
- Introduction
- Palp/Percus
- Auscultation

Palpation/Percussion
Thoracic expansion:.
- Is used to evaluate the symmetry and extent of thoracic movement during inspiration.
- Is usually symmetrical and is at least 2.5 centimeters between full expiration and full inspiration.
- Can be symmetrically diminished in ankylosing spondylitis .
- Can be unilaterally diminished in chronic fibrotic lung disease , extensive lobar pneumonia, large pleural effusions, bronchial obstruction and other disease states.
Percussion:
Percussion is the act of tapping on a surface, thereby setting the underlying structures in motion, creating a sound and palpable vibration. Percussion is used to determine whether underlying structures are fluid-filled, gas-filled, or solid. Percussion:
- Penetrates 5 - 6 centimeters into the chest cavity.
- May be impeded by a very thick chest wall.
- Produces a low-pitched, resonant note of high amplitude over normal gas-filled lungs.
- Produces a dull, short note whenever fluid or solid tissue replaces air filled lung (for example lobar pneumonia or mass) or when there is fluid in the pleural space (for example serous fluid, blood or pus).
- Produces a hyperresonant note over hyperinflated lungs (e.g. COPD ).
- Produces a tympanitic note over no lung tissue (e.g. pneumothorax ).
Diaphragmatic excursion:
- Can be evaluated via percussion.
- Is 4-6 centimeters between full inspiration and full expiration.
- May be abnormal with hyperinflation , atelectasis , the presence of a pleural effusion , diaphragmatic paralysis, or at times with intra-abdominal pathology.

- Report problem with article
- View revision history
Citation, DOI, disclosures and article data
At the time the article was created Craig Hacking had no recorded disclosures.
At the time the article was last revised Craig Hacking had the following disclosures:
- Philips Australia, Paid speaker at Philips Spectral CT events (ongoing)
These were assessed during peer review and were determined to not be relevant to the changes that were made.
- Diaphragm fluoroscopy
The fluoroscopic sniff test , also known as diaphragm fluoroscopy , is a quick and easy real time fluoroscopic assessment of diaphragmatic motor function (excursion). It is used most often to confirm absence of muscular contraction of the diaphragm during inspiration in patients with phrenic nerve palsy or breathing difficulties following stroke . Chest radiograph demonstrating a newly elevated hemidiaphragm often precedes a sniff test.
In critically unwell patients who can not attend the fluoroscopy unit in the radiology department, bedside US assessment can be used to demonstrate appropriate diaphragmatic movement with normal respiration and when asked to sniff (see case 5).
The following technique is suggested:
ask the patient to practice sniffing before the study
with the patient either standing (preferred) or supine, perform frontal fluoroscopy of the diaphragm at rest, breathing quietly through an open mouth
ask the patient to take a few quick short breaths in with a closed mouth ('sniffs') causing rapid inspiration
occasionally, repeating (3) in the lateral projection is required to evaluate the posterior hemidiaphragms
In normal diaphragmatic motion:
the diaphragm contracts during inspiration: moves downwards
the diaphragm relaxes during expiration: moves upwards
both hemidiaphragms move together
in healthy patients 1-2.5 cm of excursion is normal in quiet breathing 2
3.6-9.2 cm of excursion is normal in deep breathing 2
up to 9 cm can be seen in young or athletic individuals in deep inspiration 2
excursion in women is slightly less than men 2
In abnormal diaphragmatic motion:
the affected hemidiaphragm does not move downwards during inspiration
paradoxical motion can occur
Interpretation
Absence of diaphragmatic movement confirms phrenic nerve palsy in the appropriate clinical setting. A mass anywhere along the course of the phrenic nerve requires further workup, usually with neck and chest CT. A hilar mass due to lung cancer is the most common finding on CT and a classic exam case.
Normal diaphragmatic excursion can also be impaired in patients with:
previous diaphragmatic trauma or surgery
neuromuscular disorders
previous stroke
- 1. Nason LK, Walker CM, McNeeley MF et-al. Imaging of the diaphragm: anatomy and function. Radiographics. 2012;32 (2): E51-70. doi:10.1148/rg.322115127 - Pubmed citation
- 2. Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest. 2009;135 (2): 391-400. doi:10.1378/chest.08-1541 - Pubmed citation
- Nason L, Walker C, McNeeley M, Burivong W, Fligner C, Godwin J. Imaging of the Diaphragm: Anatomy and Function. RadioGraphics. 2012;32(2):E51-70. doi:10.1148/rg.322115127 - Pubmed
Incoming Links
- Diaphragmatic paralysis
- Phrenic nerve palsy
- Ultrasound diaphragmatic sniff test
- Left hilar mass causing phrenic nerve palsy
- Large right diaphragmatic hernia
- Hemidiaphragmatic paralysis
- Abnormal sniff test
- Normal sniff test
- Phrenic nerve palsy with positive sniff test
Promoted articles (advertising)
ADVERTISEMENT: Supporters see fewer/no ads
By Section:
- Artificial Intelligence
- Classifications
- Imaging Technology
- Interventional Radiology
- Radiography
- Central Nervous System
- Gastrointestinal
- Gynaecology
- Haematology
- Head & Neck
- Hepatobiliary
- Interventional
- Musculoskeletal
- Paediatrics
- Not Applicable
Radiopaedia.org
- Feature Sponsor
- Expert advisers

- Advanced search

Advanced Search
Assessing Diaphragmatic Function
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- For correspondence: [email protected]
- Figures & Data
- Info & Metrics
The diaphragm is vulnerable to injury during mechanical ventilation, and diaphragm dysfunction is both a marker of severity of illness and a predictor of poor patient outcome in the ICU. A combination of factors can result in diaphragm weakness. Both insufficient and excessive diaphragmatic contractile effort can cause atrophy or injury, and recent evidence suggests that targeting an appropriate amount of diaphragm activity during mechanical ventilation has the potential to mitigate diaphragm dysfunction. Several monitoring tools can be used to assess diaphragm activity and function during mechanical ventilation, including pressure-derived parameters, electromyography, and ultrasound. This review details these techniques and presents the rationale for a diaphragm-protective ventilation strategy.
- respiratory muscles
- muscle weakness
- intensive care
- diagnostic techniques
- respiratory system
- diaphragm dysfunction
- effort-induced lung injury
- Introduction
Patients who are admitted to an ICU frequently exhibit muscle weakness, and the respiratory muscles are often affected. 1 The diaphragm is the primary inspiratory muscle, and diaphragm dysfunction is both a marker of severity of illness and a predictor of poor patient outcome in the ICU. There is a clear association between diaphragm dysfunction and an increased risk of mortality or prolonged mechanical ventilation. 1 - 6 Factors related to both critical illness and ICU interventions are at the root of this problem. 7 Mechanical ventilation is associated with diaphragm injury through a variety of mechanisms referred to as myotrauma. 8 The presence of either insufficient or excessive diaphragmatic contractile effort plays a central role in this process. In addition, vigorous diaphragmatic contractions also can result in lung injury. 9 - 11 Recent evidence suggests that maintaining appropriate diaphragm activity during mechanical ventilation has the potential to prevent injury to the diaphragm. 6 These observations have drawn greater attention to the importance of diaphragm monitoring in the ICU.
Several clinical monitoring tools are available to assess diaphragm activity and function, including various respiratory pressure measurements, electromyography (EMG), and ultrasound. This paper briefly discusses the impact of critical illness on the diaphragm, with an emphasis on the effects of mechanical ventilation and diaphragm activity, and details the effect of diaphragm dysfunction on outcome. It will then discuss the relevant techniques for monitoring diaphragm function, with special reference to their application in mechanically ventilated patients.
- Respiratory Muscle Physiology
The diaphragm is a thin, dome-shaped muscle that inserts into the lower ribs, the xiphoid process, and the lumbar vertebrae, separating the thoracic and abdominal cavities. During inspiration, shortening of diaphragm muscle fibers results in a piston-like action, decreasing intrapleural pressure, drawing the lungs downwards, and increasing intra-abdominal pressure. The force generated by the diaphragm is quantified by the transdiaphragmatic pressure (P di ), which is the pressure gradient generated between the thoracic and abdominal cavities during diaphragm contraction. It is calculated from the difference between the pressure in the stomach (gastric pressure, P ga ) and the esophageal pressure (P es , as a substitute for intrapleural pressure): P di = P ga – P es . 12 - 14 Decreasing pleural pressure generates a pressure gradient that drives flow and volume into the lungs, known as the transpulmonary pressure. The transpulmonary pressure is computed as the difference between airway pressure (P aw ) and P es (ie, P aw – P es ). Of note, even though the P es is closely related to the pleural pressure, the pleural pressure varies over the lung surface due to the effects of gravity and regional mechanics. 15 This transpulmonary pressure drives alveolar ventilation and reflects the stress and strain applied to the lung by the respiratory muscles (and the ventilator).
Accessory respiratory muscles include the external intercostal, scalene, and sternocleidomastoid muscles. The external intercostal muscles pull the ribs upward and forward, increasing the lateral and anteroposterior diameters of the thorax. The scalene muscles elevate the first 2 ribs, and the sternocleidomastoids raise the sternum.
Exhalation is largely a passive process, except under conditions of increased respiratory load. 16 When the workload increases, the abdominal muscles contract during expiration, with an initial recruitment of transversus abdominis muscle and subsequent recruitment of the other abdominal muscles. 17 Expiratory abdominal muscle contraction enhances inspiratory diaphragm performance (through its length–tension relationship) and spring loads the thoracic cage to expand when the abdominal muscles relax, assisting with the inspiratory work of breathing. 18 The work of breathing during heavy loads is thus redistributed to accessory inspiratory muscles, abdominal muscles, and the diaphragm. Figure 1 summarizes the action of respiratory muscles and pressure relationships.
- Download figure
- Open in new tab
- Download powerpoint
Pressure model of the respiratory system. The locations of relevant pressures are depicted on the left. Typical tracings of respiratory pressures under assisted mechanical ventilation are shown on the right. P pl is estimated with esophageal manometry. The respiratory P mus is computed as the difference between observed P cw and ΔP es . P cw is estimated as the product of tidal volume and chest wall elastance measured during passive ventilation. P alv = alveolar pressure; P aw = airway pressure; P cw = chest wall elastic recoil pressure; P es = esophageal pressure; P ga = gastric pressure; P L = transpulmonary pressure (P aw – P es ); P mus = respiratory muscle pressure; P pl = pleural pressure. Adapted from Reference 19 .
- Causes of Diaphragm Weakness in the ICU
The causes and mechanisms leading to the observed weakness of the diaphragm in ventilated patients in the ICU have been extensively studied. We now know that there are multiple intertwined factors related to critical illness, ICU stay and therapies, and mechanical ventilation itself that are causing this weakness. The combination of these mechanisms causing diaphragm injury and weakness in the ICU is now called critical illness-associated diaphragm weakness. The precise mechanisms are thoroughly detailed in a recent review by Dres et al 7 and summarized in Figure 2 .
Schematic illustration of the mechanisms involved in the occurrence of critical illness-associated diaphragm weakness. Dashed lines represent uncertain causation; solid lines represent established causation. Adapted from Reference 7 .
Of specific interest for this article are the mechanisms of ventilator myotrauma, which are the deleterious effects of mechanical ventilation on diaphragm structure and function. Up to 4 distinct forms of myotrauma might occur during ventilation: ventilator overassistance, ventilator underassistance, eccentric (pliometric) diaphragm contractions, and excessive end-expiratory shortening. Interestingly, these mechanisms have the potential to be targeted by specific ventilation strategies, potentially mitigating the occurrence or severity of diaphragm myotrauma.
Overassistance myotrauma refers to the diaphragm atrophy resulting from excessive unloading of the respiratory muscles. 20 - 24 This form of injury is well documented in the clinical setting. It affects approximately 50% of ventilated patients and can be mitigated by preserving some degree of muscle activity during mechanical ventilation. 20 , 25 - 27
Underassistance myotrauma develops when respiratory effort is excessive because of insufficient unloading. 28 Experimental and clinical studies have demonstrated sarcomere disruption, tissue inflammation, and muscle fatigue. 29 - 31 Sepsis renders the muscle tissue particularly susceptible to this form of injury. 32 The observation that diaphragm thickness increases over time in some ventilated patients (in association with elevated respiratory effort) may reflect this edema and injury. 6
Eccentric diaphragm contractions developing during muscle fiber lengthening, that is, during the ventilator’s expiratory phase, can also cause injury (ie, eccentric myotrauma). Eccentric loading is considerably more injurious than concentric loading. This type of myotrauma can be the result of increased postinspiratory diaphragm activity in the expiratory phase (ie, expiratory braking), patient–ventilator asynchrony (particularly reverse-triggering), or even excessive accessory respiratory muscle activity moving the diaphragm cranially during inspiration. 33 , 34
Preliminary evidence also suggests the possibility that prolonged shortening of the diaphragm from elevated end-expiratory pressure may cause muscle fiber dropout and may allow longitudinal atrophy. 35 Abruptly decreasing PEEP may then put the diaphragm in a disadvantageous length–tension relationship at the beginning of inspiration. 36 The clinical relevance of this phenomenon is uncertain.
This brief summary of the mechanisms of diaphragm myotrauma suggests that routine monitoring of diaphragm activity and function might help clinicians prevent or mitigate myotrauma, potentially improving clinical outcomes.
- Monitoring Diaphragm Function and Activity
A range of techniques are available to monitor the diaphragm. Depending on the conditions under which they are measured, these techniques can be used to quantify either function (ie, force-generating capacity) or muscular contractile activity. Most tests of muscle function require a maximum volitional contractile effort from the patient. Some parameters actually reflect the performance of the respiratory system as a whole, not just the diaphragm. We will discuss the relevant techniques with special reference to their application in mechanically ventilated patients. These are summarized in Table 1 .
- View inline
Pressure-Based Monitoring Tools, EMG, and Ultrasound Parameters to Evaluate Diaphragm and Respiratory System Activity and Function
Respiratory System Pressures
Several techniques measure the pressures generated by the respiratory system as a whole or by the diaphragm alone ( Table 1 ). The maximum inspiratory pressure (P Imax ) can be measured at the airway while the patient makes a maximum inspiratory effort against a closed airway; this is frequently used as a test of respiratory muscle function. 37 A 1-way valve should be applied so that the patient can exhale but not inhale, thus minimizing lung volume to optimize the length–tension relationship of the diaphragm and maximize force generation. When both P ga and P es are recorded during this effort, maximum P di can be calculated to specifically evaluate the strength of the diaphragm. A related parameter is the pressure generated by all respiratory muscles (P mus ). By definition, P mus = (V T × E cw ) − ΔP PL , where V T is the tidal volume, Δ-P PL is the pleural pressure swing represented by Δ-P es , and E cw is the chest wall elastance. When the airway is occluded, P mus is equal to ΔP es and hence to ΔP aw .
With these techniques, it is critical to make sure that the patient is exerting maximum effort. This dependence on effort is the primary drawback of all volitional function tests. To circumvent this shortcoming, different strategies have been implemented. By stimulating the phrenic nerves with a magnetic or electric pulse (or twitch) while the patient is relaxed at end-expiration, a brief diaphragm contraction of standard magnitude is induced, independent of the patient’s effort. 37 Despite its technical challenges, this technique is the accepted standard for measuring diaphragm function in ventilated patients. 42 To obtain accurate values, a supramaximal stimulation of the phrenic nerves is needed, and positioning of the magnetic coils must be very precise. Similarly, twitch P aw can be recorded to provide a close estimate of twitch P di in ventilated patients. 42 , 43 Reference cutoff values defining diaphragm weakness are available and are summarized in Table 1 . Some have proposed lower cutoff values for twitch P aw for defining dysfunction in an ICU setting, based on the possibility of these values to better predict weaning outcome. 44
An alternative strategy to obtain maximum volitional effort for a functional measurement is to apply a 20-s airway occlusion with a 1-way valve (Marini maneuver), allowing for expiration but not inspiration. 45 The pressure obtained with this maneuver corresponds closely to the pressure obtained when patients are coached to breathe at maximum effort, provided that respiratory drive is adequate at rest (ie, P 0.1 >2 cm H 2 O).
Another technique to measure respiratory muscle strength in nonintubated patients is the sniff nasal inspiratory pressure (SNIP). Sniffing is an intuitive subconscious maneuver that elicits maximal diaphragmatic and respiratory muscle activation. Like the previously mentioned parameters, P es and transdiaphragmatic pressures can also be recorded during sniffing.
If the pressure is obtained during a tidal breath, the recorded pressure quantifies the effort exerted by the respiratory muscles or diaphragm. During inspiration, a negative deflection in esophageal pressures signifies respiratory muscle contraction. Figure 3 shows a sample of high and low inspiratory effort, documented with P es and P aw tracings. Small amounts of diaphragm activity may go unnoticed by only looking at the P es curve (ie, the amount of effort counterbalanced by chest wall recoil pressure, see Fig. 1 ), which may be detected with EMG monitoring. 46
Airway pressure (P aw ), esophageal pressure (P es ), and transpulmonary pressure (P L ) tracings of a patient with high (left) and low (right) inspiratory effort. High effort is demonstrated by a large drop in P es during inspiration.
The airway occlusion pressure (P 0.1 ), which is the pressure developed in the occluded airway 100 ms after the onset of inspiration, is an old parameter that may have a value in the assessment of a patient’s respiratory drive. 47 It can be obtained easily on most ventilators, and it is reliable in the setting of respiratory muscle weakness. 48 It correlates well with work of breathing (WOB) and the pressure–time product (PTP), 2 parameters that assess respiratory activity, so P 0.1 can reliably demonstrate excessive effort during various modes of ventilation and during extracorporeal membrane oxygenation. 49 - 52 Cutoff values indicating underassist have been proposed. Rittayamai and coworkers 51 defined the optimal threshold of P 0.1 at 3.5 cm H 2 O with a sensitivity of 92% and a specificity of 89% to detect underassist; others have set the optimal threshold for overassistance at ≤ 1.6 cm H 2 O.
The amplitude of swings in P di and P es do not fully reflect the amount of breathing effort a patient performs because the inspiratory time, frequency, and expiratory muscle activity are not taken into account. 53 The PTP is the integral of the pressure developed by the respiratory muscles during contraction (ie, P mus ) over time (specified as either per breath or per minute). When P di is measured, the specific PTP of the diaphragm can be quantified. Oxygen consumption by the respiratory muscles correlates well with the PTP, whereas it only weakly correlates with the mechanical WOB index mentioned above. 54 This could be due to the fact that PTP takes the isometric phase of muscle contraction into account.
Diaphragm EMG
Diaphragm EMG can be used to assess diaphragm activity. Either surface EMG for the costal diaphragm or esophageal recordings of the crural diaphragm can be used. Needle EMG studies are rarely used to monitor diaphragm activity for clinical monitoring, but they can be useful in the assessment of neuropathy and myopathy. The EMG-derived parameters are summarized in Table 1 .
EMG provides the best clinically available representation of the integrated neural output of the brain’s respiratory center; changes in EMG values are linearly correlated with changes in CO 2 levels. 52 , 57 A specialized nasogastric tube with electrodes positioned at the diaphragm can be used to measure the crural diaphragm electric activity (EA di ). A specific mode of ventilation called neutrally adjusted ventilatory assist uses this measurement to synchronize diaphragm EMG with the ventilator. The peak of the EA di signals per breath and EA di values during maximum inspiratory effort can be recorded. Some elements need to be taken into account to understand the relationship between diaphragm EMG, respiratory drive, and diaphragm force. One of the elements is the neuromuscular efficiency, which is the relationship or coupling between EA di and P di (ie, the pressure generated by the diaphragm). By definition, the neuromuscular efficiency index is P di /EA di (cm H 2 O/mcV). This index is patient-specific and can change over time. 58 , 59 As a result, neuromuscular efficiency can only be used to estimate the breathing effort and diaphragm function on an individual basis because reference values are nonexistent. 58 In a study by Liu et al, 60 subjects who passed a spontaneous breathing trial exhibited higher neuromuscular efficiency values than those who failed the spontaneous breathing trial.
Diaphragm ultrasound has gained popularity in the last decade because it enables clinicians to directly and noninvasively assess diaphragm activity and function. The diaphragm can be visualized in 2 ways, either in the zone of apposition or via a subcostal anterior approach. There are excellent reviews on the technical details and validity of these techniques, summarized in Table 1 . 61 , 62 When the diaphragm contracts and shortens, the muscle thickens, and this thickening can be visualized on ultrasound ( Fig. 4 ). The increase in thickness during contraction (quantified as the thickening fraction) reflects diaphragm contractile activity and correlates with other parameters of diaphragm activity, like EA di and PTP. 62 , 64 The maximum thickening fraction correlates with twitch P aw and provides an estimate of diaphragm function. 44 , 65 , 66 The technique can also be used to detect structural changes in the diaphragm during mechanical ventilation, such as diaphragm atrophy, load-induced injury, or recovery of muscle mass. 6 , 20 , 23 , 67
M-mode ultrasound images of the diaphragm measured at the zone of apposition, and measurements of the thickness (blue vertical lines) during expiration (distance 1) and inspiration (distance 2). (A) Undersupport with a thickening fraction of 150%: ([0.55 − 0.22 cm] × 100)/0.22 cm. (B) Oversupport with a thickening fraction of 4%: ([0.25 − 0.24 cm] × 100)/0.24 cm. (C) Adequate support with a thickening fraction of 38%: ([0.36 − 0.26 cm] × 100)/0.26 cm. Reprinted from Reference 63 , with permission.
As discussed earlier, a maximum inspiratory effort is required to assess diaphragm function (ie, maximum thickening fraction). A maximum inspiratory effort can be elicited by coaching or by the Marini maneuver (described above). However, because thickening results from muscular shortening, occluding the airway during the inspiratory effort can artefactually reduce thickening. Consequently, if a prolonged 20-s occlusion is applied to maximize inspiratory effort, maximal thickening should be measured only once the occlusion is released (but shortly after so that respiratory effort is still elevated).
Diaphragm excursion (motion) can be quantified when looking at the diaphragm subcostally ( Fig. 5 ). These measurements provide a well-validated method of assessing diaphragm function. Importantly, interpretation of the result is only possible during unassisted breaths because downward displacement during assisted breaths could be a result of passive lung inflation by the ventilator. Therefore, the excursion cannot be used to monitor effort during mechanical ventilation. Diaphragm weakness will result in reduced caudal excursion, and paresis will often result in cranial (paradoxical) excursion during inspiration. 67 Vigorous accessory respiratory muscle activity moving the diaphragm cranially during inspiration could theoretically give falsely low values for this parameter. 34 Cutoff values for the diagnosis of diaphragm dysfunction using these techniques are summarized in Table 1 .
B-mode (right) and M-mode (left) ultrasound images of the diaphragm with a probe positioned subcostally. A downward diaphragm excursion during inspiration (ie, towards the ultrasound probe) is visible. Reprinted from Reference 68 , with permission.
- Balancing Over- and Underassistance
There is uncertainty about the optimum range of diaphragm activity during mechanical ventilation, but the avoidance of excessive activity when possible appears to be supported by recent evidence. Several parameter values have been proposed to demonstrate underassistance, including PTP and WOB. Table 2 summarizes the possible monitoring techniques to assess patient and ventilator breath contribution and to balance over- and underassistance.
Available Parameters to Evaluate Ventilator Over- and Underassist
The diaphragm is vulnerable to injury during mechanical ventilation, and a range of factors can impact its function. Among these factors, the effects of mechanical ventilation require close attention as they are potentially avoidable. Several mechanisms link mechanical ventilation with diaphragm injury, including excessive and insufficient respiratory support, which can lead to very high or low respiratory effort. In addition to the effects on the diaphragm itself, inappropriate respiratory muscle effort is also associated with lung injury, patient–ventilator asynchrony, and poor sleep quality. Furthermore, recent evidence has indicated that diaphragm dysfunction by itself has a strong impact on patient outcome. As a consequence, the assessment of diaphragm function and activity during mechanical ventilation has gained importance in the ICU setting. Several methods are available, including pressure-based parameters, EMG, and ultrasound. Depending on their specific use, these methods can evaluate strength (ie, function) or measure activity. Using these tools, the potential to balance diaphragm activity and to combine diaphragm- and lung-protective elements in a novel ventilation strategy has emerged. Future research will need to further detail these elements and define safe margins for diaphragm activity. For those reasons, a good understanding of monitoring tools is needed, and building expertise into at least one of them is useful for the bedside clinician.
It’s amazing work you’ve performed, and we use it a lot in our daily practice. I always struggle with the precision of the measurements because we’re talking about something that’s 2 mm and so whether you have a 20% variation, let’s say it goes from 2 to 2.4 mm and sometimes the precision of your caliper is not that good. If you go just one pixel above or below you will have very different impressions. How do you take that into account when you measure, and how do you make sure that what you measure is what you see on the screen?
An excellent point, Tài. In a lot of the early work we did looked at measuring ability and reproducibility with the technique, we found that you could get very good reproducibility of end-expiratory thickness measurements, the thickness of the muscle with reproducibility of ± 0.2 mm when the technique was optimized. However, the reproducibility of the thickening fraction, because it’s really the combination and ratio of 2 different thickness measurements (and therefore combines the error of both measurements) is suboptimal; ±16–20% is what we showed. There’s no question that it’s an imperfect technique for monitoring inspiratory effort from that standpoint, you’re going to have noise. You’ll be able to distinguish between a patient who’s not making any effort, a patient making low effort like a healthy subject, a patient making elevated effort, and a patient making very high effort. But in terms of a change from 10% vs 20% I wouldn’t call that physiologically significant just given the measurement noise. Nevertheless, this does a couple of things. First of all, it means the technique really starts to shine whenever you’re able to make measurements in large groups of patients where the signal/noise ratio can become clearer. Really the exciting advance of the technique is that it allows you to assess the diaphragm in large numbers of patients because it’s so feasible. Secondly, I think it’s questionable whether this is the answer in terms of driving our monitoring of respiratory effort at the bedside. Should we be using thickening fraction to decide how much pressure support is best so that the patient is in the optimum window? Personally, I think it’s not quite feasible enough, it takes 5–10 minutes to set up, you have to find the diaphragm, and until if and when somebody develops a probe that sits there continuously and can make measurements, I’m not sure it’s sufficiently easy to implement, never mind the reproducibility. In my view, things like airway occlusion pressure or the occlusion pressure technique described here or EAdi or other pressure-based techniques for monitoring have a lot more potential to guide diaphragm-protective ventilation, but I’m still amazed by how much we’ve been able to learn from a technique that’s not perfect.
How important is the diaphragm condition at the beginning of mechanical ventilation in terms of injury. Should we use different protective mechanical ventilation strategies? Should we use more spontaneous breathing or less sedation?
This is my personal bias, but I really think this should be considered in every patient who’s on a ventilator, because in our cohort study subjects were at similar risk of diaphragm atrophy across the range of diagnoses whether it was acute hypoxemic respiratory failure or post-transplantation or all different admission diagnoses. I think it’s something we need to consider with every ventilated patient, it’s a little different than even lung protection, which primarily we think about in ARDS. Granted, we do think about lung protection in other patients. First, a diaphragm-protective approach should be considered in most patients. Second, in order to implement a diaphragm-protective approach, it’s not going to just change how we set the ventilator, it’s going to change how we apply sedation, it will change which sedatives we use, and so on. We actually just organized a consensus meeting in Milan with the Pleural Pressure Working Group to get together and think about all these kinds of issues because it’s a completely different paradigm for how to manage respiratory failure. In terms of concerns about load-induced injury, the concerns about this apply even before the patient is intubated. How much load-induced injury are patients developing while we’re sitting there trying to decide whether to intubate them and put them on a ventilator or not?
This links with breathing frequency and effort. Before mechanical ventilation initiation, usually frequency changes little while effort increases.
Exactly. That’s a very important point. Frequency really doesn’t reflect effort levels very well at all.
I think the future is interesting, so I’ll be very attentive to where this work goes in topics connected to this conference. What Lluís [Blanch] was asking about, you have this information and you showed this great figure of 3 groups, those who had a thickening at the onset of mechanical ventilation, those that maintained that thickening, and those who lost thickness. I understand it’s going to be a little bit speculative, but I’d be interested to hear your vision for what that’s going to be at the bedside. One such case may be, ‘hey this person has been dwindling on the floor because the clinician didn’t count the frequency properly, missed signs of respiratory distress and this poor soul has been working like a dog… let’s rest them for a period of time and allow their diaphragm to recover.’ Or perhaps there’s another group who will be susceptible to rapid onset diaphragmatic atrophy in the ICU. I’m interested to hear your thoughts as to where this may go.
I think we need to do a huge amount of work to understand which patients we need to be most attentive to these issues in, and what the relative balance of protecting the lung versus protecting the diaphragm is because the two issues sometimes compete. Sometimes you have a completely suppressed respiratory effort in order to minimize tidal volume, and at that point you’re sacrificing the diaphragm in order to protect the lungs. The timing of which organ you prioritize and when is an important issue that needs to get sorted out. I would say in general we should be targeting a relatively low-normal level of effort in all patients from the moment of intubation essentially. Whether or not that’s feasible is a different question because there are sometimes reasons why respiratory effort needs to be suppressed. We’re now running a pilot feasibility physiological trial where we’re trying to take subjects at a very early stage and see if we can get their respiratory effort into a protective range. The idea would be really that everyone should be breathing at a low-normal level of effort as seen in healthy subjects unless there’s some good reason why the case should be otherwise.
What is the timeframe and magnitude of recovery?
That’s an interesting question that actually needs to be described. I’ve seen data from a group in Italy as well as some of our data, where some patients will recover diaphragm thickness almost as rapidly as they lost it and in others it seems to persist. There are so much data that we haven’t had time to analyze and write it all up, but that’s an important project waiting to happen. In animal models, the muscle can recover very quickly after the reinstitution of respiratory effort. It’s probably not that big of a deal to have a patient apneic for a couple of days, but as soon as they’re allowed to breathe we should try to make sure that the muscle is active at the protective level to try and restore function. But it’s a great question that needs more study.
If the recovery potential is so high, would that argue more for protecting the lungs over the diaphragm?
I think so, for sure. There’s no question that lung injury drives mortality, we know that from ARDSnet and other trials – I’m not claiming that diaphragm injury necessarily drives mortality. We’ve found a very weak association with mortality. However, patients may survive but then will be stuck on the ventilator for a prolonged period of time because of myotrauma and then develop other nosocomial badness from the prolonged mechanical ventilation and then experience the devastating functional sequelae of critical illness – in my view, intervening on myotrauma has the potential to change long-term functional outcomes. It may not change survival per se.
The question is which is the worst asynchrony for diaphragm injury?
Ask Tài [Pham].
I have no answer, ask Laurent Brochard.
It’s hard to answer confidently, but there are several ways in which asynchrony may be injurious to the diaphragm. And probably the most important way is by inducing eccentric contractions of the muscle, these are contractions that occur while the muscle is lengthening rather than shortening. Usually when you’re using your diaphragm, the inspiring muscle is shortening and lung volume is increasing. But, for example, in reverse triggering the muscle often reaches peak contractile activity after the ventilator has already cycled into expiration, so lung volume is actually decreasing. The dome of the diaphragm is rising and then the diaphragm is forced to contract while it’s rising and that induces eccentric contractile conditions. It’s a well-established principle of exercise physiology that if you want to train a muscle you do so by injuring it and the best way to injure it is with an eccentric contraction, that’s why when you lift weights to strengthen your biceps, you really want to contract that bicep as you’re lowering the weight because that’s where the injury stimulus for hypertrophy occurs. It’s the same with the diaphragm. Of course because you’re repeating it over for hours and hours it becomes very injurious rather than having a training effect. A similar circumstance we might be familiar with eccentric contraction is after a day of skiing and the next day your quads are screaming in pain, it’s because you were having eccentric contractions in your quadriceps all day the day before. There are very limited experimental data showing that eccentric contractions are fairly injurious to the diaphragm. I’m aware of some data from a group in Toronto, who found it may be profoundly injurious to the diaphragm, but this needs to be studied more. The principle is that anything that causes an eccentric contraction, even short cycling where a patient is in the middle of an inspiratory effort and all of a sudden the ventilator stops delivering inspiratory support and the patient is stuck, that could be profoundly injurious as well.
On the subject of eccentric contraction, it is certainly injurious but beyond the type of asynchrony it’s also the magnitude of the efforts that may be important. This reverse triggering, after passive insufflation depending on the timing might have a different impact. And if the peak is before inflation, maybe it will maintain your diaphragm function by having some contractions rather than remaining totally passive. But it’s a hypothesis only; we have no proof.
I’m sure you’ve seen the idea of pacing the diaphragm. A lung pacer company has proposed a clinical trial (Percutaneous Temporary Placement of a Phrenic Nerve Stimulator for Diaphragm Pacing (RESCUE1) ClinicalTrials.gov Identifier: NCT03107949 ). What do you think about doing pacing of the diaphragm, regardless of the method by which you do it? To me it seems clear that pacing a diaphragm that has disuse atrophy is going to be completely different than pacing a diaphragm in a septic patient or a patient who has respiratory muscle weakness.
Great question. There are certainly data that show that phrenic nerve stimulation in human subjects during cardiac surgery can protect mitochondrial function in the muscle, in the diaphragm for example. So there is a lot of biological plausibility for the technique. I think the question is in whom should we do this? It needs to be in a patient in whom you otherwise cannot optimize mechanical ventilation to achieve a reasonable respiratory effort level. It’s somebody who will have to be heavily sedated for at least 24 h in order to be a patient who could benefit from the intervention. But also they can’t be pharmacologically paralyzed because this kind of phrenic nerve pacing really doesn’t achieve any effect. It’s an ongoing question of exactly who is the most likely to benefit. In the group of patients with sepsis in whom the muscle becomes very vulnerable and fragile to mechanical stresses, you might well be doing more harm than good. You’d probably have to generate a high-normal level effort levels in order to achieve much injury, pacing at a low effort level might still be safe in these patients but that’s an important subgroup that needs to be studied.
My question is regarding ineffective efforts. In pressure support, excessive assistance is accompanied with ineffective inspiratory efforts but not dyspnea. The contrary happens in conditions of insufficient assistance where there are not ineffective efforts because respiratory drive is increased but at considerable dyspnea.
I’m not sure how injurious ineffective efforts are because most of the time the effort levels during ineffective efforts are really quite small. I assume you agree with me on that?
I think like Tài pointed out it’s a dose-dependent phenomenon and if the effort levels are very small even if the contractile conditions are potentially injurious it’s probably not that big of a deal.
Maybe the point is comfort. Vitacca et al 1 tested different levels of pressure support and PEEP showed a U shape relationship between excessive dyspnea without ineffective efforts and no dyspnea with increased hyperinflation and ineffective efforts. Perhaps a balance between the two is clinically acceptable.
- Acknowledgment
We thank Jose Dianti MD, for his help in designing the figures.
- Correspondence: Ewan C Goligher MD PhD, Toronto General Hospital, 585 University Ave, Peter Munk Building, 11th Floor, Room 192, Toronto, Ontario, Canada M5G 2N2. E-mail: ewan.goligher{at}utoronto.ca
Dr Schepens is supported in part by the European Respiratory Society, Fellowship STRF October 2018. Dr Goligher is supported by an Early Career Investigator Award from the Canadian Institutes of Health Research, and he has disclosed a relationship with Getinge. Ms Fard has disclosed no conflicts of interest.
Dr Goligher presented a version of this paper at the 58th R espiratory C are Journal Conference, held June 10–11, 2019, in St Petersburg, Florida.
- Copyright © 2020 by Daedalus Enterprises
- Delemazure J ,
- Brochard L ,
- Hermans G ,
- Testelmans D ,
- Decramer M ,
- Gayan-Ramirez G
- Supinski GS ,
- Callahan LA
- Medrinal C ,
- Quesada AR ,
- Bonnevie T ,
- Goligher EC ,
- Rubenfeld GD ,
- Scales DC ,
- Herridge MS ,
- Heunks LMA ,
- Brochard LJ
- Brochard LJ ,
- Saarela O ,
- Slutsky AS ,
- Yoshida T ,
- Nakahashi S ,
- Nakamura MAM ,
- Torsani V ,
- Agostoni E ,
- Laporta D ,
- Amato MBP ,
- Grieco DL ,
- Aliverti A ,
- Duranti R ,
- Ferrigno G ,
- Kenyon CM ,
- Pedotti A ,
- De Troyer A ,
- Estenne M ,
- Van Gansbeke D ,
- Finucane KE ,
- Panizza JA ,
- Schepens T ,
- Goligher EC
- Friscia ME ,
- Rothenberg P ,
- Petrof BJ ,
- Chanques G ,
- Berthet J-P ,
- Verbrugghe W ,
- Corthouts B ,
- Parizel PM ,
- Eikermann M ,
- Gayan-Ramirez G ,
- Brumback BA ,
- Martin AD ,
- Joseph A-M ,
- Beaver TM ,
- Martin TD ,
- Jiang T-X ,
- Redenbach DM ,
- Vassilakopoulos T ,
- Walker DC ,
- Belcastro AN
- Ebihara S ,
- El Dwairi Q ,
- Hussain SN ,
- Gottfried SB ,
- Pellegrini M ,
- Hedenstierna G ,
- Segelsjö M ,
- Larsson A ,
- Perchiazzi G
- Guenther SM ,
- Lodato RF ,
- Dantzker DR
- Lindqvist J ,
- van den Berg M ,
- van der Pijl R ,
- Hooijman PE ,
- Beishuizen A ,
- Laveneziana P ,
- Albuquerque A ,
- Barreiro E ,
- Seymour J ,
- Rafferty G ,
- Demoule A ,
- Prodanovic H ,
- Molinari N ,
- Coirault C ,
- Gottesman E ,
- Boussuges A ,
- Cattapan SE ,
- Watson AC ,
- Hughes PD ,
- Louise Harris M ,
- Morawiec E ,
- Dangers L ,
- Truwit JD ,
- Piquilloud L ,
- Beloncle F ,
- Richard J-C ,
- Mancebo J ,
- Damiani F ,
- Schoene RB ,
- Alberti A ,
- Fongaro A ,
- Valenti S ,
- Albaladejo P ,
- Touchard D ,
- Subirana M ,
- Lemaire F ,
- Rittayamai N ,
- Grasselli G ,
- Suriano G ,
- Spadaro S ,
- Turrini C ,
- Pletsch-Assuncao R ,
- Caleffi Pereira M ,
- Ferreira JG ,
- Cardenas LZ ,
- de Albuquerque ALP ,
- de Carvalho CRR ,
- Bellemare F ,
- Zakynthinos S ,
- Polkey MI ,
- Bellani G ,
- Coppadoro A ,
- Patroniti N ,
- Albiero D ,
- Matamis D ,
- Soilemezi E ,
- Tsagourias M ,
- Akoumianaki E ,
- Dimassi S ,
- Detsky ME ,
- Mekontso Dessap A ,
- Lyazidi A ,
- Thille AW ,
- Similowski T ,
- Bocchino S ,
- Cabrini L ,
- Beccaria PF ,
- Zangrillo A
- Cabello B ,
- Vitacca M ,
- Bianchi L ,
- Zanotti E ,
- Vianello A ,
- Barbano L ,
In this issue

- Table of Contents
- Table of Contents (PDF)
- Cover (PDF)
- Index by author
Thank you for your interest in spreading the word on American Association for Respiratory Care.
NOTE: We only request your email address so that the person you are recommending the page to knows that you wanted them to see it, and that it is not junk mail. We do not capture any email address.
Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager

- Tweet Widget
- Facebook Like
- Google Plus One
Jump to section
Related articles, cited by....
- Open access
- Published: 14 January 2023
Using automatic speckle tracking imaging to measure diaphragm excursion and predict the outcome of mechanical ventilation weaning
- Daozheng Huang 1 , 2 na1 ,
- Feier Song 3 na1 ,
- Bangjun Luo 4 ,
- Shouhong Wang 1 ,
- Tiehe Qin 1 ,
- Zhuandi Lin 4 ,
- Tieying Hou 5 , 6 &
- Huan Ma 7
Critical Care volume 27 , Article number: 18 ( 2023 ) Cite this article
4600 Accesses
8 Citations
18 Altmetric
Metrics details
A Correspondence to this article was published on 07 August 2023
A Correspondence to this article was published on 15 June 2023
The speckle tracking ultrasound is an innovative technology enabling distinct assessment of diaphragmatic movement, yet the relative data are scarce. In this pilot study, we sought to evaluate the predictive value of the weaning outcome of automatic speckle tracking in assessing diaphragm excursion.
This is a prospective, multicenter, observational study. A total of 160 critically ill subjects underwent speckle-tracking ultrasonography of the right/left hemidiaphragm before the spontaneous breathing trial. Meanwhile, the diaphragm excursion and velocity values were measured manually by M-mode ultrasound. Patients were divided into weaning-failure and weaning-success groups. The correlation was assessed between automatic and manual measurement, and the diagnostic efficacy of automatic measured excursion and velocity for predicting weaning outcome was analyzed.
A total of 88 patients completed the follow-up of the weaning outcome. The overall incidence of weaning failure was 43.18%. There was a significant correlation between the automatic measurement of mean excursion and velocity assessed by speckle tracking imaging and manual measurement (R 0.69 and 0.65, respectively). Receiver operating characteristic (ROC) curve analysis showed that the mean excursion and diaphragmatic velocity exhibited high diagnostic values for prolonged weaning [area under the ROC curve (AUROC) 0.824 and 0.786, respectively]. The diaphragmatic excursion showed moderate diagnostic value for predicting both weaning failure and in-hospital death/withdrawal of treatment (AUROC 0.659 and 0.653, respectively).
Automatic speckle tracking analysis of the diaphragm showed high consistency with conventional manual ultrasound measures. Diaphragmatic excursion and its excursion velocity helped predict mechanical ventilation weaning failure, prolonged weaning, as well as in-hospital adverse outcomes, which served as a reliable tool in guiding clinical weaning strategy.
Automatic speckle tracking analysis of the diaphragm showed high consistency with conventional manual ultrasound measures.
Diaphragmatic excursion and its excursion velocity helped predict mechanical ventilation weaning failure, prolonged weaning, as well as in-hospital adverse outcomes.
Introduction
Ultrasound performs static structural analysis as well as dynamic motion to evaluate diaphragm functional changes, allowing direct visualization [ 1 ]. Studies have shown that the assessment of diaphragm movement, such as excursion, can help predict the success rate of weaning or prolonged weaning from mechanical ventilation (MV) [ 2 , 3 , 4 ]. At present, M-mode ultrasound is commonly used in clinical practice to assess the diaphragmatic movements and diaphragmatic velocity of contraction. It allows more accurate timing of the respiratory cycle, but is worse spatial orientation and is difficult to operate [ 1 ]. Also, since there is no real standard for M-line selection, repeatability is poor [ 5 , 6 , 7 , 8 ]. Speckle tracking ultrasound is an innovative ultrasound technique enabling distinct assessment of muscle function or organ motion, such as velocity, displacement, strain, and strain rate [ 9 , 10 ]. It tracks the movement of the tissue by tracking the speckle formed by the tissue echo signal on the B image. Through this, the physiological characteristics of the tissue can be quantitatively analyzed. Moreover, via big data training samples, the accurate diaphragm recognition model and speckle tracking algorithm are obtained.
Speckle-tracking echocardiography is a novel and mature technique for assessing myocardial function. Due to the contribution of speckle-tracking echocardiography, we intended to apply this method to diaphragm ultrasound. Despite the structural difference between the diaphragm and myocardium, there is still no readily available product for automatic speckle tracking imaging to measure diaphragmatic parameters. Furthermore, data regarding the relationship between diaphragmatic movement measured by automatic speckle tracking imaging and MV weaning failure in critically ill patients are scarce. In tQ3his pilot study, we sought to evaluate the predictive value for the weaning outcome of automatic speckle tracking in assessing diaphragm excursion and how this compares to manual methods of diaphragm excursion assessment by ultrasound.
This prospective, multicenter, observational study was conducted at the intensive care units of Guangdong Geriatric Institute and Guangzhou Panyu Central Hospital, China. All subjects’ families provided written informed consent. The study was performed following the approval of the ethics committee.
Patients were included when they met all of the following criteria: aged ≥ 18 years, received MV for > 48 h, suitable for a spontaneous breathing trial (SBT). The exclusion criteria were as follows: patients with a pre-existent neuromuscular disorder, diaphragmatic paralysis, cervical injury, pneumothorax, or mediastinal emphysema, and if the patient has a poor echogenicity or who were unable to tolerate ultrasound examination.
Ultrasound imaging and analysis
The measurements of left/right diaphragm excursion and velocity were taken on the M-mode frozen images using the ultrasound machine calibration and algorithm in a supine or semi-recumbent position before SBT by a well-trained expert. All of the patients had spontaneous breathing with the pressure support of 10–12 cm H 2 O. Diaphragm excursion was measured as previously described [ 11 ]. TE7 Diagnostic Ultrasound System (C5-2 array probe, Shenzhen Mindray Bio-medical, China) was used. Manual measurement was taken when the patient's breathing was relatively stable and the ultrasound image was steady. Under M-mode ultrasound, the exploration line was selected so that the ultrasound beam was perpendicular to the posterior diaphragm. Meanwhile, the liver, inferior vena cava, and hyperechoic diaphragm line were shown in the plane. The M-mode showed the diaphragm movement along the exploration line. Diaphragm excursion was the vertical distance from the baseline (during exhalation) to the highest point (during inhalation) of the curve. The diaphragmatic velocity of the excursion (cm/s) was calculated as diaphragmatic excursion (cm) divided by the duration of the corresponding excursion (s). An additional file showed this in more detail (Additional file 1 ).
Speckle tracking imaging
During the identification phase, the videos of the left/right diaphragm motions were saved and diaphragm excursion was detected with the cardiac transducer (SP5-1u probe, Resona7 ultrasound system, Shenzhen Mindray Bio-medical, China) (Additional file 2 : Video 1 and Additional file 3 : video 2). The automatic speckle tracking imaging was performed using independently developed patent software. During the validation phase, a 15–20-s (to cover 3–5 respiratory cycle) clip was recorded, using TE7 Diagnostic Ultrasound System (C5-2 array probe, Shenzhen Mindray Bio-medical, China). 3 regions of interest (ROI) were traced and the measurements were averaged in the offline analysis. The excursion of the ROI was calculated by the algorithm with the anatomical M-line (Fig. 1 ) (Additional file 4 ). Examples of tracking were displayed in the Additional file 8 videos 3 and Additional file 9 : video 4. The speckle tracking method for the diaphragm was as follows: (1) the recorded ultrasound data were identified through pattern recognition of deep learning; (2) The region of interest (ROI) was placed to represent each segment of the diaphragm; (3) several candidate anchor points around each ROI were selected to allow adequate tracking; (4) perform matching calculation on the front and back frames of the area where the ROI and the candidate anchor point are located; (5) the point with the highest comprehensive matching calculation coefficient is the movement direction of the ROI; (6) Obtain the motion trajectory of each ROI; 7) calculate the range of motion and thickness change.
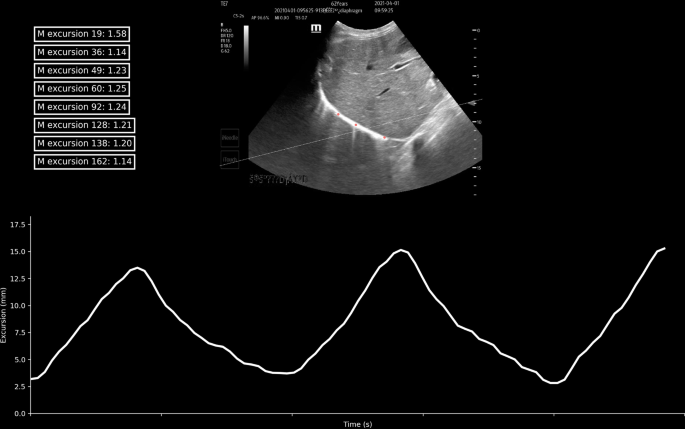
Three regions of interest (ROI) were selected to represent each segment of the diaphragm (color red). The line chart shows that the diaphragm excursion (cm) was measured by one ROI. The anatomic M-line was drawn via calculation of the first excursion (dashed line)
The criteria for weaning readiness included: (1) respiratory rate ≥ 10 and ≤ 35 breaths per min; (2) PaO 2 /FiO 2 ratio ≥ 150; (3) positive end-expiratory pressure ≤ 5–8 cm H 2 O; (4) FiO 2 < 50%; and (5) pH value > 7.25. For patients with chronic obstructive pulmonary disease, the criteria were: (1) pH > 7.30; (2) PaO 2 > 50 mmHg; and (3) hemodynamic stability with no dynamic changes of myocardial ischemia or hypotension (no vasopressors or little inotropes such as dopamine/dobutamine), in the absence of vasopressors. MV was disconnected from the patient, and an independent source of oxygen was provided through the T-piece. The attempt was targeted at least 30 min and be up to 120 min. SBT was terminated when one of the following signs occurred: (1) rapid shallow breathing index > 105; (2) respiratory rate < 10 and > 35 breaths per min; (3) heart rate > 140 beats per min or changed > 20% compared with the baseline or the new onset of arrhythmia; (4) tidal volume < 4 mL/kg; and (5) SaO 2 < 90%.
The weaning outcome was diagnosed successfully if the patient could maintain spontaneous breathing for ≥ 48 h with no need for any level of ventilator support after extubating. Otherwise, the outcome was defined as a weaning failure. Patients were divided into weaning-failure and weaning-success groups. Another classification of patients according to the weaning process includes weaning time. Prolonged weaning was defined as more than 3 times SBT failures or failure to wean within 7 days after the first SBT. Factors that are known to affect weaning outcomes were noted, such as the underlying diseases, ventilation time, weaning time, and relevant blood biochemistry findings.
Statistical analysis
Pearson’s correlation coefficient was used to assess the correlation between automatic and manual measurement of excursion and velocity. Averaged data were expressed as mean ± standard deviation for continuous variables and as absolute or relative frequencies for categorical variables. An independent sample t -test was used to compare continuous variables and the chi-square test or Fisher’s exact tests were used for categorical variables. A Bland–Altman plot was used to describe the agreement between manual and automatic measurement. Receiver operating characteristic (ROC) curve analysis was performed to determine the diagnostic efficacy of automatic measured excursion and velocity for predicting weaning outcomes. The sensitivity and specificity of automatic speckle tracking imaging in predicting the outcome of weaning were calculated and compared with that of manual measurement. All data were handled in R version 4.0.2 and P < 0.05 was considered significant and all probability values were 2-sided.
A total of 160 subjects underwent speckle-tracking ultrasonography between August 2020 and July 2021. Images were captured and visualized with all 160 subjects’ right/left hemidiaphragm before SBT. Meanwhile, the diaphragm excursion and velocity values were measured manually by M-mode ultrasound. Finally, 88 patients completed the follow-up of the weaning outcome and were enrolled in the diagnostic analysis of ultrasonic imaging assessment. Among these patients, the overall incidence of weaning failure was 43.18% (38/88). Of the 88 remaining patients, the overall mean age was 73 ± 14 years, and 59 (69.41%) were male. The mean body mass index was 22.8 kg/m 2 . Demographic factors, comorbidity, respiratory parameter, and laboratory findings did not differ significantly between the weaning-failure group and weaning-success group, except for body mass index, tidal volume, calcium concentration, albumin, hemoglobin, age, and Sequential Organ Failure Assessment score. Pre-SBT baseline characteristics of the subjects by weaning outcome are reported in Table 1 .
The mean, maximal, and minimal excursion of the diaphragm and velocity measured by speckle tracking imaging and manual assessment are listed in Table 2 . The mean automatic diaphragmatic excursion was significantly lower among patients of the weaning-failure group, as compared with the weaning-success group (1.1 vs. 1.5 cm, p = 0.0163). Diaphragmatic velocity of excursion (cm/s) were similar among patients of both group (1.0 vs. 0.9, p = 0.2437). Mean diaphragmatic excursion via manual imaging assessment was 0.9 ± 0.4 cm in the weaning-success group and 0.9 ± 0.7 cm in the weaning-failure group ( p = 0.6681). The mean diaphragmatic velocity of excursion via manual imaging assessment was 1.4 ± 0.7 cm/s in the weaning-success group compared with 1.4 ± 0.8 in the weaning-failure group, with no significant differences between groups. (Table 2 ).
Excursion and velocity were both significantly correlated with manual measurement. There was a significant correlation between the automatic measurement of mean excursion assessed by speckle tracking imaging and manual measurement (R 0.69, p < 0.0001, Fig. 2 A), while the correlation between automatic velocity and manual data was also observed (R 0.65, p < 0.0001, Fig. 2 B). Bland–Altman plot showed the representations of the agreement between manual and automatic measurements of diaphragmatic excursion (bias: − 0.5 cm; LOA: − 1.6–0.6 cm, Additional file 5 : Fig S1) and velocity (bias: 0.1 cm/s; LOA: − 1.0–1.2 cm/s, Additional file 6 : Fig. S2). Additional file 7 : Table S1 shows the ultrasonic variables between left and right diaphragm.
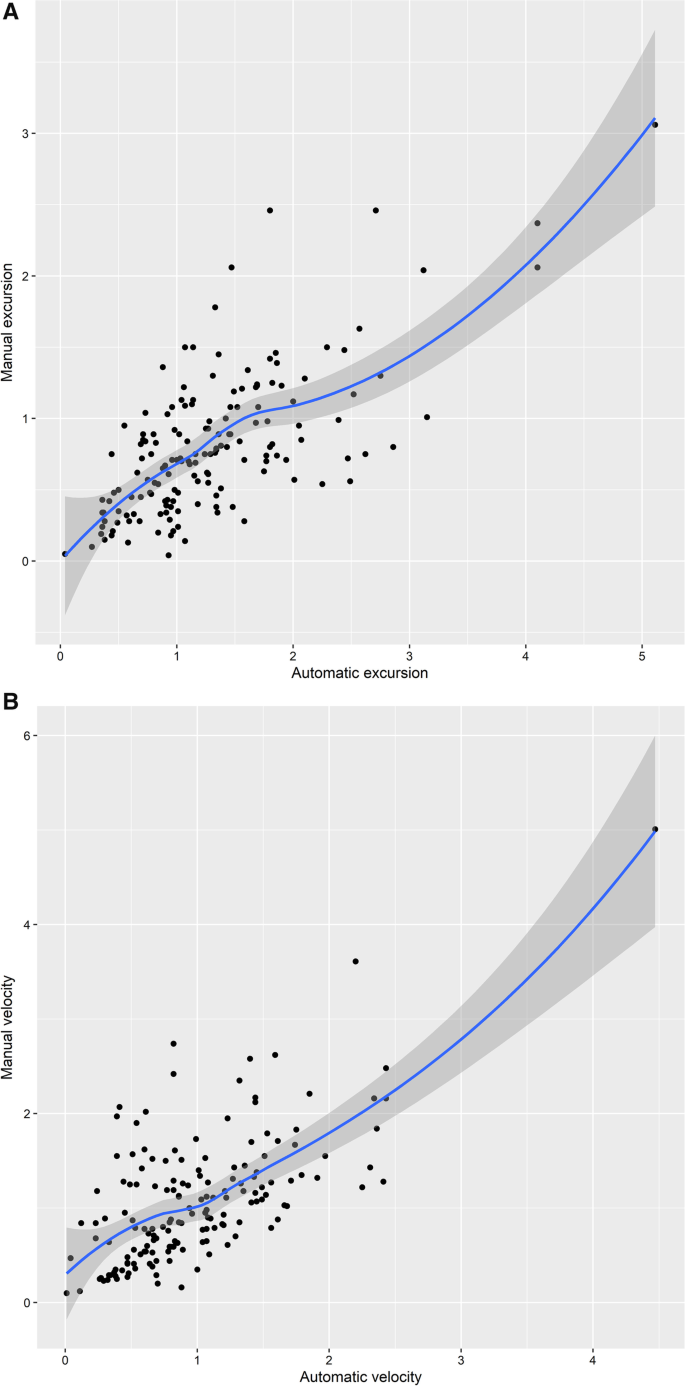
A Scatter Plot and Fitting Curve of Average Excursion of Automatic Measurement and Manual Measurement (R 0.69). B Scatter Plot and Fitting Curve of Average Velocity of Automatic Measurement and Manual Measurement (R 0.65)
The best cut-off values and area under the curve for weaning failure were calculated for automatic diaphragmatic excursion and diaphragmatic velocity. We performed a ROC curve analysis to assess the predictive value of these variables. The mean diaphragmatic excursion exhibited high diagnostic values for prolonged weaning (Fig. 3 A) with the area under the receiver operating characteristic curve (AUROC) of 0.782. The sensitivity and specificity for predicting prolonged weaning were 88.9% and 61.0%. The mean diaphragmatic velocity of excursion also exhibited moderate diagnostic values for prolonged weaning (Fig. 3 B) with an AUROC of 0.679. The sensitivity and specificity for predicting prolonged weaning were 33.3% and 100%. Besides, the diaphragmatic excursion showed moderate diagnostic value for predicting both weaning failure (Fig. 3 C) and in-hospital death/withdrawal of treatment (Fig. 3 D).
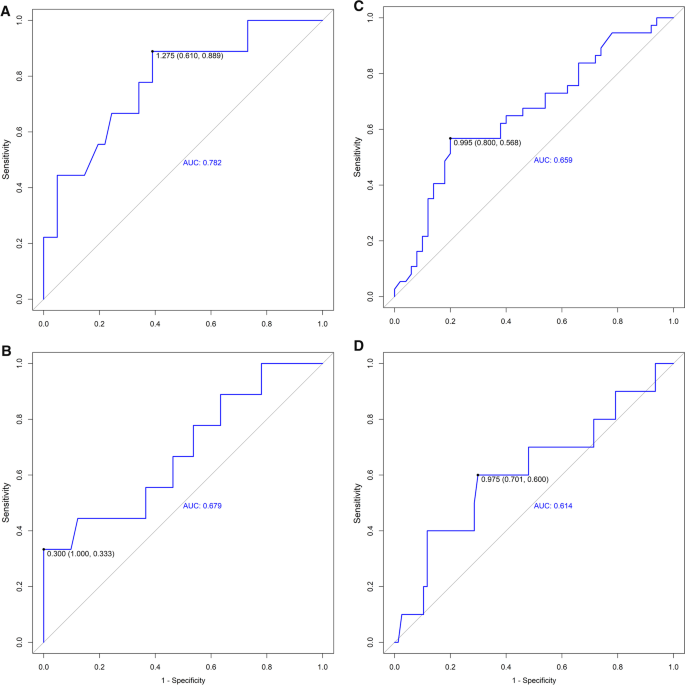
A ROC Curve Shows the Diagnostic Value for Prolonged Weaning of the Automatic Mean Excursion (Cut-Off 1.275, AUROC 0.782). B ROC Curve Shows the Diagnostic Value for Prolonged Weaning of the Automatic Mean Velocity (Cut-Off 0.300, AUROC 0.679). C ROC Curve Shows the Diagnostic Value for Weaning-Failure of the Automatic Mean Excursion (Cut-Off 0.995, AUROC 0.659). D Curve Shows the Diagnostic Value for In-hospital Death/Withdrawal of treatment of the Automatic Mean Excursion (Cut-Off 0.975, AUROC 0.614)
The diaphragm plays a significant role in ventilation, and its dysfunction can result in difficulty weaning from MV [ 4 ]. The diaphragm weakness developed rapidly in the first few days of MV [ 12 , 13 , 14 ]. Growing evidence showed that diaphragm dysfunction contributed to weaning failure and prolonged ventilation [ 11 , 13 , 15 , 16 ]. Diaphragmatic function assessment is vital in critically ill patients and this part of the population with invasive MV. It is also important to consider the factors that affect and predict the success of ventilator weaning.
Diaphragm ultrasound can currently be performed at the bedside to monitor the diaphragm movement, the diaphragm thickness, and the thickening rate [ 8 , 17 , 18 ]. However, conventional methods, such as thickness fraction, or caudal displacement assessed by M-mode have limitations, such as angle dependence and translational error. At present, there is no standard quantification for measuring diaphragmatic movement, because the moderate consistency of two-dimensional ultrasound was a bottleneck problem. Our study aimed to provide a novel quantifiable method of diaphragm function analysis.
In this study, the speckle tracking technology was used to measure the diaphragmatic excursion and its velocity. Compared to conventional ultrasound, it automatically chose three to six ROI on the diaphragm when measuring, calculated its speed or displacement in different parts of the diaphragm, and then form a general parameter, which was accurate and comprehensive to evaluate the excursion speed of the entire diaphragm. In addition, the original intention of developing the software was to standardize the measurement of the diaphragm ultrasound, including excursion and velocity.
Pesero et al. discovered the anatomical M-mode which allowed free placement of the cursor to measure diaphragmatic excursion and helped recognize diaphragmatic dysfunction since the conventional analysis line overestimated excursion in cardiac surgical patients[ 19 ]. Orde et al. proposed the use of angle-independent M-mode sonography for the assessment of diaphragm displacement, demonstrating that the cursor might not be orientated to the true direction of the diaphragm movement, leading to orientation and translation error[ 20 ]. Inspired by the previous studies, we calculated a calibration line during the automatic measurement (Additional file 8 videos 3 and Additional file 9 : video 4). Our results suggested that the automatic measurement of diaphragmatic excursion velocity was lower than that obtained by manual measurement, which might be due to the use of the anatomic M-line adjusted algorithm. The abovementioned study suggested that the diaphragmatic excursion measured by conventional M-mode was overestimated [ 19 ]. It might partially explain the result of the automatic measurement of velocity. However, it should be emphasized that this was a newly developed software, which still needed to be trained with large sample data to achieve continuous improvement. Overall, the present study exhibited a scenario where diaphragmatic kinetics assessment could be performed via automatic measurement.
In addition, the low excursion and velocity might be contributed to the timing of the ultrasound. Ultrasound was performed before SBT in the present study while the previous literature reported ultrasound data collected during the first 30 min of SBT [ 11 ]. Cammarota et al. investigated the diaphragmatic excursion velocity measured with tissue Doppler imaging at the end of the SBT [ 21 ]. The result suggested that subjects who developed both extubation failure and success experienced a greater diaphragmatic activation, compared with the result in the present study. Upon MV assistance, diaphragmatic movement and contraction might not require too much effort due to the positive pressure support. Another study indicated that the mode of ventilation affected the preservation of diaphragmatic contraction, as MV support, could partially reverse the muscle atrophy process [ 22 ]. It might be mutually verified that the diaphragmatic excursion and velocity were affected by MV. For acutely hospitalized patients ventilated more than 24 h, guideline suggested that the initial SBT be conducted with inspiratory pressure augmentation (5–8 cm H 2 O) rather than without [ 23 ]. Using low-level pressure support or continuous positive airway pressure counteracted the resistance of the breathing circuit. The initial purpose of the diaphragmatic assessment was to predict the extubation success so that to avoid the potentially hazardous effects, such as respiratory muscle fatigue or dyspnea, caused by SBT. Therefore, we chose to perform the diaphragmatic assessment before SBT. Moreover, all of the patients had spontaneous breathing with the pressure support of 10–12 cm H 2 O. We believed that it simulated SBT with inspiratory pressure augmentation, to a certain extent.
Expert consensus recommended diaphragmatic movement ultrasound measurement and emphasized the importance of context-specific or outcome-related cut-off values[ 1 ]. The results of the present study showed that the correlation between manual measurement and automatic speckle tracking measurement was high. Follow-up data on clinical adverse outcomes were collected to validate the prognostic value. In the present study, a 43.18% (38/88 patients) incidence of weaning failure was observed. ROC curve analysis showed that a mean excursion ≤ 1.3 cm, and a mean velocity ≤ 0.3 cm/s represent possible predictors for prolonged weaning. The AUROC curves for these variables were 0.782 and 0.679, respectively, our results also suggested that a mean excursion ≤ 1.0 cm was predictive of weaning failure (AUROC = 0.659), while a mean excursion ≤ 1.0 cm prognosticated in-hospital death/withdrawal of treatment (AUROC = 0.614). The cut-off value was consistent with the diagnostic criteria of diaphragmatic dysfunction[ 24 ].
The software calculations are based on an algorithm patented, which is not open to the public. The present study provided a pilot vision toward a novel measurement for diaphragm ultrasonography in research and daily practice, compared with the currently used techniques.
There are limitations to the present study. Primarily, given the small sample size in our pilot study, a larger, multicentered study could be useful to validate the role of the current software module. Second, speckle tracking could be applied to automatically measure the thickness and changing rate of the diaphragm. The rate of change in the thickness of the diaphragm, plus the excursion and velocity data that has been achieved so far, may be valuable in the evaluation of the diaphragm function. A combination of several parameters might provide multiple dimensions and enhance predictive power.
Automatic speckle tracking analysis of the diaphragm showed high consistency with conventional manual ultrasound measures. Diaphragmatic excursion and its velocity helped predict MV weaning failure, prolonged weaning, as well as in-hospital adverse outcomes. The automatic speckle tracking ultrasound imaging module served as a reliable tool to predict weaning outcomes at the bedside, holding a promising prospect in guiding clinical weaning strategy.
Availability of data and materials
The data sets generated and/or analyzed during the current study are not publicly available to protect the privacy of participants but are available from the corresponding author upon reasonable request.
The study protocol was approved by the ethics committee of Guangdong Provincial People’s Hospital/ Guangdong Academy of Medical Sciences (No. 2020-246H-1) and Guangzhou Panyu Central Hospital (No. PYRC-2021–115), and written informed consent was obtained from each patient’s family.
Haaksma ME, Smit JM, Boussuges A, Demoule A, Dres M, Ferrari G, Formenti P, Goligher EC, Heunks L, Lim EHT, et al. EXpert consensus on diaphragm ultrasonography in the critically ill (EXODUS): a Delphi consensus statement on the measurement of diaphragm ultrasound-derived parameters in a critical care setting. Crit Care. 2022;26(1):99.
Article PubMed PubMed Central Google Scholar
Zambon M, Greco M, Bocchino S, Cabrini L, Beccaria PF, Zangrillo A. Assessment of diaphragmatic dysfunction in the critically ill patient with ultrasound: a systematic review. Intensive Care Med. 2017;43(1):29–38.
Article PubMed Google Scholar
Vetrugno L, Guadagnin GM, Barbariol F, Langiano N, Zangrillo A, Bove T. Ultrasound imaging for diaphragm dysfunction: a narrative literature review. J Cardiothorac Vasc Anesth. 2019;33(9):2525–36.
Kim WY, Suh HJ, Hong SB, Koh Y, Lim CM. Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation. Crit Care Med. 2011;39(12):2627–30.
Sarwal A, Walker FO, Cartwright MS. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve. 2013;47(3):319–29.
McCool FD, Tzelepis GE. Dysfunction of the diaphragm. N Engl J Med. 2012;366(10):932–42.
Article CAS PubMed Google Scholar
Gerscovich EO, Cronan M, McGahan JP, Jain K, Jones CD, McDonald C. Ultrasonographic evaluation of diaphragmatic motion. J Ultrasound Med. 2001;20(6):597–604.
Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest. 2009;135(2):391–400.
Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, Stoylen A, Ihlen H, Lima JA, Smiseth OA, et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol. 2006;47(4):789–93.
Rubin JM, Feng M, Hadley SW, Fowlkes JB, Hamilton JD. Potential use of ultrasound speckle tracking for motion management during radiotherapy: preliminary report. J Ultrasound Med. 2012;31(3):469–81.
Huang D, Ma H, Zhong W, Wang X, Wu Y, Qin T, Wang S, Tan N. Using M-mode ultrasonography to assess diaphragm dysfunction and predict the success of mechanical ventilation weaning in elderly patients. J Thorac Dis. 2017;9(9):3177–86.
Hooijman PE, Beishuizen A, Witt CC, de Waard MC, Girbes AR, Spoelstra-de Man AM, Niessen HW, Manders E, van Hees HW, van den Brom CE, et al. Diaphragm muscle fiber weakness and ubiquitin-proteasome activation in critically ill patients. Am J Respir Crit Care Med. 2015;191(10):1126–38.
Article CAS PubMed PubMed Central Google Scholar
Jaber S, Petrof BJ, Jung B, Chanques G, Berthet JP, Rabuel C, Bouyabrine H, Courouble P, Koechlin-Ramonatxo C, Sebbane M, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011;183(3):364–71.
Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358(13):1327–35.
Hermans G, Agten A, Testelmans D, Decramer M, Gayan-Ramirez G. Increased duration of mechanical ventilation is associated with decreased diaphragmatic force: a prospective observational study. Crit Care. 2010;14(4):R127.
Laghi F, Cattapan SE, Jubran A, Parthasarathy S, Warshawsky P, Choi YS, Tobin MJ. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med. 2003;167(2):120–7.
Noh DK, Lee JJ, You JH. Diaphragm breathing movement measurement using ultrasound and radiographic imaging: a concurrent validity. Biomed Mater Eng. 2014;24(1):947–52.
PubMed Google Scholar
Rocco M, Carbone I, Morelli A, Bertoletti L, Rossi S, Vitale M, Montini L, Passariello R, Pietropaoli P. Diagnostic accuracy of bedside ultrasonography in the ICU: feasibility of detecting pulmonary effusion and lung contusion in patients on respiratory support after severe blunt thoracic trauma. Acta Anaesthesiol Scand. 2008;52(6):776–84.
Pasero D, Koeltz A, Placido R, Fontes Lima M, Haun O, Rienzo M, Marrache D, Pirracchio R, Safran D, Cholley B. Improving ultrasonic measurement of diaphragmatic excursion after cardiac surgery using the anatomical M-mode: a randomized crossover study. Intensive Care Med. 2015;41(4):650–6.
Orde SR, Boon AJ, Firth DG, Villarraga HR, Sekiguchi H. Use of angle-independent M-mode sonography for assessment of diaphragm displacement. J Ultrasound Med. 2016;35(12):2615–21.
Cammarota G, Boniolo E, Santangelo E, De Vita N, Verdina F, Crudo S, Sguazzotti I, Perucca R, Messina A, Zanoni M, et al. Diaphragmatic kinetics assessment by tissue doppler imaging and extubation outcome. Respir Care. 2021;66(6):983–93.
Grassi A, Ferlicca D, Lupieri E, Calcinati S, Francesconi S, Sala V, Ormas V, Chiodaroli E, Abbruzzese C, Curto F, et al. Assisted mechanical ventilation promotes recovery of diaphragmatic thickness in critically ill patients: a prospective observational study. Crit Care. 2020;24(1):85.
Schmidt GA, Girard TD, Kress JP, Morris PE, Ouellette DR, Alhazzani W, Burns SM, Epstein SK, Esteban A, Fan E, et al. Liberation from mechanical ventilation in critically ill adults: executive summary of an Official American College of Chest Physicians/American Thoracic Society clinical practice guideline. Chest. 2017;151(1):160–5.
Umbrello M, Formenti P, Longhi D, Galimberti A, Piva I, Pezzi A, Mistraletti G, Marini JJ, Iapichino G. Diaphragm ultrasound as indicator of respiratory effort in critically ill patients undergoing assisted mechanical ventilation: a pilot clinical study. Crit Care. 2015;19:161.
Download references
Acknowledgements
To reflect appropriate contributors to the study, we thank Mingliu Zhu, Jiaming Jiao, and Shuo Liu, who are the system research engineers from the ultrasound imaging system development department of Shenzhen Mindray Bio-Medical Electronics Co., Ltd., for technical support. We also thank Prof. Leo Heunks from the Department of Intensive Care Medicine, Erasmus University Medical Center, and Prof Lei Xu from the Department of Neurosurgery and Neurosurgical Intensive Care Unit, Chongqing University Central Hospital for their comment and advice on this article.
This research was supported by the grants from Natural Science Foundation of Guangdong Province (2021A1515011118), Start-up Funding of National Natural Science Foundation of China (No.8207120182), High-level Hospital Construction Project of Guangdong Provincial People’s Hospital (DFJH201922), and Guangzhou Health Science and Technology Project (No. 20231A010084).
Author information
Daozheng Huang and Feier Song contributed equally to this work
Authors and Affiliations
Department of Critical Care Medicine, Guangdong Provincial Geriatrics Institute, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, 510080, China
Daozheng Huang, Shouhong Wang & Tiehe Qin
Office of Organ Procurement Organizations, Medical Department, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, 510080, Guangdong, China
Daozheng Huang
Department of Emergency Medicine, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, 510080, China
Department of Critical Care Medicine, Guangzhou Panyu Central Hospital, Guangzhou, 510080, China
Bangjun Luo & Zhuandi Lin
Guangdong Clinical Laboratory Center, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, 510080, Guangdong, China
Tieying Hou
Medical Department, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, 510080, Guangdong, China
Department of Cardiology, Guangdong Provincial Cardiovascular Institute, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, 510080, China
You can also search for this author in PubMed Google Scholar
Contributions
DH was involved in the conceptualization and supervision. FS contributed to the formal analysis and writing—original draft. BL assisted in the investigation. SW contributed to the project administration. TQ contributed to the resources. ZL performed the data curation. TH was involved in the supervision. HM assisted in writing—review and editing. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Tieying Hou or Huan Ma .
Ethics declarations
Competing interests.
All authors report no conflicts of interest .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
. Process of ultrasound imaging.
Additional file 2 . Video 1 . During the identification phase, speckle-tracking technology was used to develop an automatic measurement module for diaphragmatic ultrasound, to identify the diaphragm region through pattern recognition, deep learning, and other methods. The software selected 3 regions of interest (ROI) to represent each segment of the diaphragm (color red, yellow, and blue), and automatically measure the motion trajectory, speed, excursion, and other parameters from each ROI through a certain algorithm.
Additional file 3 . Video 2 . The recording clip of ultrasound images in both Video 2 and Video 1 was identical. The software selected 6 regions of interest (ROI) to represent each segment of the diaphragm (color red, yellow, blue, pink, grey, and green).
Additional file 4
. Process of determining the anatomic M-line.
Additional file 5
. Figure S1 . Bland-Altman plot: representations of the agreement between manual and automatic measurement of diaphragmatic excursion
Additional file 6
. Figure S2 . Bland-Altman plot: representations of the agreement between manual and automatic measurement of diaphragmatic velocity.
Additional file 7
. Table S1 . Ultrasonic Variables of Automatic Speckle Tracking and Manual Measurement between left and right diaphragm.
Additional file 8 . Video 3 . During the validation phase. The software selected 3 regions of interest (ROI) to represent each segment of the diaphragm (color red). The line chart showed that the diaphragm excursion (cm), measured by one ROI, changed continuously along with the respiratory cycle, which was measured by ROI. The box in the upper left corner represented diaphragmatic excursion within every respiratory cycle (the former number was the frames in which the ultrasound image was acquired and the next number was excursion).
Additional file 9 . Video 4 . During the validation phase. The software selected 3 regions of interest (ROI) to represent each segment of the diaphragm (color red). The line chart showed that the diaphragm excursion (cm), measured by one ROI, changed continuously along with the respiratory cycle, which was measured by ROI. The box in the upper left corner represented diaphragmatic excursion within every respiratory cycle (the former number was the frames in which the ultrasound image was acquired and the next number was excursion).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Huang, D., Song, F., Luo, B. et al. Using automatic speckle tracking imaging to measure diaphragm excursion and predict the outcome of mechanical ventilation weaning. Crit Care 27 , 18 (2023). https://doi.org/10.1186/s13054-022-04288-3
Download citation
Received : 12 August 2022
Accepted : 17 December 2022
Published : 14 January 2023
DOI : https://doi.org/10.1186/s13054-022-04288-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Speckle tracking ultrasound
- Diaphragmatic excursion
Critical Care
ISSN: 1364-8535
- Submission enquiries: [email protected]
Diaphragmatic excursion: Quantitative measure to assess adequacy of expiratory phase CT chest images
Affiliations.
- 1 Department of Radiology, Icahn School of Medicine at Mount Sinai, United States; Department of Radiology, NYU Langone Health, United States. Electronic address: [email protected].
- 2 Department of Radiology, Icahn School of Medicine at Mount Sinai, United States.
- 3 Department of Medicine, Icahn School of Medicine at Mount Sinai, United States.
- 4 Department of Radiology, Icahn School of Medicine at Mount Sinai, United States; Department of Radiology, Columbia University Medical Center, United States.
- PMID: 33460955
- DOI: 10.1016/j.ejrad.2021.109527
Objective: To evaluate diaphragmatic excursion as a quantitative metric for change in lung volume between inspiratory and expiratory chest computed tomography (CT) images.
Methods: A 12-month retrospective review identified 226 chest CT exams with inspiratory and expiratory phase imaging, 63 in individuals referred with diagnosis of asthma by ICD9/10 code. Exams acquired in the supine position at 1.25 mm slice thickness in each phase were included (n = 30, mean age = 62, M = 15, F = 15). Diaphragmatic excursion was calculated as the difference between axial slices through the lungs on inspiration and expiration, using the lung apex as the cranial bound, and the hemidiaphragm caudally. Inspiratory and expiratory lung and tracheal volumes were calculated through volumetric segmentation. Tracheal morphology was assessed at 1 cm above the level of the aortic arch, and 1 cm above the carina.
Results: Inspiratory and expiratory lung volumes were higher in men (mean I = 5 + 1.6 L, E = 3.1 + 1.2 L) than women (mean I = 3.6 + 0.8 L, E = 2.4 + 0.7 L), p = .005 and p = .047, respectively. Average inspiratory and expiratory tracheal volumes were higher in men (I = 61 + 17 mL, E = 43 + 14) than women (I = 44 + 14, E = 30 + 8), p = .006 and p = .005. Average change in lung and tracheal volume between inspiratory and expiratory scans did not significantly differ between men and women. Average diaphragmatic excursion was 2.5 cm between inspiratory and expiratory scans (2.7 cm in men, 2.3 cm in women; p = .5). There was a strong positive correlation between diaphragmatic excursion and change in lung (r = .84) and tracheal volume (r = .79). A moderate correlation was also found between change in tracheal volume and change in lung volume (r = 0.67). Change in tracheal morphology between inspiratory and expiratory imaging was associated with change in tracheal volume at both 1 cm above the aortic arch (p = .04) and 1 cm above the carina (p = .008); there was no association with diaphragmatic excursion or lung volume.
Conclusions: Diaphragmatic excursion is a quantitative measure of expiratory effort as validated by both lung and tracheal volumes in asthma patients, and may be more accurate than qualitative assessment based on tracheal morphology.
Keywords: Air trapping; Asthma; Computed tomography; Diaphragmatic excursion; Expiratory; Lung.
Copyright © 2021 Elsevier B.V. All rights reserved.
- Exhalation*
- Lung / diagnostic imaging
- Lung Volume Measurements
- Middle Aged
- Retrospective Studies
- Tomography, X-Ray Computed*
- Research article
- Open access
- Published: 27 January 2023
Clinical values of diaphragmatic movement in patients with chronic obstructive pulmonary disease
- Taehwa Kim 1 , 2 na1 ,
- Sungchul Huh 3 na1 ,
- Jae Heun Chung 1 , 2 ,
- Yun Seong Kim 1 , 2 ,
- Ra Yu Yun 3 , 4 ,
- Onyu Park 5 &
- Seung Eun Lee ORCID: orcid.org/0000-0002-4266-7722 1 , 2
BMC Pulmonary Medicine volume 23 , Article number: 33 ( 2023 ) Cite this article
2088 Accesses
1 Altmetric
Metrics details
The limitation of activity due to dyspnea in chronic obstructive pulmonary disease (COPD) patients is affected by diaphragmatic dysfunction and reduced lung function. This study aimed to analyze the association between diaphragm function variables and forced expiratory volume in the first second (FEV1) and to estimate the clinical significance of diaphragm function in the correlation between COPD severity and lung function.
This prospective, single-center, cross-sectional observational study enrolled 60 COPD patients in a respiratory outpatient clinic. Data for baseline characteristics and the dyspnea scale were collected. Participants underwent a pulmonary function test (PFT), a 6-minute walk test (6MWT), and diaphragm function by ultrasonography.
The right excursion at forced breathing showed the most significant correlation with FEV1 ( r = 0.370, p = 0.004). The cutoff value was 6.7 cm of the right diaphragmatic excursion at forced breathing to identify the FEV1 above 50% group. In the group with a right diaphragmatic excursion at forced breathing < 6.7 cm, modified Medical Research Council (mMRC), St. George's Respiratory Questionnaire and the total distance of 6MWT showed no difference between groups with FEV1 under and above 50% ( p > 0.05). In the group with ≥ 6.7 cm, mMRC and the total distance of 6MWT showed a significant difference between FEV1 under and above 50% ( p = 0.014, 456.7 ± 69.7 m vs. 513.9 ± 60.3 m, p = 0.018, respectively).
The right diaphragmatic forced excursion was closely related to FEV1, and analysis according to the right diaphragmatic forced excursion-based cut-off value showed a significant difference between both groups. When the diaphragm function was maintained, there was a lot of difference in the 6MWT’s factors according to the FEV1 value. Our data suggest that diaphragmatic function should be performed when interpreting PFT.
Peer Review reports
Introduction
The most common complaint in respiratory diseases regardless of the disease type is dyspnea [ 1 ]. COPD is characterized by worsening dyspnea during movement [ 2 ]. COPD restricts various activities of daily living due to shortness of breath, leading to poor quality of life and increased mortality and morbidity [ 3 ]. There are many causes of dyspnea; however, for patients with stable COPD, a major contributor is the weakening of the respiratory muscles, excluding conditions such as acute infectious diseases [ 4 ].
The diaphragm is the main respiratory muscle, particularly the inspiratory muscles. The weakness of the diaphragm in COPD has been extensively studied. Some studies have reported a significant reduction in diaphragmatic excursion in patients with COPD [ 5 , 6 , – 7 ]. Lung hyperinflation-associated shortening of the diaphragm has traditionally been considered a major cause of diaphragmatic weakness [ 8 ]. Also, there were previous studies about diaphragmatic thickness. Diaphragmatic thickness was a factor related to weaning and prognosis in patients under mechanical ventilation [ 9 , 10 ]. Recently, several studies have reported the clinical value of diaphragm ultrasonography according to COPD severity, and even compared to traditional methods, the diagnostic value of ultrasonography has proven to be reliable and useful [ 11 ]. Ultrasonography is also commonly used in medical facilities because it can be carried out anywhere, has no associated radiation risk, and can be used to adequately visualize the structure of the diaphragm [ 12 ].
Furthermore, 6MWT is an important tool for assessing exercise capacity and functional status in patients with COPD. Diaphragmatic weakness can impair physical performance, especially the 6MWT [ 13 , 14 ]. A previous study reported that pulmonary function was significantly correlated with the 6MWT in patients with severe and very severe COPD [ 15 ]. The relationship between 6MWT and PFT is a matter of connecting and understanding the respiratory muscles. PFT is used to measure the volume and flow rate of the lungs, and 6MWT is an important test for evaluating the exercise capacity and functional status of patients.
When we summarize the above, PFT correlates with 6MWT in COPD patients [ 15 ]. 6MWT can evaluate physical performance of COPD patients. Physical performance can also reflect diaphragmatic weakness [ 13 , 14 ]. Therefore, PFT correlates with 6MWT, 6MWT reflects physical performance, and physical performance was associated with diaphragmatic weakness. This relationship of PFT and diaphragmatic weakness can be expressed as follows for the patient. If the pulmonary function expressed by PFT is good, or if case which the power and strength of the respiratory muscles are good when PFT remains the same, breathing is more stable. Therefore, understanding the physiological principles of the respiratory muscle performance that establish the relationship these and compensate for this is important for managing the patient’s condition. Through this study, a review of the correlation between the PFT reflecting the 6MWT and diaphragm ultrasound features of respiratory muscle may be helpful to understand the physiological principles of patients with COPD.
Thus, this study aimed to analyze diaphragm movement characteristics using ultrasonography in patients with COPD and clarify its association with pulmonary function.
Study design and methods
Study design and participants.
This single-center, prospective, cross-sectional observational study recruited participants from a tertiary hospital outpatient respiratory clinic between April 2020 and April 2021. The inclusion criteria were: 1) patients 18 years old or older diagnosed with COPD by a pulmonologist; COPD diagnostic criterion was a post-bronchodilator FEV1/forced vital capacity (FVC) ratio < 0.70 based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD), 2) patients who could maintain the required posture for diaphragm function measurement by ultrasonography and stable breathing during the examination such as 6MWT. Patients unable to cooperate with the examination and unstable patients requiring immediate medical intervention were excluded. Patients with interstitial lung disease featured on chest computed tomography (CT) that could affect diaphragm movement were also excluded.
Sixty-nine patients were enrolled, six of whom with combined interstitial lung disease on CT were excluded. Two patients were lost to follow-up, and one died before all examinations were completed. Finally, 60 patients completed all examinations for the study protocol and were included in the analysis.
All patients provided informed consent before participating in the study. Each patient’s clinical information was collected from four domains: pulmonary function, exercise capacity, body composition, and diaphragm function. Pulmonary function was evaluated through spirometry, MIP, and maximal expiratory pressure (MEP). Exercise capacity and body composition were assessed using the 6MWT and bioelectrical impedance analysis (BIA). Diaphragm dysfunction is defined as loss of muscle contractility [ 16 ]. To evaluated diaphragm dysfunction, we was assessed using ultrasonography in both the M-mode and B-mode for excursion and thickness, respectively.
Assessments
For patients who had performed a PFT within 1 month of participating in the study, the previous results were used and no retesting was performed. Patients who had no available PFT results within 1 month of participating in this study were reevaluated after enrollment. The Carefusion Vmax 20 (VIASYS Healthcare Inc. Sensormedics; Yorba Linda, CA, USA) was used for PFTs and FEV1, FVC, diffusing capacity of the lungs for CO, and total lung capacity were measured using the body plethysmography test. Regarding spirometry, the patients sat in a small booth and breathed into a mouthpiece. One technical expert from the Department of Respiratory Medicine conducted all the tests to maintain the consistency of the results.
MIP (PONY FX, COSMED Inc.; Rome, Italy) and MEP (PONY FX, COSMED Inc.; Rome, Italy) were measured in the sitting position using a portable mouth pressure meter. Three consecutive MIP and MEP measurements were taken, and the best result was recorded. The PFT was measured in a sitting position. A flanged mouthpiece was applied to the short and rigid tube of the measuring instrument and air leakage was checked around the mouthpiece before testing. The test was performed by an experienced examiner who has conducted the test for more than 8 years. MIP was measured by exhaling as deep as possible and inhaling as hard as possible for at least 1.5 s. MEP was measured by inhaling as deep as possible and exhaling as hard as possible for at least 1.5 s. Both measurements were made three times, and patients recovered to normal breathing patterns with at least a minute of break between measurements. The highest of the three measurements was recorded [ 17 ].
The 6MWT was performed according to the American Thoracic Society standards under the direction of a well-trained respiratory therapist at a 30 m indoor walking course [ 18 ]. Patients were encouraged by the instructor every minute and were allowed to rest or quit the test at any point. We measured the total distance and peripheral saturation with the portable oxygen meter. The patients’ body compositions were estimated indirectly using the BIA from a supine position (InBody S10, InBody, Co. Ltd., Seoul, Korea).
Diaphragm function was assessed using ultrasonography (LOGIQ E9, GE Healthcare; Chicago, IL, USA) obtained from both supine and sitting positions. It is generally accepted that there are positional differences in diaphragm contractility. The effects of gravitational loading on the diaphragm length-tension and body position-mediated changes in intra-abdominal pressure may explain the differences found. Not only that there is also a difference in the excursion between right and left. The excursion of the right diaphragm shows a lower value than that of the left diaphragm because the liver in the abdominal cavity restricts the movement of the right diaphragm. We also measured the diaphragm function in two positions based on this information. The supine position involved lying on the back or with the face upward while the sitting position was semi-seated (45–60 degrees). Both M-mode and B-mode imaging were used to evaluate diaphragmatic excursion and thickness, respectively. The mid-clavicular line and the liver were used as anatomical landmarks on the right side and the spleen on the left side to visualize the diaphragm in the M-mode. B-mode ultrasonography was used to measure the diaphragmatic thickness at the bilateral zone of apposition [ 19 ]. The diaphragm thickness was measured during quiet spontaneous breathing without peak inspiratory or expiratory maneuvers. The diaphragmatic thickness fraction was calculated as the difference between thickness at the end of inspiration and thickness at the end of expiration divided by thickness at the end of expiration x 100. The diaphragmatic excursion was measured as follows. The highest position of the diaphragm movement taken by the M-mode was considered to be the end-expiratory phase, whereas the lowest position was considered as the end-inspiratory phase.
The dyspnea scale used St. George's Respiratory Questionnaire (SGRQ) and the modified Medical Research Council scale (mMRC scale). The SGRQ is a self-administered questionnaire with 76 items [ 20 ]. This can identify the patient’s symptoms and the activities of daily life. mMRC scale is most commonly used in the assessment of dyspnea in chronic respiratory diseases and is a very useful and unrecognized dyspnea scale [ 21 ].
Statistical analysis
The data were analyzed using IBM SPSS (version 27.0; Chicago, IL, USA). The level of significance was set at p < 0.05. Descriptive statistics, including numbers, percentages, means, and standard deviations, were used to summarize each variable (demographics, PFTs, 6MWT, and diaphragmatic ultrasound results). The results were analyzed by independent t-test, cross-analysis, and frequency analysis. The correlation between the variables was analyzed by Pearson’s Correlation Coefficient, which confirmed the linear relationship between two variables using a scatterplot. The cut-off value was calculated using the receiver operating characteristic (ROC) curve analysis. The reference plane was 0.5 or more in the ROC curve, and the p -value < 0.05; hence, this result was adopted. Consequently, the cut-off value was confirmed when sensitivity and specificity were plotted in a line chart, which is the point where the two graphs meet.
Ethics statement
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed throughout this study. The study procedures were reviewed and approved by our Pusan National University Yangsan Hospital Institutional Review Board [IRB No. 05–2020-217].
FEV1 and diaphragm function
We assessed whether diaphragm function was associated with FEV1 (Fig. 1 ). In the total group analysis, both diaphragmatic excursion and thickness were associated with FEV1. However, the diaphragmatic excursion was more associated with FEV1 than thickness. Diaphragmatic excursion during forced breathing and in the supine position had a greater association with FEV1 than breathing at rest and in the sitting position. Additionally, when comparing the right and left under the same conditions, the right was more significant during forced breathing and in the supine position ( r = 0.370, p = 0.004,). Moreover, diaphragmatic thickness at right end-expiration was associated with FEV1. In summary, right ( r = 0.370, p = 0.004) and left ( r = 0.257, p = 0.048) diaphragmatic excursion during forced breathing in the supine position and diaphragmatic thickness at right end-expiration ( r = 0.310, p = 0.016) were significantly associated with FEV1.
Correlation between forced expiratory volume in 1 s and diaphragm function Right forced excursion, and left forced excursion in the supine position and right end-expiratory thickness were correlated to forced expiratory volume in 1 s
Diaphragmatic function and BMI (body mass index)
To evaluate the function of the diaphragm muscle [ 22 ], the diaphragmatic excursion was measured at rest and during forced expiration (Supplement Table 1 ). In 60 patients, diaphragmatic excursion at rest in the supine position was 3.5 cm ± 1.2 on the right side and 3.5 cm ± 1.2 on the left side. During forced breathing, diaphragmatic excursion in the supine position was 6.9 cm ± 2.0 on the right side and 7.6 cm ± 1.6 on the left side. The total percent body fat was 24.2% ± 6.9. Segmental lean mass analysis was performed by direct segmental multi-frequency BIA. The lean mass was 90.5% ± 9.7 on the right arm, 88.1% ± 9.2 on the left arm, 94.5% ± 5.8 on the trunk, 95.7% ± 131.3 on the right leg, and 9.51% ± 8.8 on the left leg.
Cutoff value-associated characteristics
The ROC curve analysis of the diaphragm function variables was performed to identify the cutoff value for differentiating between FEV1 ≥ 50% and < groups. The cutoff value was ≤ 6.7 cm on the right diaphragmatic excursion at forced breathing with an area under the curve of 0.5 or more and p -value was 0.043. Right diaphragmatic excursion during forced breathing was less than the cut-off value of 6.7 cm for 26 patients and ≥ 6.7 cm for 43 patients (Table 1 ). There were no differences in age, sex, or smoking history between the two groups. The dyspnea scales such as mMRC, SGRQ, and GOLD were not significantly different between both groups. There were no differences in body mass index, percent body fat, or lean mass of the right or left legs between the groups. However, among the pulmonary function indicators, there were significant differences between the two groups. Specifically, FEV1, FVC, and MIP were significantly different (< 6.7 cm group vs. ≥ 6.7 cm group, FEV1: 49.2% ± 16.2 vs. 59.5% ± 17.2, p = 0.021; FVC: 76.2% ± 19.1 vs. 86.0% ± 15.5, p = 0.032; MIP: 67.4 cm H 2 O ± 25.1 vs. 86.5 cm H 2 O ± 28.7, p = 0.010). Concerning the 6MWT, there was a significant difference in SpO2 before 6MWT and the number of interruptions (SpO2 before 6MWT: 94.1% ± 2.7 vs. 95.3% ± 1.6, p = 0.038; number of interruptions: 4 [15.4%] vs. 0 [0%], p = 0.018). The left diaphragmatic excursion during forced breathing was also different between the two groups (6.8 cm ± 1.5 vs. 7.6 cm ± 1.3, p = 0.022) as well as the diaphragmatic thickness during right end-inspiration (0.3 cm ± 0.1 vs. 0.4 cm ± 0.1, p = 0.006). In addition, the ROC ≥ 6.7 cm group left diaphragmatic excursion was also measured with a value greater than that of the ROC < 6.7 cm group.
Subgroup characteristics according to FEV1
To identify the clinical significance of diaphragm function with the relationship between lung function, and COPD severity, the two groups classified as a right diaphragmatic excursion at 6.7 cm of forced breathing were further divided into groups based on FEV1 (< 50% or ≥ 50%) (Table 2 ). There were significant differences in age (65.0 ± 7.8 years vs. 72.7 ± 6.2 years, p = 0.011), the GOLD score ( p < 0.001), FEV1/FVC (40.1% ± 14.7 vs. 55.%4 ± 11.4, p = 0.007), peak expiratory flow rate (183.3 L/min ± 80.4 vs. 275.8 L/min ± 113.8, p = 0.027), SpO2 after the 6MWT (85.9% ± 6.5 vs. 91.5% ± 2.2, p = 0.011), and left diaphragmatic excursion during forced breathing (6.2 cm ± 1.6 vs. 7.4 cm ± 1.0, p = 0.038).
When the group with the right diaphragmatic excursion ≥ 6.7 cm was further divided into subgroups according to FEV1 (< 50% or ≥ 50%) and analyzed, mMRC, GOLD score, FEV1/FVC, MIP, peak expiratory flow rate, 6MWT, SpO2 before and after the 6MWT, and right diaphragmatic thickness at end-expiration subgroups were significantly different between the two groups.
This study contains the following: 1) evidence that FEV1 is significantly correlated with diaphragm movement, 2) cutoff values for diaphragm movement in patients with COPD, and 3) evidence to support the claim that the function of the diaphragm should be considered when interpreting the patient’s condition based on their FEV1.
First, FEV1 was significantly correlated with diaphragm movement. Studies on the relationship between the diaphragm and pulmonary function in patients with COPD are ongoing and have consistently reported that the severity of COPD and diaphragm function are closely related. Some previous studies have evaluated the direct relationship between FEV1 and diaphragm function [ 23 , 24 ].
The results of this study is also consistent with those of previous studies showing that diaphragm movement and FEV1 are related. However, beyond the findings of previous results [ 23 ], in our study, diaphragmatic excursion and thickness were found to be more correlated to FEV1 on the right side than on the left side.
Like the previous study that the thickness of the diaphragm is related to the ventilator weaning mechanical ventilation [ 9 , 10 ], this result has confirmed that the right diaphragm thickness was significantly related not only to the weaning of the ventilator and the prognosis of the patient but also to FEV1.
Second, we provided a cutoff value for a right diaphragmatic forced excursion in patients with COPD. Although there are studies that have presented a reference [ 23 ] value for healthy persons, the significant contribution of this study is the proposed reference value for patients with COPD.
We analyzed the correlation using Pearson’s correlation coefficient and confirmed the factors of diaphragmatic function-related components side (right, left), thickness, and excursion that were most-related to FEV1. Among them, Rt. forced excursion (supine), Lt. forced excursion (supine) and Rt. end-expiratory thickness showed meaningful p -value in association with FEV1. In addition, these three factors were analyzed in the linear relationship with the scattered plot and showed a proportional relationship between FEV1. Finally, when all factors related to the diaphragmatic function were analyzed, the right forced excursion was statistically determined as the most meaningful factor in relation to FEV1. We also obtained the cut-off value of 6.7 cm through the ROC curve.
The range in diaphragmatic excursion values varies considerably depending on the patient’s condition. A previous study has suggested normal values based on sex and the side of the diaphragm using healthy individuals. When breathing deeply, the right diaphragmatic excursion was 7 cm ± 1.1 in men and 5.7 cm ± 1 in women ( p < 0.001) and the left diaphragmatic excursion were 7.5 cm ± 0.9 and 6.4 cm ± 1 in men and women, respectively ( p < 0.01) [ 23 ]. In our study, we also assessed excursion during deep breathing to provide a cut-off value for patients with COPD.
When analyzed by dividing them into two groups based on a cut-off value, the following evaluation factors showed significant differences ( p < 0.05): FEV1, FVC, MIP, left forced excursion, right diaphragmatic thickness during end-inspiration, 6MWT, the SpO2 before and after 6MWT, and interruption of the 6MWT.
These factors can be broadly divided into PFT-related and performance-related factors. As mentioned above, PFT-related factors such as MIP, left diaphragmatic forced excursion and right diaphragmatic thickness during end-inspiration were lower in the < 6.7 cm group. Moreover, the SpO2 level before the 6MWT was lower in the < 6.7 cm group, the overall 6MWT was shorter, and there were many interruptions in the 6MWT. These factors might reflect activity as a performance evaluation factor. Although generalizability is limited given the few patients and the fact that all the participants were outpatients who could walk; these results may reflect an actual patient’s status. However, these findings are intended for patients who can walk, suggesting that the cut-off value of 6.7 cm may be reliable in this population.
Finally, results concerning the degree of pulmonary function and correlations with the diaphragmatic movement were noteworthy. The two groups were analyzed based on the right diaphragmatic forced excursion (6.7 cm) and divided into subgroups based on FEV1 (< 50% vs. ≥ 50%). As a result, in the group that had maintained diaphragm function (≥ 6.7 cm), the MIP, portable peak flow meter, 6MWT, SpO2 before and after the 6MWT, and right diaphragmatic thickness at end-expiration were different between the two FEV1 groups. In summary, the difference between the two FEV1 groups was large when diaphragm function was maintained; when it was not maintained, there were no differences between the two FEV1 groups. Therefore, even in patients who maintained their FEV1 > 50%, when diaphragm function deteriorated, the patient’s 6MWT, SpO2 before and after the 6MWT were less predictable (they either deteriorated or were maintained). The patients whose FEV1 decreased < 50%, if the diaphragm function was maintained, the 6MWT could be better than that in patients with an FEV1 ≥ 50% and a reduced diaphragm function.
In conclusion, when interpreting a patient’s condition based on FEV1, it is important to assess diaphragm function, since the effect of the FEV1 value on the patient depends on how well the diaphragm function has been maintained.
In this study, when the diaphragm function was maintained, there were significant differences in MIP, peak expiratory flow rate, 6MWT, SpO2 before and after the 6MWT, and right diaphragmatic thickness at end-expiration according to FEV1 in patients with COPD. Even if the diaphragm function was not maintained, because there are still differences in the FEV1, it may be beneficial to consider diaphragmatic function measured by right diaphragm excursion as an additional indicator of function beyond the FEV1. Therefore, it can be clinically helpful to check whether the diaphragm is functioning properly when determining a patient’s condition based on FEV1.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Chronic obstructive pulmonary disease
Pulmonary function test
- 6-minute walk test
Forced expiratory volume in the first second
Maximal inspiratory pressure
International Classification of Diseases 11TH
Forced vital capacity
Global Initiative for Chronic Obstructive Lung Disease
Computed tomography
Maximal expiratory pressure
Bioelectrical impedance analysis
Modified Medical Research Council
Receiver operating characteristic
Body mass index
St. George's Respiratory Questionnaire
Niedermeyer J. Dyspnea in airway and pulmonary diseases. Internist. 2015;56(8):882–9.
Article CAS Google Scholar
Antoniu SA. Descriptors of dyspnea in obstructive lung diseases. Multidisciplinary respiratory medicine. 2010;5(3):216–9.
Article Google Scholar
Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27(2):397–412.
Decramer M. Respiratory muscles in COPD: regulation of trophical status Verhandelingen. Koninklijke Academie voor Geneeskunde van Belgie. 2001;63(6):577–602 discussion −4.
CAS Google Scholar
Corbellini C, Boussuges A, Villafañe JH, Zocchi L. Diaphragmatic mobility loss in subjects with moderate to very severe COPD may improve after in-patient pulmonary rehabilitation. Respir Care. 2018;63(10):1271–80.
Crimi C, Heffler E, Augelletti T, Campisi R, Noto A, Vancheri C, et al. Utility of ultrasound assessment of diaphragmatic function before and after pulmonary rehabilitation in COPD patients. Int J Chronic Obstruct Pulmon Dis. 2018;13:3131–9.
He L, Zhang W, Zhang J, Cao L, Gong L, Ma J, et al. Diaphragmatic motion studied by M-mode ultrasonography in combined pulmonary fibrosis and emphysema. Lung. 2014;192(4):553–61.
Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med. 2003;168(1):10–48.
Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brace D, et al. Evolution of diaphragm thickness during mechanical ventilation. Impact Inspiratory Effort Am J Respirat Cri Care Med. 2015;192(9):1080–8.
Goligher EC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med. 2018;197(2):204–13.
Boussuges A, Rives S, Finance J, Brégeon F. Assessment of diaphragmatic function by ultrasonography: current approach and perspectives. World J Clin Cases. 2020;8(12):2408–24.
Sarwal A, Walker FO, Cartwright MS. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve. 2013;47(3):319–29.
Criner G. 6-minute walk testing in COPD: is it reproducible? Eur Respir J. 2011;38(2):244–5.
Hernandes NA, Wouters EF, Meijer K, Annegarn J, Pitta F, Spruit MA. Reproducibility of 6-minute walking test in patients with COPD. Eur Respir J. 2011;38(2):261–7.
Chen H, Liang BM, Tang YJ, Xu ZB, Wang K, Yi Q, et al. Relationship between 6-minute walk test and pulmonary function test in stable chronic obstructive pulmonary disease with different severities. Chin Med J. 2012;125(17):3053–8.
Google Scholar
Minami T, Manzoor K, McCool FD. Assessing diaphragm function in Chest Wall and neuromuscular diseases. Clin Chest Med. 2018;39(2):335–44.
ATS/ERS Statement on Respiratory Muscle Testing. Am J Respir Crit Care Med. 2002;166(4):518–624.
ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7.
Matamis D, Soilemezi E, Tsagourias M, Akoumianaki E, Dimassi S, Boroli F, et al. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med. 2013;39(5):801–10.
Jones PW, Quirk FH, Baveystock CM. The St George's respiratory questionnaire. Respir Med. 1991;85 Suppl B(25-31):discussion 3-7.
Launois C, Barbe C, Bertin E, Nardi J, Perotin JM, Dury S, et al. The modified Medical Research Council scale for the assessment of dyspnea in daily living in obesity: a pilot study. BMC pulmonary medicine. 2012;12:61.
Dhungana A, Khilnani G, Hadda V, Guleria R. Reproducibility of diaphragm thickness measurements by ultrasonography in patients on mechanical ventilation. World J Critical Care Med. 2017;6(4):185–9.
Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest. 2009;135(2):391–400.
Rocha FR, Brüggemann AK, Francisco DS, Medeiros CS, Rosal D, Paulin E. Diaphragmatic mobility: relationship with lung function, respiratory muscle strength, dyspnea, and physical activity in daily life in patients with COPD. Jornal brasileiro de pneumologia : publicacao oficial da Sociedade Brasileira de Pneumologia e Tisilogia. 2017;43(1):32–7.
Download references

Acknowledgements
Abstract has been published/presented in the Korean tuberculosis and respiratory society, the Korean tuberculosis and respiratory society fall academic presentation | 129 volume 0342 ~ 343, total 2 PAGES, 2021
https://journal.kstudy.com/ISS_Detail.asp?key=3921544&tname=kiss2002&code=YqldZWtoSqVtJTNEOTEnMSUmN/B%20Z%20xLJTNEVHJpZSUmNbNj2bRU4XB/JTNEMA ==
This study was supported by the Research Institute for Convergence of Biomedical Science and Technology (30–2020-003), Pusan National University Yangsan Hospital. The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Taehwa Kim and Sungchul Huh contributed equally to this work.
Authors and Affiliations
Division of Respiratory, Allergy and Critical Care Medicine, Department of Internal Medicine, Pusan National University Yangsan Hospital and Pusan National University School of Medicine, Geumo-ro 20, Beomeo-ri, Yangsan-si, Gyeongsangnam-do, 50612, Republic of Korea
Taehwa Kim, Jae Heun Chung, Yun Seong Kim & Seung Eun Lee
BioMedical Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, South Korea
Department of Rehabilitation Medicine, Rehabilitation Hospital, Pusan National University Yangsan, Yangsan, South Korea
Sungchul Huh & Ra Yu Yun
Pusan National University School of Medicine, Yangsan, South Korea
College of Nursing, Pusan National University, Pusan National University Yangsan Hospital, Yangsan, South Korea
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: TK, SEL. Data acquisition and analysis: TK, OP, RYY, SH, JHC, SEL. Data interpretation: TK, RYY, SH, JHC, SEL. Validation: TK, JHC. Writing – original draft: SH, TK. Writing – review: SEL, JHC, YSK. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Seung Eun Lee .
Ethics declarations
Ethics approval and consent to participate.
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) [ 17 ]. The study was approved by Pusan National University Yangsan Hospital (PNUYH) Institutional Review Board (IRB No. 05–2020-217) and individual consent for this retrospective analysis was waived.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest or funding sources to declare.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Kim, T., Huh, S., Chung, J.H. et al. Clinical values of diaphragmatic movement in patients with chronic obstructive pulmonary disease. BMC Pulm Med 23 , 33 (2023). https://doi.org/10.1186/s12890-022-02220-7
Download citation
Received : 25 April 2022
Accepted : 02 November 2022
Published : 27 January 2023
DOI : https://doi.org/10.1186/s12890-022-02220-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cut-off value
BMC Pulmonary Medicine
ISSN: 1471-2466
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Decomposition Rates and Community Structure of Arthropods in the Litter of Invasive Solidago gigantea Do Not Support the Home-Field Advantage Hypothesis
- Published: 19 July 2022
- Volume 53 , pages 328–334, ( 2022 )
Cite this article
- E. N. Ustinova 1 ,
- M. N. Maslov 1 ,
- S. N. Lysenkov 1 &
- A. V. Tiunov 2
166 Accesses
Explore all metrics
Decomposition rates of an invasive plant litter in native-species communities can be slower, since decomposers are not adapted to the litter of the invasive species. We have compared rates of plant decomposition and the structure of arthropod communities during the incubation of the litter of the invasive giant goldenrod Solidago gigantea (Asteraceae) and three native species ( Urtica dioica , Cirsium arvense , and Chamaenerion angustifolium ) in the biotopes with dominance of local and invasive plant species. Our results suggest that the arthropod community involved in decomposition of S. gigantea and other species is not species specific and does not provide a higher or lower rate of decomposition of the invasive species. Neither the rate of litter decomposition, nor the structure and diversity of arthropod communities support the home-field advantage hypothesis.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others
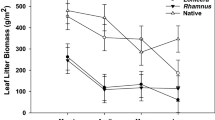
Exotic tree and shrub invasions alter leaf-litter microflora and arthropod communities
Gerald R. Woodworth, Jennifer N. Ward & David E. Carr
Increased litter decomposition rates of exotic invasive species Hieracium pilosella (Asteraceae) in Southern Patagonia, Argentina
Karen Braun, M. B. Collantes, … J. A. Anchorena
Do fire and seasonality affect the establishment and colonisation of litter arthropods?
Diego Anjos, Estevao Alves-Silva & Sérvio Pontes Ribeiro
Gholz, H.L., Wedin, D.A., Smitherman, S.M., et al., Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition, GCB , 2000, vol. 6, no. 7, pp. 751–765.
Google Scholar
Ayres, E., Steltzer, H., Simmons, B.L., et al., Home-field advantage accelerates leaf litter decomposition in forests, Soil Biol. Biochem ., 2009, vol. 41, no. 3, pp. 606–610.
Article CAS Google Scholar
Veen, G.F., Freschet, G.T., Ordonez, A., and Wardle, D.A., Litter quality and environmental controls of home-field advantage effects on litter decomposition, Oikos , 2015, vol. 124, no. 2, pp. 187–195.
Article Google Scholar
Ehrenfeld, J.G., Effects of exotic plant invasions on soil nutrient cycling processes, Ecosystems , 2003, vol. 6, no. 6, pp. 503–523.
Allison, S.D. and Vitousek, P.M., Rapid nutrient cycling in leaf litter from invasive plants in Hawai’i, Oecologia , 2004, vol. 141, no. 4, pp. 612–619.
Ashton, I.W., Hyatt, L.A., Howe, K.M., et al., Invasive species accelerate decomposition and litter nitrogen loss in a mixed deciduous forest, Ecol. Appl ., 2005, vol. 15, no. 4, pp. 1263–1272.
Samye opasnye invazionnye vidy Rossii (TOP-100) (The most Dangerous Invasive Species in Russia (TOP-100)), Dgebuadze, Yu.Yu., Petrosyan, V.G., and Khlyap, L.A., Eds., Moscow: KMK, 2018.
Berg, B. and McClaugherty, C., Plant Litter: Decomposition, Humus Formation, Carbon Sequestration , Berlin: Springer-Verlag, 2008.
Book Google Scholar
R Core Team. R: A language and environment for statistical computing, R Foundation for Statistical Computing , Vienna, 2020.
Perez, G., Aubert, M., Decaëns, T., et al., Home-Field Advantage: A matter of interaction between litter biochemistry and decomposer biota, Soil Biol. Biochem ., 2013, vol. 67, pp. 245–254.
Ayres, E., Steltzer, H., Berg, S., and Wall, D.H., Soil biota accelerate decomposition in high-elevation forests by specializing in the breakdown of litter produced by the plant species above them, J. Ecol ., 2009, vol. 97, no. 5, pp. 901–912.
Canty, A. and Ripley, B., Boot: Bootstrap R (S-Plus) functions (R package version 1.3-9), R Foundation for Statistical Computing , Vienna, 2013.
Oksanen, J., Kindt, R., Legendre, P., et al., The vegan package, Community Ecology Package , 2007, vol. 10, pp. 631–637.
McTee, M.R., Lekberg, Y., Mummey, D., et al., Do invasive plants structure microbial communities to accelerate decomposition in intermountain grasslands?, Ecol. Evol ., 2017, vol. 7, no. 24, pp. 11227–11235.
Gießelmann, U.C., Martins, K.G., Brändle, M., et al., Lack of home-field advantage in the decomposition of leaf litter in the Atlantic Rainforest of Brazil, Appl. Soil Ecol ., 2011, vol. 49, pp. 5–10.
John, M.G.S., Orwin, K.H., and Dickie, I.A., No ‘home’ versus ‘away’ effects of decomposition found in a grassland–forest reciprocal litter transplant study, Soil Biol. Biochem ., 2011, vol. 43, no. 7, pp. 1482–1489.
Gartner, T.B. and Cardon, Z.G., Decomposition dynamics in mixed-species leaf litter, Oikos , 2004, vol. 104, no. 2, pp. 230–246.
Hättenschwiler, S., Tiunov, A.V., and Scheu, S., Biodiversity and litter decomposition in terrestrial ecosystems, Annu. Rev. Ecol. Evol. Syst ., 2005, vol. 36, pp. 191–218.
Shilenkova, O.L. and Tiunov, A.V., Soil-litter nitrogen transfer and changes in δ13C and δ15N values in decomposing leaf litter during laboratory incubation, Pedobiologia , 2013, vol. 56, no. 3, pp. 147–152.
Gergócs, V., Rétháti, G., and Hufnagel, L., Litter quality indirectly influences community composition, reproductive mode and trophic structure of oribatid mite communities: a microcosm experiment, Exp. Appl. Acarol ., 2015, vol. 67, no. 3, pp. 335–356.
Stefanowicz, A.M., Stanek, M., Nobis, M., and Zubek, S., Species-specific effects of plant invasions on activity, biomass, and composition of soil microbial communities, Biol. Fertil. Soils , 2016, vol. 52, no. 6, pp. 841–852.
Wang, C., Jiang, K., Zhou, J., and Wu, B., Solidago canadensis invasion affects soil N-fixing bacterial communities in heterogeneous landscapes in urban ecosystems in East China, Sci. Total Environ ., 2018, vol. 631, pp. 702–713.
Zhang, C.B., Wang, J., Qian, B.Y., and Li, W.H., Effects of the invader Solidago canadensis on soil properties, Appl. Soil Ecol ., 2009, vol. 43, nos. 2–3, pp. 163–169.
Scharfy, D., Güsewell, S., Gessner, M.O., and Venterink, H.O., Invasion of Solidago gigantea in contrasting experimental plant communities: effects on soil microbes, nutrients and plant-soil feedbacks, J. Ecol ., 2010, vol. 98, no. 6, pp. 1379–1388.
Sterzyńska, M., Shrubovych, J., and Nicia, P., Impact of plant invasion ( Solidago gigantea L.) on soil mesofauna in a riparian wet meadows, Pedobiologia , 2017, vol. 64, pp. 1–7.
Ustinova, E.N., Schepetov, D.M., Lysenkov, S.N., and Tiunov, A.V., Soil arthropod communities are not affected by invasive Solidago gigantea Aiton (Asteraceae), based on morphology and metabarcoding analyses, Soil Biol. Biochem ., 2021, vol. 159, art. ID 108288.
Download references
ACKNOWLEDGMENTS
We thank the experts who carried out taxonomic identification of soil arthropods: Collembola—A.Yu. Korotkevich (Moscow State Pedagogical University, Zoology and Ecology Department); Oribatida—V.D. Leonov (Institute of Ecology and Evolution, Russian Academy of Sciences); Mesostigmata—M.S. Bizin (Institute of Ecology and Evolution, Russian Academy of Sciences).
Author information
Authors and affiliations.
Moscow State University, 119991, Moscow, Russia
E. N. Ustinova, M. N. Maslov & S. N. Lysenkov
Institute for Ecology and Evolution, Russian Academy of Sciences, 119071, Moscow, Russia
A. V. Tiunov
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to E. N. Ustinova .
Additional information
Translated by T. Kuznetsova
Rights and permissions
Reprints and permissions
About this article
Ustinova, E.N., Maslov, M.N., Lysenkov, S.N. et al. Decomposition Rates and Community Structure of Arthropods in the Litter of Invasive Solidago gigantea Do Not Support the Home-Field Advantage Hypothesis. Russ J Ecol 53 , 328–334 (2022). https://doi.org/10.1134/S1067413622040063
Download citation
Received : 28 December 2021
Revised : 03 February 2022
Accepted : 07 February 2022
Published : 19 July 2022
Issue Date : August 2022
DOI : https://doi.org/10.1134/S1067413622040063
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- decomposition of plant residues
- home-field advantage
- invasive plants
- field experiment
- Find a journal
- Publish with us
- Track your research
- Streaming Media 101
- Wowza Streaming Engine
- Live Streaming Bootcamp
- Video Quality Metrics
- AV1 encoding
- Video lessons
About Jan Ozer
Streaming learning center where streaming professionals learn to excel.

- How to Build an Encoding Ladder: What You Need to Know
- Protected: Norsk Studio: A No-Code Interface for Media Workflows
- Simplifying Streaming Workflows with Norsk: An Interview with Dom Robinson
- The Business Case for Norsk: Interview with id3as CMO Eric Schumacher-Rasmussen
- Norsk for Broadcasters: Interview with id3as’ Steve Strong
- New Five Star Review for Video Quality Metrics Course
Review of Moscow State University Video Quality Measurement Tool
Jan Ozer January 19, 2015 Blogs Leave a comment 519 Views
Related Articles

New Lesson Helps Students Choose the Best Codec for Streaming
March 18, 2023
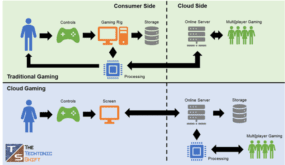
What is Cloud Gaming (and Why You Care)
February 11, 2023
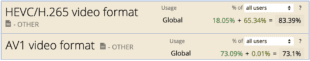
HEVC Passes AV1 on CanIUse
December 24, 2022
Tutorial: Producing Live-Streamed Events with the Roland V-02HD MK II
December 19, 2021
A recent consulting project necessitated that I add objective quality measurements to the analysis. The tool I used was the Moscow Statue University Video Quality Measurement Tool (VQMT) , which enables comparisons using Peak Signal to Noise (PSNR), Structured Similarity Index (SSIM), and the new Video Quality Metric (VQM), which I found most useful.
In this review on the Streaming Media Europe site, I discuss how VQMT works and why it’s so useful. If you do a lot of encoder or codec comparisons, you’ll find this $999 tool as invaluable as I did.

Tags Streaming production

Take the Bitmovin Video Developer Survey
Contribute to the one of the most valuable sources of industry data by completing the …
Speech-to-text In Premiere Pro – Fast, Easy, Accurate, and Free
This video tutorial teaches you how to convert speech-to-text in Premiere Pro. I’ve been using …

Streaming Media 101: Training for App & Player Development/Testing Professionals
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Diagnostics (Basel)

Ultrasonographic Assessment of Diaphragmatic Function and Its Clinical Application in the Management of Patients with Acute Respiratory Failure
Marina saad.
1 Division of Respiratory Diseases, Ospedale Luigi Sacco, Polo Universitario, ASST Fatebenefratelli-Sacco, 20157 Milano, Italy
Stefano Pini
Fiammetta danzo.
2 Department of Biomedical and Clinical Sciences (DIBIC), Università degli Studi di Milano, 20157 Milano, Italy
Francesca Mandurino Mirizzi
Carmine arena.
3 Servizio di Cardiologia, Centro Ospedaliero Militare di Milano, Esercito Italiano, 20147 Milano, Italy
Francesco Tursi
4 UOS di Pneumologia, Ospedale di Codogno, ASST Lodi, 26845 Codogno, Italy
Dejan Radovanovic
Pierachille santus, associated data.
Not applicable.
Acute respiratory failure (ARF) is a common life-threatening medical condition, with multiple underlying etiologies. In these cases, many factors related to systemic inflammation, prolonged use of steroids, and lung mechanical abnormalities (such as hyperinflation or increased elastic recoil due to pulmonary oedema or fibrosis) may act as synergic mechanisms leading to diaphragm dysfunction. The assessment of diaphragm function with ultrasound has been increasingly investigated in the emergency department and during hospital stay as a valuable tool for providing additional anatomical and functional information in many acute respiratory diseases. The diaphragmatic ultrasound is a noninvasive and repeatable bedside tool, has no contraindications, and allows the physician to rapidly assess the presence of diaphragmatic dysfunction; this evaluation may help in estimating the need for mechanical ventilation (and the risk of weaning failure), as well as the risk of longer hospital stay and higher mortality rate. This study presents an overview of the recent evidence regarding the evaluation of diaphragmatic function with bedside ultrasound and its clinical applications, including a discussion of real-life clinical cases.
1. Introduction
Acute respiratory failure (ARF) is a common life-threatening medical condition, with multiple underlying etiologies. Diaphragm muscle dysfunction is increasingly recognized as an important element that can present in patients with ARF or who are critically ill [ 1 , 2 ]. As of today, there are various static and dynamic imaging techniques used for the assessment of diaphragm function [ 3 ]. Static imaging techniques can be used to assess the position, shape, and dimensions of the diaphragm and include chest radiography [ 4 ], brightness mode (B-mode) ultrasound [ 5 ], computed tomography (CT) [ 6 ], and static magnetic resonance imaging (MRI) [ 7 ]. Dynamic imaging techniques are used to assess diaphragm motion in one or more directions, and include fluoroscopy [ 8 ], motion-mode (M-mode) ultrasonography [ 5 , 9 ], and dynamic MRI [ 10 ].
As compared with standard diagnostic approaches, chest ultrasound has been proven to improve the overall diagnostic accuracy in critical care settings, as well as in the emergency department [ 11 ]. In this context, the assessment of diaphragm function with ultrasound has been increasingly investigated in the emergency department [ 12 , 13 , 14 ] and during hospital stay [ 15 , 16 ] as a valuable tool for providing additional anatomical and functional information in many acute respiratory diseases [ 17 ].
The diaphragmatic ultrasound is a noninvasive and repeatable bedside tool, has no contraindications [ 15 ], and allows the physician to rapidly assess the presence of diaphragmatic dysfunction; this evaluation helps in estimating the need for mechanical ventilation (and its risk of failure), as well as the risk of longer hospital stay and higher mortality rate [ 13 , 14 , 15 , 16 ].
An overview of the recent evidence regarding the evaluation of diaphragmatic function with bedside ultrasound and its clinical applications is hereby presented, including a discussion of real-life clinical cases.
2. Diaphragm Function Assessment
The diaphragm is an ample dome-shaped muscle which separates the thoracic cavity from the abdomen. It is comprised of three different portions: the central tendon, which is a non-contractile, fibrous structure; the costal portion, which inserts to the rib cage and thoracic vertebrae; and the crural portion, which inserts to the upper three lumbar vertebrae [ 3 ]. The area in which the diaphragmatic fibers connect to the rib cage is called the zone of apposition [ 18 ].
The diaphragm is the main respiratory muscle. During inspiration, its contraction lowers and widens the inferior part of the thorax, thus lowering the intrathoracic pressure (as well as increasing the abdominal pressure) and allowing the insufflation of the lungs. During exhalation, the relaxation of the diaphragm allows the elastic recoil of the lungs to increase pleural pressure, thus removing air from the lungs [ 19 ]. In healthy individuals during resting breathing, inspiration is an active process, while exhalation is passive and does not require muscle activity [ 20 ].
Assessing the anatomy and function of the diaphragm can be an auxiliary or, in many conditions, an invaluable tool to diagnose, characterize, and monitor both acute and chronic respiratory diseases. Ultrasonography is particularly promising for this purpose, due to its availability, fast execution, repeatability, low costs, and safety; moreover, it can be easily deployed bedside, which is very helpful in the acute or critical care setting [ 3 , 18 , 19 , 20 , 21 ].
Ultrasonography of the diaphragm was first described at the end of the 1960s, but the true cornerstone of its implementation in the clinical practice was the seminal study by Wait and collaborators in 1989 [ 15 ]. Wait introduced a technique to measure the thickness of the diaphragm by applying a high-frequency ultrasound probe on the apposition zone of the diaphragmatic dome [ 22 ].
The technique to perform diaphragmatic ultrasonography has been currently revised in an Expert Consensus statement, relatively to its adoption in the Critical Care setting [ 23 ]. The diaphragm can be visualized by deploying high-frequency (10–15 MHz) probes between the seventh and the ninth intercostal space on the median anterior axillary line, transversal to the ribcage. Since the thickness of the diaphragm is variable over the surface of the muscle, a precise note regarding the position of the probe is essential to ensure the repeatability of this measure on the same patient, or to compare the values found in different patients.
The diaphragm can be visualized as a three-layered band, which includes (from the outer to the inner layer) the hyperechogenic diaphragmatic pleura, the relatively hypoechogenic muscle, and the hyperechogenic peritoneal pleura ( Figure 1 ).

Thickness of the right hemi-diaphragm at the zone of opposition ( left panel) and diaphragm thickening during maximal inspiration ( right panel) in a healthy woman in her thirties while semi-recumbent. Left panel: note the two hyperechoic lines (arrows) representing the peritoneum (empty arrow) and parietal pleura (filled arrow). The measured thickness (D1) is 1.5 mm. Right panel: the same diaphragmatic region during maximal inspiration from resting volume to total lung capacity. Diaphragmatic thickening is examined in motion mode (M-Mode). During full inspiration, thickness increases from 1.6 (D1) to 4.2 mm (D2), equal to a thickening fraction of 162%. Own image.
The distance between the inner margins of the diaphragmatic and peritoneal sheets is defined as the thickness of the diaphragm, the normal median (interquartile range) value of which is highly variable, with the majority of measurements ranging 3.3 (1.3–7.6) mm. Thickness is lower in female than in male patients, and is positively correlated to height and body weight, but independent of age [ 24 , 25 ]. Moreover, it should be noted that diaphragm thickness is increased in standing and sitting position compared with the recumbent position [ 26 ], and that it is greater at the lower intercostal spaces [ 27 ]. Diaphragm thickness at rest in healthy patients is highly variable, ranging from 1.2 to 11.8 mm among individuals, with group mean values of 1.6 to 3.4 mm and a lower limit of normal (LLN) in adults ranging from 0.80 to 1.60 mm [ 21 ].
Variations of the thickness of the diaphragm during the respiratory cycle are inversely proportional to variations of its transverse surface, which results from its contractile activity. Since the volume of the muscle is constant, the shortening and flattening of the diaphragm during contraction is associated by a proportional increase in its thickness ( Figure 1 ) [ 22 ]. The percentage by which the thickness increases from the end of exhalation to the end of inspiration is called the thickening fraction, while the ratio between the end-inspiratory and the end-expiratory thickness is called the thickening ratio [ 28 ]. Similar to diaphragmatic thickness, the thickening fraction also shows wide variability in different individuals, with reference values ranging from 60% to 260% (left hemidiaphragm) and from 57 to 200% (right hemidiaphragm) while seated [ 28 ].
Assessment of the diaphragmatic thickening fraction shows good intra- and inter-observer repeatability also in mechanically ventilated patients [ 16 , 29 , 30 ].
It has been demonstrated that, in sedated and mechanically ventilated patients, passive inflation of the lungs causes an increase in the diaphragmatic thickness only when a volume of air superior to 50% of the total lung capacity is insufflated; therefore, at lower lung volumes, the thickening fraction reflects the diaphragmatic activity rather than passive alterations of the diaphragm induced by chest inflation, hence the utility of this assessment even in ventilated patients [ 16 , 27 ].
The thickening fraction has been widely studied as an indicator of the contractile efficiency of the diaphragm [ 31 ], and consequently as a possible predictor of the outcome of a weaning trial in mechanically ventilated patients [ 32 , 33 ]. A pilot study also found a correlation between the thickening fraction and the response to non-invasive support with continuous positive airway pressure (CPAP) in patients hospitalized with SARS-CoV2 pneumonia [ 34 ]. However, results from clinical study exploring the usefulness of the thickening fraction as a predictor of the outcome in patients undergoing invasive or non-invasive ventilation for acute respiratory failure are often inconclusive [ 35 , 36 ].
In fact, despite the fact that some studies have found a positive correlation between the thickening fraction and the pressure output of the diaphragm [ 37 , 38 ], the correlation between these measurements is usually weak [ 39 , 40 ], highlighting the need for further studies to validate the clinical applications of this technique [ 38 ].
Ultrasonography also allows the measurement of the diaphragmatic cranio–caudal excursion during the respiratory cycle. This requires employing curvilinear, low-frequency probes (2–6 MHz), which can be positioned just below the right costal arch, on the right midclavicular line, and directed upwards and laterally, so that the liver acts as an acoustic window and the ultrasound beam reaches the dome of the diaphragm perpendicularly. The diaphragm will be visualized as a bright, hyperechogenic line which lays over the profile of the liver. After having obtained a good visualization of the diaphragm in 2D brightness (B) mode, ultrasonography will be switched to movement (M) mode, in order to visualize the diaphragmatic respiratory excursions over time [ 41 ] ( Figure 2 ). The same procedure can also be executed by pointing the probe towards the left midclavicular line, thus using the spleen as an acoustic window, or on the right or left mid axillary line, even if visualization of the diaphragm is often suboptimal [ 9 ]. The maximum diaphragmatic excursion can be measured as the difference between the position of the diaphragm at functional residual capacity (FRC) and total lung capacity (TLC), while tidal excursion is the difference between the positional FRC and the end-inspiratory position during resting breathing. Ultrasonography assessment of the diaphragmatic excursion has been proven to have a good inter- and intra-observer reproducibility ( Table 1 ) [ 24 , 26 ].

Visualization of the diaphragmatic dome from the right subcostal view in brightness (B) mode ( a ) and of the diaphragmatic excursion in motion (M) mode ( b ) in a healthy subject. Own image.
Normal reference values of diaphragm ultrasound measurements expressed as medium (5–95° percentile). Adapted from: Goligher EC. Diaphragm Ultrasound. In: Magder S, Malhotra A, Hibbert KA, Hardin CC (Editors). Cardiopulmonary Monitoring. Basic Physiology, Tools, and Bedside Management for the Critically Ill. Springer Nature Switzerland AG 2021.
Diaphragmatic ultrasonography can be limited by a poor acoustic window, for example in obese patients, in the presence of marked abdominal meteorism, or in patients with massive contraction of abdominal muscles during exhalation. Moreover, complete visualization of the diaphragm over the entire respiratory cycle may be impaired in patients with ribcage anomalies. As a result, diaphragmatic excursion may be hardly measurable in up to 28% of patients [ 42 ]. Interpreting the diaphragmatic excursion is also complicated by mechanical ventilation, since it is impossible to discriminate if the lowering of the dome may be due to passive inflation of the lungs by means of the ventilator, rather than by muscular activity [ 37 ].
Due to the challenges of the ultrasound assessment of the diaphragm, the learning curve of this procedure is likely to require significant practice before being reliably implemented in the clinical setting. A randomized controlled educational study performed in eight Italian University hospitals in 2019 showed that integrating theoretical formation with practical training was able to achieve much better results in educating medical students or residents to perform diaphragmatic ultrasound [ 43 ]; the authors suggested that 25 supervised examinations could be sufficient to achieve sufficient familiarity to employ the technique in the critical care setting, similar to what has been observed regarding the training to perform lung ultrasound [ 44 ]. A study performed in 2018 involving the measurement of the diaphragmatic thickening fraction showed that intra-observer reproducibility significantly increased over the first ten patients and only slightly improved afterwards [ 29 ].
Both the thickening fraction and the excursion can be used as indexes of diaphragmatic contractility, and thus should be included in the functional evaluation of the diaphragm both in acute and chronic conditions. These parameters, and particularly the thickening fraction, have been explored as non-invasive predictors of ventilation or weaning failure in mechanically ventilated patients in the ICU setting, as discussed above [ 16 , 25 , 42 , 45 , 46 , 47 , 48 ]. There is no established consensus yet regarding the superiority of either diaphragmatic thickening fraction or excursion as a predictor of clinical outcomes in the intensive care setting, with some studies suggesting a superiority of the former [ 49 , 50 ] or latter [ 51 , 52 , 53 ] technique. A systematic review published in 2021 concluded that the thickening fraction had slightly lower sensitivity but higher specificity than the diaphragmatic excursion in predicting successful weaning from mechanical ventilation [ 54 ], but the analysis was potentially hindered by the significant heterogeneity between the studies. However, a more recent systematic review concluded for the superiority of the thickening fraction both in terms of sensitivity, specificity, and diagnostic Odds Ratio [ 33 ].
The gold standard procedure to assess inspiratory efforts is measuring the diaphragmatic pressure–time product (PTPdi) by means of gastric and esophageal balloons; experimental studies have demonstrated that the diaphragmatic thickening fraction has a significant, but often-underwhelming, correlation with PTPdi [ 37 , 39 , 55 , 56 ], while, to date, no study has found a significant correlation between diaphragmatic excursion and PTPdi [ 37 ].
3. Clinical Application of Diaphragm Ultrasound
3.1. clinical case one.
B.M., an obese (body mass, index, BMI, 31 kg/m 2 ) 75-year-old female with no smoking history, was admitted to the emergency department in October 2021 for progressive dyspnea and constrictive chest pain worsened in the last days. Her past medical history included moderate aortic–stenosis and mitral insufficiency, permanent atrial fibrillation, and arterial hypertension. Her chronic pharmacological therapy consisted of a beta-blocker, DOACs, and statin.
A doppler echocardiography revealed a worsening of her mitral insufficiency conditioning pulmonary edema. Accordingly, she was admitted to the cardiology unit, where she received a cardiopulmonary resuscitation for cardiac arrest. Subsequently, she underwent cardiac surgery that consisted of single coronary artery bypass, mitral valvuloplasty, and aortic valve replacement with a bio-prothesis.
She was then transferred to the intensive care unit (ICU), where she was mechanical ventilated. After 3 days, a first trial of extubation was performed, but reintubation was necessary after 6 h. After a week, she underwent percutaneous tracheostomy. Her clinical course in the ICU was complicated by ventilator-associated pneumonia (VAP), with isolation of P. mirabilis on broncho-alveolar lavage treated with ceftazidime, and by methicillin-resistant S. aureus (MRSA) septicemia, treated with daptomycin. Her weaning from tracheostomy was complicated by fever and a further increase in bronchial secretions, which occurred a week after starting antibiotic therapy. A chest HRCT was performed, revealing a mild right pleural effusion, associated with atelectasis of the lower lobe and elevation of the right hemidiaphragm. Given the recent heart surgery, which might have been complicated by phrenic nerve injury, and the presence of right hemidiaphragm elevation, diaphragm ultrasound was performed. Right hemidiaphragm dysfunction was confirmed by the absence of diaphragmatic excursion, in association with reduced muscle thickness and absence of diaphragmatic thickening ( Figure 3 ). Phrenic nerve injury and right hemidiaphragm paresis were then confirmed by electromyography.

Ultrasound evaluation of B.M.’s right hemidiaphragm during resting breathing; upper image, end-exhalation, lower image, end-inhalation. B-mode and M-mode were used for the evaluation of the diaphragm excursion: it can be easily noted that there were no diaphragm movements during resting breathing.
3.2. Clinical Case Two
D.G., a 76-year-old former smoker (25 PKY), with a past medical history of arterial hypertension and a recent diagnosis of pheochromocytoma, was admitted to the intensive care unit (ICU) for acute respiratory failure which occurred after a right adrenalectomy to remove the pheochromocytoma in June 2022. Due to the high risk of cardiocirculatory arrest, he was intubated and mechanically ventilated for 3 days. After extubation, the patient was transferred to our high-dependency respiratory unit, where he was treated with non-invasive ventilation (NIV) and high-flow nasal oxygen for a relapse of respiratory distress and hypoxic respiratory failure. The chest X-ray showed the presence of bilateral inflammatory infiltrates and right hemidiaphragm elevation ( Figure 4 ). Considering the subsequent difficult weaning from NIV, conditioned by the rapid onset of rapid shallow breathing and early respiratory fatigue, we decided to rule out the presence of diaphragmatic dysfunction. The chest ultrasound revealed the presence of a significant elevation of the right hemidiaphragm, associated with pleural effusion and partial atelectasis of the basal lung parenchyma. Although right hemidiaphragm excursion was reduced (hypomobility), the diaphragmatic thickness was within normal limits, the estimated end-expiratory thickness of the right hemidiaphragm being within 2.9 and 4.7 mm. The latter finding allowed us to exclude a diagnosis of diaphragmatic dysfunction; this assessment was later confirmed by the electromyography (EMG) of the diaphragm, that excluded a damage of the phrenic nerve. The patient was therefore treated with large spectrum antibiotic therapy, systemic steroids, and continued with NIV cycles with progressive reduction of the support pressure until weaning from the ventilatory support was achieved. A chest X-ray performed in 2015 and brought by patients’ relatives by the end of the hospital stay, showed a right hemidiaphragm elevation, confirming the pre-existence of the anatomical alteration.

Chest X-ray performed in our unit after clinical worsening of the patient, which documented the presence of right hemidiaphragm elevation.
3.3. Case Discussion
Patients admitted to the emergency department or to the intensive care unit because of acute respiratory failure have considerably high in-hospital and one-year mortality rates [ 57 ]. Diaphragm muscle dysfunction can be involved in many cases of ARF. Diaphragm dysfunction can present as weakness, paralysis, and eventration [ 11 ]; ultrasound evaluation allows the identification or exclusion of diaphragm weakness and paralysis by the presence of abnormalities of thickness, thickening, and motion, as presented in the previous clinical cases. Therefore, ultrasound assessment of the diaphragm might play a central role in the clinical and therapeutical management of the patient.
Diaphragm ultrasound has been already successfully used in patients admitted to the emergency department with ARF, and particularly in patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD) [ 12 , 13 ].
Marchioni and colleagues evaluated the usefulness of early and noninvasive US diaphragm evaluation in patients admitted for acute exacerbation of COPD. They reported that the presence of diaphragm dysfunction on admission, defined as a change in diaphragm thickness less than 20%, was associated with a sixfold increased risk of NIV failure within the first 48 h and a fivefold higher risk of dying during the follow up [ 13 ].
Moreover, Bobbia and colleagues proved that diaphragmatic excursion is feasible and reproducible (at both intra and inter observer level) [ 12 ]. They also reported that, in patients presenting with acute dyspnea or ARF admitted to the emergency department, an excursion higher than 2.3 cm at admission was not correlated with the necessity of NIV [ 12 ].
Diaphragm dysfunction can present also as a complication of thoracic surgery, particularly after coronary artery bypass graft (CABG) [ 58 ]. In these cases, the presence of diabetes, hypertension, and obesity are risk factors for post-operative diaphragm dysfunction [ 59 ]. The consequences of diaphragm dysfunction can be severe, resulting in an increase in mechanical ventilation duration, risk of re-intubation, as presented in our clinical case, and higher risk of mortality [ 60 , 61 ].
To prevent the occurrence of post-surgery diaphragmatic impairment, it has been speculated that diaphragm ultrasound might also help the assessment of pre-operative risk, in addition to the EuroSCORE II (European System for Cardiac Operative Risk Evaluation), in patients undergoing cardiac surgery [ 62 ].
More recently, Chen and colleagues analyzed the use of bedside diaphragm ultrasound in septic patients and acute respiratory failure in ICU [ 63 ]. They observed that the presence of diaphragm dysfunction, defined as reduced excursion, occurred earlier than diaphragm atrophy in septic patients. The severity of the diaphragm dysfunction was also correlated with the severity of the disease and proved to be a predictor of poor outcomes in ICU patients.
Several risk factors for diaphragmatic dysfunction during ARF have been identified ( Table 2 ) [ 60 , 64 ]. Acute diaphragm injury and weakness may result from sepsis, trauma, systemic inflammation, or mechanical ventilation [ 65 ]. Demoule and colleagues identified sepsis as a major independent risk factor for diaphragm dysfunction on ICU admission [ 66 ]. Diaphragm weakness may also be a pre-existent condition and precipitate respiratory failure [ 47 ].
Risk factors for diaphragmatic dysfunction.
Formenti and colleagues demonstrated how ultrasound characteristics of the diaphragm and skeletal muscles such as rectus femoris and intercostals are strongly associated with ICU unfavorable outcomes in intubated patients with COVID-19 and ARDS [ 67 ]. More specifically, muscle echogenicity at ICU admission, quantitatively defined by a greyscale analysis, was significantly lower in survivors in respect to patients that died during the ICU stay, indicating that monitoring of diaphragm architecture in association with function can potentially become a reliable predictor of ICU survival and a possible indicator of the need for early introduction of pharmacological and non-pharmacological therapies capable of preserving muscular architecture and fitness in patients exposed to invasive mechanical ventilation. The same group has recently demonstrated that ICU-acquired weakness cannot be predicted by short-term variations that occur in diaphragmatic thickness in ventilated patients [ 68 ]. In fact, the role of diaphragmatic ultrasound to assess ICU-acquired diaphragmatic dysfunction has been lately investigated as a possible tool for predicting weaning failure in mechanically ventilated patients. The process of weaning is complex and challenging, and the occurrence of weaning failure depends upon numerous factors, among which are respiratory muscle weakness and fatigue [ 69 ]. Respiratory muscle weakness, and in particular diaphragmatic dysfunction, can develop secondary to pre-existing conditions (such as neuromuscular disorders, malnutrition, static and dynamic hyperinflation, endocrine disturbances) and to ventilator-associated muscle dysfunction, sepsis-associated neuro-myopathy, ICU-acquired paresis, and disturbances related to disease and treatment such as severity of respiratory failure and medications [ 69 ], and can derive from an increased resistive and elastic load, or from a deficiency of the contractile properties of the muscle. Therefore, ultrasound-guided evaluation of diaphragmatic performance has been proposed as a tool to guide the readiness for a weaning trial and predict its outcome. Al Tayar et al. showed that, compared with the electrical activity of the diaphragm, diaphragmatic thickening fraction performed better as a predictor of weaning failure [ 70 ]. Two-dimensional speckle tracking to detect diaphragm longitudinal strain has been studied as an index of diaphragmatic dysfunction capable of predicting weaning outcome. Despite acceptable sensitivity and fair specificity, longitudinal strain did not perform better that the rapid shallow breathing index in predicting liberation from mechanical ventilation [ 71 ]. Finally, Palkar and colleagues have proposed the excursion–time index of the diaphragm to estimate the diaphragmatic respiratory work and, consequently, its potential role as a predictor of weaning failure. The authors suggested that an increase in or the maintenance of the excursion–time index (the product of diaphragmatic excursion and inspiratory time) following the suspension of assisted ventilation during a spontaneous breathing trial can be a reliable predictor of weaning outcome [ 48 ]. Despite its promise, to date these indexes have not been validated with the gold standard measurements of diaphragmatic performance (e.g., pressure time product of the diaphragm), and still cannot properly reflect the contribution of the diaphragm or the involvement of accessory respiratory muscles during the different phases of a weaning process.
4. Conclusions
The diaphragm is the main muscle involved in ventilation and whole-body homeostasis. In patients with respiratory impairment or acute respiratory distress due to different etiologies (AECOPD, sepsis, thoracic surgery, pneumoniae, etc.), many factors related to systemic inflammation, prolonged use of steroids, and lung mechanical abnormalities (such as hyperinflation or increased elastic recoil due to pulmonary oedema or fibrosis) may act as synergic mechanisms leading to diaphragm dysfunction [ 72 ]. This explains the heterogeneity of clinical conditions that can be associated with diaphragm abnormalities, as previously described. On the other hand, diaphragmatic impairment is a negative prognostic factor in both chronic and acute respiratory diseases, possibly increasing mortality, length of hospitalization, and risk of treatment failure [ 73 , 74 ]. Since diaphragm ultrasound has proved to be a feasible diagnostic tool in subjects with acute respiratory conditions, including ARF, acute dyspnea, sepsis, or prolonged use of steroids [ 63 ], it can be easily understood how useful it can be for the physician to evaluate the diaphragm with ultrasonography in an emergency setting, ICU, or during hospital stay.
In conclusion, assessment of diaphragmatic anatomy and function by bedside ultrasonography should be encouraged as an integral part of clinical practice in respiratory care.
Acknowledgments
Funding statement.
This research received no external funding.
Author Contributions
Conceptualization, D.R., M.S., P.S. and P.S.; methodology, D.R. and P.S.; writing—original draft preparation, D.R., M.S., S.P., F.D., F.M.M. and P.S.; writing—review and editing, D.R., M.S., S.P., F.D., F.M.M., C.A., F.T. and P.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.

IMAGES
VIDEO
COMMENTS
Diaphragmatic excursion. Diaphragmatic excursion is the movement of the thoracic diaphragm during breathing. Normal diaphragmatic excursion should be 3-5 cm, but can be increased in well-conditioned persons to 7-8 cm. This measures the contraction of the diaphragm. It is performed by asking the patient to exhale and hold it.
A thorough respiratory assessment consists of inspection, palpation, percussion, and auscultation in conjunction with a comprehensive health history. Use a systematic approach and compare findings between left and right so the patient serves as his own control. ... Measure the distance between the marks to determine diaphragmatic excursion ...
Diaphragmatic excursion: Can be evaluated via percussion. Is 4-6 centimeters between full inspiration and full expiration. May be abnormal with hyperinflation, atelectasis, the presence of a pleural effusion, diaphragmatic paralysis, or at times with intra-abdominal pathology.
Over the last 25 years, numerous studies have supported the advantage of ultrasonography (US) in the assessment of diaphragmatic function. Various ultrasonographic methods, such as measurement of diaphragmatic excursions by two-dimensional (BD)[1,2] or M-mode[3,4] and changes in diaphragm thickness during inspiration, have been proposed. In ...
The fluoroscopic sniff test, also known as diaphragm fluoroscopy, is a quick and easy real time fluoroscopic assessment of diaphragmatic motor function (excursion).It is used most often to confirm absence of muscular contraction of the diaphragm during inspiration in patients with phrenic nerve palsy or breathing difficulties following stroke.Chest radiograph demonstrating a newly elevated ...
Furthermore, diaphragmatic excursion is an index for respiratory muscle fatigue during the SBT. Some authors had reported a lower accuracy for diaphragmatic excursion compared to most of the available data and suggested that this lower accuracy is due to the heterogeneity of the patients included in the meta-analyses , . Therefore, separate ...
Diaphragm excursion (motion) ... As a consequence, the assessment of diaphragm function and activity during mechanical ventilation has gained importance in the ICU setting. Several methods are available, including pressure-based parameters, EMG, and ultrasound. Depending on their specific use, these methods can evaluate strength (ie, function ...
Introduction. The diaphragm is the main muscle of respiration [].Diaphragmatic excursion is 1-2 cm during tidal breathing and 7-11 cm during deep inspiration [].The assessment of diaphragmatic function is important for diagnosis and follow up of various physiologic and pathologic conditions [].Several methods exist for the evaluation of diaphragmatic function.
Diaphragmatic excursion is a quantitative measure of expiratory effort as validated by both lung and tracheal volumes in asthma patients, and may be more accurate than qualitative assessment based on tracheal morphology.
Ultrasonography assessment of the diaphragmatic excursion has been proven to have a good inter- and intra-observer reproducibility [24,26]. Diaphragmatic ultrasonography can be limited by a poor acoustic window, for example in obese patients, in the presence of marked abdominal meteorism, or in patients with massive contraction of abdominal ...
The speckle tracking ultrasound is an innovative technology enabling distinct assessment of diaphragmatic movement, yet the relative data are scarce. In this pilot study, we sought to evaluate the predictive value of the weaning outcome of automatic speckle tracking in assessing diaphragm excursion. This is a prospective, multicenter, observational study.
Diaphragmatic excursion is a quantitative measure of expiratory effort as validated by both lung and tracheal volumes in asthma patients, and may be more accurate than qualitative assessment based on tracheal morphology. Diaphragmatic excursion: Quantitative measure to assess adequacy of expiratory phase CT chest images ...
On the other hand, ultrasonographic assessment of excursions of the right diaphragm shows high intra- and interobserver reliability . Reduced movements of the diaphragm are a major risk factor for increased mortality in patients with COPD . However, the relationship between diaphragmatic mobility and DLH remains unclear in patients with COPD.
Diaphragmatic function and BMI (body mass index) To evaluate the function of the diaphragm muscle [], the diaphragmatic excursion was measured at rest and during forced expiration (Supplement Table 1).In 60 patients, diaphragmatic excursion at rest in the supine position was 3.5 cm ± 1.2 on the right side and 3.5 cm ± 1.2 on the left side.
The C/N ratio in different litter types differed significantly at the end of the incubation period depending on the litter type (p < 0.001; litter of all four plant species differed significantly; the minimal C/N ratio was determined in the nettle biotope, maximum, in the fireweed) and biotope (p < 0.001; C/N ratio on average was higher in the nettle biotope than in the other three) as well as ...
A recent consulting project necessitated that I add objective quality measurements to the analysis. The tool I used was the Moscow Statue University Video
Ultrasound assessment of diaphragmatic excursion was done by experienced ultrasonologists. Diaphragmatic excursion for patients was measured on GE make, Voluson S8 series ultrasound machine. The assessment was done in supine position using M-mode and B-mode techniques in quiet and deep breathing scenarios.
* Address correspondence to: Maria V. Sura, National Center for Health Technology Assessment, POB 88, Moscow 117335, Russia. VALUE IN HEALTH REGIONAL ISSUES 2 (2013) 284- 289 Technical evaluation is a preliminary stage of processing the
Welcome to the 16th European Congress of Psychology 2019. that will take place in Moscow, from the 2nd to the 5th of July. The Russian Psychological Society organizes the XVI EFPA Congress that will bring together scientists and practitioners of psychology from all over the world. It is very significant that a country with such a long tradition ...
In most previous studies, diaphragm ultrasonography was used to assess DE max, i.e., the measurement of the excursion of the right hemidiaphragm, as used in this study, and diaphragm thickness that assessed the length and thickness of the zone of apposition of the diaphragm against the rib cage [35, 36].
2. Diaphragm Function Assessment. The diaphragm is an ample dome-shaped muscle which separates the thoracic cavity from the abdomen. It is comprised of three different portions: the central tendon, which is a non-contractile, fibrous structure; the costal portion, which inserts to the rib cage and thoracic vertebrae; and the crural portion, which inserts to the upper three lumbar vertebrae [].