How to Troubleshoot Temperature Excursions?
Download our free temperature excursions poster below detailing everything from this blog including:
- Warning signs of a temperature excursion
- Actions items to take during an excursion event
- Preventative measures you can take
As a primer, we’ve detailed below everything you need to know about temperature excursions so you can be prepared if one happens inside your healthcare facility.
What Is a Temperature Excursion?
In the United States, the CDC defines a temperature excursion as "any break in the cold chain of a pharmaceutical product" . The "cold chain", is a series of refrigerated production , storage and distribution activities. These processes ensure that vaccines and other pharmaceuticals remain at the correct temperature. Maintaining proper temperatures and avoiding excursions ensures that materials remain safe and effective.
Meanwhile, according to the European Compliance Academy , a temperature excursion is defined as: " a deviation from the labeled storage condition of a product for any duration " .
This includes excursions during transportation or distribution.

As a result of this degradation, there will be a generation of impurities in the product. Degradation products like this are not wanted in the manufacturing chain. Additionally, they present a threat to patients' health.
As such, the raw materials needed to produce pharmaceuticals need to be maintained as well . Active pharmaceutical ingredients (API) need proper temperatures during the manufacturing process. If not properly handled, they can lose potency, effectiveness and safety before they're even distributed.
Deviating from optimal storage conditions can result in significant changes in the API. This includes degradation, decay, polymerization, and an increase in impurity levels.
Reasons Why Temperature Excursions Occur
Air handling units (AHU) maintain the required temperature in pharmaceutical factories. So, the design and capacity of these units reflect the API manufacturers temperature requirements during the production process. Regardless, temperature excursions are unavoidable. Even in the manufacturing area and during transportation.

- An insufficient number of air handling units to maintain the desired temperature conditions
- A leaked or ruptured air duct
- Mechanical failure in air handling units
- Unprecedented temperature fluctuations.
- Power outages interrupting the AHU's operation
- Poor adherence to good manufacturing practices (GMP) on the production floor
- Poor staff oversight of temperatures/ Poor Quality Control
- Extreme weather conditions and poor or absent SOPs for handling them

Effects Of Temperature Excursion
A temperature excursion affects product quality in two potential ways. But, the impacts of high temperatures are different than those of temperatures that are too low. Though the result is the same nonetheless - a compromised product.
Products sensitive to high temperatures experience degradation that can cause a decrease in the active ingredient content of the product .
This happens due transformations in the affected, degraded components within. The transformation could be oxidative, hydrolytic, or some other form. As a result, much more toxic variants of the compound begin to appear. The amount of degradation/toxicity is dependent on length and severity of the temperature excursion, which can result in:
- Components becoming discolored.
- Changes in dissolution rates
- Separation of emulsions
Products sensitive to a low temperature usually get damaged by phase changes caused by the freezing process . As a result, the physical atomic structures of the chemicals experience permanent changes.
Products with large quantities of water, like creams or biologicals, are especially prone. Often, they'll lose their properties after experiencing excessive freeze-thaw or temperature cycles. Because water can easily change temperature, the presence of ice quickly damages product.
How To Measure Temperature Excursions
One responsibility of pharmaceutical manufacturers is guaranteeing every batch is a safe and effective high quality product. One of the ways to make this happen is through continuous temperature monitoring. By monitoring the temperature range, they can catch any excursions that occur. After which, they can take the necessary steps to address them immediately.
The Center for Disease Control recommends using a continuous temperature monitoring device (TMD) to track excursions . The recommended TMD is a digital data logger (DDL) that has the following characteristics:
- Ability to record the logged temperature values to a computer system for review.
- The digital data logger's calibration status must be regularly verified, with up-to-date software. This ensures accurate information about storage conditions.
The following information is required when documenting temperature excursions:
- The date and time the temperature excursion occurred
- An inventory of affected products
- The storage unit air temperatures. Including the minimum and maximum temperatures observed during the temperature excursion, if available
- Ambient temperature (also referred to as “room temperature”).
- A general description of the event, such as the length of the temperature event. The digital data logger should provide this data.
- A list of any other items in the storage unit
- Documentation of any problems with the storage unit
All the data should be compiled, and a copy given to the distributor and recipient of the affected product(s).


How To Avoid and Combat Temperature Excursions
Simply put, any temperature excursion is a result of prolonged exposure to air that is room temperature or above. For end users of pharmaceuticals, the most likely scenario for them to experience a temperature excursion is because they’ve left the door open to a medical refrigerator/medical freezer or it has stopped running.
If Your refrigerator/freezer temperature has dropped below 35°F (2.0°C) for 15 or more minutes consecutively:
- Check the Placement of the Thermometer probe – Place in the middle and monitor temperature in 30-minute intervals
- Adjust Refrigerator/Freezer Temperature - Change temperature of appliance, if possible, to a warmer setting. Monitor and record = temperature every 30 minutes for next 2 hours.
- If temperatures remain out of range — implement facilities’ relocation plan. Immediately call vaccine manufacturer/distributor.
If your Refrigerator has risen above 46°F (7.7°C) or Freezer has risen above 5°F(-15°C) for 60 minutes or more consecutively:
- Check your power supply — if your area is experiencing a power outage estimated to last 2 or more hours and your facility does NOT have a dedicated backup power source for your cold storage appliances, implement emergency relocation plan
- Check the door to the storage unit — ensure nothing is preventing door from securely closing. If so, adjust accordingly and shut door completely.
- Adjust the refrigerator/freezer temperature — Change temperature of appliance to a cooler temperature is possible. Monitor and record temperature every 30 minutes for next 2 hours.
- If temperatures remain out of range — implement facilities’ relocation plan. Immediately call vaccine manufacturer/distributor. In short, unless total failure of the appliance has occurred, the most common solutions to these problems are:
- A door alarm to ensure staff always close it
- A backup power solution to ensure any medical and pharmaceutical refrigerators continue to operate as normal.
While door alarms are fairly easy to source tools, a reliable and powerful backup power system for refrigerators can be harder to find.
Luckily, battery backup systems offer instant and automatic power for medical appliances as soon as the power goes out. As a result, no staff needs to be on-site to keep track of or start the generator and vaccines will continue to remain safe—with no extra work required.
Additionally, their vertical, cabinet-like design and leak-proof batteries mean they can be installed in even the tightest spaces and oriented in anyway to make them fit. Plus, if your medication or vaccine room is truly tight on space, a hardwired backup power unit can instantly supply remote power to your appliance—directly via the outlet its already plugged into.
Regardless of what kind of system is the best fit, they ensure that your entire stock of vaccines are protected from a sudden loss of power (and the resulting temperature excursions) by guaranteeing a seamless transition from utility power to backup power.

- Over a weekend
- Or even for a whole week.
So, to protect your facility from tens of thousands of dollars in lost vaccine stock, speak to a Medi-Products battery backup expert.
They’ll help design you a system that both meets your power needs and will fit inside your facility—for a much lower cost than what your vaccines are worth. So a backup power system pays for itself the first time your power goes out.
Designing a system for you is as easy as taking a picture of your appliance’s nameplate, and a photo of the room where it’s in.
Then, you just email both photos to our Product experts, and we’ll provide you with multiple options for backup power protection.
For more information contact:
1.800.7653237
Battery Backup for Vaccine Refrigerators and Freezers.

Our powerful battery backup systems will instantly power multiple appliances during a power outage. These custom sized systems can provide power for up to 72 hours of runtime!
POPULAR QUESTIONS
Blog categories.
- Healthcare Compliance (29)
- Healthcare Management (26)
- Healthcare Storage (22)
- Healthcare Design (21)
- Vaccine Storage (20)
- Backup Generators (18)
- Power Outages (18)
- Energy-Efficient Solutions (1)
VISIT OUR LEARNING CENTER

We would like to hear from you
Our sales and technical support staff are available 8-5 EST, Mon-Fri

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Saudi Pharm J
- v.25(2); 2017 Feb
Temperature excursion management: A novel approach of quality system in pharmaceutical industry
Quality of pharmaceutical product largely depends upon the environment controls during its storage and handling. Each pharmaceutical product should be handled and stored under specified storage condition labelled on product information data sheet or product pack. Hence the temperature excursions during receipt of raw materials, manufacturing of pharmaceutical products and distribution should be managed during entire product life cycle with holistic approach. The research is based on primary data and exploratory study through literature review. The temperature excursion may be observed during transportation of raw materials manufacturing as well as distribution of pharmaceutical products, which have potential to deteriorate the product quality. Temperature excursion in pharmaceutical industry should be recorded and reported to the manufacturer for further investigation and risk analysis. The concept of temperature excursions, its reasons, consequences and handling mechanism should be well understood to ensure the concerted efforts under the aegis of Quality Management System. Based on the reasons and consequences of temperature excursions during pharmaceutical operations, a system based quality management has been envisaged through this study. The concept and procedure to handle temperature excursion have evolved after this study which shall be useful to pharmaceutical industry as well as to medicine distributors and consumers.
1. Introduction
The pharmaceutical product quality largely depends upon the storage environmental conditions. Natural reasons or human negligence could create uncalled-for situation causing temperature excursions. The most important environmental parameter having significant potential to impact quality of pharmaceutical product is temperature. If the temperature excursions are not handled systematically, there shall be an adverse impact on product quality.
There is a growing need to manage the environment excursions during pharmaceutical operations and its impacts on quality of products. In an era of Quality by Design (QbD) for pharmaceutical products, the attention is paid towards inbuilt quality instead of inspected quantity ( Roy et al., 2012 ). As manufacturers have extensive knowledge about critical product and process parameters and quality attributes, the impact assessment has to be extended to temperature excursions. The temperature and relative humidity (RH) beyond limit shall lead to product degradation rate and microbial growth. This concept is the theoretical basis for the pharmaceutical guidelines that provide recommendations for long-term, intermediate, and accelerated storage conditions and for establishing shelf life periods or expiry dates of products ( Scrivens, 2012 ).
Pharmaceutical regulatory bodies expect strict adherence of Good Manufacturing Practices (GMP) and Good Distribution Practices (GDP) during plant manufacturing and product distribution processes. GMP and GDP are deemed as synonyms of Quality System in pharmaceutical business. Since temperature excursions are observed during raw material receipt, manufacturing operation and distribution of pharmaceutical products, there is a need of holistic approach of quality system which shall be based on both GMP and GDP.
1.1. Research methodology
The following instruments have been used to generate data for the study:
- (a) A survey has been conducted amongst pharmaceutical professionals to understand their experience regarding environmental condition during pharmaceutical manufacturing and that during distribution process.
- (b) The guidance papers issued by drug regulatory agencies and related literature and scientific search engines such as Google were searched for pharmaceutical supply chain risk management studies in English language. Searching through databases was done with different keywords: supply chain risk, Good Distribution Practices, Quality Risk Management, and pharmaceutical. Searching in each database was adapted to databases characteristics and additionally pharmaceutical risk. The result studies and meeting abstracts were screened at 4 steps and exclusion process was based on consensus of both the authors.
1.2. Data and analysis
The research survey study amongst pharmaceutical professionals in India reveals that the records of environment condition (EC) monitoring during manufacturing and distribution operations follow a contrast trend (refer Chart 1 ). The survey alludes that deployment and monitoring of data logger results during manufacturing as per GMP are in place, whereas that during distribution operation is not so methodological.

Trend of Environment Condition (EC) monitoring practices during manufacturing and distribution operations.
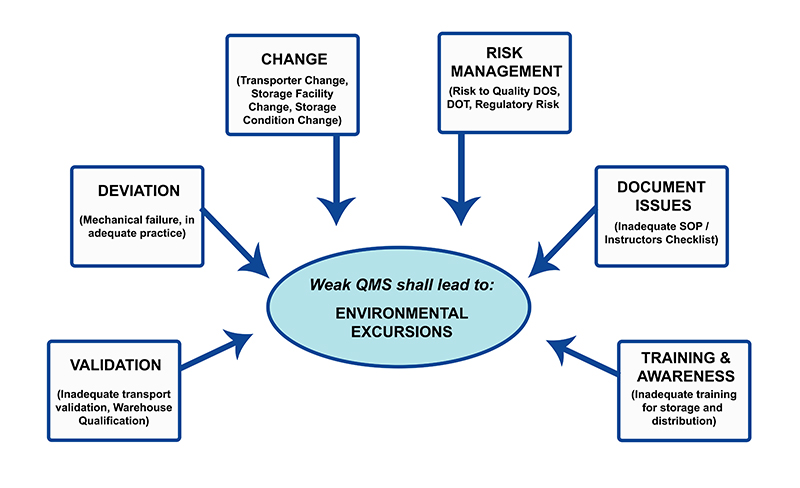
As a part of Quality Management System (QMS), pharmaceutical industry has identified a number of core quality elements which are followed at manufacturing site level. Few of such core elements of plant quality system may be listed as,
- a. Documents and Record Control: There shall be good documentation practices in organization to ensure the document and online records are adequately maintained.
- b. Deviation Control: Incidences leading to departure from documented and approved instructions shall be recorded and evaluated for potential impact on product quality.
- c. Change Control: The changes to an approved design, equipment or system in pharmaceutical facility shall be adequately reviewed and validated.
- d. Validation Master Plan: The validation master plan shall exhibit management philosophy, strategy and commitment of organization towards validations of processes and qualification of equipment.
- e. Quality Risk Management: Quality risk to product shall be identified and evaluation shall be made to estimate the severity, occurrence and detectability. A robust quality risk management.
- f. Training and Awareness: The organization shall develop and implement robust training programme for personnel engaged in GMP operations.
- g. Market Complaint Handling system: There shall be a documented procedure to receive, log and investigate each market complaint to further facilitate necessary corrective and preventive action.
- h. Recall Management, etc.: There shall be documented procedure to handle the recall or market returned goods.
It is observed that inadequate QMS components have direct impact on consistency of storage conditions with respect to temperature and humidity. The temperature excursion is a common notion which signified the general environmental excursions (see Fig. 1 ).

Effect of inadequate Quality System element can cause Environmental Excursions (EC).
A research survey amongst pharmaceutical professionals finds that the core quality assurance aspects are followed by each manufacturing site as a part of cGMP but during distribution practices (out of plant) the above quality elements are not followed meticulously. The environmental excursion management is interlinked with Product Complaint Management, Quality Risk Management, Deviation Management and Change Control Management, sometimes as a cause or else as an effect.
2. Discussions – facets of temperature excursion
Quality and environmental excursions are two important aspects of pharmaceutical operational excellence. An integrated approach for managing the quality system should include the temperature excursion management. The overall temperature excursion management can be laid down in following steps.
2.1. Understanding temperature excursion
The environmental condition during pharmaceutical business is defined by temperature (T) and relative humidity (RH). The temperature and relative humidity are used and monitored during manufacturing processes particularly when the product intermediates are exposed to environment. During packaged conditions temperature is measured, monitored and controlled. Any spike in measured value of T and RH shall be construed as environmental excursion. During manufacturing the temperature is always maintained below 27 °C, and whenever any deviation from this limit is observed, the case is thoroughly investigated and impact on product quality is mitigated.
According to European Compliance Academy, a temperature excursion is the deviation from the labelled storage condition of a product for any duration whether during transportation or distribution. Studies indicate that if there is exposure of product or intermediate beyond specified environmental limits for substantial time, there shall be generation of impurities as result of product degradation. Such degradation products are not only regarded as undesired but also shall have adverse reaction to the patient’s health.
The temperature excursion phenomena are also applicable during manufacturing and transportation of active pharmaceutical ingredients (API) prior to receipt at pharmaceutical manufacturing site. Deviation against storage condition can lead to significant qualitative change in API, such as degradation, decomposition, polymerization and impurity level increase.
2.2. Storage temperature and humidity limits
Specified directions are stated in some monographs with respect to temperature and humidity at which official articles shall be stored and distributed (including the shipment of articles to the customer), when stability data indicate that storage and distribution at a lower or higher temperature and humidity produce undesired results.
Cold chain products are those products which have to be necessarily stored under cold condition (refer Table 1 ). For products from class of cold chain, the storage condition is maintained at 2–8 °C. Similarly the relative humidity shall be maintained below 60% and 40% depending upon the hygroscopic nature of product.
Typical storage conditions.
United States Pharmacopoeia (USP) has described the different labelling terminologies related to temperature conditions. The general chapter of USP clarified various storage labelling conditions.
2.3. Measurement devices
Temperature and Humidity measuring devices (popularly known as data loggers) are available in market, which log the temperature at a defined (preset) interval that can be downloaded in computer system for review, evaluation, investigation and record. Periodic verification of calibration status of temperature data loggers and upgradation of software is the prerequisite of uninterrupted and accurate information about product storage condition.
Calibration and periodic verification of measurement device are keys of correct recording. In addition to that periodic cleaning of measuring device and appropriate precautions should be taken to avoid blockage or damage of sensor. It is recommended to use a temperature measurement reference instrument which is of higher accuracy than the device to be checked. The temperature and relative humidity sensor should be placed on the hottest spot, concluded after temperature mapping of the area.
2.4. Locations where environmental or temperature excursions may take place
To manage the temperature excursion related issue, it is important to know the places where temperature excursion can occur. The deviations can be observed against the temperature limits not only at manufacturing sites or during transportation or distribution rather, it can be caused at the end of business i.e., retail outlets and drug shops (see Fig. 2 ).

Locations wherein excursions can occur.
2.5. Reason of temperature excursion
In pharmaceutical manufacturing plant and storage area, the specified temperature is maintained with help of air handling units (AHU). The design and capacity of AHU are selected on the basis of volume of work area or manufacturing cubical.
2.5.1. Temperature excursions in manufacturing area caused due to following reasons (not limited to)
- a. Inadequate number of air handling unit (AHU) installed which are actually required to maintain the desired temperature conditions inside manufacturing shop floor.
- b. Leakage or rupture from air duct, resulting in insufficient cooling effect.
- c. Mechanical failure in air handling units (AHU), such as breakdown.
- d. Power failure making the AHU operation defunct.
- e. Lack of quality system and weak discipline of Good Manufacturing Practices (GMP) on production shopfloor.
- f. General awareness about the consequences of not maintaining temperature within limit and undue keenness to prevent power consumption.
- g. Extreme weather changes and obsolete contingency plan to handle unprecedented temperature fluctuations.
2.5.2. During transportation of pharmaceutical product, following reasons (not limited to) cause temperature excursion
- a. Unexpected delay in transportation due to which the temperature control cannot be maintained effectively.
- b. Product pallets are kept in hot zones of airport or shipping yards.
- c. Reefer containers or refrigerated control vans are not deployed for transportation.
- d. The transport agency fails to maintain the planned transport condition.
- e. Higher cost to maintain temperature within limits and other business issues.
- f. Power failure due to short longevity of power bank during longer travel time.
- g. Good Distribution Practices (GDP) understanding amongst supply chain personnel about the adverse impact on product quality.
2.6. Consequences of temperature excursion
The storage condition for product is assigned on the basis of scientific studies to avoid deterioration during product life cycle. If the temperature excursion is not taken due care the following negative impacts are commonly noticed :
- a. Loss of assay.
- b. Increase of impurity.
- c. Separation of layers of liquid products.
- d. Change in dissolution pattern of solid dosage.
- e. Discolouration of products.
Achieving the stability by design for solid dosage pharmaceutical products shall require establishment of limits for storage temperature (T) and relative humidity (RH). Within these prescribed temperature (T) and relative humidity (RH) limit, the kinetics of degradation of a pharmaceutical product can be estimated using and extended Arrhenius model ( Porter, 2013 ).
An excursion can have a significant impact on quality of products, which can be investigated as a part of therapeutic drug properties. As a consequence of excursion, the following impact can be observed (see Fig. 3 ).

Impact on quality and therapeutic drug properties due to temperature.
2.7. Review of sensitivity of drug products towards temperature excursions
To control temperature excursions is often complicated because there is no way to predict the condition that the product will be exposed to. The management of temperature excursion becomes more important particularly for the drug products, which are sensitive towards temperature conditions. Due care against potential excursions is mandatory during the course of drug manufacturing as well as during distribution process.
2.7.1. Product sensitive towards higher temperature
Drug product sensitive to higher temperature excursions can depreciate the intended action by receiving the thermal energy. This may instigate,
- • Transformation of degraded ingredients because of oxidation, hydrolysis, decomposition, polymerization. As longer as the time of exposure of product to unspecified temperature, higher would be the impact on quality.
- • Modification of the drug dissolution pattern (either higher or lower) in case of solid dosage drug product.
- • Separation of emulsions.
2.7.2. Product sensitive towards lower temperature
The adverse impact on product quality is not observed only due to exposure to higher temperature. Drug product sensitive to lower temperature can also depreciate the intended action by losing their therapeutic characters. This may cause,
- • Lattice positions dislocation due to extremely lower temperature.
- • Change in property of biological products.
2.7.3. Cold chain products
A deficiency in monitoring and maintenance system usually affects the products’ therapeutic properties and causes quality risks such as lack of effect, intoxications. In case of cold chain products the challenge of temperature excursions is bigger, because there is a task to preserve the adequate storage and temperature conditions throughout the product life cycle. Quality Assurance personnel must make sure that conditions of storage are observed at any time during manufacturing, transport and distribution.
2.7.4. In case of lyophilized drug products
Lyophilization process is commonly used for pharmaceuticals/biopharmaceuticals to improve the stability and shelf life. A disruption of the initial freezing rate due to temperature excursion can potentially lead to incomplete crystallization of crystalline excipients or heterogeneous moisture distribution in the lyophilized products. Temperature excursion during drying can lead to collapse or there could be melt back related negative impact on product quality.
3. Result – solution strategy and model for temperature excursion management
3.1. solution strategy against excursions.
In view of significant impact on product quality due to environmental excursions, the pharmaceutical industry should establish a documented programme for ‘Temperature Excursion Management’. As a part of this programme, the storage condition database and standard operating procedure (SOP) are required. The details of strategy are as under.
3.1.1. Development of storage condition database
To handle the environment excursion, the strategic planning, effective packaging and well documented procedure are recommended. Development of storage database of pharmaceutical product is useful to assign the standard storage condition.
The storage conditions suitable for product are assigned through the following information:
- a. At the product development stage, the semi finished product and final pharmaceutical product dosage are subjected to challenging conditions (i.e., forced conditions) to observe the potential impact on quality attributes.
- b. Hold time studies are carried out to establish the database for allowable time period at specified storage condition without impacting quality. The quality attributes of product intermediates include chemical, microbiological and pharmacological determinants at various time points of stability.
- c. Studies of the long term (i.e., real time) and accelerated condition stability study data of each product formula form an assurance for the storage condition that shall be suitable to product safety. The real time stability data are generated in laboratory to assess the change in quality attributes of product throughout the expiration date. The accelerated stability data are generated to evaluate the impact on quality of product under slightly stressed environmental condition.
A freeze/thaw study for multiple cycles should be conducted to specify the effect of freezing, if any, and the subsequent thawing. Samples from different layers (top, middle and bottom) of container are drawn for analysis at the end of the cycle (see Fig. 4 ).
- a. Know your formula.
- b. Carryout Quality Risk Analysis.
- c. Establish Stability Study Protocol.
- d. Evaluate Stability Study Data.
- e. Improve Product Formula.
- f. Redefine Product Formula.

Temperature Management and Control Cycle.
3.1.2. Thermal packing during transportations
Packaging configuration should include the provision of thermal packing and display of appropriate storage condition. Caution notes to avoid storage outside the specified conditions shall help supply chain personnel to protect the product quality.
The storage condition should be effectively displayed on the packaging of pharmaceutical product. The packaging configuration card must contain the details of data loggers.
3.1.3. Ten step procedure to deal with temperature excursions
The temperature excursion has regulatory implications as well as business impact due to impact on quality.
A standard operating procedure (SOP) compromising of following steps should be established and adherence should be ensured through adequate training to concerned personnel:
- i. The provision of technical agreement should be laid down in SOP. Technical agreement between manufacturer and distributor should clearly assign the responsibility of notifying the details of environmental excursions during transit, storage and distribution process. A contract is a written agreement between two or more interested parties which creates obligations that are enforceable by law. Before services are provided by a vendor, a contract must be put in place and executed by the parties.
- – Active Pharmaceutical Ingredient (API) supplier – Pharmaceutical product manufacturer.
- – Active Pharmaceutical Ingredient (API) supplier – transporter, cargo.
- – Pharmaceutical product manufacturer – transporter, cargo, etc.
- ii. Product characteristics database should be accessible to all concerned personnel for ready reference. Such database should clearly mention the storage instruction and precautions prominently highlighted on packs of product. Ideally larger display of storage condition on shipment pack shall alert the distribution agencies to avoid potential excursions.
- (a) manufacturing facility with special attention on environmental controls,
- (b) transport media (motor van, shipping containers, air cargo, etc.),
- (c) replenishment and handling devices,
- (d) storage warehouse in loaded conditions,
- (e) distribution centres, and
- (f) drug seller’s outlet and pharmacy.
- iv. Standard Operating Procedure (SOP) should be in place for each critical steps of manufacturing and distribution that may have trigger temperature excursions. Adequate training (in the form of personalized, awareness or document read) should be imparted to all operation executives. The key personnel across the pharmaceutical business should be aware about the SOP on investigation, handling and managing the temperature excursions.
- v. Records related to temperature excursions and duration should be regularly reviewed and approved to ascertain whether an excursion may have occurred and a systematic investigation should be performed. This should be formally signed off physically or electronically.
- vi. Investigation report against temperature excursions and duration should be notified immediately to the responsible person (as per the technical agreement) or manufacturer in a timely fashion.
- vii. Product quality risk analysis should be carried out against each case of temperature excursions. As a part of risk evaluation, the specimen complaint sample and control sample (retained by manufacturer) may be simultaneously analysed by using the validated analytical method. Due focus should be there to estimate the loss of assay as well as increase in impurity in sample impacted due to temperature excursion.
- viii. The stability data should be available with manufacturer against each excursion to evaluate and justify that there is no impact on product quality due to the excursions. The stability study under accelerated condition and freeze–thaw study are the relevant to evaluate the impact on product quality in a scientific manner.
- An accelerated stability study programme in line with ICH:Q1 guidelines should be carried out.
- A typical freeze–thaw study comprises of estimating the quality impact due to storage of product at extremely low and high temperatures, such as −20 and +50 °C for a duration up to 12 days in multiple cycles depending upon the proposed route, time and length of travel ( Adadevoh, 2002 , GCC Guidelines, 2007 ).
- ix. The statistics of temperature excursion cases should be evaluated periodically by quality professionals and in case of recurring observation of excursions are noticed from a particular facility, that particular mode or facility should be subjected to requalification.
- x. Appropriate corrective actions should be taken to avoid the recurrence of temperature excursions, if any. Modify the storage and transportation conditions on the basis of quality risk management programme.
4. Conclusion
Temperature excursion is a general term that represents the environmental excursions. There is a need of holistic approach to handle the temperature excursions starting from raw material manufacturing site to medicine retailers shop to protect quality of product. The temperature excursion at any stage of pharmaceutical business operation must be reported as soon as possible and investigated appropriately. The consequences of deviation against temperature and humidity limits should be studied appropriately by quality assurance personnel. The risk of temperature excursions cannot be ruled out, but it can be minimized through effective system. Alternative is to use thermal resistant packaging and stringent control measures during transit and shipment, to avert the undesired quality impact on pharmaceutical product. The systematic approach to handle the issues related temperature excursions becomes inevitable for pharmaceutical manufacturers.
Peer review under responsibility of King Saud University.
- Adadevoh, K., 2002. Short-term, Freeze Thaw and Shipping Studies. ARMWG RFP11 Report.
- The GCC Guidelines for Stability Testing of Drug Substances and Pharmaceutical Products, 2007, Edition Two (1428 H – 2007G).
- Porter William R. Degradation of pharmaceutical solids accelerated by changes in both relative humidity and temperature and combined storage temperature and storage relative humidity (T × h) design space for solid products. J. Valid. Technol. 2013; 19 (2) [ Google Scholar ]
- Roy Souvik, Ruitberg Chiristian, Sethuraman Ananth. Troubleshooting during the manufacturing of lyophilized drug product – being prepared for the unexpected. Am. Pharm. Rev. 2012 [ Google Scholar ]
- Scrivens G. Mean kinetic relative humidity: A new concept for assessing the impact of variable relative humidity on pharmaceuticals. Pharm. Technol. 2012; 36 (11) [ Google Scholar ]
Comprehensive Temperature Excursion Management Program for the Commercial Distribution of Biopharmaceutical Drug Products
Affiliations.
- 1 Biopharmaceutical Product Sciences, GlaxoSmithKline, 1250 S Collegeville Road, Collegeville, Pennsylvania 19425. Electronic address: [email protected].
- 2 Biopharmaceutical Product Sciences, GlaxoSmithKline, 1250 S Collegeville Road, Collegeville, Pennsylvania 19425.
- PMID: 32315663
- DOI: 10.1016/j.xphs.2020.04.006
Biopharmaceutical drug products may be exposed to temperatures outside of the intended storage temperature range (typically 2-8°C) during commercial distribution due to uncontrolled variables and unexpected events. Pharmaceutical companies are expected to ensure that product quality and stability are not negatively impacted by temperature excursions defined as being acceptable for the product. It is imperative that all firms involved in the distribution understand key elements of the temperature excursion management program in place to overcome the challenges of global distribution and comply with regulatory requirements. Proactive implementation of a comprehensive temperature excursion management program is expected to help achieve successful commercial distribution. In this article, important aspects related to the key elements of a comprehensive temperature excursion management program are summarized, including standard stability testing, regulatory expectations related to the justification of temperature excursions, thermal cycling studies to assess and support potential temperature excursions (including how/when thermal cycling study data is used to support temperature excursions), good distribution practices to minimize temperature excursions and use of theoretical methods/mathematical simulation models to assess temperature excursions. A comprehensive temperature excursion management program is expected to ensure product quality and help minimize, assess, and justify temperature excursions more efficiently, ensure regulatory compliance and avoid business impact caused by the loss of products or inadequate supply.
Keywords: Biopharmaceutical drug product; Commercial distribution; Good distribution practices; Mean kinetic temperature; Regulatory expectations; Stability testing; Temperature excursion; Thermal cycling; mAb.
Copyright © 2020 American Pharmacists Association®. Published by Elsevier Inc. All rights reserved.
- Biological Products*
- Pharmaceutical Preparations*
- Temperature
- Biological Products
- Pharmaceutical Preparations
Explore solutions built for your industry
Our customer-proven solutions monitor medications and food inventories for some of the most recognizable names in the industries of healthcare, food service, and transportation, and logistics. See how our solutions adapt to your industry needs.
BY INDUSTRY
- Retail Grocery
- Food Service
- K-12 Education
- Conveniences Stores
- Supply Chain Monitoring
BY USE CASE
- COVID-19 Solutions
- Pharmacy Monitoring
- VFC Monitoring
- Food Safety Monitoring
- Asset Monitoring
- Advanced Analytics
SYSTEM COMPONENTS
- System Overview
- Cloud Dashboard
- Digital Checklists
- Sensors & Data Loggers
- Implementation Services
Festival Foods Protects its Profits & People with SmartSense
Share SmartSense Solutions with your team.
Resource Center
Work smarter. Explore our videos, webinars, and customer stories.
Learn how our Sensing-as-a-Service solutions can fit your business.
Review technical specifications for our solutions.
- Customer Videos
- Customer Stories
- Thought Leadership
- Media Coverage
- Wireless Sensors
- Installation
- See all categories
- Contact Support
Questions? Call +1 (866) 806-2653 to speak to our experts.
Questions contact us..
Call +1 (866) 806-2653 to speak with our experts or get started with a demo.
SmartSense was created to use the power of the Internet of Things (IoT) to help our customers protect the assets most critical to the success of their business.
Create the future of IoT by joining our team.
CONNECT. PROTECT. RESULTS.
- Leadership Team
- In the News
Select your login
- Help Center
- COVID-19 Monitoring
- SCHEDULE DEMO
- There are no suggestions because the search field is empty.
June 21, 2017
6 Steps for Handling Temperature Excursions
Written by SmartSense | Pharmacy Safety
What is a temperature excursion? In the pharmaceutical industry , it refers to any temperature reading outside recommended ranges from the manufacturer’s package insert. Why is it important? Because out-of-range storage temperatures or inappropriate conditions for any vaccine can negatively impact the efficacy, and will require immediate action.
Fortunately, the Centers for Disease Control (CDC) have developed best practices to handle this emergency situation. If there is any question about whether vaccines may have been exposed to a temperature excursion due to the unit becoming too cold or too hot, take the following steps.
Step 1: Notify Supervisors
Any staff member who hears an alarm, receives an alert message, or notices a temperature excursion should notify the vaccine coordinator immediately or report the problem to a supervisor.
Step 2: Quarantine Vaccines
If a vaccine has been compromised, quarantine it immediately. Label exposed vaccines, “DO NOT USE,” and place them separately from other vaccines in the storage unit. Do NOT discard the compromised vaccines.
Step 3: Document the Event
The vaccine coordinator, supervisor, or if necessary, the person reporting the problem, should document the event . Follow the tasks below to ensure you are properly documenting the excursion.
- Name of the person completing the report
- Date and time of the temperature excursion
- Inventory of affected vaccines
- Description of the event
- Minimum and maximum storage unit temperature and room temperature during the time of the event
- Length of time vaccine may have been affected
- Listing of items in the unit (including water bottles) other than vaccines
- Any problems with the storage unit and/or affected vaccines before the event
- Other relevant information

Step 4: Get Guidance
Contact your immunization program or vaccine manufacturer for additional guidance on whether to use affected vaccines and for information about whether patients will need to be recalled for re-vaccinations. Be prepared to provide documentation of the event (e.g., temperature log data).

Step 5: Implement SOPs
Implement your facility’s Standard Operating Procedures (SOPs) to adjust the unit temperature to the appropriate range. At a minimum, check the temperature monitoring device to make sure it is appropriately placed in the center of the fridge.
Step 6: Wrap Up
Complete your documentation of the event, including the following:
- What happened to affected vaccines
- What you did with the vaccine and when
- Whom you have contacted and instructions received
- What you have done to prevent a similar future event
You never know when a temperature excursion may happen. If you have not yet incorporated these best practices into your monitoring program, now is a good time to start!
Subscribe to Our Blog!
Subscribe to our blog to get weekly email updates about quality and safety issues important to the pharmaceutical industry.
Subscribe to the SmartSense Blog
Stay up-to-date on the evolution of IoT connectivity.
Learn how our complete critical environment monitoring solution will help you connect and transform your business.
Call +1 (866) 806-2653 to speak with our industry experts or get started by requesting a demo.
- Terms of Service
- Return Policy
- Privacy Policy
- Cookie Policy
- System Status
- LEADING MINDS NETWORK
- GxP Services
- Book a Demo
- Jetzt Demo buchen
- Login / Register
Search our website
- Production & Cleanroom
- Lab & Equipment
- Cryogenic Storage & Shipment
- Dry Ice Transport
Cold Chain in Transport
- Warehouse & Cold room
- Direct-to-Patient Shipment
- Clinical Trials
- Pharmacy & Hospital
- WHO Vaccines
- Server Room
- Cold Chain Monitoring
- MONITORING OF ROOMS & EQUIPMENT
- Gxp Services
- All Solutions
- liberoMANAGER
- LIBERO Cx multi-use
- LIBERO Cx single-use
- ECOLOG-PRO xG
- ECOLOG Unlimited
- Temperature Data Loggers
- Produktion & Reinraum
- Labor & Geräte
- Kryolagerung & -Versand
- Trockeneis-Transport
- Kühlkette im Transport
- Lagerhaus & Kühlraum
- Direct-to-Patient Versand
- Klinische Studien
- Apotheke & Krankenhaus
- WHO-Impfstoffe
- Lebensmittel
- KÜHLKETTEN ÜBERWACHUNG
- ÜBERWACHUNG VON RÄUMEN UND GERÄTEN
- ALLE PRODUKTE
- Temperatur Datenlogger

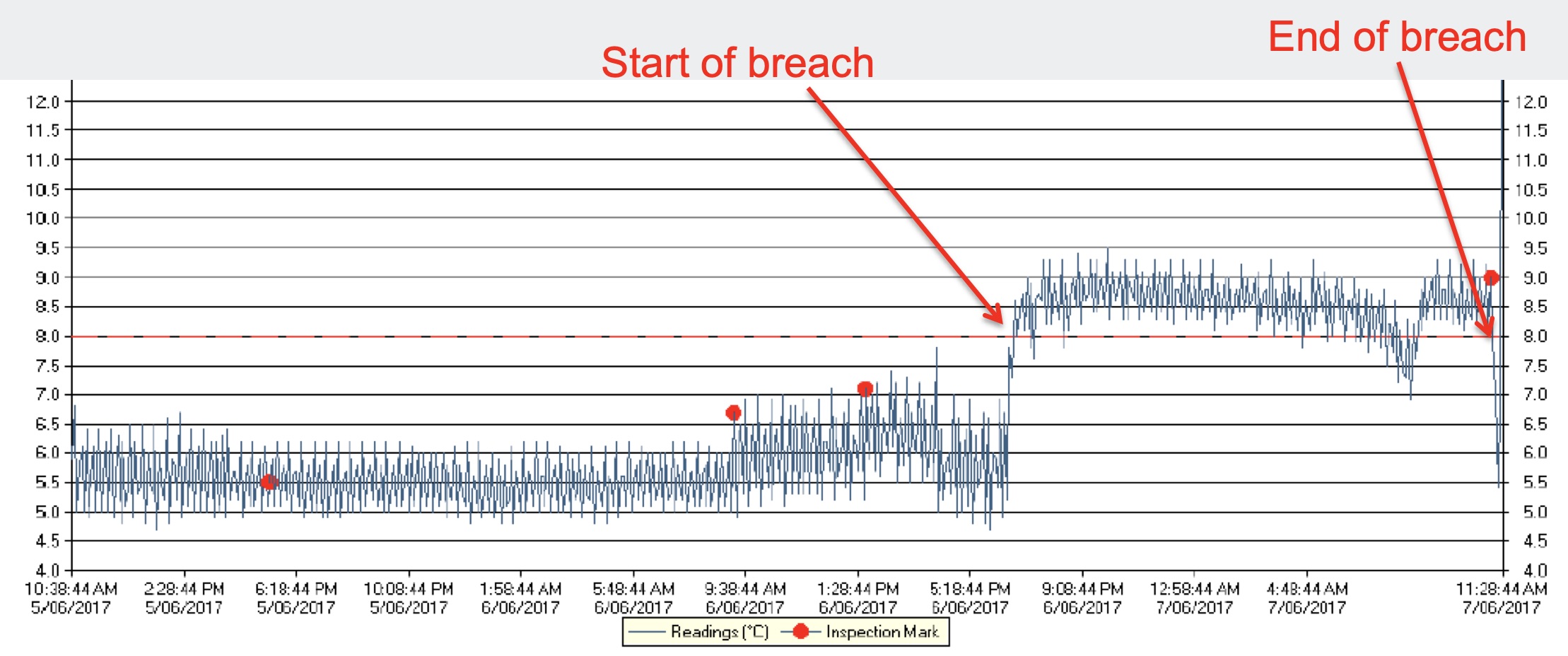
Documenting Temperature Excursions
It is the responsibility of every pharmaceutical manufacturer to guarantee every medicine they deliver to a patient is of the highest quality. Maintaining the quality of a pharmaceutical product requires adhering to strict precautions, including storing or transporting the medication at specified temperatures.
If a shipment is complete, a decision needs to be made to release or quarantine the product. The following data must be available:
Complete measurement record from a calibrated sensor
Start time stamp (clear date and time)
Stop time stamp (clear date and time)
Stability budget (assessment criteria) with clear conditions of temperature zones/limits and allowed times
Sometimes additional criteria are defined, like number of allowed excursions or number of freeze-thaw cycles. As soon as all the data is available, the assessment can be performed a clear OK (= release) or ALARM (=quarantine) decision can be made. Information between stakeholders usually takes place via email or SMS.
A temperature excursion could result in patients ending up with unsafe products that may cause adverse reactions. Therefore, every pharmaceutical manufacturer must ensure all products are kept in the specified conditions until they reach the patient.
So, what are the right tools to use to guarantee that patients are getting the right quality and, most importantly, safe medicine?
Temperature Excursion Allowance: A GxP Compliant Solution
By programming stability information into data loggers, you can prevent products from being discarded prematurely.
What Are Temperature Excursions and Why Do They Even Matter?
Under the World Health Organization (WHO) Model Guidance, temperature excursion is “an excursion event in which a Time Temperature Sensitive Pharmaceutical Product (TTSPP) is exposed to temperatures outside the range(s) prescribed for storage and/or transport. Temperature ranges for storage and transport may be the same or different, as they are based on individual product stability data.”
Like many products, some medicinal products are sensitive to temperature and need to be stored and/or transported within a limited temperature range until expiration. However, unlike a flower that has lost the vibrancy and aromas, it isn't easy to tell when pharmaceutical products have lost their quality or efficacy.
Whenever temperature-sensitive pharmaceutical products are exposed to temperatures above or below the specified range, they experience temperature excursions. These excursions can affect their potency or result in an adverse reaction to the patient’s health. A temperature excursion may occur at the manufacturing site, in transit, or at the repository.
The Role of Regulators
Both the European and United States Pharmacopeia outline that pharmaceutical manufacturers are accountable for a product’s quality until it reaches the patient. While temperature conditions at manufacturing and packaging sites are usually under strict control (GMP regulation), this control is likely to decrease as soon as the product is dispatched for transportation.
As such, manufacturers should guarantee the safety, efficacy, and quality of the product until its final use. When temperature excursions occur, they have to take the required measures.
5 Examples of False Excursions and How to Correct Them
Temperature alarms may not be a final result. Sometimes there are false/positive excursions that can be corrected by a cold chain database. From the experience of millions of pharma shipments analyzed in our database, we know that less in than 10 percent of all cases, a true temperature alert (excursion outside defined shipping conditions) is found.
Typically, half of the temperature excursion cases are caused by a late stop or other mistakes that happen at destination – which could be considered false excursions.
The following are examples of common causes of false alarms and their interventions:
Early start, sensor measures too early (before product has been loaded or conditioned)
Intervention: Cold chain database can reassess the data using the correct time stamps.
No temperature values available due to sensor failure (sensor not started or no sensor added)
Intervention: If the shipment contains more than one device, it might be possible to use this data to release the entire shipment.
Minor temperature excursion during shipment
Intervention: Cold chain database can reassess the data using the stability data of the product.
Wrong sensor setting triggers a temperature alarm
Intervention: Cold chain database can reassess the data using the correct stability data.
Late stop at destination, sensor continues to measure (at room temperature)
In every case, it is important to have a two-level process in place whereby both logistics and quality review excursions before the product is released.
How to Document Temperature Excursions
Manufacturers that wish to follow GDP and GMP regulations are required to have procedures in place to document, investigate, and handle temperature excursions. The approach below outlines best practices for the documentation of temperature excursions at the manufacturing site, during transportation, or in warehouse storage.
Collect the following information:
- Details of the person completing the report
- Date and time of the temperature excursion
- Inventory of affected products
- Storage unit temperature (including minimum/maximum temperatures during the time of the event, if available)
- Room temperature (if available)
- General description of the event (i.e., what happened) • The length of the exposure if using a digital data logger (DDL)
- List of other items in the unit
- Any problems with the storage unit or affected products before the event
- Other relevant information
The distributor and recipient of the affected product(s) should receive a notification should a temperature excursion occur.
7 Components of a Cost-Saving CAPAs for Temperature Excursions
In case of a temperature excursion, GMP and GDP regulations require a Corrective Action & Preventive Action (CAPA). It is a structured process, which investigates and identifies root causes of problems and defines corrective action to prevent recurrences.
To create a useful CAPA, ask these questions:
Who found the temperature excursion, when, and where?
What is the scope of the case (shipment number, delivery, handling unit, pallet, product, batch)?
What is the severity of the excursion?
What was the label/transport condition?
What was the highest (or lowest) temperature measure?
What was the number of excursion hours (can the product still be released based on stability budget)?
What was the root cause of the excursion?
What are corrective actions to eliminate this specific problem?
Have similar cases happened before? Are there patterns in the data?
Can we define preventative actions to make sure similar root causes are eliminated?
For a reliable CAPA process in cold chain management, a database is necessary where all data is available in a structured and well-documented way.
Practical Guide: Cold Chain Logistics Choices
Learn how the choice of transportation mode & equipment affect products, & how to best handle temperature excursions & subsequent CAPAs.
Related Articles
Stability budget explained, making the right cold chain logistics and packaging choices ..., get everything under control with the right software, let's talk about temperature monitoring .
Schedule a call with our experts today. We're here to support your cold chain monitoring project and help ensure it is successful.

- Monitoring for Rooms & Equipment
- Product Catalog
- ECOLOG Connected Monitoring
- LIBERO Stability Monitoring
- INDEPENDENT Monitoring
- News & Events
- Compliance & whistleblower system
- Leading Minds Network
we prove it.

- Privacy Policy
- Legal Notice
- Terms of Use
- General terms and conditions
- General terms of conditions and purchase
Copyright © ELPRO-BUCHS AG 2024. All Rights Reserved.
Thermo Fisher Scientific is the recipient of five CDMO leadership awards for 2024.
Managing Temperature Excursions: Key Pointers That Must Be Addressed
The maintenance of cold chain product integrity across the entire supply chain demands rigorous processes and cold chain expertise of the highest caliber—from packaging, handling, storage and distribution of temperature sensitive Investigational Medicinal Products (IMP), all the way to the investigator site.
Part 4 clearly outlines what happens when a temperature excursion occurs. Top tips are given on the best course of action to take.
Discover what to do when a temperature excursion occurs and get summary recommendations for sponsors on how to handle cold chain or temperature sensitive IMP across the supply chain, up until delivery to the investigator site.
Ready to discuss your project?
Connect with us
Thermal Cycling Study
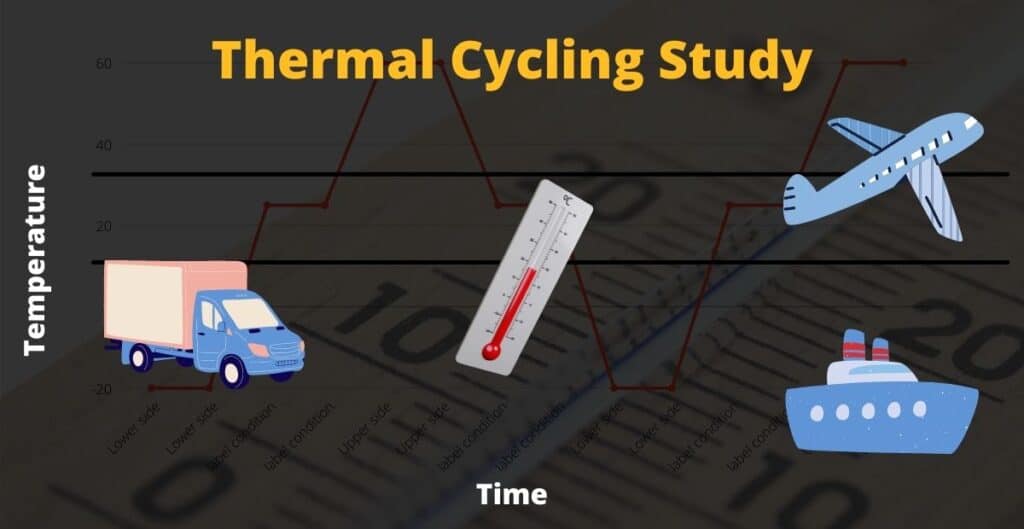
What is a thermal cycling study?
A thermal cycling study is done to determine the impact of temperature excursion on the edge and outside of a product’s labeled storage conditions. The excursion is simulated below and above the product’s labeled storage conditions for multiple cycles, and the impact on the product quality will be evaluated because of thermal cycling.
An example of thermal cycling is illustrated in the following diagrams.
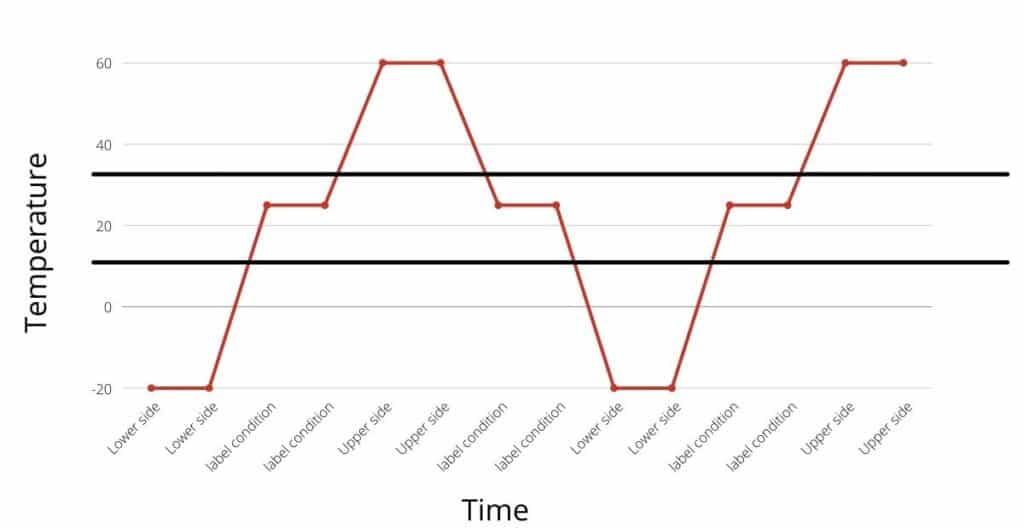
How is thermal cycling study different from excursion studies?
A thermal cycling study is done by simulating upper and lower side excursion multiple times and assessing its impact on product quality. Whereas, in the temperature excursion study, the one-sided excursion is simulated without cycling.
What are the reference guidelines?
There is no direct reference in any of the regulatory guidelines regarding testing to determine the suitability of transport conditions for sensitive products; however, you can find general instructions in various guidance.
ICH Q1A and WHO guidance provide general direction to perform stress testing. The study’s objective is to evaluate the impact of stress conditions (temperature, humidity, oxidation, pH, and light) on drug products.
ICH Q1A and WHO also suggest that the data from accelerated stability studies can be used to evaluate the effect of short-term excursions higher or lower than the label storage conditions that may occur during the shipping of drug products.
The guidelines also direct for the drug substance to be stored in a freezer: “…testing on a single batch at an elevated temperature (e.g., 5°C ± 3°C or 25°C ± 2°C) for an appropriate time period should be conducted to address the effect of short term excursions outside the proposed label storage condition, e.g., during shipping or handling.”
Similarly, for the drug products to be stored in a freezer, “In the absence of an accelerated storage condition for drug products intended to be stored in a freezer, testing on a single batch at an elevated temperature (e.g., 5°C ± 3°C or 25°C ± 2°C) for an appropriate time period should be conducted to address the effect of short term excursions outside the proposed label storage condition.”
ICH Q5C directs for the stability of Biotechnological/ Biological Products that “Studies under stress conditions may be useful in determining whether accidental exposures to conditions other than those proposed (e.g., during transportation) are deleterious to the product.”
Work had done by Bishara and Seevers has now been included in the Parenteral Drug Association (PDA) documents. In this document, temperature excursions and temperature cycling conditions are proposed.

Why is thermal cycling study required?
Drug products are stored in the controlled environment in the facility, whereas when the products are transported in a commercial environment, it is a different scenario. Temperature conditions and excursions in the facility can be easily controlled; however, it may not be always possible during transportation. The impact on drug product due to unforeseen transport events could not be covered during the long-term and accelerated stability studies. Hence, the purpose of the thermal cycling study is to consider anticipated extreme challenges, including expected ambient temperature variation and shipment duration. In addition, the study will demonstrate the robustness of the product against usual excursions experienced during transportation.
How will it be helpful to justify excursions?
The drug product manufacturer’s responsibility is to ensure that the drug product reaches the patient without loss of therapeutic properties.
It is unpredictable that what types of excursion may occur during the transportation of pharmaceutical products, how long and how many times. For example, accidental temperature excursion may occur, which is beyond the pharmacopoeial acceptable excursion range. In such scenarios, the drug product would no longer be acceptable for patient consumption without sufficient scientific data.
Thermal cycling study results will help to determine product behavior and product quality impact when it experiences different environmental conditions during the transportation condition. In addition, the study data will help to understand the effect of accidental excursions on the drug product quality throughout the shelf life of the drug product.
How to design the thermal cycling study?
While designing the protocol for thermal cycling study, various factors should be considered such as product development data, Route of transportation, possible extreme conditions, and product strengths.
a. Support of product development data:
During the development of drug substances and drug products, R&D performs stress studies to determine the impact of various stress conditions on drug product, such as temperature, humidity, light, and oxygen. In this article, we will mainly discuss thermal stress. Suppose developmental studies show study product does not get degraded with extreme stress conditions. Those data may support accidental excursion during transportation, or these development data can support the design of thermal cycling study in terms of duration and extremes of stress condition. When development data shows the degradation of the product at specific stress conditions, there is no point in covering such situations during the study even though that could be expected excursion during transportation. In such a scenario, transportation facilities should be designed to maintain storage conditions so that such excursions would not occur.
b. Route of transportation:
The simplest way to design the study is to map the transportation routes from starting to recipient locations and identify the route’s seasonal temperature conditions and time required to reach from starting location to recipient locations. Typically two seasons are mapped, winter and summer. Consideration should be made for the day and night temperatures of the route. The external environmental temperature data can be collected using previous transportations or collected from historical meteorological data.
If one location is the Northern hemisphere and the other is the Southern hemisphere, the transportation route’s actual condition should be used. Such types of seasonal conditions are called summer-winter or winter-summer conditions. For example, a product is supplied to three different destinations. Temperature conditions should be mapped at the ambient environment to understand the potential excursions during transportation for respective destinations.
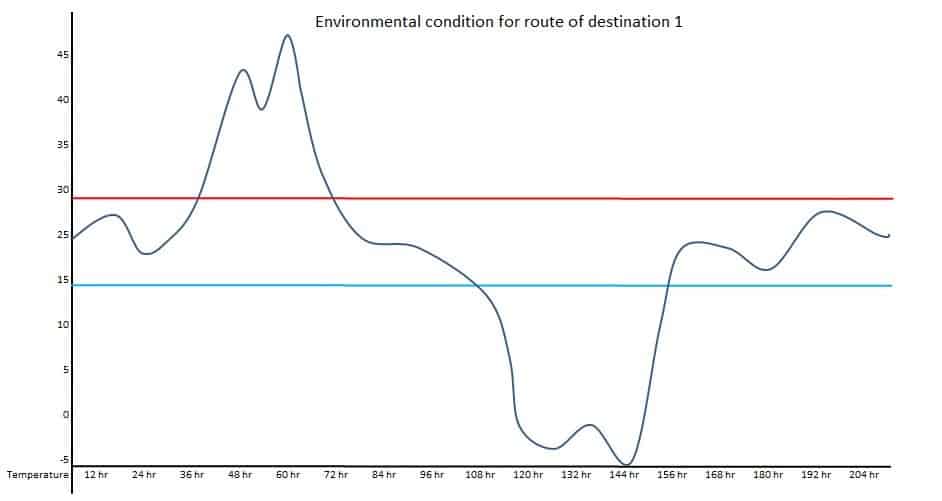
Data of above diagrams are summarized in following table.
Observed upper extreme temperature: 47 Deg. C
Observed lower extreme temperature: -5 Deg. C
Number of time upper side excursion during one run: 5 times
Number of time upper side excursion during one run: 2 times
Maximum total duration of upper side excursion: 55 hr
Maximum total duration of lower side excursion: 48 hr
Maximum duration of transportation: 228 hr
The above data can provide potential worst-case conditions during the transportation for the selected season. The study design for thermal cycling can be done based on the above data, such as total duration of the study, a number of excursion cycles, upper and lower extreme temperature, duration of each cycle, etc.
As per PDA Technical Report No. 39 (2007) suggest the following example for thermal cycling study for different storage conditions:
The above approach can be modified based on collected data from the field study data presented in Table: 1 for the storage condition of Controlled Room Temperature, 20 to 25°C. [Opinion of the author of this article]
c. Consideration of extremes :
There are natural temperature ranges that could be defined. It will be very unusual to have temperatures below −100°C or over 100°C in a natural environment. In Antarctica, temperature minima have been measured at −89°C. Temperature maxima have been measured at +58°C in Libya and in Death Valley. But even if a product is stored in a closed environment under the sun, it will not be heated over 100°C.
d. Consideration of product and strength selection :
The bracketing approach with higher and lower strength can be considered as one of the approaches. Product strengths to be bracketed can be defined using the Quality Risk Management principle.
Conclusion:
Systemically designed thermal cycling study will provide valuable insight about product robustness under stress condition and accidental exposure of product with high or low temperature can be scientifically justified. Based on the study performed, Product-Specific Transportation Control Strategy Document can be prepared for to determine the Effect of Temperature Excursions as follows:

Temperature Excursion Management – A Novel Approach Of Quality System In Pharmaceutical Industry
Authors: nirmal kumar, ajey jha.
Quality of pharmaceutical product largely depends upon by the environment controls during its storage and handling. Each pharmaceutical product should be handled and stored under specified storage condition labelled on product information data sheet or product pack. Hence the temperature excursions during receipt of raw materials, manufacturing of pharmaceutical products and distribution should be managed during entire product life cycle with holistic approach. The research is based on primary data and exploratory study through literature review. The temperature excursion may be observed during transportation of raw materials manufacturing as well as distribution of pharmaceutical products, which have potential to deteriorate the product quality. Temperature excursion in pharmaceutical industry should be recorded and reported to the manufacturer for further investigation and risk analysis. The concept of temperature excursions, its reasons, consequences and handling mechanism should be well understood to ensure the concerted efforts under the aegis of Quality Management System. Based on the reasons and consequences of temperature excursions during pharmaceutical operations, a system based quality management has been envisaged through this study. The concept an d procedure to handle temperature excursion have evolved after this study which shall be useful to pharmaceutical industry as well as to medicine distributors and consumers.
GMP; Temperature excursions management; Formulation; Quality by Design; Degradation products
Citation: Kumar, N., Jha, A., Temperature excursion management – A novel approach of quality system in pharmaceutical industry, Saudi Pharmaceutical Journal(2016),doi: http://dx.doi.org/10.1016/j.jsps.2016.07.001
Received: 12 December 2015, Accepted: 1 July 2016, Available online: 9 July 2016
Copyright: © 2016 Production and hosting by Elsevier B.V. on behalf of King Saud University.Open Access funded by King Saud University
Temperature excursion is a general term that represents the environmental excursions. However, there is a need of holistic approach to handle the temperature excursions starting from raw material manufacturing site to medicine retailers shop to protect quality of product. The temperature excursion at any stage of pharmaceutical business operation must be reported as soon as possible and investigated appropriately. The consequences of deviation against temperature and humidity limits should be studied appropriately by quality assurance personnel. The risk of temperature excursions cannot be ruled out, but it can be minimized through effective system. Alternative is to use thermal resistant packaging and stringent control measures during transit and shipment, to avert the undesired quality impact on pharmaceutical product. The systematic approach to handle the issues related temperature excursions become inevitable for pharmaceutical manufacture.

- Skip to main content
- Skip to primary sidebar
- Skip to footer
Colors of engineering
02/26/2018 | Redazione Fandis | Leave a Comment
The effects of thermal excursion in enclosures: condensation
In electrical panels, temperature changes and condensation phenomena are among the main causes of malfunctioning of electrical and electronic equipment. In addition to a correct ventilation, it’s therefore necessary to provide anti-condensation heaters, to raise the temperature of the panel up avoiding the so-called dew point, over which is reduced the risk of condensation.
Why does condensation form inside enclosures?
Electrical and electronic components inside the electrical panel generate heat during their operation: when the temperature rises, water particles, naturally contained in the air, especially in the humid areas, are at their gaseous state, but when temperature drops and in contact with a colder surface (such as the walls of the panel, for example), the vapor condenses back to its liquid state in form of drops. It’s the same phenomenon that can be observed on a glass, blowing on it.
What are the cases in which anti-condensation heaters should be installed?
During the winter, in areas affected by a strong temperature excursion between night and day (as in hot countries or desert), or in humid countries, the risk of condensation is much higher!
Therefore, it’s important to maintain ideal climatic conditions to ensure a long life for the components installed in the electrical panel, considering the use of an anti-condensation heater already from the production / installation phase.
The Fucsis H series anti-condensation heaters from Fandis
The electric resistances installed in the Fandis’ Fucsis H-series anti-condensation heaters guarantee a temperature rising inside the electrical panel, preventing dew point and condensation from forming, protecting electrical and electronic components. Fandis anti-condensation heaters allow the maintenance of an ideal internal temperature, working in combination with a thermostat or hygrostat to constantly monitor temperature and humidity levels. They are also available with built-in forced convection fan, to ensure a proper air circulation and a more accurate distribution of heat in the enclosure and with Touch Safe plastic protection, avoiding burns in case of accidental contact.
To learn more, visit our website fandis.com and discover our products for electrical panels, or leave a comment to this article. One of our technicians will answer you as soon as possible.
Articoli correlati
Reader interactions, leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Notify me of follow-up comments by email.
Notify me of new posts by email.
Fandis S.p.A.
Via per Castelletto, 65/69 28040 Borgo Ticino (NO) Italy
Tel. +39 0321 963232 Fax. +39 0321 963296 Email [email protected]
Who is Fandis
Fandis presents itself to the global market as a modern, customer-oriented and highly versatile production reality, thanks to an important cognitive and experiential heritage of a technical and commercial nature. Today it operates in different business areas, offering effective and tailor-made solutions for every type of customer application need.
Vai al sito Fandis
Your name (required)
Your email (required)
Your message
I accept the data protection terms and conditions

Industry News
Temperature excursion management in pharmaceutical storage.
AKCP 06.2020 Articles , Blog

The quality of pharmaceuticals relies on environmental controls during their storage and handling. Every pharmaceutical item ought to be taken care of and stored under manufacturer-recommended storage conditions marked on the data information sheet or packaging.
Temperature Excursion Management in Pharmaceutical Storage is important during receipt of raw materials, manufacturing, and distribution of drugs.
System failures or human carelessness can cause circumstances leading to temperature excursions . The most significant environmental condition having the capacity to affect the nature of the pharmaceutical product is temperature. If the temperature excursions are not taken care of efficiently, there will be an adverse impact on product quality . There is a developing need to monitor for environmental excursions during pharmaceutical logistics to negate effects on the quality of the product. Quality Management System (QMS) should be implemented to avoid temperature deviations during the storage, transport, and distribution of pharmaceuticals.
Pharmaceutical Temperature Excursion : An integrated approach for managing the quality system should include temperature excursion management. The overall temperature excursion management can be laid down in the following steps:
- Storage temperature and humidity limits: Specified directions are stated for pharmaceutical products with reference to the temperature and humidity at which articles shall be stored, transported, and distributed. Pharmaceutical supply chain products require cold storage or climate-controlled storage to meet the manufacturer’s recommendations. For products from the class of cold chain, the storage condition is maintained at 2°C–8 °C. Similarly, the relative humidity shall be maintained below 60% and above 40% depending upon the hygroscopic nature of the product. When environmental stability data indicates there has been temperature deviation with storage and distribution at a lower or higher temperature and humidity there are protocols in place that may require the disposal of the product, or at the least taken out of circulation until tested and verified.
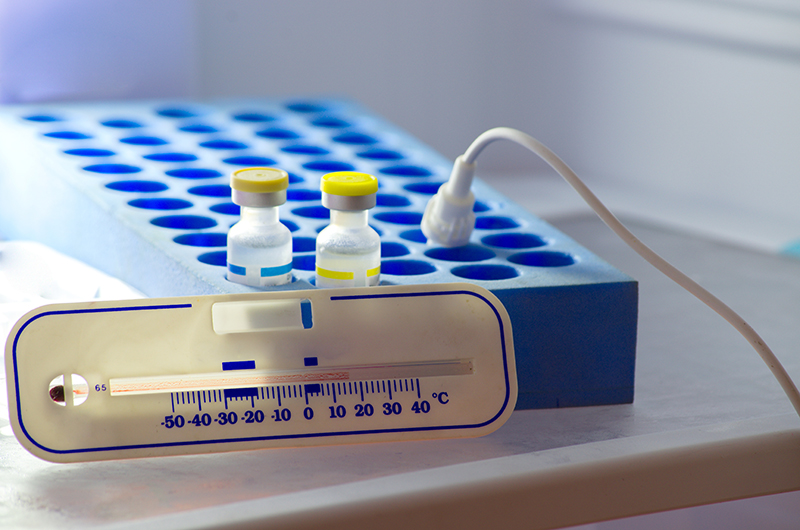
- Measurement devices: Temperature and Humidity measuring devices (popularly referred to as data loggers) are available. They log the temperature at a preset interval which will be downloaded to a computer system or QMS for review, evaluation, and recording. Periodic verification of the calibration status of temperature data loggers and upgrading of software is a prerequisite for uninterrupted and accurate information about product storage conditions. The temperature and relative humidity sensor should be placed on the hottest spot, concluded after temperature mapping of the area.
- Reason for Temperature excursion: In pharmaceutical factories and cargo areas, the required temperature is maintained with help of air handling units (AHU). The design and capacity of AHU are selected on the temperature required to be maintained.
Temperature excursions in a manufacturing area are caused due to the following reasons (not limited to):
- An inadequate number of air handling units (AHU) were installed to maintain the desired temperature conditions inside the manufacturing shop floor.
- Leakage or rupture from the air duct, resulting in an insufficient cooling effect.
- Mechanical failure in air handling unit (AHU). unprecedented temperature fluctuations.
- Power failure makes the AHU operation defunct.
- Lack of quality system and weak discipline of Good Manufacturing Practices (GMP) on the production shop floor.
- General awareness about the consequences of not maintaining the temperature within limits.
- Extreme weather changes and obsolete contingency plans to handle
Temperature excursions during transport are caused due to the following reasons:
- An unexpected delay in transportation due to which the temperature control cannot be maintained effectively
- Product pallets are kept in hot zones of airports or shipping yards.
- Reefer containers or refrigerated control vans are not deployed for transportation.
- The transport agency fails to maintain the planned transport condition.
- Higher cost to maintain the temperature within limits.
- Power failure due to short longevity of power bank during longer travel time.
- Good Distribution Practices (GDP) understanding amongst supply chain personnel about the adverse impact on product quality.
Consequences Of Temperature Excursion
The storage condition for the product is assigned based on scientific studies into the deterioration of the product during its life cycle. If the temperature excursion isn’t addressed immediately, the subsequent negative impacts are common:
- Loss of product.
- Decreased efficacy.
- Separation of layers in liquid products.
- Change in dissolution pattern of solid dosage.
- Discoloration of products.
Control of Temperature Excursions
To handle the temperature excursion strategic planning , effective packaging, and well-documented procedures are recommended. The development of a QMS database of pharmaceutical products is beneficial to assign quality storage conditions.
The storage conditions suitable for the product are assigned through the following tests:
At the merchandise development stage, the semi-finished product and final pharmaceutical product dosage are subjected to challenging conditions to monitor the potential impact on quality attributes.
- Hold time studies are administered to determine the allowable period of time at a specified storage condition without impacting quality. the standard attributes of product intermediates include chemical, microbiological and pharmacological determinants at various time points of stability.
- Accelerated condition stability study data of the product form an assurance for the storage condition that shall be suitable to product safety. The time stability data is generated in the laboratory to assess the product’s change in quality and attributes throughout the expiration date. The accelerated stability data is generated to gauge the impact on the quality of the product under a stressed condition.
- A freeze/thaw study for multiple cycles should be conducted to specify the effect of freezing, if any, and therefore the subsequent thawing. Samples from different layers (top, middle)
Thermal Packing During Transportations:
Packaging of pharmaceutical products for transport should include the supply of thermal packing and display of appropriate storage conditions. Caution notes to avoid storage outside the specified conditions shall help supply chain personnel protect the merchandise quality. The storage condition should be effectively displayed on the packaging of the pharmaceutical product . The packaging configuration card must contain the small print of knowledge loggers.
Procedure To Minimize Temperature Excursions: The temperature excursion has regulatory implications as well as an impact on business operations. A standard procedure (SOP) should be established and adherence ensured through adequate training to concerned personnel. An operational checklist of the Integrated Quality Management System (QMS) approach should include the qualification status of a manufacturing facility with special attention to environmental controls during storage and transportation.
Control of Temperature Excursion By Using AKCP Wireless Temperature and Humidity Sensor:
With AKCP wireless environmental monitoring solution, you can monitor temperature excursions with real-time alerts, data logging , and reporting.
A monitoring system gives an accurate picture of the temperature and humidity conditions of drug storage . Have confidence in the quality and safety of pharmaceutical products. End-to-end monitoring prevents the loss of thousands of dollars of perished products
Wireless Tunnel radio technology penetrates even thick secure storage and refrigerated cabinets. Battery-powered sensors with a 10-year battery life guarantee easy installation.
Industry Articles and News
Homepage blog 37.

Importance of PUE on Data Center Costs
How does PUE affect your data center costs? IMproving PUE is not only good for the environment but for your OpEx.
Find out more
Homepage Blog 35

Understanding Data Center Containment
Containment in the data center means the prevention of hot and cold air mixing. This improves cooling efficiency and lowers costs.
Homepage Blog 22

How Containment Saved Energy for Saudi Aramco Data Centers
Case Study : Lufthansa Technik Warehouse

Lufthansa Technik, the maintenance arm of the German airline Lufthansa, have selected AKCP monitoring devices for use in several of their maintenance hubs worldwide. In the business of aircraft maintenance time plays a crucial role, and so does the timely supply of the mechanical, consumable and expendable spare parts.

- Cambrex.com
- Client Login

Thermal Cycling / Freeze Thaw Study
By Stephen Delaney
Thermal Cycling gives an indication as to how a product will react to adverse conditions, usually encountered during transportation.
Adverse Conditions
Temperature sensitive medicinal products should be transported in a manner which ensures product quality is not adversely affected. Transportation of product typically takes place within a commercial environment. Factors such as traffic events and unforeseen weather conditions can delay shipments and have a deleterious effect on the product.
The range encountered during transportation may differ from that which is specified for long term storage, ascertained from stability studies. To understand the effect these excursions may have on a product, Thermal Cycling studies are completed. Also known as freeze thaw studies, they are used to evaluate the potential effects of temperature deviations that may occur during the transport process. The storage conditions and the lengths of studies chosen should be sufficient to cover storage, shipment and subsequent use (ICH Q1 A 2.1.7)
Thermal Cycling Process
An example of a study, would follow the following process:
- The samples in our example have a recommended storage condition of 20°C to 25°C
- Samples are placed on stability storage at -20°C for 48 hours.
- Samples are then transferred to 40°C / 75%rH for 48 hours.
- Procedure is repeated for a total of 3 cycles.
Q1 Scientific can customise Thermal Cycling/Freeze Thaw studies to clients specific requirements.
Can we help you?
Contact us to find out more about how we can help you with your Thermal Cycling/Freeze Thaw studies or call +353 51 355 977.
Join our live webinars
If you are reading this article, then you may also be interested in our new webinar series . Following feedback from clients, we have recently curated a series of webinars designed for those working in the pharmaceutical, medical device and life sciences sectors with responsibility for designing and managing stability studies. If would like to gain some key insights into stability study design see our upcoming webinar series .
Privacy Overview
Necessary cookies are absolutely essential for the website to function properly. This category only includes cookies that ensures basic functionalities and security features of the website. These cookies do not store any personal information.
Any cookies that may not be particularly necessary for the website to function and is used specifically to collect user personal data via analytics, ads, other embedded contents are termed as non-necessary cookies. It is mandatory to procure user consent prior to running these cookies on your website.
Email: sales.en@vackerglobal.com
- Disposable USB Temperature & Humidity Data Logger
- Temperature Data Loggers
- Temperature and Humidity Data Logger
- Disposable, Single use USB temperature data logger
- Dry Ice Data logger
- Freezer & Refrigerator Temperature Monitoring System
- Temperature Monitoring System for Cold Room, Walk in Chillers, Warehouses
- Temperature recorder with Printer for vehicle
- Temperature tracking system for Vehicles, Vans and Trucks
- SMS Temperature tracking for long haul trucks
- Temperature Data Logger with Printer
- 9 Channel Data Logger MSR255
- 3 Axis Data Logger MSR165
- 4 Channel Data Logger MSR160
- Bluetooth Data Logger MSR145WD
- 9 Channel Mini Data Logger MSR145
- Dehumidifier Capacity Calculation
- Portable Desiccant Industrial Dehumidifiers for Cold Room
- Marine dehumidifier
- Desiccant dehumidifier Stationary
- Condensation dehumidifiers portable
- Condensation dehumidifiers & dryers stationary
- Dehumidifiers for Swimming pools
- Dehumidifier for home and office use
- Commercial & Industrial Air Humidifier for Increasing Humidity
- Basement dehumidifiers
- Dehumidifier Calculation in Excel
- Multifunction data loggers by Graphtec, Japan
- Infrared food thermometer with probe
- Food core thermometer,Insertion Thermometer
- Data Center Temperature Monitoring
- Wireless Temperature Monitoring
- Infrared Thermometer
- Remote Temperature sensor
- Thermocouple
- High Temperature Industrial Data Logger
- Environment Monitoring
- Agricultural Monitoring
- Temperature Mapping Validation, Study and Qualification
- Temperature Mapping of Racks/Panels
- Infrared (IR) Thermal Imaging
- Cold Storage Rooms
- Temperature Qualification
- FAQ – Temperature Mapping and Monitoring
- Get A Quote
- iMini plus pdf Temperature Data logger
- iMini Temperature and Humidity Data loggers
- Temperature Monitoring & Alert system
- iMini single use USB data logger
- Search for: Search Button
Eliminate The Risk Of Temperature Excursions With Thermal Mapping Study For Pharmaceutical Business
Pharmaceutical products are sensitive to fluctuations in temperature. Their storage and transportation need precisely monitored conditions to preserve their quality. Service providers in this sector should administer the same. Because, in case of any lapse, changes in the drug can be lethal to countless. This is where Vacker’s thermal mapping study services become so crucial.
Temperature Mapping is an integral part of cold chain management. It is an efficient way of ensuring that drugs maintain their integrity. This technology enables the concerned distributors to regulate and control the temperature to create a suitable environment for quality pharmaceutical products. Let’s learn more about the process.
Starting with the basics: What is a thermal excursion?
- According to the World Health Organization , a temperature excursion occurs when a temperature-sensitive product in the pharmaceutical industry receives exposure to temperatures beyond the recommended range.
- Temperature excursions can severely hamper the stability of drugs, causing physical and chemical changes in medicines. There is no room for error because of obvious reasons.
- Since temperature is a natural element and is difficult to control, our temperature mapping and validation services come to the rescue.
What is thermal mapping study and validation?
- Temperature mapping is the process of analyzing and examining whether the temperature-control systems, that are supposed to keep the pharmaceutical products safe, can do what they are supposed to.
- It helps to understand whether a storage or transportation unit can uniformly maintain temperature levels. You can identify any abnormality or potential source of concern through a thermal mapping study.
- Thermal validation is another related process aimed at finetuning and ensuring that the equipment, machines, and physical spaces that store or execute temperature control can carry out their function flawlessly.
- Both the processes eliminate the chances of temperature excursions by precisely monitoring all the equipment involved in creating the proper environment for the safe storage and transportation of pharmaceutical products.
Temperature mapping and validation conducted by us is one-of-a-kind. Apart from the quality of service provided, we will also equip you with the following additional information to eliminate temperature excursions—
- Presence of hot spots and ways to restore uniformity
- Number of people and pieces of machinery that should ideally be in a given area
- Behavioral difference between hot and cold months
- The period for which products will be safe after a power failure
A Final Note:
Temperature excursion is the arch-nemesis of the pharmaceutical industry. If you want to manage a cold chain successfully or make yourself known as a reliable distributor, Vacker’s thermal mapping and validation is the way to go. Visit our detailed explanation of thermal validation and mapping study here. We also have a downloadable guide that provides an easy understanding of the entire process too. Let us help you execute your obligations as a part of the pharmaceutical industry successfully.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
Recent Posts
- Introduction to the Concept of Thermal Mapping
- Cold Chain Management: Ensuring Quality with Temperature Data Loggers
- Enhancing Efficiency and Accuracy with Temperature Data Logger
- Monitoring Warehouse Temperatures With Remote Temperature Sensor : How Well Does It Work?
- Why Do We Need Thermal Mapping Study in Pharmaceuticals?
- September 2023
- December 2022
- November 2022
- October 2022
- September 2022
- February 2022
- February 2019
- October 2018
- September 2018
- November 2017
- October 2017
- September 2017
- August 2017
- December 2016
- October 2016
- September 2016
- August 2016
- February 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- February 2015
- January 2015
- November 2014
- October 2014
- September 2014
- February 2014
- January 2014
- December 2013
- November 2013
- October 2013
- air quality monitoring
- Alert system
- ATM monitoring system
- button cell data logger
- cargo temperature logger
- Cheap Cold room
- clean room monitoring
- climate meter
- Cold Chain Auditing
- Cold Chain Consultancy
- cold chain management
- cold chain packaging
- cold chain solutions
- Cold Chain validation
- cold room dehumidifier
- concrete thermometer
- cooling box
- data center monitoring
- Data logger
- dehumidifier
- Electric Medical Cooler Box
- Electronic Temperature Indicator
- freezer with monitoring
- Gel Packs and Ice Packs
- humidity data logger
- Humidity Indicator Plug
- Humidity monitoring
- indicator strips and labels
- insulated box
- IR Thermometer
- Low Cost Cool Storage Rooms
- Mean Kinetic Temperature
- Mean Kinetic Value
- Measuring Instruments
- Meter supplier Africa
- Miscellaneous
- Monitoring systems Africa
- Particle Analysis
- portable freezer
- portable refrigerator
- Pressure data logger
- Qualification
- Refrigerator temperature monitoring
- Remote Temperature Monitoring
- RFID Data Logger
- server room monitoring
- Single use Temperature Humidity Data Logger
- strip chart recorder
- Systems for HAAD UAE
- temperature & humidity recorder
- temperature chart recorder
- Temperature controller
- temperature data logger
- Temperature display LED Panel
- Temperature insulated cover
- Temperature Mapping
- Temperature Mapping & Validation
- Temperature mapping FMCG
- temperature monitoring
- temperature qualification
- Temperature recording
- Thermal Blanket
- thermohygrometer
- transportation solutions
- USB-single-use-data-logger
- Validation & Qualification
- voltage data logger
- WiFi Temperature recording & Monitoring system
- Wireless Data Logger
- Wireless Temperature monitoring system
- RKM Building, Hor Al Anz, Deira 92438 Dubai, United Arab Emirates
- [email protected]
- +971 4 266 1144
- Vacker LLC Disclaimer
- Vacker LLC Privacy Policy
- Temperature Data Loggers / Recorders
- Refrigerator & Freezer Monitoring
- Cold Room / Warehouse Monitoring
- Shipping / Cold Chain Monitoring
- Multichannel data loggers by MSR Switzerland
- Dehumidifiers & Humidifiers
- Food Processing & HACCP Thermometer
- Infrared Thermal Imaging camera
- Temperature Sensor
- Temperature Mapping Study & Validation
Quick Links

An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

- Digg
Latest Earthquakes | Chat Share Social Media
Paleoenvironmental and paleoecological dynamics of the U.S. Atlantic Coastal Plain prior to and during the Paleocene-Eocene Thermal Maximum
We studied the rapid paleo-environmental changes and the corresponding biotic responses of benthic foraminifera of a shallow shelf site during the late Paleocene and the Paleocene-Eocene Thermal Maximum (PETM). The PETM is globally characterized by a negative δ 13 C excursion in marine and terrestrial sediments. Isotope data from the Atlantic Coastal Plain from the South Dover Bridge core, Maryland, show an additional small δ 13 C excursion just below the base of the PETM: the “pre-onset excursion” (POE). The benthic foraminiferal and coupled grain-size record of the late Paleocene indicates a well-oxygenated, current-dominated environment with a stable, high food supply. During the POE, bottom currents become subdued and finer-grained sediment accumulation increased. These changes are partially reversed after the end of the POE. Before the PETM the river influence increases again, food supply becomes more pulsed and the benthic taxa, typically connected to the PETM, start to appear in those gradually warming conditions. During the PETM, the environment shifts to a river-dominated one, with strongly reduced currents. The low-diversity PETM fauna thrives under episodic low-oxygen conditions, caused by river-induced stratification, while the Paleocene assemblage nearly vanishes from the record. Gradually the environment begins to recover, the grain size shows an uptick in bottom currents and pre-PETM foraminifera become more abundant again, indicating increased oxygen levels and a more stable food supply. While the overall environmental shifts at South Dover Bridge fit within the observations across the shelf, the POE related insights are so far unique. Our bathymetric reconstructions show an outer neritic paleodepth (∼100 m) during the Paleocene, with a modest sea level rise in the core phase of the PETM, which is subsequently reversed during the recovery phase.
Citation Information
Related content, marci m robinson, ph.d., research geologist.

COMMENTS
Establish a Study Program: This should include temperature excursion studies, thermal cycling studies [5], transport studies, mathematical model (Figure 2) [11, 12], and freeze-thaw cycles as needed. Figure 2. Relationship between time and temperature as described by the mathematical model. It shows how much time a product can be placed at a ...
How/When Thermal Cycling Study Data is Used to Support Temperature Excursions. Thermal cycling study data (from development studies and in some cases from formal thermal cycling on cGMP drug product batches) and associated impact toward product quality and stability may be included in a New Drug Application (NDA). Pharmaceutical companies may ...
Maintaining proper temperatures and avoiding excursions ensures that materials remain safe and effective. Meanwhile, according to the European Compliance Academy, a temperature excursion is defined as: "a deviation from the labeled storage condition of a product for any duration ". This includes excursions during transportation or distribution.
This paper has covered how HATS testing can be used to determine the impact of simulated temperature assembly and rework thermal excursions on long term field life. In particular, the data has shown a complex relationship between higher temperature preconditioning and subsequent temperature cycling to failure (CTF).
Temperature excursion in pharmaceutical industry should be recorded and reported to the manufacturer for further investigation and risk analysis. The concept of temperature excursions, its reasons, consequences and handling mechanism should be well understood to ensure the concerted efforts under the aegis of Quality Management System.
pectations related to the justification of temperature excursions, thermal cycling studies to assess and support potential tempera-ture excursions (including how/when thermal cycling study data is used to support temperature excursions), GDPs to minimize tem-perature excursions and use of theoretical methods/mathematical
In this article, important aspects related to the key elements of a comprehensive temperature excursion management program are summarized, including standard stability testing, regulatory expectations related to the justification of temperature excursions, thermal cycling studies to assess and support potential temperature excursions (including ...
Step 3: Document the Event. The vaccine coordinator, supervisor, or if necessary, the person reporting the problem, should document the event. Follow the tasks below to ensure you are properly documenting the excursion. Name of the person completing the report. Date and time of the temperature excursion.
Excursions must be evaluated and investigated if they exceed 24 hours in duration and if there is any impact on the quality of the products, additional actions may be necessary. ... affords the same thermal challenge to a drug substance or drug product as would be experienced over a range of both higher and lower temperatures for an equivalent ...
The approach below outlines best practices for the documentation of temperature excursions at the manufacturing site, during transportation, or in warehouse storage. Collect the following information: Details of the person completing the report. Date and time of the temperature excursion. Inventory of affected products.
The maintenance of cold chain product integrity across the entire supply chain demands rigorous processes and cold chain expertise of the highest caliber—from packaging, handling, storage and distribution of temperature sensitive Investigational Medicinal Products (IMP), all the way to the investigator site. Part 4 clearly outlines what ...
The objective of this article is to summarize important aspects to establish a comprehensive global temperature excursion management program, including standard stability testing, regulatory expectations related to the justification of temperature excursions, thermal cycling studies to assess and support potential temperature excursions ...
A thermal cycling study is done to determine the impact of temperature excursion on the edge and outside of a product's labeled storage conditions. The excursion is simulated below and above the product's labeled storage conditions for multiple cycles, and the impact on the product quality will be evaluated because of thermal cycling.
The temperature excursion may be observed during transportation of raw materials manufacturing as well as distribution of pharmaceutical products, which have potential to deteriorate the product quality. ... Alternative is to use thermal resistant packaging and stringent control measures during transit and shipment, to avert the undesired ...
How/When Thermal Cycling Study Data is Used to Support Temperature Excursions. Thermal cycling study data (from development studies and in some cases from formal thermal cycling on cGMP drug product batches) and associated impact toward product quality and stability may be included in a New Drug Application (NDA). Pharmaceutical companies may ...
The effects of thermal excursion in enclosures: condensation - From Blog. This site uses cookies or third-party cookies necessary for some operation and uses described in our cookie policy. If you want to learn more or opt out all or some cookies, see the cookie policy. By closing this banner, scrolling through this page, clicking on a link or ...
Temperature Excursion Management in Pharmaceutical Storage. The quality of pharmaceuticals relies on environmental controls during their storage and handling. Every pharmaceutical item ought to be taken care of and stored under manufacturer-recommended storage conditions marked on the data information sheet or packaging.. Temperature Excursion Management in Pharmaceutical Storage is important ...
Thermal cycling Shipping excursion Biologics Best practices Drug product Regulatory expectations Introduction ICH Guidelines Q1A(R2) and Q5C address information to be sub-mitted in a core stability data package in registration applications for new investigational drug substances and products, to provide evi-
The excursion study is followed by storage at the long-term storage condition • Patient convenience period needs to be included at the long-term storage condition, if applicable. A cycling approach (versus a block excursion) reduces the risk if multiple temperature excursions are experienced during the same shipment. Study Setup •
Waste2Fiber facility will use a proprietary thermal method to separate wind blade materials for reuse and will have a processing capacity of 6,000 tons of material/year. ... Or what if there are no excursions, but the post-process thermal analysis of specimens taken from the part laminate show a lower-than-required thermal glass transition ...
Thermal Cycling involves rotating samples between sub zero and ambient conditions. The range encountered during transportation may differ from that which is specified for long term storage, ascertained from stability studies. To understand the effect these excursions may have on a product, Thermal Cycling studies are completed. Also known as ...
The temperature excursion may be observed during transportation of raw materials manufacturing as well as distribution of pharmaceutical products, which have potential to deteriorate the product quality. ... Drug product sensitive to higher temperature excursions can depreciate the intended action by receiving the thermal energy. This may ...
Temperature excursion is the arch-nemesis of the pharmaceutical industry. If you want to manage a cold chain successfully or make yourself known as a reliable distributor, Vacker's thermal mapping and validation is the way to go. Visit our detailed explanation of thermal validation and mapping study here. We also have a downloadable guide ...
Thermal runaway refers to a situation where an increase in temperature changes the conditions in a system in a way that causes a further increase in temperature. With an increase in temperature, the reaction rate also increases and this escalates the overall system temperature even further. In other words, it's a sort of chain reaction where ...
We studied the rapid paleo-environmental changes and the corresponding biotic responses of benthic foraminifera of a shallow shelf site during the late Paleocene and the Paleocene-Eocene Thermal Maximum (PETM). The PETM is globally characterized by a negative δ13C excursion in marine and terrestrial sediments. Isotope data from the Atlantic Coastal Plain from the South Dover Bridge core ...