caused by diarrhea can cause serious complications, including organ damage, shock or coma. Symptoms of dehydration include a very dry mouth, intense thirst, little or no urination, dizziness, or extreme weakness.
Watch what you eat
The general rule of thumb when traveling to another country is this: Boil it, cook it, peel it or forget it. But it's still possible to get sick even if you follow these rules.
Other tips that may help decrease your risk of getting sick include:
- Don't consume food from street vendors.
- Don't consume unpasteurized milk and dairy products, including ice cream.
- Don't eat raw or undercooked meat, fish and shellfish.
- Don't eat moist food at room temperature, such as sauces and buffet offerings.
- Eat foods that are well cooked and served hot.
- Stick to fruits and vegetables that you can peel yourself, such as bananas, oranges and avocados. Stay away from salads and from fruits you can't peel, such as grapes and berries.
- Be aware that alcohol in a drink won't keep you safe from contaminated water or ice.
Don't drink the water
When visiting high-risk areas, keep the following tips in mind:
- Don't drink unsterilized water — from tap, well or stream. If you need to consume local water, boil it for three minutes. Let the water cool naturally and store it in a clean covered container.
- Don't use locally made ice cubes or drink mixed fruit juices made with tap water.
- Beware of sliced fruit that may have been washed in contaminated water.
- Use bottled or boiled water to mix baby formula.
- Order hot beverages, such as coffee or tea, and make sure they're steaming hot.
- Feel free to drink canned or bottled drinks in their original containers — including water, carbonated beverages, beer or wine — as long as you break the seals on the containers yourself. Wipe off any can or bottle before drinking or pouring.
- Use bottled water to brush your teeth.
- Don't swim in water that may be contaminated.
- Keep your mouth closed while showering.
If it's not possible to buy bottled water or boil your water, bring some means to purify water. Consider a water-filter pump with a microstrainer filter that can filter out small microorganisms.
You also can chemically disinfect water with iodine or chlorine. Iodine tends to be more effective, but is best reserved for short trips, as too much iodine can be harmful to your system. You can purchase water-disinfecting tablets containing chlorine, iodine tablets or crystals, or other disinfecting agents at camping stores and pharmacies. Be sure to follow the directions on the package.
Follow additional tips
Here are other ways to reduce your risk of traveler's diarrhea:
- Make sure dishes and utensils are clean and dry before using them.
- Wash your hands often and always before eating. If washing isn't possible, use an alcohol-based hand sanitizer with at least 60% alcohol to clean your hands before eating.
- Seek out food items that require little handling in preparation.
- Keep children from putting things — including their dirty hands — in their mouths. If possible, keep infants from crawling on dirty floors.
- Tie a colored ribbon around the bathroom faucet to remind you not to drink — or brush your teeth with — tap water.
Other preventive measures
Public health experts generally don't recommend taking antibiotics to prevent traveler's diarrhea, because doing so can contribute to the development of antibiotic-resistant bacteria.
Antibiotics provide no protection against viruses and parasites, but they can give travelers a false sense of security about the risks of consuming local foods and beverages. They also can cause unpleasant side effects, such as skin rashes, skin reactions to the sun and vaginal yeast infections.
As a preventive measure, some doctors suggest taking bismuth subsalicylate, which has been shown to decrease the likelihood of diarrhea. However, don't take this medicine for longer than three weeks, and don't take it at all if you're pregnant or allergic to aspirin. Talk to your doctor before taking bismuth subsalicylate if you're taking certain medicines, such as anticoagulants.
Common harmless side effects of bismuth subsalicylate include a black-colored tongue and dark stools. In some cases, it can cause constipation, nausea and, rarely, ringing in your ears, called tinnitus.
- Feldman M, et al., eds. Infectious enteritis and proctocolitis. In: Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 11th ed. Elsevier; 2021. https://www.clinicalkey.com. Accessed May 25, 2021.
- LaRocque R, et al. Travelers' diarrhea: Microbiology, epidemiology, and prevention. https://www.uptodate.com/contents/search. Accessed May 26, 2021.
- Ferri FF. Traveler diarrhea. In: Ferri's Clinical Advisor 2023. Elsevier; 2023. https://www.clinicalkey.com. Accessed April 28, 2023.
- Diarrhea. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/digestive-diseases/diarrhea. Accessed April 27, 2023.
- Travelers' diarrhea. Centers for Disease Control and Prevention. https://wwwnc.cdc.gov/travel/yellowbook/2020/preparing-international-travelers/travelers-diarrhea. Accessed April 28, 2023.
- LaRocque R, et al. Travelers' diarrhea: Clinical manifestations, diagnosis, and treatment. https://www.uptodate.com/contents/search. Accessed May 26, 2021.
- Khanna S (expert opinion). Mayo Clinic. May 29, 2021.
- Symptoms & causes
- Diagnosis & treatment
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
Please note: This information was current at the time of publication but now may be out of date. This handout provides a general overview and may not apply to everyone.

Am Fam Physician. 1999;60(1):135-136
See related article on traveler's diarrhea .
What is traveler's diarrhea?
Traveler's diarrhea is a kind of diarrhea you might get when you're traveling in less developed countries. Many countries in Africa, Asia and Central and South America are risky places for travelers' diarrhea. It's usually caused by eating food or drinking water that is contaminated with bacteria. The illness may also cause nausea, vomiting and cramps. Fortunately, travelers' diarrhea is usually mild, and recovery is usually quick.
If you're planning a trip to a developing country, talk to your family doctor first about what you can do to stay healthy on your trip. Your doctor can give you advice about things you can do to help prevent illnesses like traveler's diarrhea. Your doctor also may prescribe an antibiotic for you to take along with you, in case you get traveler's diarrhea.
What can I do to prevent traveler's diarrhea?
You can help prevent traveler's diarrhea by being very careful about the foods you eat and the beverages you drink when you're traveling.
Do these things:
Tie a ribbon around the water faucet handle so you'll remember not to use tap water.
Keep safe, bottled water by the sink to use for drinking and tooth brushing.
Always clean your hands before you eat, but use prepackaged hand wipes or antiseptic gel to clean your hands, not just tap water.
Don't do these things:
Don't drink tap water. Don't even use it for brushing your teeth.
Don't use ice unless you know it was made from boiled or filtered water.
Don't eat raw vegetables or salads.
Don't eat unpasteurized dairy products.
Don't eat any fruits unless you peel them yourself.
Don't buy food and drinks from street vendors.
Don't go swimming in streams and lakes because of the risk of water pollution.
What foods and drinks are safe to eat in developing countries?
Coffee and tea made with boiled water are safe. Carbonated soft drinks (without ice), beer and wine are safe. Tap water that has been boiled, filtered or purified with iodine is safe to use. Most of the time, though, it's easier to buy purified bottled water for drinking than to purify the tap water.
It's safe to eat foods that are thoroughly cooked and served piping hot. Fresh breads and most dry foods are safe to eat.
What should I do if I get traveler's diarrhea?
When you're packing for your trip, take along some loperamide just in case you get travelers' diarrhea. This medicine usually stops diarrhea quickly. You may know this drug by its brand name—Imodium A-D. Take two tablets right after your first bout of diarrhea. Then take one tablet after each episode of diarrhea. Don't take more than four tablets a day.
How will I know if I need to take an antibiotic for traveler's diarrhea?
If loperamide doesn't stop your diarrhea, you may need to take an antibiotic to get rid of the infection. Your doctor might prescribe an antibiotic for you to take with you on your trip, just in case.
Several antibiotics can be used to treat traveler's diarrhea. One antibiotic commonly used for traveler's diarrhea is ciprofloxacin (brand name: Cipro). Often, only a few doses are needed to clear up the infection. (You shouldn't take Cipro if you're pregnant or under 18.)
If the diarrhea stops 12 hours after you took the first dose of the antibiotic, you probably don't need to take any more of the medicine. But see a doctor if your diarrhea hasn't stopped after you've been taking antibiotics for three days.
What should I do so I don't get dehydrated when I have traveler's diarrhea?
One of the biggest problems caused by diarrhea is dehydration. You can prevent dehydration by drinking lots of clear liquids, such as water, juices and soft drinks. Remember to keep drinking safe, purified water.
You can also drink a product that's made just to prevent and treat the dehydration caused by diarrhea. It's called “oral rehydration” mix. You can buy it in drug stores. It comes in a powder. You mix it with safe water and drink it. You can take some packets of oral rehydration mix with you on your trip, just in case.
What are danger signs that mean I have severe diarrhea?
If you're having diarrhea and you notice blood in your stool or you also have a high fever, you may have a severe infection. Stop taking loperamide and start taking an antibiotic. See a doctor if you don't get better in a day or two.
Traveler's diarrhea can turn a great vacation into a bad vacation. But taking a few simple precautions and knowing what to do if travelers' diarrhea strikes can make the difference in your trip.
Continue Reading
More in afp.
Copyright © 1999 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
- Skip to content
- Accessibility help
Diarrhoea - prevention and advice for travellers: Scenario: Diarrhoea - prevention and advice for travellers
Last revised in September 2023
Covers the prevention of travellers' diarrhoea, and advice for people who are at risk of travellers' diarrhoea.
Scenario: Diarrhoea - prevention and advice for travellers
From age 1 month onwards.
How should I assess the risk of acquiring travellers' diarrhoea before travelling?
- Low-risk areas (fewer than 7% of travellers experience travellers' diarrhoea) include Western Europe, the USA, Canada, Japan, Australia, and New Zealand.
- Intermediate risk areas (8–20% of travellers experience travellers' diarrhoea) include southern Europe, Israel, South Africa, and some Caribbean and Pacific islands.
- High-risk areas (more than 20% of travellers experience travellers' diarrhoea) include Africa, Latin America, the Middle East, and most parts of Asia.
- For up-to-date risk assessment of individual countries and the requirement for strict food, water, and personal hygiene precautions , see the National Travel Health Network and Centre (NaTHNaC) website ( www.nathnac.org ).
- Local amenities and sanitation — in areas with low standards of hygiene and sanitation and poor control over the safety of food, drink, and drinking water, the risk of acquiring travellers' diarrhoea is high.
- How the person will be travelling — backpackers, campers, adventurers, and passengers on cruise ships are at increased risk.
- When the person will be travelling — the peak incidence of travellers' diarrhoea is in the warmer months. Lower rates occur in winter.
- What the person eats — for example, risks are associated with food from buffets, restaurants, and street vendors, and certain types of food such as raw fish and seafood, salads, and meat and poultry that have been inadequately cooked. For more information, see Advice on food and drink .
- Review the person's susceptibility to traveller's diarrhoea and their risk of complications. For more information on which groups of people are considered to be at higher risk, see the section on Risk factors .
- Enquire about access to medical amenities — this is particularly important for those travelling to remote areas with few or no medical amenities (especially trekking and camping).
- Discuss whether visits to high-risk areas are essential (particularly if the person is at increased risk of complications) and whether they are suitably prepared (for example, if backpacking through remote areas).
Basis for recommendation
The recommendations about how to assess a person's risk of acquiring travellers' diarrhoea are largely based on expert opinion in a World Health Organisation (WHO) publication International Travel and Health [ WHO, 2012 ], the BMJ Best Practice guideline Traveller's Diarrhoea [ BMJ Best Practice, 2021 ], the Centres for Disease Control (CDC) guidance Travelers' diarrhea - Preparing International Travelers [ CDC, 2023a ], and the guidance on Travellers' diarrhoea from the National Travel Health Network and Centre (NaTHNaC) [ NaTHNaC, 2023 ].
How should I manage someone at low or intermediate risk of travellers' diarrhoea?
- Advise the person that most travellers' diarrhoea is caused by the consumption of contaminated food or water.
- Reassure the person that no special precautions (other than basic hygiene measures) are required when travelling to locations with a high standard of hygiene.
- Provide information on food hygiene and safe drinking water if the person is travelling to locations with lower standards of hygiene and sanitation.
- Offer advice regarding self-management and when to seek medical advice if the person develops diarrhoea during their trip.
The recommendations on managing people at low or intermediate risk of travellers' diarrhoea are largely based on expert opinion regarding general advice to offer travellers in a World Health Organisation (WHO) publication International Travel and Health [ WHO, 2012 ], the American College of Gastroenterologists guideline Diagnosis, Treatment, and Prevention of Acute Diarrheal Infections in Adults [ Riddle et al, 2016 ], the BMJ Best Practice guideline Traveller's Diarrhoea [ BMJ Best Practice, 2021 ], the Centres for Disease Control (CDC) guidance Travelers' diarrhea - Preparing International Travelers [ CDC, 2023a ] and the guidance on Travellers' diarrhoea from the National Travel Health Network and Centre (NaTHNaC) [ NaTHNaC, 2023 ].
How should I manage someone at high risk of travellers' diarrhoea?
- For further information, see advice on food and drink .
- Warn travellers about the risk of food- and water-borne infections and how to avoid potentially contaminated recreational water. Contaminated recreational water can be found in inadequately treated pools, water playgrounds, hot tubs, spas, and freshwater or seawater. Potential illness-causing contaminants include human faeces, animal waste, sewage, or wastewater runoff.
- Antibiotic prophylaxis may be appropriate for certain high-risk travellers. For more information, see the section on prophylactic antibiotics .
- The use of bismuth subsalicylate and probiotics for prophylaxis is not recommended.
- Antidiarrhoeal drugs (such as loperamide) should not be taken prophylactically.
- Consider whether 'stand by' antibiotic treatment (to use if affected) is appropriate. For more information, see the section on 'stand-by' antibiotics .
- Inform the person that there are no universal vaccines to cover all the infections which may cause travellers' diarrhoea. However, travellers to risky areas must seek advice about appropriate vaccination against other infections that cause gastrointestinal effects, such as cholera, hepatitis A, and typhoid. For further information on vaccines recommended for overseas travel, extended holidays, or working overseas, see the CKS topic on Immunizations - travel .
- For further information, see advice on managing travellers' diarrhoea .
The recommendations on managing people at high risk of travellers' diarrhoea are largely based on expert opinion in a World Health Organisation (WHO) publication International Travel and Health [ WHO, 2012 ], the American College of Gastroenterologists guideline Diagnosis, Treatment, and Prevention of Acute Diarrheal Infections in Adults [ Riddle et al, 2016 ], the BMJ Best Practice guideline Traveller's Diarrhoea [ BMJ Best Practice, 2021 ], the Centres for Disease Control (CDC) guidelines Travelers' diarrhea - Preparing International Travelers [ CDC, 2023a ] and Food and water precautions - Preparing International Travelers [ CDC, 2023c ] and the guidance on Travellers' diarrhoea from the National Travel Health Network and Centre (NaTHNaC) [ NaTHNaC, 2023 ].
What food and drink measures are recommended for preventing travellers' diarrhoea?
- Hands should always be washed with soap and water before handling and consuming food (particularly after contact with raw meat and uncooked food), using the toilet, or caring for an ill person.
- If soap and water are not available, an alcohol-based hand sanitiser can be used as an alternative until it is possible to wash the hands. Note that hand sanitiser is not effective when hands are greasy or visibly dirty and has limited effectiveness against Cryptosporidium and norovirus.
- Raw fish and seafood should be avoided, as well as shellfish, and any meat or poultry that has not been thoroughly cooked.
- Risks are associated with food from buffets, restaurants, and street vendors. Food from these sources should be avoided, if it has neither been kept hot, nor refrigerated or kept on ice.
- Cooked food that has been in contact with raw food, or contains raw or uncooked eggs (such as homemade mayonnaise), should be avoided.
- If hygiene and sanitation are likely to be inadequate, avoid salads, uncooked vegetables, and unpasteurised fruit juices. Fruits and vegetables that can be peeled by the end-user are suitable choices (fruits and vegetables with damaged skins should be avoided).
- Ice should be avoided, unless it has been made from safe water.
- Unpasteurised (raw) milk or cheese should be avoided.
- Commercially prepared carbonated drinks, pasteurised drinks, fruit juices, and alcoholic beverages are usually safe provided they are in factory-sealed containers. The surface of the container should be wiped clean and dried if the drink is to be consumed directly from the container.
- Tap water should be avoided for drinking, preparing food, making ice, cooking, and brushing teeth, unless there is reasonable certainty it is safe.
- Bottled water is the safer choice for drinking water. The seal must not have been tampered with.
- Water should be boiled (for at least 1 minute) if its safety for drinking is in doubt.
- Other options include micropore filtering and the use of disinfectant preparations. These might be more relevant for people travelling to areas where there is little or no access to safe drinking water.
- A fact sheet on Food and Water Hygiene is available on the Travel Health Pro website ( travelhealthpro.org.uk ).
The recommendations on food and drink precautions to reduce the risk of travellers' diarrhoea are based on expert opinion in the Centres for Disease Control (CDC) guidance on Travelers' diarrhea - Preparing International Travele rs [ CDC, 2023a ] and Travelers' diarrhea - Food & Water precautions [ CDC, 2023c ] and the guidance on Travellers' diarrhoea from the National Travel Health Network and Centre (NaTHNaC) [ NaTHNaC, 2023 ].
The CDC states that [ CDC, 2023a ]:
- Poor hygiene practices in local restaurants and underlying hygiene and sanitation infrastructure deficiencies are likely the largest contributors to the risk for travellers' diarrhoea.
- While measures such as avoiding certain food and food establishment types and ensuring food is thoroughly cooked, can help to reduce the risk of travellers' diarrhoea, it is acknowledged that some of these measures may be difficult to follow while travelling, and that some food safety factors are out of the traveller's control. Risk can therefore never be fully eliminated.
What should I advise about self-management of travellers' diarrhoea?
- That most episodes of travellers' diarrhoea are short-lived and self-limiting, and last a few days.
- To consider purchasing sachets of oral rehydration salts before travelling as they may not be available at the destination.
- For infants, breastfeeding should not be interrupted.
- Hydration must be maintained, for example with boiled, treated, or sealed bottled water. Most otherwise healthy adults can stay hydrated by eating and drinking as normal.
- Alcohol and other drinks and beverages with a diuretic effect (such as coffee and tea) and overly sweet drinks (in large quantities) should be avoided.
- For more severe symptoms, or people prone to complications from dehydration, oral rehydration powders diluted into clean drinking water can help to correct electrolyte imbalances.
- Immediate medical attention is required if children show signs of dehydration such as restlessness or irritability, great thirst, sunken eyes, and dry skin with reduced elasticity.
- Loperamide is usually considered the standard treatment when rapid control of symptoms is required — for example during travelling where toilet amenities are limited or unavailable.
- Bismuth subsalicylate is more suitable for mild diarrhoea; loperamide has a faster onset of action and is more effective than bismuth subsalicylate for controlling diarrhoea and cramping.
- Bismuth subsalicylate is not suitable for people with aspirin allergy, renal insufficiency, gout, severe enteric disease or HIV (risk of bismuth absorption), or people who are taking an anticoagulant such as warfarin, or pregnant or breastfeeding women. Darkened tongue and stools are common adverse effects.
- Advise the person not to use loperamide or bismuth subsalicylate (for example Pepto-Bismol ® ) if they have blood or mucous in the stool and/or high fever or severe abdominal pain.
- Loperamide should not be used in children younger than 12 years of age because of concerns that it may cause intestinal obstruction.
- Bismuth subsalicylate must not be used in children younger than 16 years of age because of the possible association between salicylates and Reye's syndrome.
- Stools are blood-stained, profuse and watery, or there is high or persistent fever or severe abdominal pain.
- Severe diarrhoea occurs in infants or elderly people, or a child is unwell with travellers' diarrhoea and symptomatic treatment is needed.
- The person becomes dehydrated — particularly in infants and children (restlessness or irritability, very thirsty, sunken eyes, and dry skin with reduced elasticity); or it is difficult to maintain adequate hydration.
- Diarrhoea does not begin to improve within 24–36 hours despite self-treatment.
- There is a medical comorbidity (for example immunosuppression or gastrointestinal disorder).
- NHS: www.nhs.uk — Access to healthcare abroad .
- The National Travel Health Network and Centre (NaTHNaC): www.nathnac.net .
- The 'Fit for travel' website provided by NHS Scotland: www.fitfortravel.scot.nhs.uk .
- The World Health Organization: www.who.int .
The recommendations on self-management of travellers' diarrhoea are largely based on expert opinion in a World Health Organisation (WHO) publication International Travel and Health [ WHO, 2012 ], the American College of Gastroenterologists guideline Diagnosis, Treatment, and Prevention of Acute Diarrheal Infections in Adults [ Riddle et al, 2016 ], the US consensus Guidelines for the prevention and treatment of travellers' diarrhea: a graded expert panel report [ Riddle, 2017 ], the BMJ Best Practice guideline Traveller's Diarrhoea [ BMJ Best Practice, 2021 ], the Centres for Disease Control (CDC) guideline Travelers' diarrhea - Preparing International Travelers [ CDC, 2023a ], the guidance on Travellers' diarrhoea from the National Travel Health Network and Centre (NaTHNaC) [ NaTHNaC, 2023 ], as well as a review article Traveler's diarrhea in children: New insights and existing gaps [ Ashkenazi, 2020 ].
Symptomatic treatment for adults
- Expert consensus guidance suggests that, given the concerns regarding potential adverse effects of antibiotics and antimicrobial resistance patterns, increasing fluid intake and treatment with loperamide or bismuth subsalicylate may be sufficient for most people with mild travellers' diarrhoea [ Riddle, 2017 ].
- Loperamide reduces the frequency of bowel movements, taking 1–2 hours to reach its therapeutic effect, and appears to have antisecretory properties. It has a well-established safety profile and is usually well-tolerated [ Steffen, 2015 ; Riddle, 2017 ; CDC, 2023a ].
- A systematic review found a small amount of data comparing loperamide with antibiotics and found no proof of antibiotics being more effective for the treatment of travellers' diarrhoea. The review authors therefore advised antibiotics only for severe illness and noted the value of loperamide for symptom control, particularly given increasing antimicrobial resistance [ Laaveri, 2016 ].
- Loperamide should be avoided in people with fever or bloody diarrhoea, and/or active inflammatory bowel disease; loperamide can inhibit peristalsis, resulting in complications such as ileus, megacolon, and toxic megacolon [ EMC, 2023a ; CDC, 2023a ; NaTHNaC, 2023 ]
Bismuth subsalicylate
- Bismuth subsalicylate is well tolerated, with usually mild adverse effects in adults, although it is slower to act than loperamide; may be less effective; cannot be used in some of groups of people, and has the potential for interactions with other medications [ Moore, 2015 ; Giddings, 2016 ; Riddle, 2017 ].
- A meta-analysis of data from 11 studies (including the study by DuPont discussed below, total n=840 participants with acute diarrhoea) found that compared to those receiving placebo, people treated with bismuth subsalicylate were 3.7-fold more likely to self-report symptomatic relief [ Brum, 2020 ].
Symptomatic treatment for children not recommended
- Rehydration is the mainstay of self-management for travellers' diarrhoea in young children; parents and carers should be aware that medical assistance should be sought if a child becomes unwell with travellers' diarrhoea, particularly if they are not tolerating fluids or are showing signs of dehydration [ NaTHNaC, 2023 ].
- Loperamide is only licensed for use in children older than 12 years and there is limited data on its use in children younger than 12 years old [ EMC, 2023a ].
- Bismuth salicylate (Pepto-Bismol ® ) is not licensed for use in children younger than 16 years of age because of the possible association between salicylates and Reye's syndrome [ Ashkenazi, 2020 ; CDC, 2023a ]
When to seek medical assistance
- The recommendations on when to seek medical assistance are in line with guidance issued by the American College of Gastroenterology and an expert panel [ Riddle et al, 2016 ; Riddle, 2017 ], information published by NaTHNaC [ NaTHNaC, 2023 ], and expert opinion [ Leder, 2015 ; Moore, 2015 ; Giddings, 2016 ] [ Barrett, 2018 ; Ashkenazi, 2020 ].
When should I consider prescribing a prophylactic antibiotic for travellers' diarrhoea?
- Do not routinely prescribe prophylactic antibiotic treatments for travellers' diarrhoea.
- The risk of the person developing travellers' diarrhoea (see Assessing risk (before travelling) ).
- The potential harm to the person if they were to develop travellers' diarrhoea.
- The benefits and risks of antibiotic treatment.
- Access to medical assistance while travelling.
- Have increased susceptibility to infection or are immunocompromised.
- People with chronic gastrointestinal disease (such as Crohn's disease, or ulcerative colitis).
- People with an ileostomy or colostomy.
- People with other diseases (such as malignancy, type 1 diabetes mellitus, renal disease, stroke, congestive heart failure, or sickle cell disease) in whom a diarrhoeal illness might severely impact on their health.
- Have a history of significant long-term morbidity after an enteric infection (for example, reactive arthritis).
- Are undertaking critical trips in which a short bout of diarrhoea could severely impact the purpose of the trip (for example, athlete, politician, professional musician, lecturer).
- May not have access to adequate medical treatment if they experience complicated travellers' diarrhoea.
The recommendations on when to consider prophylactic antibiotics for travellers' diarrhoea are largely based on expert opinion in a World Health Organisation (WHO) publication International Travel and Health [ WHO, 2012 ], the American College of Gastroenterologists guideline Diagnosis, Treatment, and Prevention of Acute Diarrheal Infections in Adults [ Riddle et al, 2016 ], the US consensus Guidelines for the prevention and treatment of travellers' diarrhea: a graded expert panel report [ Riddle, 2017 ], the BMJ Best Practice guideline Traveller's Diarrhoea [ BMJ Best Practice, 2021 ], the Centres for Disease Control (CDC) guidelines Travelers' diarrhea - Preparing International Travelers [ CDC, 2023a ] and Perspectives: Antibiotics in Travelers' Diarrhea - Balancing Benefit & Risk [ CDC.2023 ] as well as the guidance on Travellers' diarrhoea from the National Travel Health Network and Centre (NaTHNaC) [ NaTHNaC, 2023 ].
Avoiding routine prophylaxis
- Antibiotic prophylaxis has been shown in studies to provide up to 90% protection against developing travellers' diarrhoea. However, for most people the potential risks of prophylaxis with antibiotics outweigh the benefits [ CDC, 2023a ].
- For most people, travellers' diarrhoea is mild and self-limiting.
- Antibiotic prophylaxis offers no protection against non-bacterial causes of travellers' diarrhoea (such as viruses and parasites).
- Removal of normally protective microflora from the bowel with prophylactic antibiotics can increase the risk of infection with resistant bacteria.
- Use of prophylactic antibiotics reduces the choices for treatment if required for travellers' diarrhoea.
- Taking antibiotic prophylaxis may give the traveller a false sense of security, which might lead to neglect of food and water precautions.
- The increasing prevalence of antibiotic resistance is of concern (including to fluoroquinolones).
- No antibiotics are licensed in the UK for the prophylaxis of travellers' diarrhoea.
- Antibiotic treatment can lead to adverse effects and routine use of antibiotics for prophylaxis could expose the person to risks greater than that of the illness.
When to consider prophylaxis
The groups of travellers for whom antibiotic prophylaxis may be considered are based on recommendations from the WHO [ WHO, 2012 ], the CDC [ CDC, 2023a ], a BMJ Best Practice guideline [ BMJ Best Practice, 2021 ] an expert panel report [ Riddle, 2017 ], as well as in a number of review articles [ Barrett, 2016 ; Giddings, 2016 ; DuPont, 2017 ; Taylor, 2017 ; Barrett, 2018 ; Ashkenazi, 2020 ].
- CKS notes that there is some inconsistency in the expert opinion relating to groups of people who may be eligible for prophylaxis.
Seeking specialist advice about prophylaxis
- Planning prophylactic treatment is complex due to the large number of potential pathogens, their geographical variation and resistance patterns, and case-specific factors including comorbidities [ Zaidi and Wine, 2015 ].
- Fluoroquinolones were historically used as the first-line choice for prophylaxis. However, resistance among Campylobacter and Shigella species has been observed globally and fluoroquinolones are associated with cartilage damage, tendon rupture, and C. difficile -associated diarrhoea [ CDC, 2023a ]. In addition, use of fluoroquinolones for prophylaxis limits therapeutic options if travellers' diarrhoea develops [ Taylor, 2017 ].
- Rifaximin is a non-absorbable antibiotic for which there is evidence of efficacy (although it does not prevent all cases of travellers' diarrhoea), with a favourable safety profile. It is not associated with antimicrobial resistance in travellers and does not appear to modify the gut microflora, but Campylobacter is not susceptible, meaning it only offers moderate protection in South and South East Asia [ Riddle et al, 2016 ; Riddle, 2017 ; Taylor, 2017 ].
- Azithromycin is lacking an evidence base to support its use as a prophylactic agent for travellers' diarrhoea. It is also useful as a therapeutic option for travellers' diarrhoea, particularly in South and South East Asia, and in cases of dysentery, [ Leder, 2015 ; Giddings, 2016 ; Riddle, 2017 ; Taylor, 2017 ; Barrett, 2018 ].
- Taking these factors into account, along with the uncertainty regarding the overall benefit of prophylactic antibiotic treatment, and the inconsistent opinion on which groups of people are eligible for prophylaxis, CKS has recommended seeking specialist advice if considering prescribing antibiotic prophylaxis for the prevention of travellers' diarrhoea.
When should I prescribe a 'stand-by' antibiotic for treatment of travellers' diarrhoea?
- Do not routinely prescribe 'stand-by' (to be used if affected) treatments for travellers' diarrhoea.
- Travelling to high-risk locations (especially where access to medical assistance is poor or not available, or there is doubt about the safety and purity of antibiotics available at their destination).
- At high risk of severe illness.
- The National Travel Health Network and Centre (NaTHNaC) provides a telephone advice line for health professionals advising travellers with complex itineraries or specialist health needs. For more information, see the NaTHNaC website ( www.nathnac.net ).
The recommendations on when to consider 'stand-by' antibiotic treatment for travellers' diarrhoea are largely based on expert opinion in the American College of Gastroenterologists guideline Diagnosis, Treatment, and Prevention of Acute Diarrheal Infections in Adults [ Riddle et al, 2016 ], the US consensus Guidelines for the prevention and treatment of travellers' diarrhea: a graded expert panel report [ Riddle, 2017 ], the BMJ Best Practice guideline Traveller's Diarrhoea [ BMJ Best Practice, 2021 ], the Centres for Disease Control (CDC) guidelines Travelers' diarrhea - Preparing International Travelers [ CDC, 2023a ] and Perspectives: Antibiotics in Travelers' Diarrhea - Balancing Benefit & Risk [ CDC.2023 ] as well as the guidance on Travellers' diarrhoea from the National Travel Health Network and Centre (NaTHNaC) [ NaTHNaC, 2023 ].
Not routinely offering a 'stand-by' antibiotic
- Most cases of travellers' diarrhoea are mild and self-limiting and will resolve without treatment [ Giddings, 2016 ; Riddle, 2017 ].
- Risks of antibiotic treatment for travellers' diarrhoea include changes to the host microbiome, and the acquisition of multidrug resistant bacteria. In particular, travellers to South and Southeast Asia who take antibiotics for travellers' diarrhoea are at risk for colonization with extended-spectrum β-lactamase–producing Enterobacteriaceae (ESBL-PE), which may be harmful to some, for example older people and those prone to urinary tract infections [ CDC.2023 ].
- NaTHNaC advises that since travellers who take antibiotics may acquire organisms that are resistant to antibiotics such multi-drug resistant (MDR) Enterobacteriaceae or Clostridium difficile , the prescribing of antibiotics for standby treatment of travellers' diarrhoea should be carefully considered. Groups that may be eligible include people at high risk of developing severe illness, or those visiting high-risk areas in remote locations where access to medical care is limited [ NaTHNaC, 2023 ].

Seeking specialist advice if considering a 'stand-by' antibiotic
- Azithromycin — although it is not licensed for the treatment of travellers' diarrhoea [ EMC, 2023b ], azithromycin can be useful if there is severe travellers' diarrhoea, dysentery, high fever, travel to south and south East Asia, and if rifaximin has been used as prophylaxis [ Leder, 2015 ; Riddle, 2017 ; Barrett, 2018 ; CDC, 2023a ]. Azithromycin is well-tolerated by most people, and has a more convenient dosing schedule compared with quinolones [ Leder, 2015 ; Riddle, 2017 ]. Prescribers should note, however, that enteropathogens with increased azithromycin resistance have been documented in several countries [ CDC, 2023a ].
- Rifaximin — this antibiotic is licensed for the treatment of travellers' diarrhoea that is not associated with fever, bloody diarrhoea, blood or leucocytes in the stools [ EMC, 2020 ]. Although there is evidence that rifaximin is effective for treating travellers' diarrhoea compared with placebo and has similar efficacy to fluoroquinolones in non-invasive travellers' diarrhoea [ Steffen, 2015 ; Riddle, 2017 ], it is not effective for treating diarrhoea caused by invasive enteric pathogens such as Salmonella, Shigella, or Campylobacter [ EMC, 2020 ].
- Note: although fluoroquinolones were historically first-line therapy for empiric treatment of travellers; diarrhoea, or to treat specific pathogens, there is increasing fluoroquinolone resistance in Campylobacter, Shigella , and Salmonella species, particularly in South and South East Asia, where a significant proportion of travellers' diarrhoea is due to Campylobacter [ Leder, 2015 ; Riddle, 2017 ; Taylor, 2017 ; CDC, 2023a ]. In addition, the prescribing of fluoroquinolones is now not generally recommended due to risks of multiple adverse reactions including aortic tears, hypoglycaemia, mental health side effects, and tendinitis and tendon rupture [ CDC, 2023a ; NaTHNaC, 2023 ].
The content on the NICE Clinical Knowledge Summaries site (CKS) is the copyright of Clarity Informatics Limited (trading as Agilio Software Primary Care) . By using CKS, you agree to the licence set out in the CKS End User Licence Agreement .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cochrane Database Syst Rev
Antibiotic treatment for travellers' diarrhoea
Using a pilot system we have categorised this review as: Current question ‐ update pending (See "Published notes" section for an explanation).
Traveller's diarrhoea is a syndrome frequently encountered in persons crossing an international boundary. Diarrhoea can lead to significant discomfort and interference with travel plans. Bacterial pathogens are a frequent cause of this syndrome. Several antibiotics have been tested for efficacy in reducing the duration and severity of the illness.
The aims of this review were to assess the effects of antibiotics on traveller's diarrhoea in relation to duration of illness, severity of illness, and adverse effects of medications.
Search methods
The Cochrane Collaboration Trials Register, MEDLINE, and EMBASE were searched. Additional trials were identified by hand searching. Content experts were contacted.
Selection criteria
All trials in any language in which travellers older than 5 years were randomly allocated to treatment for acute non‐bloody diarrhoea with antibiotics and where the causative organism is not known at allocation.
Data collection and analysis
Two reviewers assessed trial quality and extracted data.
Main results
Twenty published studies met inclusion and quality criteria for inclusion. Twelve studies were placebo‐controlled.
A meta‐analysis for the primary outcome was not feasible. All of the 10 trials reported a significant reduction in duration of diarrhoea in participants treated with antibiotics compared with placebo.
Data from two trials demonstrated a small reduction for antibiotic treated patients in the number of unformed stools passed per each 24 hour period from randomisation up to 72 hours.
Data from six trials demonstrated a greater number of participants being cured of diarrhoea by 72 hours (odds ratio 5.90, 95% confidence interval 4.06 to 8.57).
Data regarding side effects were available from five trials. There was wide variation in the prevalence of side effects reported in different trials. Persons taking antibiotics experienced more side effects than those taking placebo (odds ratio 2.37, 95% confidence interval 1.50 to 3.75).
Authors' conclusions
Antibiotic treatment is associated with shorter duration of diarrhoea but higher incidence of side‐effects. Trials generally do not report duration of post‐treatment diarrhoea using time‐to‐event analyses, and should do.
No update planned
Many trials of antibiotics in travellers' diarrhoea have been conducted and demonstrate shorter illness following antibiotic treatment. Travellers' diarrhoea is a self‐limiting illness. Therefore an update is not a current priority for the CIDG.
Plain language summary
Antibiotic treatment reduces duration and severity of travellers' diarrhoea.
Using a pilot system we have categorised this review as: Current question ‐ update pending. (See "Published notes" section for an explanation).
Diarrhoea is a common problem for travellers, particularly travellers going from developed to less developed nations. The illness is frequently caused by a bacterial infection. Although the illness is unlikely to result in death, diarrhoea can disrupt travel plans and lead to severe or incapacitating symptoms. The review showed that antibiotic treatment shortens the duration and severity of diarrhoea. Persons taking antibiotics reported more side effects, but most side effects were minor, or resolved on stopping the antibiotic.
Travellers' diarrhoea is an acute syndrome occurring in people who have crossed a national boundary and who experience watery or unformed bowel motions which are passed more frequently than usual, sometimes accompanied by nausea, vomiting, abdominal pain or cramping. Recovery within five days is normal, although in about 10% of cases symptoms will persist beyond seven days ( DuPont 1993 , Farthing 1992 ). The syndrome is most commonly caused by intestinal bacteria known as enterotoxigenic Escherichia coli (ETEC), although other bacteria including Campylobacter, Shigella, and Salmonella species have all been frequently incriminated ( Black 1986 , Gorbach 1975 , Taylor 1991 ). Travellers' diarrhoea lasting for 14 days or more is sometimes termed "persistent diarrhoea" and is considered separately as the causes and management differ from the usual travellers' illness. Some 10% of travellers with diarrhoea develop dysentery, defined as diarrhoea mixed with visible blood, indicating a potentially serious condition which differs from travellers' diarrhoea and warrants separate investigation and management. Mortality is rare in simple travellers' diarrhoea although the condition is potentially serious in the very young and the elderly, who may not tolerate fluid loss, as well as in children and younger adults. Those with an impaired immune response, reduced gastric acidity, and some forms of heart disease may also be considered at risk of more severe effects from travellers' diarrhoea ( BSSI 1996 ).
The magnitude of the problem is considerable. With the advent of modern air travel, between 1950 and 1990 there was an increase from approximately 25 million to 425 million persons travelling worldwide ( Hundt 1996 ). Travellers' diarrhoea has been commonly reported to occur in 30‐60% of travellers to developing countries, in contrast to reported incidence rates of 5% or less in travellers to Europe and North America ( Cartwright 1997 ).
The incidence of the syndrome appears to vary according to the itinerary, to the season in which travel occurs, and to factors surrounding eating behaviour and style of travel, with the younger adult more at risk than the middle‐aged ( Steffen 1983 , Mattila 1993 ). Nevertheless, diarrhoea still occurs in the well‐informed and prepared traveller ( McIntosh 1997 ), including those who stay exclusively in "five star" accommodation.
As the international traveller is often on a restricted schedule because of pre‐planned tour itineraries or business demands, even a day lost to illness can be unexpectedly disruptive, creating a demand for prompt relief. Medical treatment may not always be easily accessible to the traveller because of factors such as an unfamiliar health system, language barriers, remoteness, and time constraints, so it is an attractive option for the traveller to carry self‐treatment for some common conditions.
The mainstay of treatment for diarrhoea of all degrees of severity is adequate replacement of fluids and electrolytes. Moreover, the use of antibiotics to treat diarrhoea has been tested in a number of clinical trials ( Katelaris 1995 , NIH‐CDCS 1985 ).
This review was designed to examine the effectiveness of antibiotic treatment for acute diarrhoea. We also considered adverse effects reported in trials, as the aim of treatment is termination of diarrhoea and relief of symptoms, rather than reduction in a more serious clinical endpoint. We did not examine several other important issues, such as cost of treatment, which may be better evaluated in other ways.
Several related reviews are planned, including assessment of the effectiveness of non‐antibiotic treatments, and of combination treatments for diarrhoea.
To assess the effects of antibiotics on diarrhoea in relation to: (a) duration of illness; (b) severity of illness; and (c) adverse effects.
Criteria for considering studies for this review
Types of studies.
All trials in any language in which travellers are randomly allocated to a treatment for non‐bloody diarrhoea lasting not more than 14 days and where the causative organism is not known at allocation; to include placebo controlled trials and comparison trials.
Types of participants
Adults and children 5 years or older who develop acute non‐bloody diarrhoea lasting not more than 14 days (i.e. to exclude dysentery and persistent diarrhoea at randomisation).
Travellers are defined as people who are travelling outside their usual country of residence, and are resident in the country of study for less than six months.
Types of interventions
1. Any antibiotic therapy versus placebo. 2. Any antibiotic versus another antibiotic.
Treatments were self‐administered or issued by a physician according to a study protocol with no prior knowledge of bacterial culture results.
Other treatments were acceptable within intervention and control groups, so long as both groups remain comparable in this respect; examples include fluid replacement, dietary advice, and malaria prophylaxis.
Types of outcome measures
Duration of diarrhoea, as assessed by time to last unformed stool.
Number of loose stools passed per 24 hour period: 0‐24 hours; 25‐48 hours; 49‐72 hours; > 72 hours.
Tolerability
Number of subjects who report any side effect of treatment.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press or in progress).
The topic search terms used for searching trial registers and databases were: "travel$" for traveler, traveller, "diarr$" for "diarrhea", "diarrhoea", "diarrea", "reisediarrhoe", and "turista".
The Cochrane Infectious Diseases Group (CIDG) Specialized Register was searched for relevant trials up to September 2003. Details of the CIDG methods and the journals hand‐searched are published in The Cochrane Library in the section on Collaborative Review Groups.
The Cochrane Central Register of Controlled Trials (CENTRAL) published in The Cochrane Library (Issue 3, 2004) was searched.
The following databases were searched using the topic search terms using the topic search terms in combination with the Cochrane highly sensitive search strategy for identifying trials as outlined in Appendix 5c of the Cochrane Handbook: MEDLINE (1966 to September 2003); and EMBASE (1988 to September 2003).
Additional trials were identified through hand searching of abstracts from the biennial International Society for Travel Medicine Conferences and other selected meetings including the annual meetings of the American Gastroenterological Society.
We contacted content experts in the field who have conducted and published randomised controlled trials on travellers' diarrhoea, aiming to identify any remaining published or unpublished trials. We also attempted to get original data from North American trialists, but these data could not be retrieved. Finally, the reference lists in all identified studies were scanned in an effort to identify missing trials.
A full text version of each article identified through the various search strategies was independently assessed by two reviewers in terms of its quality in the following categories (for details see the Policy Statement of the Cochrane Infectious Diseases Group): allocation concealment; generation of allocation sequence; inclusion of all randomised participants.
Data extraction was performed for outcome variables.
Calculation of treatment effects was performed using Cochrane statistical software, RevMan version 4.04. Results of dichotomous variables are expressed as Mantel Haenszel odds ratio (OR) and 95% confidence interval (95% CI). Continuous outcomes are expressed as mean difference (MD) and 95% CI.
Description of studies
Thirty‐three potentially eligible trials of antibiotic treatment for diarrhoea were identified. Twenty five studies were identified by searching MEDLINE, 11 by searching EMBASE (two unique), and four by hand searching of abstracts. An additional two unpublished articles were found through consultation with a content expert.
Six studies are awaiting further evaluation (See ' Characteristics of studies awaiting classification '). This includes three trials published in German language journals. Two studies are unpublished trials about which the principal investigator provided methodological details, but requested that the data not be included. One trial was available only in abstract form, and further data are awaited. Two studies were excluded for not meeting inclusion criteria and three trials did not meet predefined quality assessments (see ' Characteristics of excluded studies '). Of the remaining studies, two were duplicate publications of prior reports, and were considered together with the original study. This left a total of 20 studies which were included in the present analysis (see ' Characteristics of included studies ').
Seven trials ( Christensen 1988 , DuPont 1992 , Ericsson 1983 , Mattila 1993 , Salam 1994 , Wistrom 1989 , Wistrom 1992 ) were studies of a single antibiotic agent against a placebo agent. Five trials ( DuPont 1982 , DuPont 1992b , Ericsson 1987 , Ericsson 1990 , Steffen 1993 ) were comparisons of multiple agents, or multiple regimens of a single agent, and included a placebo group. Five studies ( DuPont 1984 , Ericsson 1992 , Ericsson 1997 , Kuschner 1995 , Thornton 1992 ) were direct comparisons of one antibiotic against another antibiotic, or multiple regimens of different durations using the same antibiotic, without use of a placebo group. Three studies ( Ericsson 1986 , Petrucelli 1992 , Taylor 1991 ) compared an antibiotic against an antibiotic‐antimotility agent combination.
Eleven studies were conducted in Mexico, of which 10 were among US adult students attending summer classes in Guadalajara, Mexico. Two studies were based in Thailand, and one each in Belize, Egypt, The Gambia, India, and Morocco. Two studies included people travelling to various destinations in developing nations.
The travellers in these studies were students (10 studies), military personnel (5 studies), "package" tourists (2 studies), resort guests (1 study), independent travellers (1 study), or volunteers recruited for the purpose of the study (1 study).
Inclusion criteria were fairly uniform across the studies, with all of the studies but three ( Christensen 1988 , Salam 1994 , Steffen 1993 ) requiring participants to have a minimum of three loose stools per 24 hours before presentation or a smaller number of stools in a shorter period, such as two stools in 8 hours, and have evidence of an enteric illness, such as abdominal cramps, nausea, vomiting, and low grade fever. Inclusion criteria were not explicitly stated in one study ( Christensen 1988 ), and a single loose stool was sufficient for inclusion in two studies ( Salam 1994 , Steffen 1993 ).
The agents evaluated in these trials included an oral flouroquinolone (ciprofloxacin, norfloxacin, or ofloxacin) in 12 studies ( DuPont 1992b , Ericsson 1987 , Ericsson 1997 , Kuschner 1995 , Mattila 1993 , Petrucelli 1992 , Salam 1994 , Steffen 1993 , Wistrom 1989 , Wistrom 1992 ), trimethoprim/ sulfamethoxazole in six studies ( DuPont 1982 , Ericsson 1986 , Ericsson 1987 , Ericsson 1990 , Ericsson 1992 , Thornton 1992 ), and in one study each for the following agents: ampicillin ( DuPont 1984 ), azithromycin ( Kuschner 1995 ), aztreonam ( DuPont 1992 ), bicozamycin ( Ericsson 1983 ), furazolidone ( DuPont 1984 ), pivmecillinam ( Christensen 1988 ), and trimethoprim alone ( DuPont 1982 ).
Risk of bias in included studies
All of the studies were randomised studies. Only one study reported on the method of randomisation used ( DuPont 1982 ).
Eighteen studies were double‐blinded, with one being single blinded ( Ericsson 1997 ), and one study ( Christensen 1988 ) not reporting whether blinding was used.
Inclusion of all randomised patients
Included trials had losses to follow up or excluded patients from efficacy analysis that were less than 10% of randomised participants in all cases. Few studies reported an intention to treat analysis ( Ericsson 1990 , Ericsson 1992 , Ericsson 1997 ).
Effects of interventions
Data from studies comparing antibiotic treatment with placebo are presented in the summary analysis and in Table 2 . A total of 1474 patients were included in placebo‐controlled studies.
A meta‐analysis of the primary outcome, duration of diarrhoea after initiation of antibiotic treatment, or time to last unformed stool, was not performed. Of 12 placebo‐controlled studies identified, 10 studies ( DuPont 1982 , DuPont 1992 , DuPont 1992b , Ericsson 1983 , Ericsson 1987 , Ericsson 1990 , Mattila 1993 , Salam 1994 , Steffen 1993 , Wistrom 1989 ) reported data for this outcome. However, only three of these ( DuPont 1992b , Ericsson 1983 , Salam 1994 ) reported mean and standard deviation (SD) time to last unformed stool. One other study reported mean and a P value for a statistical test from which an imputed pooled SD could be derived ( Wistrom 1992 ). The remaining six studies reported statistical comparisons that used non‐parametric tests. In five cases, trials used the Wilcoxon test and one used the Mann‐Whitney test.
In all 10 trials, a statistically significant reduction in time to last unformed stool was reported for participants receiving antibiotic treatment when compared with placebo, with the exception of the five day ofloxacin arm in DuPont 1992b . The data from the trials from which mean and SD could be obtained or imputed are presented in Table 2 .
For the secondary efficacy endpoint of number of patients cured by 72 hours after randomisation, data from six trials ( DuPont 1982 , Ericsson 1983 , Mattila 1993 , Salam 1994 , Steffen 1993 , Wistrom 1989 ) were available. Treatment with antibiotics resulted in a greater number of participants being cured of diarrhoea by 72 hours (OR 5.90, 95% CI 4.06 to 8.57, Analysis 1.1 ).

Comparison 1 Antibiotic versus placebo, Outcome 1 Number cured at 72 hours.
For the secondary endpoint of change in severity (number of unformed stools per 24 hour time interval), two trials ( Ericsson 1983 , Salam 1994 ) demonstrated a small reduction for antibiotic treated patients in the number of unformed stools passed per each 24 hour period from randomisation up to 72 hours (see Analysis 1.2 ). For the first 24 hours after randomisation, the difference was ‐1.59 stools (95% CI ‐2.66 to 0.52). For the period 25‐48 hours, the difference was ‐2.10 stools (95% CI ‐2.78 to ‐1.42). For the third post‐randomisation day, the difference was ‐1.38 stools (95% CI ‐1.94 to ‐0.82).

Comparison 1 Antibiotic versus placebo, Outcome 2 Severity (number of unformed stools / 24 hour period).
For the secondary endpoint of prevalence of any reported side effect of treatment, data from five trials ( DuPont 1982 , DuPont 1992 , DuPont 1992b , Ericsson 1983 , Steffen 1993 ) were available. There were more side effects reported by persons taking antibiotics than those taking placebo (OR 2.37, 95% CI 1.50 to 3.75, Analysis 1.3 ). There was significant variation in the incidence of side effects reported in different trials. Elimination of trials with the largest weight did not affect the overall result. Side effects reported were all noted to be not clinically serious, or resolved on withdrawal of the drug. Studies did not report examination for Clostridium difficile exotoxin in participants with recurrent diarrhoea. No studies reported fatalities or participants needing to be hospitalised after randomisation.

Comparison 1 Antibiotic versus placebo, Outcome 3 Side effects.
We examined the use of oral antibiotics for acute episodes of diarrhoea in travellers. The principal finding of this review was that a meta‐analysis of the primary outcome was not feasible. The pooled results of the secondary endpoints demonstrate a significant beneficial effect of antibiotic treatment in terms of the proportion of persons taking antibiotics who were cured of diarrhoea by 72 hours of treatment, and in reducing the severity of illness. There were significantly more adverse effects reported in those taking antibiotics than those taking placebo. However, most of these side effects were minor, and resolved either spontaneously or on stopping the antibiotic. The planned analyses of data for comparative trials of antibiotics and combination treatment were not carried out, principally because of concerns that the skewed nature of published data would imply a revision of the review methods.
Ten eligible trials reported summary measures based on mean, but only three reported both mean and SD. The ratios of mean and SD in these three trials also suggested that data for the primary outcome might be skewed; mean < 1.6 SD. In one trial, a pooled SD could be calculated. The other six trials used non‐parametric tests to compare the means from treatment and control groups. From this we infer that this was also because the data were not normally distributed, yet it was not stated clearly in any trials. For these reasons meta‐analysis for the primary outcome data was not feasible.
One major problem with the analyses presented in these trials was that the primary outcome was not treated as a time‐to‐event outcome. The proper analysis of such data would require time to event analysis, such as Kaplan‐Meier life table methods, comparing groups using the log rank test. If such an analysis can be undertaken, it would strengthen the reliability of the conclusions.
The trials examined in this systematic review used similar entry criteria, with three exceptions ( Christensen 1988 , Salam 1994 , Steffen 1993 ). Participants could be regarded as having had "classic travellers' diarrhoea" ( Steffen 1999 ), or moderate to severe diarrhoeal illness to have been included in these studies. Part of the reason for this uniformity is that many of the studies were performed in the same geographic location, using similar protocols, in consistent participant populations.
Implications for practice
Available trial data demonstrate that antibiotic treatment is effective in reducing the duration of post‐treatment diarrhoea, and severity of diarrhoea. However, this is at the price of an increased chance of side effects from antibiotic treatment.
Implications for research
The quality of information included in reports of therapeutic trials must meet certain minimum criteria. It is no longer accepted practice for authors to omit confidence interval data or measures of variation of the mean from reports of statistical testing. It is also crucial that data be appropriately analysed. The primary outcome for this review is ideally represented in a time‐to‐event analysis. Thorough statistical review of papers before publication should prevent such errors.
Protocol first published: Issue 3, 1998 Review first published: Issue 3, 2000
2012, Issue 2: Review status: Current question ‐ update pending. New studies have been identified; a new author team is required for this review.
The Cochrane Infectious Diseases Group assesses Review Status using a pilot system to help the reader understand whether the review concerns a current question, and is up to date. We report on:
1. The question the review addresses. Is it a:
- Historical question , where the intervention or policy has been superseded by new medical developments (such as a new drug); or a
- Current question , which is still relevant to current policy or practice.
2. Whether the review is up to date. Is the review:
- Up to date ;
- Update pending ; or
- No update intended .
We then provide comment of the review status, to help explain the categories selected.
Acknowledgements
Dr Paul Garner for general guidance, and Dr Paula Williamson for statistical advice. Dr HL DuPont provided access to unpublished trials, which are awaiting further analysis.
Data and analyses
Comparison 1, characteristics of studies, characteristics of included studies [ordered by study id].
ETEC, enterotoxigenic Escherichia coli; TMP, trimethoprim; SMX, sulfamethoxazole.
Characteristics of excluded studies [ordered by study ID]
Characteristics of studies awaiting assessment [ordered by study id], contributions of authors.
Not stated.
Sources of support
Internal sources.
- Liverpool School of Tropical Medicine, UK.
External sources
- Department for International Development, UK.
Declarations of interest
We certify that we have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of the review (e.g. employment, consultancy, stock ownership, honoraria, expert testimony).
References to studies included in this review
Christensen 1988 {published data only}.
- Christensen OE, Tuxen KK, Menday P. Treatment of travellers' diarrhoea with pivmecillinam [letter] . J Antimicrob Chemother 1988; 22 :570‐1. [ PubMed ] [ Google Scholar ]
DuPont 1982 {published data only}
- DuPont HL, Ericsson CD, Reves RR, Galindo E. Antimicrobial therapy for travelers' diarrhea . Rev Infect Dis 1986; 8 ( Suppl 2 ):S217‐22. [ PubMed ] [ Google Scholar ]
- DuPont HL, Reves RR, Galindo E, Sullivan PS, Wood LV, Mendiola JG. Treatment of travelers' diarrhea with trimethoprim / sulfamethoxazole and with trimethoprim alone . N Engl J Med 1982; 307 :841‐4. [ PubMed ] [ Google Scholar ]
- Dupont HL, Ericsson CD, Galindo E, Dupont MW, Mendiola Gomez J. Antimicrobial therapy of travellers' diarrhoea . Scand J Gastroenterol Suppl 1983; 84 :99‐105. [ PubMed ] [ Google Scholar ]
DuPont 1984 {published data only}
- DuPont HL, Ericsson CD, Galindo E, Wood LV, Morgan D, Bitsura JA, Mendiola JG. Furazolidone versus ampicillin in the treatment of traveler's diarrhea . Antimicrob Agents Chemother 1984; 26 :160‐3. [ PMC free article ] [ PubMed ] [ Google Scholar ]
DuPont 1992 {published data only}
- DuPont HL, Ericsson CD, Mathewson JJ, Cabada FJ, Conrad DA. Oral aztreonam, a poorly absorbed yet effective therapy for bacterial diarrhea in US travelers to Mexico . JAMA 1992; 267 :1932‐1935. [ PubMed ] [ Google Scholar ]
DuPont 1992b {published data only}
- DuPont HL, Ericsson CS, Mathewson JJ, DuPont MW. Five versus three days of ofloxacin therapy for traveler's diarrhea: a placebo ‐ controlled study . Antimicrob Ag Chemo 1992; 36 :87‐91. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Ericsson 1983 {published data only}
- Ericsson CD, DuPont HL, Sullivan P, Galindo E, Evans DG, Evans DJ Jr. Bicozamycin, a poorly absorbable antibiotic, effectively treats travelers' diarrhea . Ann Intern Med 1983; 98 :20‐5. [ PubMed ] [ Google Scholar ]
Ericsson 1986 {published data only}
- Ericsson CD, Johnson PC, DuPont HL, Morgan DR. Role of a novel antidiarrheal agent, BW942C, alone or in combination with trimethoprim‐sulfamethoxazole in the treatment of traveler's diarrhea . Antimicrob Agents Chemother 1986; 29 :1040‐6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Ericsson 1987 {published data only}
- Ericsson CD, Johnson PC, Dupont HL, Morgan DR, Bitsura JA, Cabada FJ. Ciprofloxacin or trimethoprim‐sulfamethoxazole as initial therapy for travelers' diarrhea, A placebo‐controlled, randomized trial . Ann Intern Med 1987; 106 :216‐20. [ PubMed ] [ Google Scholar ]
Ericsson 1990 {published data only}
- Ericsson CD, DuPont HL, Mathewson JJ, West MS, Johnson PC, Bitsura JA. Treatment of traveler's diarrhea with sulfamethoxazole and trimethoprim and loperamide . JAMA 1990; 263 :257‐61. [ PubMed ] [ Google Scholar ]
Ericsson 1992 {published data only}
- Ericsson CD, Nicholls‐Vasquez I, DuPont HL, Mathewson JJ. Optimal dosing of trimethoprim‐sulfamethoxazole when used with loperamide to treat traveler's diarrhea . Antimicrob Agents Chemother 1992; 36 :2821‐4. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Ericsson 1997 {published data only}
- Ericsson CD, DuPont HL, Mathewson JJ. Single dose ofloxacin plus loperamide compared with single dose or three days of ofloxacin in the treatment of traveler's diarrhea . J Travel Med 1997; 4 :3‐7. [ PubMed ] [ Google Scholar ]
Kuschner 1995 {published data only}
- Kuschner RA, Trofa AF, Thomas RJ, et al. Use of azithromycin for the treatment of campylobacter enteritis in travelers to Thailand, an area where ciprofloxacin resistance is prevalent . Clin Infect Dis 1995; 21 :536‐41. [ PubMed ] [ Google Scholar ]
Mattila 1993 {published data only}
- Mattila L, Peltola H, Siitonen A, Kyronseppa H, Simula I, Kataja M. Short term treatment of traveler's diarrhea with norfloxacin: a double‐blind, placebo‐controlled study during two seasons . Clin Infect Dis 1993; 17 :779‐82. [ PubMed ] [ Google Scholar ]
Petrucelli 1992 {published data only}
- Petruccelli BP, Murphy GS, Sanchez JL, et al. Treatment of traveler's diarrhea with ciprofloxacin and loperamide . J Infect Dis 1992; 165 :557‐60. [ PubMed ] [ Google Scholar ]
Salam 1994 {published data only}
- Salam I, Katelaris P, Leigh‐Smith S, Farthing MJ. Randomised trial of single‐dose ciprofloxacin for travellers' diarrhoea . Lancet 1994; 344 :1537‐9. [ PubMed ] [ Google Scholar ]
Steffen 1993 {published data only}
- Steffen R, Jori R, DuPont HL, Mathewson JJ, Sturchler D. Efficacy and toxicity of fleroxacin in the treatment of traveler's diarrhea . Am J Med 1993; Vol 94 ( suppl 3A ):S182‐186. [ PubMed ] [ Google Scholar ]
- Steffen R, Jori R, DuPont HL, Mathewson JJ, Sturchler D. Treatment of travellers' diarrhoea with fleroxacin: a case study . J Antimicrob Chemo 1993; 31 :767‐776. [ PubMed ] [ Google Scholar ]
Taylor 1991 {published data only}
- Taylor DN, Sanchez JL, Candler W, Thornton S, McQueen C, Echeverria P. Treatment of travelers' diarrhea: ciprofloxacin plus loperamide compared with ciprofloxacin alone. A placebo‐controlled, randomized trial . Ann Intern Med 1991; 114 :731‐4. [ PubMed ] [ Google Scholar ]
Thornton 1992 {published data only}
- Thornton SA, Wignall SF, Kilpatrick ME, Bourgeois AL, Gardiner C, Batchelor RA, Burr DH, Oprandy JJ, Garst P, Hyams KC. Norfloxacin compared to trimethoprim / sulfamethoxazole for the treatment of travelers' diarrhea among U.S. military personnel deployed to South America and West Africa . Mil Med 1992; 157 :55‐8. [ PubMed ] [ Google Scholar ]
Wistrom 1989 {published data only}
- Wistrom J, Jertborn M, Hedstrom SA, Alestig K, Englund G, Jellheden B, Norrby SR. Short‐term self‐treatment of travellers' diarrhoea with norfloxacin: a placebo‐controlled study . J Antimicrob Chemother 1989; 23 :905‐13. [ PubMed ] [ Google Scholar ]
Wistrom 1992 {published data only}
- Wistrom J, Gentry LO, Palmgren AC, Price M, Nord CE, Ljungh A, Norrby SR. Ecological effects of short‐term ciprofloxacin treatment of travellers' diarrhoea . J Antimicrob Chemother 1992; 30 :693‐706. [ PubMed ] [ Google Scholar ]
References to studies excluded from this review
Bouree 1987 {published data only}.
- Bouree P, Kouchner G, Ponti M. Double‐blind study of traveller's diarrhoea using Nifuroxazide . Trans R Soc Trop Med Hyg 1987; 81 :859. [ PubMed ] [ Google Scholar ]
Chagnon 1996 {published data only}
- Chagnon A. Travelers' diarrhea . Revue du Praticien 1996; 46 ( 2 ):189‐95. [ PubMed ] [ Google Scholar ]
Hill 1961 {published data only}
- Hill JA. Diarrhoea in Aden ‐ a therapeutic trial . Trans R Soc Trop Med Hyg 1961; 55 :355‐360. [ PubMed ] [ Google Scholar ]
Richards 1970 {published data only}
- Richards DA. A controlled trial in travelers' diarrhoea . Practitioner 1970; 204 :822‐824. [ PubMed ] [ Google Scholar ]
Steffen 1988 {published data only}
- Steffen R, Heusser T, Schopp A, DuPont H. Efficacy and side‐effects of six agents in the self‐treatment of traveler's diarrhea . Travel Medicine International 1988; 6 :153‐157. [ Google Scholar ]
References to studies awaiting assessment
Anonymous 1995 {published data only}.
- No authors listed. Single‐dose ciprofloxacin in the treatment of traveller's diarrhoea [Ciprofloxacin‐einmaldosis zur behandlung der reisediarrhoe]. Deutsche Medizinische Wochenschrift 1995; 120 ( 25‐6 ):943. [ Google Scholar ]
Bruns 1995 {published data only}
- Bruns R, Raedsch R. Therapy of traveller's diarrhea [Therapie der reisediarrhoe]. Medizinische Welt 1995; 46 ( 12 ):591‐6. [ Google Scholar ]
DuPont 1994 {published data only (unpublished sought but not used)}
- Otsuka Pharmaceuticals, study no 106‐92‐212.
DuPont 1994b {published data only (unpublished sought but not used)}
- Otsuka Pharmaceuticals study no 106‐92‐209.
DuPont 1997 {published data only}
- DuPont HL, Ericsson CD, Mathewson JJ, Palazzini E, DuPont MW, DeLa Cabada FJ. Rifaximin ‐ a nonabsorbed antimicrobial in the therapy of travelers' diarrhea in Guadalajara, Mexico . The Fifth International Conference on Travel Medicine . March 1997.
Ziegenhagen 1992 {published data only}
- Ziegenhagen DJ, Raedsch R, Kruis W. Traveler's diarrhea in Turkey. Prospective randomized therapeutic comparison of charcoal versus tannin albuminate/ethacridine lactate [Reisediarrho in der Turkei. Prospektiv randomisierter therapievergleich kohle versus tanninalbuminat/ethacridinlactat]. Medizinische Klinik 1992; 87 ( 12 ):637‐9. [ PubMed ] [ Google Scholar ]
Additional references
- Black RE. Pathogens that cause traveler's diarrhea in Latin America and Africa . Rev Infect Dis 1986; 8 ( Suppl 2 ):S131‐S135. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Farthing M, Feldman R, Finch R Fox R, Leen C, Mandal B Moss P, Nathwani D, Nye F, Ritchie L, Todd W, Wood M. The management of infective gastroenteritis in adults. A consensus statement by an expert panel convened by the British Society for the Study of Infection . J Inf 1996; 33 :143‐152. [ PubMed ] [ Google Scholar ]
Cartwright 1997
- Cartwright RY, Chahed M. Foodborne diseases in travellers . World Health Statistics Quarterly 1997; 50 :102‐110. [ PubMed ] [ Google Scholar ]
DuPont 1993
- DuPont HL, Ericsson CD. Prevention and treatment of traveler's diarrhea . N Engl J Med 1993; 328 :1821‐1827. [ PubMed ] [ Google Scholar ]
Farthing 1992
- Farthing M, et al. Treatment and prevention of traveller's diarrhoea . Gastroenterology International 1992; 5 :162‐175. [ Google Scholar ]
Gorbach 1975
- Gorbach SL, Kean BH, Evans DG, Evans D, Bessudo D. Traveler's diarrhea and toxigenic Escherichia coli . N Engl J Med 1975; 292 :933‐936. [ PubMed ] [ Google Scholar ]
- Hundt A. Impact of tourism development on the economy and health of Third World Nations . J Travel Med 1996; 3 :107‐112. [ PubMed ] [ Google Scholar ]
Katelaris 1995
- Katelaris PH, Farthing MJ. Traveler's diarrhea: clinical presentation and prognosis . Chemotherapy 1995; 41 Suppl 1 :40‐7. [ PubMed ] [ Google Scholar ]
McIntosh 1997
- McIntosh IB, Reed JM, Power KG. Traveller's diarrhoea and the effect of pre‐travel health advice in general practice . Br J Gen Pract 1997; 47 :71‐75. [ PMC free article ] [ PubMed ] [ Google Scholar ]
NIH‐CDCS 1985
- [No authors listed]. Travelers' diarrhea. NIH Consensus Development Conference . JAMA 1985; 253 ( 18 ):2700‐4. [ PubMed ] [ Google Scholar ]
Steffen 1983
- Steffen R, Linde F, Gyr K, Schar M. Epidemiology of Diarrhea in Travelers . JAMA 1983; 249 :1176‐1180. [ PubMed ] [ Google Scholar ]
Steffen 1999
- Steffen R, Collard F, Tornieporth N, et al. Epidemiology, etiology, and impact of traveler's diarrhea in Jamaica . JAMA 1999; 281 :811‐17. [ PubMed ] [ Google Scholar ]

COMMENTS
Travelers' diarrhea (TD) is the most predictable travel-related illness. Attack rates range from 30%-70% of travelers during a 2-week period, depending on the destination and season of travel. ... Antibiotic treatment is advised (single-dose regimens may be used) • Azithromycin is preferred • Fluoroquinolones or rifaximin1 can be used ...
Travelers' diarrhea (TD) is the most common travel-related illness, ... agent because of its low global resistance against invasive pathogens and tolerable safety profile. 38-41 A single-dose antibiotic regimen can be tried initially and continued daily for up to 3 days if symptoms are not resolved within 24 hours. Therefore, pharmacists ...
Destination is the most significant risk factor for developing traveler's diarrhea. 1 - 4 Regions with the highest risk are Africa, South Asia, Latin America, and the Middle East. Travelers ...
Episodes of travelers' diarrhea are nearly always benign and self-limited, but symptoms may disrupt planned activities and result in health care visits for some travelers . There is a growing recognition that travelers' diarrhea and its self-treatment abroad are associated with the acquisition of organisms harboring antibiotic resistance [ 5-10 ].
Antibiotics (eg, ciprofloxacin, azithromycin) for moderate to severe diarrhea. The mainstay of treatment of traveler's diarrhea is fluid replacement and an antidiarrheal medication such as loperamide. The dosage of loperamide for children < 12 years of age is weight-based. An alternative for adults is diphenoxylate/atropine.
Travelers' diarrhea is the most common illness in persons traveling from resource-rich to resource-limited regions of the world. Among travelers to such areas, ... Evaluating Ambulatory Therapy of Travelers' Diarrhea (TrEAT TD) Study: A Randomized Controlled Trial Comparing 3 Single-Dose Antibiotic Regimens With Loperamide. Clin Infect Dis 2017 ...
Single-dose antibiotic regimens may be used to treat moderate or severe travelers' diarrhea (Strong recommendation, high level of evidence). Summary of the Evidence The decision to treat TD with non-specific anti-diarrheal medications and/or an antimicrobial agent is based on assessment of the severity of illness and the effects it will have ...
Benefit. For the past 30 years, randomized controlled trials have consistently and clearly demonstrated that antibiotics shorten the duration of illness and alleviate the disability associated with travelers' diarrhea (TD). Treatment with an effective antibiotic shortens the average duration of a TD episode by 1-2 days, and if the traveler ...
Lifestyle and home remedies. If you do get traveler's diarrhea, avoid caffeine, alcohol and dairy products, which may worsen symptoms or increase fluid loss. But keep drinking fluids. Drink canned fruit juices, weak tea, clear soup, decaffeinated soda or sports drinks to replace lost fluids and minerals.
Treatment. Mild traveler's diarrhea can usually be managed with the judicious use of antimotility agents such as loperamide (Imodium A-D), in a dosage of two 2-mg tablets initially, then one ...
Travelers' diarrhea is a common ailment in persons traveling to resource-limited destinations overseas. Estimates indicate that it affects nearly 40% to 60% of travelers depending on the place they travel, and it is the most common travel-associated condition. Bacterial, viral, and parasitic infections can cause symptoms, though bacterial sources represent the most frequent etiology. While ...
Traveler's diarrhea is an infection characterized by diarrhea, nausea, and vomiting that commonly occur in travelers to areas of the world with poor water purification. Traveler's diarrhea can be caused by bacteria, parasites, or viruses. Organisms that cause the disorder are usually acquired from food or water, especially in countries where ...
The mainstay of treatment of traveler's diarrhea is fluid replacement and an antimotility drug such as loperamide. For adults and children ≥ 12 years of age, the loperamide dosage is 4 mg orally initially, followed by 2 mg orally for each subsequent episode of diarrhea (maximum of 6 doses/day or 16 mg/day). An alternative for adults is ...
Some antibiotics administered in a once-a-day dose are 90% effective at preventing travelers' diarrhea; however, antibiotics are not recommended as prophylaxis. Routine antimicrobial ... 2 262 mg tablets every 30 minutes for up to eight doses in a 24-hour period, which can be repeated on a second day. If diarrhea persists despite therapy ...
In the meta-analysis performed by Alajbegovic et al., the fluoroquinolone antibiotics, such as ciprofloxacin and norfloxacin, given for prevention showed a high degree of protection (). 51 However, many of the studies were performed in the 1980s and 1990s before fluoroquinolone resistance was detected. It is likely that if the studies were repeated today in Asia, fluoroquinolone antibiotics ...
The use of bismuth subsalicylate or loperamide may be considered. For moder-ate travelers' diarrhea, antibiotics such as fluoroquinolones, azithromycin, and rifaximin may be used. ... (regular strength) every 30 to 60 minutes until diarrhea subsides or eight doses have been taken . The recommended dose for children 10 to 11 years, 6 to 9 ...
Traveler's diarrhea is a digestive tract disorder that commonly causes loose stools and stomach cramps. It's caused by eating contaminated food or drinking contaminated water. Fortunately, traveler's diarrhea usually isn't serious in most people — it's just unpleasant. When you visit a place where the climate or sanitary practices are ...
Several antibiotics can be used to treat traveler's diarrhea. One antibiotic commonly used for traveler's diarrhea is ciprofloxacin (brand name: Cipro). Often, only a few doses are needed to clear ...
The recommendations on managing people at low or intermediate risk of travellers' diarrhoea are largely based on expert opinion regarding general advice to offer travellers in a World Health Organisation (WHO) publication International Travel and Health [], the American College of Gastroenterologists guideline Diagnosis, Treatment, and Prevention of Acute Diarrheal Infections in Adults [Riddle ...
Many trials of antibiotics in travellers' diarrhoea have been conducted and demonstrate shorter illness following antibiotic treatment. Travellers' diarrhoea is a self‐limiting illness. ... Patients kept daily diary of symptoms, signs, and medication doses: Participants: 447 Swedish travellers on short duration trips (1‐6 weeks) to ...
Gastrointestinal infection that occurs either during travel or shortly after returning home is easily identified as traveler's diarrhea. How common is traveler's diarrhea? Traveler's diarrhea is the most common travel-related illness. It affects between 30% and 70% of travelers, depending on the destination and the season.