- Search Menu
- Advance articles
- AHFS First Release
- AJHP Voices
- AJHP Residents Edition
- Top Twenty-Five Articles
- ASHP National Surveys of Pharmacy Practice in Hospital Settings
- Medication Safety
- Pharmacy Technicians
- Specialty Pharmacy
- Emergency Preparedness and Clinician Well-being
- Author Guidelines
- Submission Site
- Open Access
- Information for Reviewers
- Self-Archiving Policy
- Author Instructions for Residents Edition
- Advertising and Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Editorial Board
- Permissions
- Journals on Oxford Academic
- Books on Oxford Academic

- < Previous

Allowable room temperature excursions for refrigerated medications: A 20-year review
- Article contents
- Figures & tables
- Supplementary Data
Lucas E Orth, Amanda S Ellingson, Sara F Azimi, Joseph T Martinez, Amal A Alhadad, Brenda C Tran, Chase W Allen, Cecilia T Nguyen, Tony Duong, Jordan S Burkdoll, Jenny Yoo, Allison B Blackmer, Meghan N Jeffres, Allowable room temperature excursions for refrigerated medications: A 20-year review, American Journal of Health-System Pharmacy , Volume 79, Issue 15, 1 August 2022, Pages 1296–1300, https://doi.org/10.1093/ajhp/zxac118
- Permissions Icon Permissions
The aim of this review was to build upon previous literature describing the maximum duration for which refrigerated medications can tolerate room temperature excursions while maintaining stability and potency.
During a 12-month period ending in June 2021, the prescribing information and published monographs from multiple pharmacy compendia were reviewed for all medications and biologic products approved by the US Food and Drug Administration (FDA) for human use since January 2000. Products that were subsequently withdrawn from the US market were excluded. When temperature excursion data was unavailable in published form, product manufacturers were surveyed via telephone and/or email. Acceptable storage information for all products for which storage is recommended at temperatures below room temperature (20-25 °C [68-77 °F]) was compiled and arranged in tabular format.
Of the 705 products or formulations approved by FDA during the predefined time period, 246 were identified as requiring storage at temperatures below room temperature. After review of available prescribing information and manufacturer communications, if applicable, acceptable periods of excursion to temperatures at room temperature or higher were identified for 214 products (87%).
Information related to acceptable periods of room temperature excursion was compiled for a total of 214 products approved for US distribution since 2000. The included tables may increase patient safety and decrease medication loss or related expenditures.
Email alerts
Citing articles via.
- Recommend to Your Librarian
Affiliations
- Online ISSN 1535-2900
- Print ISSN 1079-2082
- Copyright © 2024 American Society of Health-System Pharmacists
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Explore solutions built for your industry
Our customer-proven solutions monitor medications and food inventories for some of the most recognizable names in the industries of healthcare, food service, and transportation, and logistics. See how our solutions adapt to your industry needs.
BY INDUSTRY
- Retail Grocery
- Food Service
- K-12 Education
- Conveniences Stores
- Supply Chain Monitoring
BY USE CASE
- COVID-19 Solutions
- Pharmacy Monitoring
- VFC Monitoring
- Food Safety Monitoring
- Asset Monitoring
- Advanced Analytics
SYSTEM COMPONENTS
- System Overview
- Cloud Dashboard
- Digital Checklists
- Sensors & Data Loggers
- Implementation Services
Festival Foods Protects its Profits & People with SmartSense
Share SmartSense Solutions with your team.
Resource Center
Work smarter. Explore our videos, webinars, and customer stories.
Learn how our Sensing-as-a-Service solutions can fit your business.
Review technical specifications for our solutions.
- Customer Videos
- Customer Stories
- Thought Leadership
- Media Coverage
- Wireless Sensors
- Installation
- See all categories
- Contact Support
Questions? Call +1 (866) 806-2653 to speak to our experts.
Questions contact us..
Call +1 (866) 806-2653 to speak with our experts or get started with a demo.
SmartSense was created to use the power of the Internet of Things (IoT) to help our customers protect the assets most critical to the success of their business.
Create the future of IoT by joining our team.
CONNECT. PROTECT. RESULTS.
- Leadership Team
- In the News
Select your login
- Help Center
- COVID-19 Monitoring
- SCHEDULE DEMO
- There are no suggestions because the search field is empty.
June 21, 2017
6 Steps for Handling Temperature Excursions
Written by SmartSense | Pharmacy Safety
What is a temperature excursion? In the pharmaceutical industry , it refers to any temperature reading outside recommended ranges from the manufacturer’s package insert. Why is it important? Because out-of-range storage temperatures or inappropriate conditions for any vaccine can negatively impact the efficacy, and will require immediate action.
Fortunately, the Centers for Disease Control (CDC) have developed best practices to handle this emergency situation. If there is any question about whether vaccines may have been exposed to a temperature excursion due to the unit becoming too cold or too hot, take the following steps.
Step 1: Notify Supervisors
Any staff member who hears an alarm, receives an alert message, or notices a temperature excursion should notify the vaccine coordinator immediately or report the problem to a supervisor.
Step 2: Quarantine Vaccines
If a vaccine has been compromised, quarantine it immediately. Label exposed vaccines, “DO NOT USE,” and place them separately from other vaccines in the storage unit. Do NOT discard the compromised vaccines.
Step 3: Document the Event
The vaccine coordinator, supervisor, or if necessary, the person reporting the problem, should document the event . Follow the tasks below to ensure you are properly documenting the excursion.
- Name of the person completing the report
- Date and time of the temperature excursion
- Inventory of affected vaccines
- Description of the event
- Minimum and maximum storage unit temperature and room temperature during the time of the event
- Length of time vaccine may have been affected
- Listing of items in the unit (including water bottles) other than vaccines
- Any problems with the storage unit and/or affected vaccines before the event
- Other relevant information

Step 4: Get Guidance
Contact your immunization program or vaccine manufacturer for additional guidance on whether to use affected vaccines and for information about whether patients will need to be recalled for re-vaccinations. Be prepared to provide documentation of the event (e.g., temperature log data).
Step 5: Implement SOPs
Implement your facility’s Standard Operating Procedures (SOPs) to adjust the unit temperature to the appropriate range. At a minimum, check the temperature monitoring device to make sure it is appropriately placed in the center of the fridge.
Step 6: Wrap Up
Complete your documentation of the event, including the following:
- What happened to affected vaccines
- What you did with the vaccine and when
- Whom you have contacted and instructions received
- What you have done to prevent a similar future event
You never know when a temperature excursion may happen. If you have not yet incorporated these best practices into your monitoring program, now is a good time to start!
Subscribe to Our Blog!
Subscribe to our blog to get weekly email updates about quality and safety issues important to the pharmaceutical industry.
Subscribe to the SmartSense Blog
Stay up-to-date on the evolution of IoT connectivity.
Learn how our complete critical environment monitoring solution will help you connect and transform your business.
Call +1 (866) 806-2653 to speak with our industry experts or get started by requesting a demo.
- Terms of Service
- Return Policy
- Privacy Policy
- Cookie Policy
- System Status

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

1.2: Introduction to Pharmacology
- Last updated
- Save as PDF
- Page ID 10623

- Carl Rosow, David Standaert, & Gary Strichartz
- Massachusetts Institute of Technology via MIT OpenCourseWare
A drug is a chemical agent which can affect living processes. For purposes of this course we will mainly be talking about small molecules which affect cellular processes. Most of these are Xenobiotics (Gr. xenos - stranger) chemicals that are not synthesized by the body, but introduced into it from outside. There is inevitably a certain amount of ambiguity in this definition: Is oxygen or water a drug? How about Vitamin C in a glass of orange juice? How about an injection of Vitamin C to treat scurvy?
Pharmacology (Gr. pharmakon - a drug or poison, logos - word or discourse) is the science dealing with actions of drugs on the body ( pharmacodynamics ) and the fate of drugs in the body ( pharmacokinetics ). It overlaps with pharmacy , the science of preparation of drugs; much of it deals with therapeutics , the treatment of disease (by whatever means). Toxicology is the branch of pharmacology dealing with the "undesirable" effects of drugs on biological processes (in the case of a nerve gas the bad effect may be a desired one).
In order for a drug to work, it must enter the body and somehow be distributed in such a way that it gets to its site of action. In most cases the site of action is a macromolecular "receptor" located in the target tissue. Most drug effects are temporary, because the body has systems for drug detoxification and elimination. We will consider these issues broadly for now and go into more depth in individual lectures. As you read, refer to the figure below:

Overview of Pharmacokinetics - "What the body does to the drug"
- The drug may enter the body in a variety of ways: as an oral liquid, pill, or capsule; as an inhaled vapor or aerosol; absorbed through intact skin or a mucous membrane; injected into muscle, subcutaneous tissue, spinal fluid, or directly into the bloodstream. As we shall see, the physical properties of the drug and the specific way it is prepared greatly influence the speed of absorption.
- If the drug is given orally and swallowed, it must be absorbed from the GI tract into the portal circulation. If it is absorbed from the skin, mouth, lungs or muscle it will go directly into the systemic circulation. If drug is injected directly into the bloodstream (e.g., intravenous injection), 100% of it is available for distribution to tissues. This is not usually the case for other modes of administration. For example, drug which is absorbed via the portal circulation must first pass through the liver which is the primary site of drug metabolism (biotransformation). Some of the drug may therefore be metabolized before it ever reaches the systemic blood. In this case," first-pass " metabolism reduces the bioavailability to less than 100%.
- Once the drug is in the bloodstream a portion of it may exist as free drug, dissolved in plasma water. Some drug will be reversibly taken up by red cells and some will be reversibly bound to plasma proteins. For many drugs, the bound forms can account for 95-98% of the total. This is important because it is the free drug which traverses cell membranes and produces the effect. It is also important because protein-bound drug can act as a reservoir which releases drug slowly and thus prolongs its action.
- The unbound drug may then follow its concentration gradient and distribute into peripheral tissues. In some cases, the tissue contains the target site and in others the tissue is not affected by the drug. Sites of non-specific binding act as further reservoirs for the drug. This total volume of distribution determines the equilibrium concentration of drug after a specified dose.
- Tissue-bound drug eventually reenters the bloodstream where it perfuses the liver and kidneys. The liver metabolizes most drugs into inactive or less active compounds which are more readily excreted. These metabolites and some of the parent compound may be excreted in the bile and eventually may pass out of the body in the feces. Alternatively, some of the drug may be reabsorbed again, farther down the GI tract (the so-called enterohepatic cycle). Any biotransformed drug which is not excreted in bile passes back into the systemic circulation.
- Parent drug and metabolites in the bloodstream may then be excreted: most are filtered by the kidney, where a portion undergoes reabsorption, and the remainder is excreted in the urine. Some drugs are actively secreted into the renal tubule. Another route of excretion is the lung: Drugs like alcohol and the anesthetic gases are eliminated by this route. Smaller amounts of drug are eliminated in the sweat, tears and breast milk.
- Biotransformation may sometimes produce metabolites with a great deal of activity. Occasionally, we administer a parent drug which is inactive (a pro-drug) and only the metabolite has activity. [How might this be useful?]
Overview of Pharmacodynamics - "What the drug does to the body"
As stated above, the majority of drugs bind to specific receptors on the surface or interior of cells, but there are many other cellular components and non-specific sites which can serve as sites of drug action.
- Water can be a target. Osmotic diuretics like mannitol are not reabsorbed by the kidney, and the osmotic load they create in the renal tubule obligates the loss of water. Laxatives like magnesium sulfate work in the intestine by the same principle.
- Hydrogen ions can be targets. Ammonium chloride is sometimes used to acidify the urine. When it is taken orally, the liver metabolizes ammonium ion to urea, while the chloride is excreted in the urine. The loss of Cl- obligates the loss of H+ in the urine, thus the pH is lowered.
- Metal ions can be targets. Chelating agents like EDTA may be used to bind divalent cations like Pb++. Metal ions are most frequently drug targets in cases of poisoning.
- Enzymes are targets of many therapeutically useful drugs. Drugs may inhibit enzymes by competitive, non-competitive, or irreversible blockade at a substrate or cofactor binding site. Digitalis glycosides increase myocardial contractility by inhibiting the membrane enzyme, Na+-K+ - ATPase. Antimicrobial and antineoplastic drugs commonly work by inhibiting enzymes which are critical to the functioning of the cell. In order to be effective, these drugs must have at least someselective toxicity toward bacterial or tumor cells. This usually means that there is a unique metabolic pathway in these cells or some difference in enzyme selectivity for a common metabolic pathway. An example of this is the inhibition of folate synthesis by sulfonamides. These drugs are effective antibacterial agents because the bacteria depend upon folate synthesis, while the host doesn't. This example will be covered in detail in one of our case discussions.
- Nucleic acids are targets for antimetabolites and some antibiotics. In the case of 5- fluorouracil, the compound acts as a counterfeit substitute for uracil and becomes incorporated into a faulty mRNA. Antisense oligonucleotides are another very specific way to interfere with a restricted part of the genome.
- Some drugs, like general anesthetics, appear to act by non-specific binding to a macromolecular receptor target. These drugs are thought to alter the function of membrane proteins, in part, by disordering the structure of the surrounding lipid membranes. Their lack of specificity is reflected in very low chemical structural requirements. The general anesthetics include compounds as chemically diverse as nitrogen, xenon, halogenated ethers, and steroids. They exhibit very little stereoselectivity, that is, there are not marked differences in anesthetic activity between enantiomers.
- Finally, we have the drugs which act by binding to specific receptors . As you will see in lectures 2 and 6, these drugs have both high structural specificity and stereoselectivity, i.e. relatively small changes in chemical structure can radically alter the activity of these drugs.
Let us finish with some important definitions. These are concepts which we will return to repeatedly throughout the course.
- Agonist is a drug which binds to its "receptor" and produces its characteristic effect. A drug may be a full agonist or partial agonist , depending on the maximal effect it produces. An antagonist binds to the receptor without causing an effect, thereby preventing an active substance from gaining access. Antagonists, like enzyme inhibitors, may be competitive, non-competitive or irreversible.
- Dose-Response . The sine qua non of drug effect. Simply put, as the dose of drug increases, the response should increase. [What if the response increases, then decreases as the dose is raised?] The curve generated is usually sigmoidal when effect is plotted against log dose (Dr. Strichartz will discuss the theoretical basis for this). Effect may be measured as a graded variable (change in blood pressure, force of contraction) or as a quantal variable (number dead/alive). The slope of the curve is characteristic of the particular drug-receptor interaction. When two drugs act by the same receptor mechanism, we expect to see two parallel log-dose response curves.
- ED 50 . The median effective dose, or the dose which produces a response in 50% of subjects. If the response is death (lethality) we call it the LD 50 . The EC50 refers to concentration rather than dose. Similar abbreviations are used for other response levels: ED 99 , LD 1 , etc.
- Potency . A terribly misused word – the lay public uses it to mean “effectiveness.” The potency of a drug refers to the dose (actually the molar concentration) required to produce a specific intensity of effect. [We usually specify the ED50, why?] If the ED50of drug A and B are 5 and 10 mg, respectively, the Relative Potency of A is twice that of B. Relative potency specifically applies to the comparison of drugs which act by the same mechanism, and therefore have parallel dose-response curves.
- Efficacy . Also called Maximal Efficacy or Intrinsic Activity . This is the maximum effect of which the drug is capable. A potent drug may have a low efficacy, and a highly efficacious drug may have a low potency. For the clinician, efficacy is much more important than potency (within limits). Who cares if the pill contains 5 or 10 mg of drug?
- Affinity . This refers to the strength of binding between drug and receptor. It is quantified by the dissociation constant kD (covered in the next lecture).
- Selectivity . This refers to the separation between desired and undesired effects of a drug. In the ideal case, a drug is completely specific , and an effective dose does not elicit any undesired effect. Penicillin is an example of a highly selective drug, since it works specifically by inhibiting cell wall synthesis, and (other than allergic responses) it has very little effect on human cells at normal doses. Unfortunately, many therapeutic agents, like digoxin and theophylline, produce dose-related side effects near their therapeutic dose range. For some drugs like cancer chemotherapeutic agents, their selectivity is their dose-limiting property, i.e., they are given to kill tumor cells until they produce toxicity in normal cells as well.
- Therapeutic Window . For every drug, there exists some concentration which is just barely effective (the Effective Concentration ) and some dose which is just barely toxic (the Toxic Concentration ). Between them is the therapeutic window where most safe and effective treatment will occur.
- Therapeutic Index . This is the ratio of toxic to effective doses at the level of 50% response: TD 50 /ED 50 . In animal toxicology studies, it is usually the LD 50 /ED 50 . Another measure sometimes utilized is the Certain Safety Factor , which is TD 1 /ED 99.

- ‹ Prev
- Next ›
1.1 Introduction
1.2 origins and antecedents, 1.3 the emergence of pharmacology as a science, 1.3.1 chemistry makes its entry, 1.3.2 pathology and physiology lay important foundations, 1.3.3 the receptor concept is established, 1.3.4 many chemical mediators are identified, 1.4 receptors and drug targets, 1.5 pharmacology in drug discovery, 1.6 pharmacology today, 1: what is pharmacology.
- Published: 25 Oct 2017
- Special Collection: RSC eTextbook Collection Product Type: Textbooks
- Open the Chapter PDF for in another window
- Get permissions
- Cite Icon Cite
H. Rang, in Pharmacology for Chemists: Drug Discovery in Context, ed. R. Hill, T. Kenakin, and T. Blackburn, The Royal Society of Chemistry, 2017, pp. 1-13.
Download citation file:
- Ris (Zotero)
- Reference Manager
Pharmacology is the study of the action of drugs on living systems – neatly paraphrased as the chemical control of physiology and pathology. It lies at the interface of chemistry and biology. Drugs , in this context, are chemicals of known structure that are administered as external agents – whether deliberately or accidentally – to the organism, and produce an observable effect on its function. This chapter provides an introduction to pharmacology, describing a brief history of the discipline.
Pharmacology is the study of the action of drugs on living systems – neatly paraphrased as the chemical control of physiology and pathology. It lies at the interface of chemistry and biology. Drugs , in this context, are chemicals of known structure that are administered as external agents – whether deliberately or accidentally – to the organism, and produce an observable effect on its function. Living organisms are, of course, complex chemical machines, which produce and use many of their own chemicals as a means of controlling their own functions. Not surprisingly, exposure to other chemicals ( i.e. drugs) from the outside world is liable to confuse and subvert the internal signals, and that in essence is what pharmacology is all about. An understanding of pharmacology plays an essential role in the discovery and application of drugs as therapeutic agents, where the aim is to provide benefit to individuals by the alleviation of symptoms and disabilities, improved prognosis, prolongation of life, or disease prevention. A drug that does none of these things, even though it has been exquisitely engineered to interrupt what was thought to be a key step in the pathogenesis of the disorder, is of no use as therapy, though it may prove to be a valuable research tool.
Pharmacology comprises two main components, namely pharmacodynamics , which is concerned with the effects that drugs produce on living systems ( i.e. what the drug does to the body), and pharmacokinetics , which describes the mechanisms by which the drug is absorbed, distributed, metabolised and excreted ( i.e. what the body does to the drug). To explain fully the effects of a drug in an intact organism, both need to be understood.
Given the extreme chemical complexity of living organisms, and the delicately balanced regulatory mechanisms that have evolved over millions of years to allow organisms to survive environmental threats, it is not surprising that the intrusion of a foreign chemical is, in general, more likely to do harm than good. † The aim of drug discovery research is to find those few compounds that – against all odds – can deliver benefit to individuals affected by disease. Therapeutic benefit depends not only on choosing the right compound, but delivering it in the right dose, to the right patient, by the best route, at the right time and under the right circumstances. These important aspects are the concern of the subdiscipline of clinical pharmacology.
When developed as therapeutic agents, drugs are incorporated into medicines , which normally include other substances to enable them to be administered as pills, solutions for injection, skin patches, aerosols or other dosage forms ( Box 1.1 ).
Pharmacology. The study of the action of drugs on living systems. Clinical pharmacology is a branch of pharmacology concerned with the action of drugs used clinically to treat patients.
Drug. A substance of known chemical structure that produces a functional effect when added exogenously to a living system. Many endogenous chemical mediators that regulate normal physiological functions in higher animals can also be administered as drugs, but most drugs are synthetic chemicals or natural products not found in higher animals.
Medicine. A preparation containing one or more drugs, designed for therapeutic use. Medicines usually contain additional materials to improve their suitability for clinical use, for example as an injectable solution, a pill to be swallowed, or an ointment for topical application.
Therapeutic efficacy. The disease-related benefit to humans that a medicine produces. The benefit may be:
relief of symptoms or disabilities associated with disease;
improved prognosis, i.e. slowing or reversal of the progress of the disease;
prolongation of life;
prevention of disease.
The word “pharmakon” in ancient Greek, could mean a medicine or a poison; in the ancient world there was little distinction. Attempts to heal the sick by the use of “medicines” – largely herbal and mineral in origin, and based on spiritualist dogma rather than science – was in the hands of spiritual healers, and the tradition survives to the present day, carried on by “medicine men” and “witch doctors”, whose stock-in-trade is not only to heal the sick, but also to inflict harm on enemies. Compilations of such remedies go back thousands of years and it was the task of healers to produce them according to closely guarded recipes. Such traditional practices, based on dogma rather than science, remain popular to this day, despite the fact that evidence that they deliver benefit is generally weak or nonexistent. Prior to the 19th century, even though “medicines” based on traditional dogma had been catalogued and used for thousands of years, the understanding of drug action in terms of scientific principles – in other words, the emergence of pharmacology – was impossible. Chemistry had not yet advanced to the point of defining compounds in terms of their structure; physiology and pathology could not yet describe the functioning and malfunctioning of the body; traditional teaching emphasised the importance of esoteric procedures for concocting herbal preparations as a prerequisite for their clinical use. There were, however, a few earlier breaks with tradition – discoveries that found application in modern medicine. For example, Thomas Sydenham (1624–89) described in 1666 the use of opium (containing morphine) to control pain and “Jesuit's bark” (containing quinine) to control “intermittent fever” ( i.e. malaria); William Withering (1741–99) described the use of foxglove (digitalis) to treat “dropsy” (heart failure) in 1785. But until the nineteenth century these preparations were generally assumed to owe their properties to the vital forces possessed by “organic” substances. Chemistry did not come into it, nor did any understanding of biological mechanism.
Materia medica – the description of natural products and their medicinal uses, based on beliefs passed from generation to generation down the ages – began to be commercialised in the mid-17th century by the apothecary's trade, the forerunner of the modern pharmaceutical industry, its aim being to satisfy the demand for medicines containing ingredients that were difficult to obtain, and prepared in an approved manner. At the same time, the “Age of Enlightenment”, a gradual shift from dogma to science in the practice of medicine began to grow, when luminaries such as Robert Boyle (in chemistry) and William Harvey (in physiology) started to use evidence based on careful observation and experiment, as opposed to received wisdom, as a basis for understanding the natural world. The idea of living organisms as machines governed by the same physical laws as everything else in the material world, and of chemistry as the underlying basis of every substance and structure, slowly took root over the next two centuries, and dogmatic beliefs began to be challenged by discoveries based on empirical observations, many of which have stood the test of time.
Pharmacology as a distinct biomedical discipline began in 1847, when Buchheim (1820–1879) established the first university department with that name in Dorpat. His was a bold vision, for at that time the medicines in use were mainly plant extracts of unknown composition and a few, mostly poisonous, inorganic compounds, such as salts of mercury and arsenic. Complicated mixtures were recommended, prepared and administered in accordance with elaborate rituals. Vomiting, sweating, diarrhoea and fever commonly resulted, and were regarded as evidence of the treatment's effectiveness in ridding the body of harmful “toxins”. A famous quotation from an eminent contemporary physician, Oliver Wendell Holmes in 1860 dismissed them thus: “If all the materia medica, as currently used, could be thrown into the sea, it would be the better for mankind, and the worse for the fishes”. 1 It is certainly true that the “remedies” in use at that time were not based on any understanding of how they produced their effects, or of the underlying pathological dysfunction that needed to be corrected (beyond the “toxins” notion mentioned above), and the idea of testing their therapeutic efficacy rarely surfaced. Nevertheless, Buchheim saw that the challenge, then as now, was to understand better the mechanisms by which they produced their effects in order to put their medicinal uses on a rational, and hopefully effective, basis. Even though organic chemistry had hardly come into being, he had the vision to see that physiology, pathology and chemistry were all advancing rapidly in the very fertile scientific environment of the mid-nineteenth century, to a point where a new interdisciplinary science could emerge.
One of the essential foundations of pharmacology – the use of structural formulae to define chemical compounds – did not exist until the middle of the nineteenth century. A key figure was August Kekule, a German chemist who described both the tetravalent nature of carbon and the aromatic ring structure of benzene, two essential principles that allowed accurate structural formulae of organic molecules to be produced for the first time. The idea that the biological effects of plant extracts was likely to result from their chemical constituents rather than mysterious vital forces was implicit in the work of Buchheim and other pharmacological pioneers in the nineteenth century, but it was not until 1905 that a German pharmacist, Serturner, isolated crystals of morphine from opium poppies, tested it on himself and nearly died – the first irrefutable evidence that opium worked by chemistry, not by magic. Serturner's achievement was followed quickly by others who similarly extracted and purified chemical compounds from medicinal plants and showed them to possess distinctive pharmacological properties. Studying how such plant-derived substances as nicotine, atropine, curare, strychnine and ergot alkaloids, produce their effects, and relating them to the emerging knowledge of physiology and pathology, gave pharmacology the scientific foundations that it needed. But still, at this time, synthetic compounds, as opposed to natural products, played only a very minor part.
The other essential foundations of pharmacology, namely physiology and pathology, also flourished in the nineteenth century. Some key milestones are worth noting. The cell theory, proposed by the German pathologist Rudolf Virchow (1821–1902), identified the cell as the fundamental unit of all living organisms, and proposed that cellular dysfunction – cells dying, dividing, migrating, or otherwise functioning incorrectly – was the basic cause of disease. Louis Pasteur (1822–1896), a French chemist, proposed the germ theory of infection in 1878, having famously demonstrated the role of air-borne micro-organisms in fermentation. He showed that cholera could be transferred from chicken to chicken by inoculation with fresh, but not “stale” material. In fact, inoculation with stale material actually protected against future infection, so Pasteur inadvertently discovered the phenomenon of immunisation.
The first physiological studies aimed at pinning down the site of action of poisons were performed by Francois Magendie (1783–1855) in Paris, who showed that the convulsant action of strychnine was due to its action on the spinal cord, rather than elsewhere in the brain, nerves or muscles. His pupil, Claude Bernard (1813–1878) used a similar anatomical approach to pinpoint the paralysing effect of the arrow-poison curare to the junction between motor nerve and muscle fibre.
The realisation that drugs act very specifically at precise anatomical sites led in later years to the search for cellular components and actual molecules involved. A particularly important stage in the development of modern pharmacology was the emergence of the receptor concept, pharmacology's Big Idea (reviewed by Rang, 2006 2 ) of a “lock and key” mechanism by which drug molecules act on specific cellular molecules to produce their effects. This idea had been expressed, in a philosophical way, centuries earlier: “Did we but know the mechanical affections of the particles of rhubarb, hemlock, opium and a man……we should be able to tell beforehand that rhubarb will purge, hemlock kill and opium make a man sleep…….” 3 (John Locke, 1690, Essay concerning human understanding). These “mechanical affections”, which we would today call chemical interactions, are what pharmacology is all about. The Cambridge physiologist, J. N. Langley (1852–1925), first used the term “receptive substance” in 1878 to describe the hypothetical endogenous substance in salivary glands with which pilocarpine (which causes salivary secretion) and atropine (which blocks pilocarpine's action) combine and compete with each other for binding. A. V. Hill (1886–1977), a student in Langley's laboratory, applied the Law of Mass Action to describe quantitatively the interaction of drug and receptor molecules, and this quantitative approach was further developed by later pharmacologists. We now know that specific receptors exist in all cells and tissues, and are key players in the numerous chemical signalling pathways used by all living organisms to control their physiological functions. The subversion of these signalling pathways by introducing alien chemicals is the basis is modern pharmacology. Understanding pharmacology in this mechanistic way, underpinned now through the application of molecular biological approaches and by detailed knowledge of the structure and function of receptor molecules, has become crucial for the discovery of new therapeutic drugs.
Receptors form one important class of targets for therapeutic drugs. Enzymes, transporter molecules, ion channels, etc . are other types of target, described elsewhere in this book. Identifying such drug targets, and explaining how drugs are able to act on them to influence the function of the cells and tissues that express them, is a central theme in modern pharmacology, and an important starting point for drug discovery and therapeutic innovation.
The idea that internal secretions – chemical substances liberated into the bloodstream by organs such as the thyroid gland, testis and liver – play an important physiological role, emerged in the 17th century, and slowly gained ground over the next 200 years. The term “hormone” was coined in 1905 by Bayliss and Starling, who showed that the duodenum, in response to gastric acid production, produced a substance “secretin” that caused the pancreas to release digestive enzymes. Around the same time, many physiologists described the effects of removing individual glands – adrenal, thyroid, pituitary, pancreas, etc. – and of injecting various gland extracts, on different physiological functions, though chemical techniques for isolating and identifying the mediators involved were not yet available. The realisation that the release of chemical transmitters is the mechanism by which nerve cells communicate with each other, and with other cells and tissues came initially from work by Dale and Loewi, who identified acetylcholine as the transmitter released by parasympathetic nerve endings (winning the Nobel Prize in 1936), and the identification of other chemical mediators became – and remains – a major focus of pharmacology. Many large families of mediators are now recognised. These include:
low molecular weight amines, such as acetylcholine, noradrenaline, histamine, dopamine, 5-hydroxytryptamine;
peptides, such as insulin, oxytocin and angiotensin;
protein mediators, such as growth hormone, interferon and a wide variety of cytokines;
lipid mediators, such as prostaglandins and leukotrienes;
steroids, such as oestrogens and adrenocortical hormones;
amino acids, such as glutamate, glycine and γ-amino butyric acid (GABA);
purines, such as adenosine, ADP and ATP;
small molecules such as nitric oxide, carbon monoxide and hydrogen sulfide.
New members of each of these groups of mediators are still being discovered, particularly in the protein group, where modern techniques in molecular biology, cell biology and genomics have made a big impact in recent years.
From a pharmacological perspective, this profusion of mediators gives rise to many potential drug targets. Each mediator acts by binding to a specific recognition site located on a protein – the receptor – through which it produces its physiological effects. The four main types of receptor are:
G-protein coupled receptors (GPCRs);
ligand-gated ion channels;
kinase-linked receptors;
nuclear receptors.
Apart from nuclear receptors, most receptors are proteins that span cell membranes, accessible to mediators acting on the extracellular surface, and controlling events within the cell.
Most mediators act on more than one receptor, producing different effects on different cells. GPCRs are a particularly large and important type; approximately 350 GPCRs for endogenous mediators have been identified in the human genome, and roughly half the drugs in clinical use target GPCRs. Ligand-gated ion channels, activated by transmitters such as acetylcholine and glutamate, are important mainly in mediating fast synaptic neurotransmission. Kinase-linked receptors, located on the cell surface, respond to mediators such as growth factors, cytokines and insulin. Activation of the intracellular kinase moiety of the receptor protein initiates a cascade of protein phosphorylation reactions within the cell, culminating in the functional response. Nuclear receptors are intracellular proteins, responding to mediators such as steroids and thyroid hormones that are able to enter the cell; these receptors act by controlling gene expression in the nucleus.
Drug targets are the endogenous molecules (mostly proteins) to which drug molecules bind as the first step in producing their pharmacological effects. The various type of receptors for endogenous mediators, described above, constitute one important class of drug targets, but other types of functional protein, and also DNA, are also important.
The pathologist, Paul Ehrlich (1854–1915), was impressed by the ability of chemical dyes to stain biological specimens in a very specific way, and argued that this selective binding to particular cell types might be used as a basis for finding drugs that would bind to and kill pathogenic organisms. Arsenic, in various forms, had been used as a poison and a medicine for thousands of years, so Ehrlich, working with an organic chemist, embarked on the first systematic attempt at drug discovery by chemical synthesis. They made and tested hundreds of organic arsenic compounds, based on aniline dyes (the constituents of many of the biological stains that had engaged Ehrlich's attention) as possible treatments for trypanosomiasis (sleeping sickness, a common and serious infectious tropical disease). From this came, in 1907, Compound 606, named Salvarsan, the first effective drug for this disorder, and the beginning of the era of antimicrobial chemotherapy – arguably the biggest therapeutic success story to date.
Synthetic chemistry had given rise to clinically useful drugs before this, though by serendipity rather than design. Diethyl ether was discovered in the 16th century (known then as “sweet oil of vitriol” because it was made from alcohol and sulfuric acid) and gained notoriety in the 19th century as a party drug (“ether frolics”). Nitrous oxide (laughing gas) had similar origins, and the ability of both of these agents to produce reversible insensibility led in the mid-19th century to their introduction as surgical anaesthetic agents – a vital breakthrough that allowed surgery to develop from agonising butchery to humane intervention. It was in the late 18th century that chemistry began to take over from alchemy, and the production and purification novel compounds of known structure became possible. But understanding and determination of chemical structure, and synthetic methods were still very limited, and it was not until the end of the 19th century that synthetic chemistry really took off, and some of the products were discovered to have medical uses. Among the earliest drugs that came from this were the local anaesthetic, procaine (1905), and the sedative, barbital (1907), both forerunners of important classes of clinically used drugs.
Chemistry-led drug discovery grew rapidly in the 20th century, and quickly became the leading source of new therapeutic agents, and, as a byproduct, new research tools that proved valuable in the study of physiological and pathological processes. Intervening in metabolic pathways, by synthesising “antimetabolites” – analogues of endogenous metabolites – was an approach followed for many years by the highly successful drug discovery team led by Hitchings (1905–98) and Elion (1918–99), working at Burroughs Wellcome in the USA. Earlier, Domagk (1895–1964) in Germany had developed sulfonamides, the first effective antibacterial drugs, which were later shown to work by inhibiting the synthesis of folic acid, a metabolite essential for bacterial growth. Hitchings and Elion sought other inhibitors by making and testing a range of purine and pyrimidine analogues, which acted as inhibitors of the enzyme dihydrofolate reductase. Their antimetabolite approach, begun in 1944, generated not only antibacterial drugs, but also a range of other chemotherapeutic agents, active against protozoa and human cancers. The same sulfonamide-based chemical lineage later gave rise to novel diuretics (acetazolamide, chlorothiazide), antidiabetic drugs (sulfonylureas) and antihypertensive drugs (diazoxide) – an extraordinary example of chemical inventiveness leading to important new therapeutic drug classes. Domagk was awarded the Nobel Prize in 1939, Hitchings and Elion in 1988.
Analytical chemistry later also played an important role in providing tools for identifying the signalling molecules – hormones, neurotransmitters, inflammatory mediators, etc . – that play such a major role in physiological regulation, and whose dysfunction commonly leads to disease. Chemists became very successful at inventing new drugs by synthesising analogues and derivatives of known structures. An intuitive sense – hard to pin down – possessed by successful medicinal chemists, guiding them to the kind of structures likely to yield clinically useful drugs, was an important driver of these inventions. The compounds would be handed over to biologists for testing on animals, and anything that looked interesting could be further tested and developed as a medicine. This compound-led strategy sustained a successful drug industry for many years. Nevertheless, natural products continue to be a fruitful source of new useful drugs, most notably in the discovery of penicillin, relying on the inventiveness of evolution rather than of human chemists.
From the mid-20th century “target-led” drug discovery began to rival the compound-led approach. The coming together of chemistry, physiology and pathology under the banner of pharmacology drew attention to the importance of “drug targets”, namely the endogenous molecules – in most cases proteins – to which drugs bind in order to produce their effects. Such protein targets are of many kinds, including enzymes, receptors for endogenous mediators, transporter molecules, etc . and new ones are constantly being identified. James Black (1924–2010), a British pharmacologist working in industry, was a leader in this new target-led approach. Selecting the recently identified β-adrenoceptor as a promising target for treating cardiovascular disease, he and his team developed the first β-adrenoceptor antagonist, pronethalol, to be approved for clinical use (1965). Pronethalol was quickly withdrawn owing to adverse effects, to be followed by practolol (which had even more severe toxicity), and finally by propranolol (1973), which proved to be a valuable treatment for a range of cardiovascular and other disorders, and is still widely used. Black's team went on following this approach with another major success, the first H 2 -histamine receptor antagonist, cimetidine (1975) used to treat gastric and duodenal ulcers. For this work he won the Nobel Prize in 1988. The example set by these early target-led drug discovery projects was quickly followed by pharmaceutical companies worldwide, and became the main source of new therapeutic drugs up to the late 1990s – a particularly fruitful period for drug discovery. Target-led drug discovery began with no knowledge of the molecular nature of the targets in question, the chemistry being led mainly by knowledge of the chemical nature of the relevant physiological mediators. From the 1980s, when receptors and other drug targets began to be isolated as proteins, sequenced and cloned, these new molecular approaches gave a big boost to drug discovery, both by identifying and characterising the many subtypes of receptors, transporters, enzymes and other targets, and also by providing a range of much faster and more powerful methods by which chemical leads could be screened and tested. The subdiscipline of molecular pharmacology, which emerged at this time, grew rapidly in importance, and gained a powerful boost when the human genome sequence was published in 2003. The use of genomic techniques to identify and characterise human drug targets is now a necessary part of most drug discovery projects (covered in later chapters).
As we have seen, pharmacology arose through the convergence of medicine, chemistry, pathology and physiology, its purpose being to throw light on how medicines and poisons produce their effects. Biochemistry and molecular and cell biology joined the party as these newer disciplines emerged in the 20th century. One important spin-off from molecular biology was the emergence of biopharmaceuticals in the form of protein-based therapeutic agents, such as insulin, growth hormone and a variety of monoclonal antibodies, produced by genetically engineered bacteria or eukaryotic cells as an alternative to drugs made by synthetic chemistry. Biopharmaceuticals now constitute about one-third of newly approved therapeutic agents. Since the sequencing on the human genome in 2003 genomics has had a major impact on pharmacology and drug discovery, mainly by providing abundant new information about potential new disease-relevant human drug targets, and also paving the way for “personalised” therapeutics that aims to take into account an individual's genetic make-up as a guide to maximising the efficacy and reducing drug side effects. The multidisciplinary nature of pharmacology, present throughout its history, remains its abiding characteristic, and has grown in complexity as biomedical science has progressed. Pharmacology, you could say, is sustained by hybrid vigour, rather than intellectual purity.
As well as being a key driver of drug discovery and development, pharmacology figures in many other aspects of modern life. A few examples follow:
Drugs prescribed by clinicians are often ineffective in a significant proportion of patients, and commonly cause adverse effects. 4,5 Selecting the right drug, the right dose, and where possible in the right patient, which can significantly diminish these problems, is the domain of clinical pharmacologists. The emergence of genomics-based personalised medicine is a likely to provide powerful new tools for clinical pharmacologists.
Pharmacological knowledge is essential in the design and conduct of clinical trials of new medicines, which have to be conducted according to strict protocols governing standards of ethics, experimental design and statistical analysis in order to pass scrutiny by regulatory authorities as a condition of approval of the new drug for clinical use.
Widely consumed “social” drugs, including alcohol, nicotine and caffeine have been subjected to extensive pharmacological research, the results of which provide the basis for official advice regarding their possible health risks.
Drug abuse and addiction are serious problems in many countries, and present difficult challenges for prevention, remediation, legislation and policing; understanding the pharmacological properties of abused substances, including the mechanisms by which they produce psychological reward, as well as dependence and harm, is essential in planning rational control measures. The continuing emergence of new synthetic “street drugs” is a particular problem for pharmacologists and legislators.
Drugs in sport present problems of a different kind, mainly concerned with detection, where understanding of the routes of metabolism and excretion of banned compounds, coupled with sensitive analytical methods, is the basis of most of the control measures that are used.
We do not expect to correct a fault in, say, the navigation system of an aircraft by spraying a chemical into the works, so it may seem remarkable that physiological malfunction can sometimes be put right by a circulating drug. What makes it possible is that living systems, unlike electronic ones, deploy chemical signalling to control their function, providing points of attack for chemical interventions.
- Campaigning and outreach
- News and events
- Awards and funding
- Privacy policy
- Journals and databases
- Locations and contacts
- Membership and professional community
- Teaching and learning
- Help and legal
- Cookie policy
- Terms and conditions
- Get Adobe Acrobat Reader
- Registered charity number: 207890
- © Royal Society of Chemistry 2023
This Feature Is Available To Subscribers Only
Sign In or Create an Account

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
1. Introduction to Pharmacology
Pharmacology: the study of interaction of drugs with living systems.
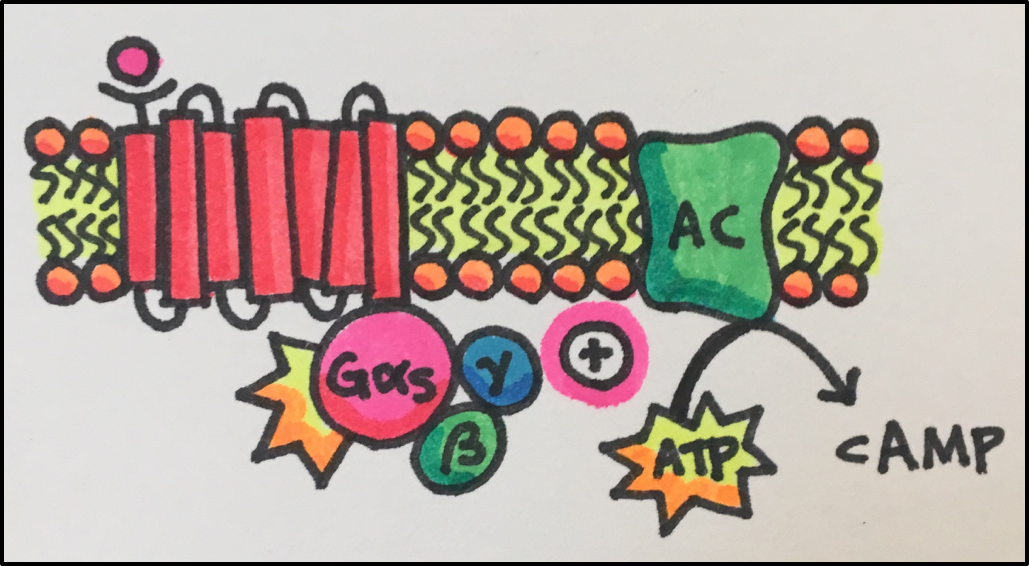
Sub-Disciplines of Pharmacology
- Drug-Receptor Interactions
- Dose-Response Relationships
- Signal Transduction
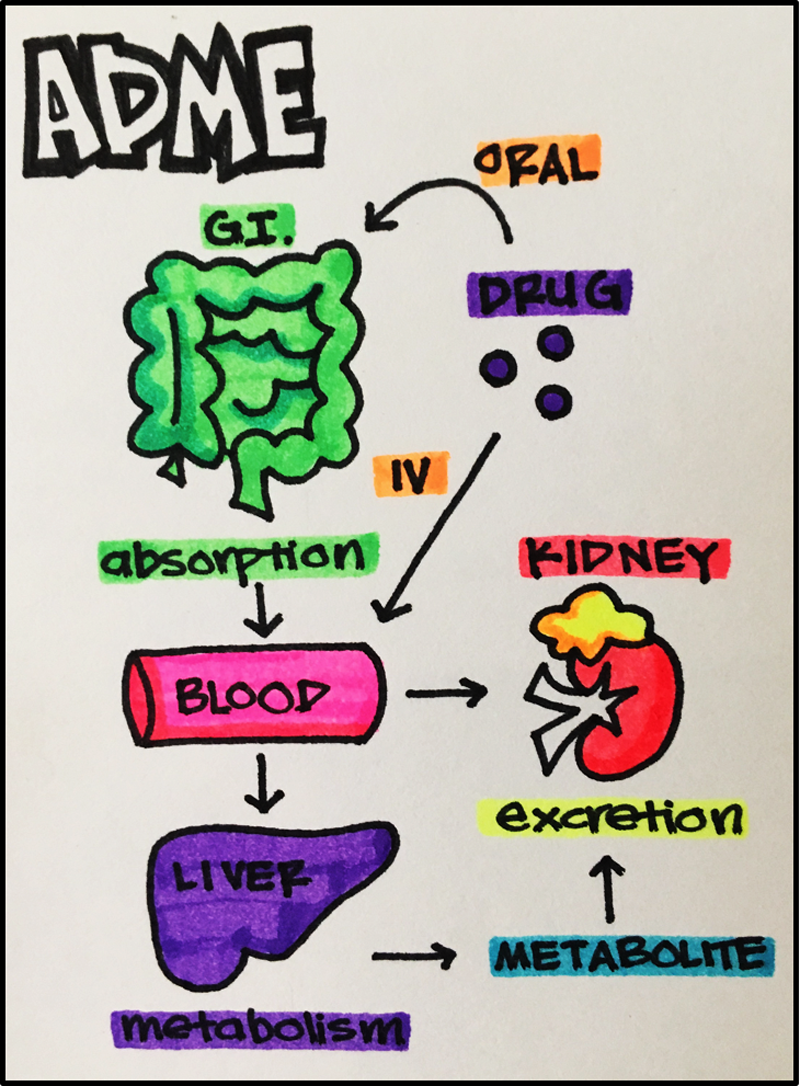
- A bsorption
- D istribution
- M etabolism
- Rate of Drug Metabolism
- Drug-Induced Toxicity
- Drug-Induced Allergies
Pharmacology and the Pharmacist
Key Questions you should be asking as a Pharmacist :
- Where is the molecular site of action ?
- What are the body function changes caused by a drug (pharmacodynamics)?
- What is the relationship between the Dose vs. Effect ?
- How does a drug produce its effect ?
- What is the fate of the drug once it enters the body (pharmacokinetics)?
- What is the interplay between genetic makeup and drug response ?
Example: Beta 1 Blocker: Metoprolol Succinate (oral)
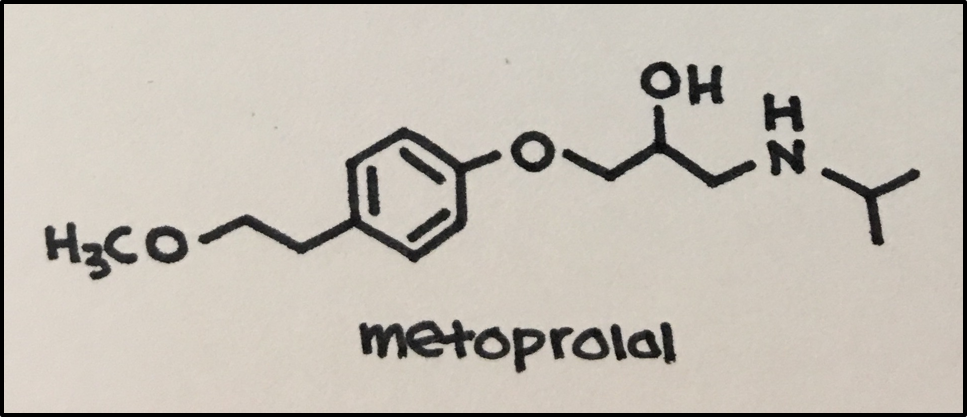
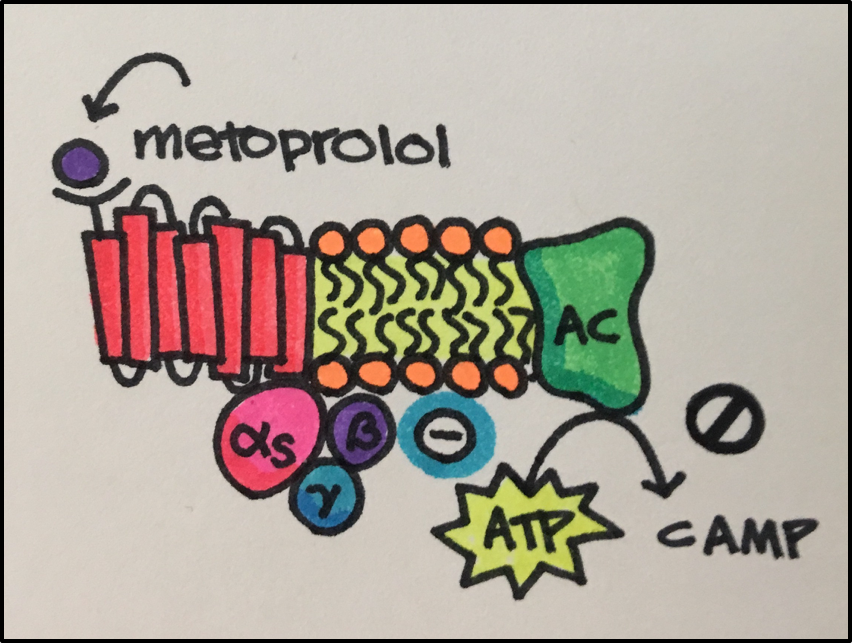
Drug Action: selective binding to cardiac muscle beta 1 adrenergic receptors that respond to norepinephrine (at higher doses, also inhibits bronchial and vascular smooth muscle by acting on beta 2 adrenergic receptors) to inhibit the binding of norepinephrine.
Drug Effect: reduced inotropic effect (contractility) and chronotropic effect (heart rate)
Fate of the Drug (pharmacokinetics): 12% protein binding and distribution 5.6 L/kg: hepatic metabolism (CYP2D6 mainly): <5% renal excretion: t 1/2 3-7 hours
Principles of Pharmacology - Study Guide Copyright © by Edited by Dr. Esam El-Fakahany and Becky Merkey, MEd is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
- Pharmacology 2024 Become a member today
- Honorary Fellows
- Committee Chairs - Vice Presidents
- Aims & objectives
- Strategic Plan 2022-2024
- Annual review & accounts
Policy positions and statements
- Consultation responses
- Open consultations
- Policy statements
- About pharmacology
- Explore pharmacology
- What is clinical pharmacology
- History of pharmacology
- Lives remembered
Our campaigns
- Personalised Prescribing
- Focus on Pharmacology
- Equity, diversity & inclusion (EDI) in pharmacology
- Clinical Pharmacology Skills Alliance (CPSA)
- Films on pharmacology
Our response and support
How you can help, resources and trusted information, society news and updates.
- COVID-19 safety at our events

Junior School (ages 7-11)
Senior school (ages 11-16), students (ages 16+), undergraduate, postgraduate and early career, clinical pharmacology: for medical students, beyond medical school, school teacher or careers advisor, pharmacology educator, where can pharmacology take me.
- Case Studies
Finding Funding
Jobs & opportunities.
- Latest jobs
- Internships & placements
- Advertise a job
- Work with us
- Volunteer with us
Latest News
- Events calendar
- Propose an event
Sponsor our meetings & events
Abstracts guidance & advice.
- Code of conduct for meetings and events
- List an event
About BPS education
Teaching pharmacology, society training opportunities, ambassadors scheme, educational resources, clinical pharmacology, prescribing and patient safety, animal research, why partner with the british pharmacological society, partnership case studies, our principles for working with commercial third-party partners, get in touch.
- Author Guidelines
Pharmacology Matters
British journal of pharmacology, british journal of clinical pharmacology, pharmacology research & perspectives, guide to pharmacology, become a peer reviewer, society membership, prizes, awards and grants.
- Awards Terms & Conditions
- Care Support Bursary
- Gary Price Early Career Industry Travel Bursary
- Society Meetings Bursary
Our Community
What is pharmacology, pharmacology is the study of how medicines work and how they affect our bodies..
/History-of-the-Society/11-16-Pharmacology-and-Pharmacy.jpg.aspx?width=800&height=565)
Interested in learning more?
Why not find out more what pharmacologists do, and where a career in pharmacology can take you ? Or explore the important impact pharmacology has around the world ?
Share this page
- Sponsor our meetings & events
- Abstracts guidance & advice
Pharmacology Hall of Fame
Individuals who have have been recognised by the membership for their key roles in the development of pharmacology.
Find out more

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
1.6 Excretion
Open Resources for Nursing (Open RN)
Excretion is the final stage of a medication interaction within the body. The body has absorbed, distributed, and metabolized the medication molecules – now what does it do with the leftovers? Remaining parent drugs and metabolites in the bloodstream are often filtered by the kidney, where a portion undergoes reabsorption back into the bloodstream, and the remainder is excreted in the urine. The liver also excretes byproducts and waste into the bile. Another potential route of excretion is the lungs. For example, drugs like alcohol and the anesthetic gases are often eliminated by the lungs. [1]
Critical Thinking Activity 1.6a
When providing care for a patient who has chronic kidney disease, how does this disease impact medication excretion?
Note: Answers to the Critical Thinking activities can be found in the “Answer Key” sections at the end of the book.
Routes of Excretion
Now let’s further discuss the various routes of excretion from the body.
The most common route of excretion is the kidney. As the kidneys filter blood, the majority of drug byproducts and waste are excreted in the urine. The rate of excretion can be estimated by taking into consideration several factors: age, weight, biological sex, and kidney function. Kidney function is measured by lab values such as serum creatinine, glomerular filtration rate (GFR), and creatinine clearance. If a patient’s kidney function is decreased, then their ability to excrete medication is affected and drug dosages must be altered for safe administration.
As the liver filters blood, some drugs and their metabolites are actively transported by the hepatocytes (liver cells) to bile. Bile moves through the bile ducts to the gallbladder and then on to the small intestine. During this process, some drugs may be partially absorbed by the intestine back into the bloodstream. Other drugs are biotransformed (metabolized) by intestinal bacteria and reabsorbed. Unabsorbed drugs and byproducts/metabolites are excreted via the feces. If a patient is experiencing decreased liver function, their ability to excrete medication is affected and drug dosages must be decreased. Lab studies used to estimate liver function are called liver function tests and include measurement of the ALT and AST enzymes that the body releases in response to damage or disease.
Other Routes to Consider
Sweat, tears, reproductive fluids (such as seminal fluid), and breast milk can also contain drugs and byproducts/metabolites of drugs. This can pose a toxic threat, such as the exposure of an infant to breast milk containing drugs or byproducts of drugs ingested by the mother. Therefore, it is vital to check all medications with a healthcare provider before administering them to a mother who is breastfeeding. [2]
Putting it all together…
Prescribing and administering medications in a safe manner to patients is challenging and requires a team effort by pharmacists, healthcare providers, and nurses. In addition to the factors described in this chapter, there are many other considerations for safe medication administration that are further explained in the “Legal/Ethical”chapter.
Lifespan Considerations
Neonate & Pediatrics: Young patients have immature kidneys with decreased glomerular filtration, resorption, and tubular secretion. As a result, they do not clear medications as efficiently from the body. Dosing for most medications used to treat infants and pediatric patients is commonly based on weight in kilograms, and a smaller dose is usually prescribed. In addition, pediatric patients may have higher levels of free circulating medication than anticipated and may become toxic quickly. Therefore, frequent assessment of infants and children is vital for early identification of drug toxicity. [3]
Older Adult: Kidney and liver function often decrease with age, which can lead to decreased excretion of medications. Subsequently, medication may have a prolonged half-life with a greater potential for toxicity due to elevated circulating drug levels. Smaller doses of medications are often recommended for older patients due to these factors, which is commonly referred to as “Start low and go slow.” [4]
Interactive Activity
- This work is a derivative of Principles of Pharmacology by LibreTexts licensed under CC BY-NC-SA 4.0 . ↵
- Fernandez, E., Perez, R., Hernandez, A., Tejada, P., Arteta, M., & Ramos, J. T. (2011). Factors and mechanisms for pharmacokinetic differences between pediatric population and adults. Pharmaceutics, 3 (1), 53–72. https://doi.org/10.3390/pharmaceutics3010053 ↵
The final stage of pharmacokinetics; process by which the body eliminates waste or excess.
Nursing Pharmacology Copyright © 2020 by Open Resources for Nursing (Open RN) is licensed under a Creative Commons Attribution 4.0 International License , except where otherwise noted.
Share This Book

Login into your account
Please enter username and password bellow!
Forgotten Password
Don't have an account? Register here
Our Pharmacy Blog
Common terms in pharmacology.

It’s easy to forget many of the common terms in pharmacology, not least because of the sheer variety of competing terms required to be committed to memory. This section combines the most common terms in pharmacology, serving as a convenient repository of pharmacology terms to aid future study.
- Anticancer Pharmacology
- Antimicrobial Drugs
- Cardiovascular Pharmacology
- Clinical Case Study
- Clinical Pharmacy
- General Pharmacology
- GI Pharmacology
- Immune System Pharmacology
- Nervous System Pharmacology
- Respiratory Pharmacology
- Study Tips and Tricks
Join Our Mailing List For Even More Facts!
Don't stop learning now, you may also like, tetracyclines pharmacology, dopamine agonists pharmacology, proton-pump inhibitors pharmacology.
- More from M-W
- To save this word, you'll need to log in. Log In
Definition of excursion
Did you know.
In Latin, the prefix ex- means "out of" and the verb currere means "to run." When the two are put together, they form the verb excurrere , literally "to run out" or "to extend." Excurrere gave rise not only to excursion but also to excurrent (an adjective for things having channels or currents that run outward) and excursus (meaning "an appendix or digression that contains further exposition of some point or topic"). Other words deriving from currere include corridor , curriculum , and among newer words, parkour .
Examples of excursion in a Sentence
These examples are programmatically compiled from various online sources to illustrate current usage of the word 'excursion.' Any opinions expressed in the examples do not represent those of Merriam-Webster or its editors. Send us feedback about these examples.
Word History
Latin excursion-, excursio , from excurrere
circa 1587, in the meaning defined at sense 1a
Theme music by Joshua Stamper ©2006 New Jerusalem Music/ASCAP
Get Word of the Day delivered to your inbox!
Dictionary Entries Near excursion
excursional
Cite this Entry
“Excursion.” Merriam-Webster.com Dictionary , Merriam-Webster, https://www.merriam-webster.com/dictionary/excursion. Accessed 30 Apr. 2024.
Kids Definition
Kids definition of excursion.
from Latin excursio, excursion- "a going out," from excurrere "to run out, make an excursion, extend," from ex- "out, forth" and currere "to run" — related to current
Medical Definition
Medical definition of excursion, more from merriam-webster on excursion.
Nglish: Translation of excursion for Spanish Speakers
Britannica English: Translation of excursion for Arabic Speakers
Subscribe to America's largest dictionary and get thousands more definitions and advanced search—ad free!

Can you solve 4 words at once?
Word of the day.
See Definitions and Examples »
Get Word of the Day daily email!
Popular in Grammar & Usage
More commonly misspelled words, commonly misspelled words, how to use em dashes (—), en dashes (–) , and hyphens (-), absent letters that are heard anyway, how to use accents and diacritical marks, popular in wordplay, the words of the week - apr. 26, 9 superb owl words, 'gaslighting,' 'woke,' 'democracy,' and other top lookups, 10 words for lesser-known games and sports, your favorite band is in the dictionary, games & quizzes.

- Cambridge Dictionary +Plus
Meaning of excursion in English
Your browser doesn't support HTML5 audio
- break-journey
- circumnavigation
excursion | Intermediate English
Examples of excursion, translations of excursion.
Get a quick, free translation!

Word of the Day
anonymously
without the name of someone who has done a particular thing being known or made public

Dead ringers and peas in pods (Talking about similarities, Part 2)

Learn more with +Plus
- Recent and Recommended {{#preferredDictionaries}} {{name}} {{/preferredDictionaries}}
- Definitions Clear explanations of natural written and spoken English English Learner’s Dictionary Essential British English Essential American English
- Grammar and thesaurus Usage explanations of natural written and spoken English Grammar Thesaurus
- Pronunciation British and American pronunciations with audio English Pronunciation
- English–Chinese (Simplified) Chinese (Simplified)–English
- English–Chinese (Traditional) Chinese (Traditional)–English
- English–Dutch Dutch–English
- English–French French–English
- English–German German–English
- English–Indonesian Indonesian–English
- English–Italian Italian–English
- English–Japanese Japanese–English
- English–Norwegian Norwegian–English
- English–Polish Polish–English
- English–Portuguese Portuguese–English
- English–Spanish Spanish–English
- English–Swedish Swedish–English
- Dictionary +Plus Word Lists
- excursion into something
- Intermediate Noun
- Translations
- All translations
Add excursion to one of your lists below, or create a new one.
{{message}}
Something went wrong.
There was a problem sending your report.

IMAGES
VIDEO
COMMENTS
Patients use mail delivery as a convenient alternative to acquiring medications in person. Federal laws require nonspecialty oral medications to be stored at controlled room temperature during distribution; however, no laws or regulations govern temperature requirements for medication transport among patients, which may expose medications to harmful temperature excursions.
When temperature excursion data was unavailable in published form, product manufacturers were surveyed via telephone and/or email. Acceptable storage information for all products for which storage is recommended at temperatures below room temperature (20-25 °C [68-77 °F]) was compiled and arranged in tabular format.
Pharmacodynamics refers to the effects of drugs in the body and the mechanism of their action. As a drug travels through the bloodstream, it exhibits a unique affinity for a drug-receptor site, meaning how strongly it binds to the site. Drugs and receptor sites create a lock and key system (see Figure 1.1 [ 1 ]) that affect how drugs work and ...
Step 3: Document the Event. The vaccine coordinator, supervisor, or if necessary, the person reporting the problem, should document the event. Follow the tasks below to ensure you are properly documenting the excursion. Name of the person completing the report. Date and time of the temperature excursion.
Temperature excursion queries that may arise during this stage due to improper handling (as communicated by the drug product user) may be handled by the manufacture's Medical Information department, which may have data on file to provide information in response to specific questions on temperature excursions (e.g., information based on in-use ...
There is evidence that PPG excursions are responsible for adverse outcomes, but it is predominantly short-term GV that has been strongly associated with the occurrence of hypoglycaemic episodes and in turn leads to adverse outcomes. Non-pharmacological interventions are the first-line measures for controlling excessively high PPG excursions and GV.
Pharmacology is the science of how drugs affect the body and how the body handles drugs. This chapter introduces the basic concepts and principles of pharmacology, such as drug classification, pharmacokinetics, and pharmacodynamics. Learn more about the fascinating field of pharmacology with Medicine LibreTexts, a free and open online resource for students and educators.
A comprehensive temperature excursion management program can be designed by integrating key elements (Fig. 2) into the overall management program. A comprehensive temperature excursion management program is expected to not only help minimize, assess, and justify temperature excursions, but also ensure regulatory compliance and
Pharmacology comprises two main components, namely pharmacodynamics, which is concerned with the effects that drugs produce on living systems (i.e. what the drug does to the body), and pharmacokinetics, which describes the mechanisms by which the drug is absorbed, distributed, metabolised and excreted (i.e. what the body does to the drug). To explain fully the effects of a drug in an intact ...
First pass effect. The first pass effect (also known as first-pass metabolism or presystemic metabolism) is a phenomenon of drug metabolism at a specific location in the body which leads to a reduction in the concentration of the active drug before it reaches the site of action or systemic circulation. [1] [2] The effect is most associated with ...
Drug Action: selective binding to cardiac muscle beta 1 adrenergic receptors that respond to norepinephrine (at higher doses, also inhibits bronchial and vascular smooth muscle by acting on beta 2 adrenergic receptors) to inhibit the binding of norepinephrine. Drug Effect: reduced inotropic effect (contractility) and chronotropic effect (heart rate) ...
Pharmacology is the study of how medicines work and how they affect our bodies. The word 'pharmacology' comes from the ancient Greek words pharmakon (meaning 'drug') and logia (meaning 'knowledge of'). Pharmacologists are scientists and medical doctors who research how medicines work, explore how different drugs and chemicals have ...
Pharmacology is the science of drugs and medications, including a substance's origin, composition, pharmacokinetics, pharmacodynamics, therapeutic use, and toxicology. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function. If substances have medicinal properties, they are considered ...
The definitions and unpacking of each of the 20 identified core concepts of pharmacology education are provided below. During the unpacking process, questions arose as to whether some of the concepts should be renamed. In some cases, the CC‐PEG agreed to make minor modifications following those discussions.
Remaining parent drugs and metabolites in the bloodstream are often filtered by the kidney, where a portion undergoes reabsorption back into the bloodstream, and the remainder is excreted in the urine. The liver also excretes byproducts and waste into the bile. Another potential route of excretion is the lungs.
A comprehensive temperature excursion management program is expected to not only help minimize, assess, and justify temperature excursions, but also ensure regulatory compliance and provide several benefits (e.g., avoid regulatory citations, improve efficiency, and minimize/avoid business impact caused by the loss of products or inadequate supply).
This article is the first in a series that aims to enhance the understanding of pharmacologic principles relevant to nuclear medicine. This article will deal with the introductory concepts, terminology, and principles that underpin the concepts to be discussed in the remainder of the series. The second article will build on the pharmacodynamic ...
analgesia. Decreased response to pain; condition in which painful stimuli are not consciously interpreted (perceived) as hurting; relief from pain; inhibition of the perception of pain. analgesic. Substance (synthetic or naturally occurring) that inhibits the body's reaction to painful stimuli or perception of pain.
pharmacology, branch of medicine that deals with the interaction of drugs with the systems and processes of living animals, in particular, the mechanisms of drug action as well as the therapeutic and other uses of the drug.. The first Western pharmacological treatise, a listing of herbal plants used in classical medicine, was made in the 1st century ad by the Greek physician Dioscorides.
Pharmacology Definition. Pharmacology is the study of drugs including their origins, history, uses, and properties. It mainly focuses on the actions of drugs on the body. A drug is defined a substance that is used to treat, cure, or prevent a disease or otherwise enhance physical or mental health. The word pharmacology comes from the Greek ...
excursion: [noun] a going out or forth : expedition. a usually brief pleasure trip. a trip at special reduced rates.
PHARMACOLOGY definition: 1. the study of medicines and drugs, including their action, their use, and their effects on the…. Learn more.
EXCURSION meaning: 1. a short journey usually made for pleasure, often by a group of people: 2. a short involvement…. Learn more.