Albatrosses are threatened with extinction – and climate change could put their nesting sites at risk
Postdoctoral research fellow, Department of Plant and Soil Science, University of Pretoria

Disclosure statement
Mia Momberg does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
University of Pretoria provides funding as a partner of The Conversation AFRICA.
View all partners

The wandering albatross ( Diomedea exulans ) is the world’s largest flying bird , with a wingspan reaching an incredible 3.5 metres. These birds are oceanic nomads: they spend most of their 60 years of life at sea and only come to land to breed approximately every two years once they have reached sexual maturity.
Their playground is the vast Southern Ocean – the region between the latitude of 60 degrees south and the continent of Antarctica – and the scattered islands within this ocean where they make their nests.
Marion Island and Prince Edward Island , about 2,300km south of South Africa, are some of the only land masses for thousands of kilometres in the Southern Ocean.
Together, these two islands support about half of the entire world’s wandering albatross breeding population, estimated at around 20,000 mature individuals . Every year scientists from South African universities survey Marion Island to locate and record each wandering albatross nest.
The species, listed as vulnerable by the International Union for Conservation of Nature , faces huge risks while in the open ocean, in particular due to bycatch from longline fishing trawlers. This makes it important to understand their breeding ecology to ensure that the population remains stable.

I was part of a study during 2021 to investigate which environmental variables affect the birds’ choice of nest site on Marion Island. The birds make their nests – a mound of soil and vegetation – on the ground. We looked at wind characteristics, vegetation and geological characteristics at nest locations from three breeding seasons.
Elevation turned out to be the most important variable – the albatrosses preferred a low (warmer) site and coastal vegetation. But these preferences also point to dangers for the birds from climate change. The greatest risk to the availability of nesting sites will be a much smaller suitable nesting range in future than at present. This could be devastating to the population.
Variables influencing nest site selection
Marion Island is of volcanic origin and has a rough terrain. Some areas are covered in sharp rock and others are boggy, with very wet vegetation. There is rain and strong wind on most days. Conducting research here requires walking long distances in all weathers – but the island is ideal for studying climate change, because the Southern Ocean is experiencing some of the largest global changes in climate and it is relatively undisturbed by humans.
Using GPS coordinate nest data from the entire breeding population on Marion Island, we aimed to determine which factors affected where the birds breed. With more than 1,900 nests, and 10,000 randomly generated points where nests are not present, we extracted:
elevation (which on this island is also a proxy for temperature)
terrain ruggedness
distance to the coast
vegetation type
wind turbulence
underlying geology.

The variables were ranked according to their influence on the statistical model predicting the likelihood of a nest being present under the conditions found at a certain point.
The most important variable was elevation. The majority of the nests were found close to the coast, where the elevation is lower. These areas are warmer, which means that the chicks would be less exposed to very cold temperatures on their open nests.
The probability of nests being present also declined with distance from the coast, probably because there are more suitable habitats closer to the coast.
Vegetation type was strongly determined by elevation and distance from the coast. This was an important factor, as the birds use vegetation to build their nests. In addition, dead vegetation contributes to the soil formation on the island, which is also used in nest construction.

The probability of encountering nests is lower as the terrain ruggedness increases since these birds need a runway of flat space to use for take-off and landing. During incubation, the adults take turns to remain on the nest. Later they will leave the chick on its own for up to 10 days at a time. They continue to feed the chick for up to 300 days.
Areas with intermediate wind speeds were those most likely to have a nest. At least some wind is needed for flight, but too much wind may cause chicks to blow off the nests or become too cold.
Delicate balance
Changing climates may upset this delicate balance. Human-driven changes will have impacts on temperature, rainfall and wind speeds, which in turn affect vegetation and other species distribution patterns .
By 2003, Marion Island’s temperature had increased by 1.2°C compared to 50 years before. Precipitation had decreased by 25% and cloud cover also decreased, leading to an increase in sunshine hours . The permanent snowline which was present in the 1950s no longer exists . These changes have continued in the 20 years since their initial documentation, and are likely to continue.
Strong vegetation shifts were already documented in the sub-Antarctic years ago. Over 40 years, many species have shifted their ranges to higher elevations where the temperatures remain cooler. Wind speeds have also already increased in the Southern Ocean and are predicted to continue doing so, which may have effects on the size of areas suitable for nesting.
If nesting sites move to higher elevations on Marion Island as temperatures warm, and some areas become unsuitable due to changes in vegetation or wind speeds, it is likely that the suitable nesting area on the island will shrink considerably.
Our study adds to what is known about the elements affecting nest-site selection in birds. Notably, we add knowledge of wind, an underexplored element, influencing nest-site selection in a large oceanic bird. The results could also provide insights that apply to other surface-nesting seabirds.
- Climate change
- Southern ocean
- Natural world

Project Offier - Diversity & Inclusion

Senior Lecturer - Earth System Science

Sydney Horizon Educators (Identified)

Deputy Social Media Producer

Associate Professor, Occupational Therapy
You are using an outdated browser. Please upgrade your browser to improve your experience and security.

- Buy Tickets
- Join & Give
Wandering Albatross
- Updated 28/07/23
- Read time 2 minutes
- Share this page:
- Share on Facebook
- Share on Twitter
- Share on Linkedin
- Share via Email
- Print this page

- IUCN Conservation Status VULNERABLE (VU)
- Classification Genus Diomedea Species exulans Family Diomedeidae Order Procellariiformes Class Aves
- Size Range 80 cm to 135 cm
The Wandering Albatross is the largest of the albatrosses and is the living bird with the greatest wingspan, measuring almost 3.5 m.
What do Wandering Albatrosses look like?
Identification.
The adult Wandering Albatross appears entirely white from a distance. Close up, the fine black wavy lines on the breast, neck and upper back become visible. The bill can vary in colour, but is normally yellowish-pink. The white tail is occasionally tipped with black and the back of the wing changes from black to white with age. A series of plumage phases are passed through as young birds reach full adult plumage, which can take up to nine years. Females are slightly smaller than males.
Where do Wandering Albatrosses live?
Wandering Albatrosses spend most of their life in flight, landing only to breed and feed. Distances travelled each year are hard to measure, but one banded bird was recorded travelling 6000 km in twelve days.
Distribution
The Wandering Albatross visits Australian waters from Fremantle, Western Australia to northern New South Wales between June and September each year. At other times birds roam the southern oceans and commonly follow fishing boats for several days.
What do Wandering Albatrosses eat?
Feeding and diet.
Wandering Albatrosses are often seen scavenging scraps from fishing boats, but squid and fish are the preferred foods. Galley refuse and floating waste also form part of the diet. Feeding is one of the few times that birds land, and this is mostly undertaken at night.
What are Wandering Albatrosses breeding behaviours?
Breeding behaviour/s.
Pairs of Wandering Albatrosses mate for life and breed every two years. Breeding takes place on subantarctic islands and commences in early November. The nest is a mound of mud and vegetation, and is placed on an exposed ridge near the sea. During the early stages of the chick's development, the parents take turns to sit on the nest while the other searches for food. Later, both adults hunt for food and visit the chick at irregular intervals.
Breeding Season: November.

The Australian Museum respects and acknowledges the Gadigal people as the First Peoples and Traditional Custodians of the land and waterways on which the Museum stands.
Image credit: gadigal yilimung (shield) made by Uncle Charles Chicka Madden
Animal Diversity Web
- About Animal Names
- Educational Resources
- Special Collections
- Browse Animalia
More Information
Additional information.
- Encyclopedia of Life
Diomedea exulans wandering albatross

Geographic Range
Wandering albatrosses are found almost exclusively in the Southern Hemisphere, although occasional sightings just north of the Equator have been reported. ( Birdlife International, 2006 ; Shirihai, 2002 )
There is some disagreement over how many subspecies of wandering albatross ( Diomedea exulans ) there are, and whether they should be considered separate species. Most subspecies of Diomedea exulans are difficult to tell apart, especially as juveniles, but DNA analyses have shown that significant differences exist. ( Birdlife International, 2006 ; Shirihai, 2002 )
Diomedea exulans exulans breeds on South Georgia, Prince Edward, Marion, Crozet, Kerguelen, and Macquarie islands. Diomedea exulans dabbenena occurs on Gough and Inaccessible islands, ranging over the Atlantic Ocean to western coastal Africa. Diomedea exulans antipodensis is found primarily on the Antipodes of New Zealand, and ranges at sea from Chile to eastern Australia. Diomedea exulans amsterdamensis is found only on Amsterdam Island and the surrounding seas. Other subspecies names that have become obsolete include Diomedea exulans gibsoni , now commonly considered part of D. e. antipodensis , and Diomedea exulans chionoptera , considered part of D. e. exulans . ( Birdlife International, 2006 ; Shirihai, 2002 )
- Biogeographic Regions
Wandering albatrosses breed on several subantarctic islands, which are characterized by peat soils, tussock grass, sedges, mosses, and shrubs. Wandering albatrosses nest in sheltered areas on plateaus, ridges, plains, or valleys.
Outside of the breeding season, wandering albatrosses are found only in the open ocean, where food is abundant. ( Birdlife International, 2006 ; Shirihai, 2002 )
- Habitat Regions
- terrestrial
- saltwater or marine
- Terrestrial Biomes
- savanna or grassland
- Aquatic Biomes
Physical Description
All subspecies of wandering albatrosses have extremely long wingspans (averaging just over 3 meters), white underwing coverts, and pink bills. Adult body plumage ranges from pure white to dark brown, and the wings range from being entirely blackish to a combination of black with white coverts and scapulars. They are distinguished from the closely related royal albatross by their white eyelids, pink bill color, lack of black on the maxilla, and head and body shape. On average, males have longer bills, tarsi, tails, and wings than females. ( Birdlife International, 2006 ; Shirihai, 2002 ; Tickell, 1968 )
Juveniles of all subspecies are very much alike; they have chocolate-brown plumage with a white face and black wings. As individuals age, most become progressively whiter with each molt, starting with the back. ( Birdlife International, 2006 ; Shirihai, 2002 ; Tickell, 1968 )
D. e. exulans averages larger than other recognized subspecies, and is the only taxon that achieves fully white body plumage, and this only in males. Although females do not become pure white, they can still be distinguished from other subspecies by color alone. Adults also have mostly white coverts, with black only on the primaries and secondaries. ( Birdlife International, 2006 ; Shirihai, 2002 ; Tickell, 1968 )
Adults of D. e. amsterdamensis have dark brown plumage with white faces and black crowns, and are distinguished from juveniles by their white bellies and throats. In addition to their black tails, they also have a black stripe along the cutting edge of the maxilla, a character otherwise found in D. epomophora but not other forms of D. exulans . Males and females are similar in plumage. ( Birdlife International, 2006 ; Shirihai, 2002 ; Tickell, 1968 )
Adults of D. e. antipodensis display sexual dimorphism in plumage, with older males appearing white with some brown splotching, while adult females have mostly brown underparts and a white face. Both sexes also have a brown breast band. ( Birdlife International, 2006 ; Shirihai, 2002 ; Tickell, 1968 )
With age, D. e. dabbenena gradually attains white plumage, although it never becomes as white as male D. e. exulans . The wing coverts also appear mostly black, although there may be white patches. Females have more brown splotches than males, and have less white in their wing coverts. ( Birdlife International, 2006 ; Shirihai, 2002 ; Tickell, 1968 )
- Other Physical Features
- endothermic
- homoiothermic
- bilateral symmetry
- Sexual Dimorphism
- sexes alike
- male larger
- sexes colored or patterned differently
- Average mass 8130 g 286.52 oz AnAge
- Range length 1.1 to 1.35 m 3.61 to 4.43 ft
- Range wingspan 2.5 to 3.5 m 8.20 to 11.48 ft
- Average wingspan 3.1 m 10.17 ft
- Average basal metabolic rate 20.3649 W AnAge
Reproduction
Wandering albatrosses have a biennial breeding cycle, and pairs with chicks from the previous season co-exist in colonies with mating and incubating pairs. Pairs unsuccessful in one year may try to mate again in the same year or the next one, but their chances of successfully rearing young are low. ( Shirihai, 2002 ; Tickell, 1968 )
After foraging at sea, males arrive first at the same breeding site every year within days of each other. They locate and reuse old nests or sometimes create new ones. Females arrive later, over the course of a few weeks. Wandering albatrosses have a monogamous mating strategy, forming pair bonds for life. Females may bond temporarily with other males if their partner and nest are not readily visible. ( Shirihai, 2002 ; Tickell, 1968 )
- Mating System
Copulation occurs in the austral summer, usually around December (February for D. e. amsterdamensis ). Rape and extra-pair copulations are frequent, despite their monogamous mating strategy. Pairs nest on slopes or valleys, usually in the cover of grasses or shrubs. Nests are depressions lined with grass, twigs, and soil. A single egg is laid and, if incubation or rearing fails, pairs usually wait until the following year to try again. Both parents incubate eggs, which takes about 78 days on average. Although females take the first shift, males are eager to take over incubation and may forcefully push females off the egg. Untended eggs are in danger of predation by skuas ( Stercorarius ) and sheathbills ( Chionis ). ( Shirihai, 2002 ; Tickell, 1968 )
After the chick hatches, they are brooded for about 4 to 6 weeks until they can be left alone at the nest. Males and females alternate foraging at sea. Following the brooding period, both parents leave the chick by itself while they forage. The chicks are entirely dependent on their parents for food for 9 to 10 months, and may wait weeks for them to return. Chicks are entirely independent once they fledge. ( Shirihai, 2002 ; Tickell, 1968 )
Some individuals may reach sexual maturity by age 6. Immature, non-breeding individuals will return to the breeding site. Group displays are common among non-breeding adults, but most breeding adults do not participate. ( Shirihai, 2002 ; Tickell, 1968 )
- Key Reproductive Features
- iteroparous
- seasonal breeding
- gonochoric/gonochoristic/dioecious (sexes separate)
- Breeding interval Breeding occurs biennially, possibly annually if the previous season's attempt fails.
- Breeding season Breeding occurs from December through March.
- Average eggs per season 1
- Range time to hatching 74 to 85 days
- Range fledging age 7 to 10 months
- Range time to independence 7 to 10 months
- Range age at sexual or reproductive maturity (female) 6 to 22 years
- Average age at sexual or reproductive maturity (female) 10 years
- Range age at sexual or reproductive maturity (male) 6 to 22 years
- Average age at sexual or reproductive maturity (male) 10 years
Males choose the nesting territory, and stay at the nest site more than females before incubation. Parents alternate during incubation, and later during brooding and feeding once the chick is old enough to be left alone at the nest. Although there is generally equal parental investment, males will tend to invest more as the chick nears fledging. Occasionally, a single parent may successfully rear its chick. ( Shirihai, 2002 ; Tickell, 1968 )
- Parental Investment
- provisioning
Lifespan/Longevity
Wandering albatrosses are long-lived. An individual nicknamed "Grandma" was recorded to live over 60 years in New Zealand. Due to the late onset of maturity, with the average age at first breeding about 10 years, such longevity is not unexpected. However, there is fairly high chick mortality, ranging from 30 to 75%. Their slow breeding cycle and late onset of maturity make wandering albatrosses highly susceptible to population declines when adults are caught as bycatch in fishing nets. ( Birdlife International, 2006 ; Shirihai, 2002 ; Tickell, 1968 )
- Range lifespan Status: wild 60 (high) years
- Average lifespan Status: wild 415 months Bird Banding Laboratory
While foraging at sea, wandering albatrosses travel in small groups. Large feeding frenzies may occur around fishing boats. Individuals may travel thousands of kilometers away from their breeding grounds, even occasionally crossing the equator.
During the breeding season, Wandering albatrosses are gregarious and displays are common (see “Communication and Perception” section, below). Vocalizations and displays occur during mating or territorial defense. ( Shirihai, 2002 ; Tickell, 1968 )
- Key Behaviors
- territorial
- Average territory size 1 m^2
Wandering albatrosses defend small nesting territories, otherwise the range within which they travel is many thousands of square kilometers. ( Shirihai, 2002 ; Tickell, 1968 )
Communication and Perception
Displays and vocalizations are common when defending territory or mating. They include croaks, bill-clapping, bill-touching, skypointing, trumpeting, head-shaking, the "ecstatic" gesture, and "the gawky-look". Individuals may also vocalize when fighting over food. ( Shirihai, 2002 )
- Communication Channels
- Perception Channels
Food Habits
Wandering albatrosses primarily eat fish, such as toothfish ( Dissostichus ), squids, other cephalopods, and occasional crustaceans. The primary method of foraging is by surface-seizing, but they have the ability to plunge and dive up to 1 meter. They will sometimes follow fishing boats and feed on catches with other Procellariiformes , which they usually outcompete because of their size. ( Birdlife International, 2006 ; Shirihai, 2002 )
- Primary Diet
- molluscivore
- Animal Foods
- aquatic crustaceans
Although humans formerly hunted wandering albatrosses as food, adults currently have no predators. Their large size, sharp bill, and occasionally aggressive behavior make them undesirable opponents. However, some are inadvertently caught during large-scale fishing operations.
Chicks and eggs, on the other hand, are susceptible to predation from skuas and sheathbills, and formerly were harvested by humans as well. Eggs that fall out of nests or are unattended are quickly preyed upon. Nests are frequently sheltered with plant material to make them less conspicuous. Small chicks that are still in the brooding stage are easy targets for large carnivorous seabirds. Introduced predators, including mice, pigs, cats, rats, and goats are also known to eat eggs and chicks. ( Birdlife International, 2006 ; IUCN, 2006 ; Shirihai, 2002 ; Tickell, 1968 )
- skuas ( Stercorariidae )
- sheathbills ( Chionis )
- domestic cats ( Felis silvestris )
- introduced pigs ( Sus scrofa )
- introduced goats ( Capra hircus )
- introduced rats ( Rattus rattus and Rattus norvegicus )
- introduced mice ( Mus musculus )
Ecosystem Roles
Wandering albatrosses are predators, feeding on fish, cephalopods, and crustaceans. They are known for their ability to compete with other seabirds for food, particularly near fishing boats. Although adult birds, their eggs, and their chicks were formerly a source of food to humans, such practices have been stopped. ( IUCN, 2006 ; Shirihai, 2002 )
Economic Importance for Humans: Positive
Wandering albatrosses have extraordinary morphology, with perhaps the longest wingspan of any bird. Their enormous size also makes them popular in ecotourism excursions, especially for birders. Declining population numbers also mean increased conservation efforts. Their relative tameness towards humans makes them ideal for research and study. ( Shirihai, 2002 )
- Positive Impacts
- research and education
Economic Importance for Humans: Negative
Wandering albatrosses, along with other seabirds, follow fishing boats to take advantage of helpless fish and are reputed to reduce economic output from these fisheries. Albatrosses also become incidental bycatch, hampering conservation efforts. ( Birdlife International, 2006 ; IUCN, 2006 ; Shirihai, 2002 )
Conservation Status
Diomedea exulans exulans and Diomedea exulans antipodensis are listed by the IUCN Red list and Birdlife International as being vulnerable; Diomedea exulans dabbenena is listed as endangered, and Diomedea exulans amsterdamensis is listed as critically endangered.
All subspecies of Diomedea exulans are highly vulnerable to becoming bycatch of commercial fisheries, and population declines are mostly attributed to this. Introduced predators such as feral cats , pigs , goats , and rats on various islands leads to high mortality rates of chicks and eggs. Diomedea exulans amsterdamensis is listed as critically endangered due to introduced predators, risk of becoming bycatch, small population size, threat of chick mortality by disease, and loss of habitat to cattle farming.
Some conservation measures that have been taken include removal of introduced predators from islands, listing breeding habitats as World Heritage Sites, fishery relocation, and population monitoring. ( Birdlife International, 2006 ; IUCN, 2006 ; Shirihai, 2002 )
- IUCN Red List Vulnerable More information
- US Migratory Bird Act No special status
- US Federal List No special status
- CITES No special status
Contributors
Tanya Dewey (editor), Animal Diversity Web.
Lauren Scopel (author), Michigan State University, Pamela Rasmussen (editor, instructor), Michigan State University.
the body of water between Africa, Europe, the southern ocean (above 60 degrees south latitude), and the western hemisphere. It is the second largest ocean in the world after the Pacific Ocean.

body of water between the southern ocean (above 60 degrees south latitude), Australia, Asia, and the western hemisphere. This is the world's largest ocean, covering about 28% of the world's surface.
uses sound to communicate
young are born in a relatively underdeveloped state; they are unable to feed or care for themselves or locomote independently for a period of time after birth/hatching. In birds, naked and helpless after hatching.
having body symmetry such that the animal can be divided in one plane into two mirror-image halves. Animals with bilateral symmetry have dorsal and ventral sides, as well as anterior and posterior ends. Synapomorphy of the Bilateria.
an animal that mainly eats meat
uses smells or other chemicals to communicate
the nearshore aquatic habitats near a coast, or shoreline.
used loosely to describe any group of organisms living together or in close proximity to each other - for example nesting shorebirds that live in large colonies. More specifically refers to a group of organisms in which members act as specialized subunits (a continuous, modular society) - as in clonal organisms.
- active during the day, 2. lasting for one day.
humans benefit economically by promoting tourism that focuses on the appreciation of natural areas or animals. Ecotourism implies that there are existing programs that profit from the appreciation of natural areas or animals.
animals that use metabolically generated heat to regulate body temperature independently of ambient temperature. Endothermy is a synapomorphy of the Mammalia, although it may have arisen in a (now extinct) synapsid ancestor; the fossil record does not distinguish these possibilities. Convergent in birds.
offspring are produced in more than one group (litters, clutches, etc.) and across multiple seasons (or other periods hospitable to reproduction). Iteroparous animals must, by definition, survive over multiple seasons (or periodic condition changes).
eats mollusks, members of Phylum Mollusca
Having one mate at a time.
having the capacity to move from one place to another.
the area in which the animal is naturally found, the region in which it is endemic.
generally wanders from place to place, usually within a well-defined range.
islands that are not part of continental shelf areas, they are not, and have never been, connected to a continental land mass, most typically these are volcanic islands.
reproduction in which eggs are released by the female; development of offspring occurs outside the mother's body.
An aquatic biome consisting of the open ocean, far from land, does not include sea bottom (benthic zone).
an animal that mainly eats fish
the regions of the earth that surround the north and south poles, from the north pole to 60 degrees north and from the south pole to 60 degrees south.
mainly lives in oceans, seas, or other bodies of salt water.
breeding is confined to a particular season
reproduction that includes combining the genetic contribution of two individuals, a male and a female
associates with others of its species; forms social groups.
uses touch to communicate
that region of the Earth between 23.5 degrees North and 60 degrees North (between the Tropic of Cancer and the Arctic Circle) and between 23.5 degrees South and 60 degrees South (between the Tropic of Capricorn and the Antarctic Circle).
Living on the ground.
defends an area within the home range, occupied by a single animals or group of animals of the same species and held through overt defense, display, or advertisement
A terrestrial biome. Savannas are grasslands with scattered individual trees that do not form a closed canopy. Extensive savannas are found in parts of subtropical and tropical Africa and South America, and in Australia.
A grassland with scattered trees or scattered clumps of trees, a type of community intermediate between grassland and forest. See also Tropical savanna and grassland biome.
A terrestrial biome found in temperate latitudes (>23.5° N or S latitude). Vegetation is made up mostly of grasses, the height and species diversity of which depend largely on the amount of moisture available. Fire and grazing are important in the long-term maintenance of grasslands.
uses sight to communicate
Birdlife International, 2006. "Species factsheets" (On-line). Accessed November 07, 2006 at http://www.birdlife.org .
IUCN, 2006. "2006 IUCN Red List of Threatened Species" (On-line). Accessed November 06, 2006 at http://www.iucnredlist.org .
Shirihai, H. 2002. The Complete Guide to Antarctic Wildlife . New Jersey: Princeton University Press.
Tickell, W. 1968. Biology of Great Albatrosses. Pp. 1-53 in Antarctic Bird Studies . Baltimore: Horn-Schafer.
The Animal Diversity Web team is excited to announce ADW Pocket Guides!
Read more...
Search in feature Taxon Information Contributor Galleries Topics Classification
- Explore Data @ Quaardvark
- Search Guide
Navigation Links
Classification.
- Kingdom Animalia animals Animalia: information (1) Animalia: pictures (22861) Animalia: specimens (7109) Animalia: sounds (722) Animalia: maps (42)
- Phylum Chordata chordates Chordata: information (1) Chordata: pictures (15213) Chordata: specimens (6829) Chordata: sounds (709)
- Subphylum Vertebrata vertebrates Vertebrata: information (1) Vertebrata: pictures (15168) Vertebrata: specimens (6827) Vertebrata: sounds (709)
- Class Aves birds Aves: information (1) Aves: pictures (7311) Aves: specimens (153) Aves: sounds (676)
- Order Procellariiformes tube-nosed seabirds Procellariiformes: pictures (48) Procellariiformes: specimens (15)
- Family Diomedeidae albatrosses Diomedeidae: pictures (27) Diomedeidae: specimens (6)
- Genus Diomedea royal and wandering albatrosses Diomedea: pictures (5) Diomedea: specimens (4)
- Species Diomedea exulans wandering albatross Diomedea exulans: information (1) Diomedea exulans: pictures (3)
To cite this page: Scopel, L. 2007. "Diomedea exulans" (On-line), Animal Diversity Web. Accessed April 24, 2024 at https://animaldiversity.org/accounts/Diomedea_exulans/
Disclaimer: The Animal Diversity Web is an educational resource written largely by and for college students . ADW doesn't cover all species in the world, nor does it include all the latest scientific information about organisms we describe. Though we edit our accounts for accuracy, we cannot guarantee all information in those accounts. While ADW staff and contributors provide references to books and websites that we believe are reputable, we cannot necessarily endorse the contents of references beyond our control.
- U-M Gateway | U-M Museum of Zoology
- U-M Ecology and Evolutionary Biology
- © 2020 Regents of the University of Michigan
- Report Error / Comment
This material is based upon work supported by the National Science Foundation Grants DRL 0089283, DRL 0628151, DUE 0633095, DRL 0918590, and DUE 1122742. Additional support has come from the Marisla Foundation, UM College of Literature, Science, and the Arts, Museum of Zoology, and Information and Technology Services.
The ADW Team gratefully acknowledges their support.
- Text account
- Data table and detailed info
- Distribution map
- Reference and further resources
In 1998, the total annual breeding population was estimated at 8,500 pairs, equivalent to c. 28,000 mature individuals (Gales 1998). However, current estimates are 1,553 pairs on South Georgia (Georgias del Sur) (Poncet et al. 2006), 1,800 pairs on Prince Edward Island (2008, Ryan et al. 2009), c. 1,900 pairs on Marion Island (2013, ACAP 2009), c. 340 pairs on Iles Crozet (CNRS Chinzè Monitoring Database 2010), c. 354 pairs in Iles Kerguelen (CNRS Chinzè Monitoring Database 2011), and 4 pairs on Macquarie Island (DPIWPE 2010, unpublished data), making a total of c. 6,000 annual breeding pairs. Using the same ratio as Gales (1998) for estimating the number of mature individuals, this would equate to approximately 20,100 mature individuals.
Diomedea exulans breeds on South Georgia (Georgias del Sur) (c. 18% of the global breeding population), Prince Edward Islands ( South Africa ) (c. 44% of the global population), Crozet Islands and Kerguelen Islands ( French Southern Territories ) (approximately 38% of the global population) and Macquarie Island ( Australia ) (approximately four pairs breeding per year), with a total global population of c. 8,050 pairs breeding in any given year (ACAP 2009). At South Georgia, the population declined by 1.8% per annum between 1984 and 2004 (Poncet et al. 2006), and continued to decline by 1.8% per annum between 2004 and 2015 (A. Wolfaardt in litt . 2016). The population on Crozet declined by 54% between 1970 and 1986. From the mid-1980s to late 1990s, the Crozet, Kerguelen and Prince Edward Islands populations appeared to be stable or increasing (Weimerskirch et al. 1997, Weimerskirch and Jouventin 1998, Crawford et al. 2003, Ryan et al. 2003), but declines were later detected (H. Weimerskirch in litt. 2008, Ryan et al. 2009). Overall declines are estimated to exceed 30% over 70 years. Recovery is believed to be impeded by a decline in recruitment rate (Weimerskirch et al. 2006). Non-breeding and juvenile birds remain north of 50°S between subantarctic and subtropical waters with a significant proportion crossing the Indian Ocean to wintering grounds around the southern and eastern coast of Australia (Weimerskirch et al. 2014). A significant proportion of the Crozet and Kerguelen populations disperse into the Pacific and the western coast of South America (Weimerskirch et al. 2014, 2015).
Behaviour Diomedea exulans is a biennial breeding species, although about 30% of successful and 35% of failed breeders (on average) defer breeding beyond the expected year. Adults return to colonies in November, and eggs are laid over a period of 5 weeks during December and January. Most chicks hatch in March and fledge in December. Birds usually return to colonies when 5-7 years old, though they can return when as young as 3 years old. Birds can start breeding as young as 7 or 8 years old, but more typically at 10-12 years old (Tickell 2000). Wandering Albatross typically forages in oceanic waters, however considerable time is spent over shelf areas during certain stages of the breeding season (BirdLife International 2004). Satellite tracking has revealed that juvenile birds tend to forage further north than adults (Weimerskirch et al. 2006, British Antarctic Survey, unpubl. data ), bringing them into greater overlap with longline tuna fleets which may be driving falls in recruitment rates (Weimerskirch et al. 1997). Females may also be at greater risk of being caught in tuna fisheries since they tend to forage further north than males (Nel et al. 2002, Weimerskirch et al. 2003, Pinaud and Weimerskirch 2007, Jiménez et al . 2016). It is mostly a diurnal breeder, taking most prey by surface-seizing (ACAP 2009).
Habitat Breeding Wandering Albatross nests in open or patchy vegetation near exposed ridges or hillocks (Carboneras 1992).
Diet Adults feed at sea mainly on cephalopods and fish, often following ships and feeding on offal and galley refuse (Carboneras 1992, Cherel and Klages 1998). Patagonian Toothfish Dissostichus eleginoides is the primary fish species in the diet, potentially obtained as discarded offal (Xavier et al . 2004).
Foraging range This wide-ranging species has a circumpolar distribution, and both breeding and non-breeding birds have very large foraging ranges. Satellite tracking data indicate that breeding birds forage at very long distances from colonies (up to 4,000 km) and that foraging strategies change throughout the breeding season (Froy et al. 2015). A fledgling covered 6,590 km in 28 days after leaving the colony on Marion Island (Clokie 2007).
The observed decline of this species has been shown to be driven largely by incidental catch in fisheries, which has reduced adult survival and juvenile recruitment (Rolland et a l. 2010, Pardo et al . 2017). Fisheries were responsible for a 54% decrease in numbers on the Crozet Islands between 1970 and 1986 (Weimerskirch et al . 1997). The South Georgia population is declining rapidly, but other populations (Prince Edward Islands and Crozet Islands) have shown signs of recovery. The South Georgia population disperses throughout the Southern Ocean during the nonbreeding season, although may be most at risk from longline fisheries operating in the south-west Atlantic throughout the year (Jiménez et al . 2014, 2016, Tancell et al . 2016), whereas the Crozet and Prince Edward Island populations are most vulnerable to pelagic longline fishing in the Indian Ocean and Australian region (Weimerskirch 1998, Nel et al. 2002c). The apparent recovery of populations from the Crozet and Prince Edward Islands during the early 1990s was ascribed to reduced fishing effort and relocation of fisheries away from foraging grounds, however increased effort in the late 1990s at various different localities may once again be impacting these populations (Weimerskirch et al . 1997, Nel et al . 2002b), as even low bycatch rates will affect the species due to the small population size (ACAP 2009). The Macquarie population was harvested extensively by sealers and despite recoveries in the early 20th century, it experienced subsequent declines that were also attributed to longline fisheries (de la Mare and Kerry 1994). Additionally, chicks are vulnerable to the accumulation of anthropogenic debris and fishing hooks, which may kill a small number annually (Nel and Nel 1999, Phillips et al. 2010, 2016).
The impacts of predation by introduced species are severe for some breeding populations. On Kerguelen, in some years, certain colonies have suffered complete breeding failure due to chick predation by cats Felis catus (H. Weimerskirch in litt . 2008). House mice Mus musculus have been recorded attacking Wandering Albatross chicks on Marion Island since 2003 and continue to affect up to 1% of the population (Dilley et al . 2015). There has been extensive habitat loss and degradation at South Georgia (Islas Georgias del Sur) due to the activities of Antarctic Fur Seals Arctocephalus gazelle (ACAP 2009).
Text account compilers Stattersfield, A., Stuart, A., Sullivan, B., Symes, A., Fjagesund, T., Hermes, C., Calvert, R., Anderson, O., Martin, R., Moreno, R., Nel, D., Small, C.
Contributors Crawford, R., Weimerskirsch, H., Cooper, J., Ryan, P.G., Croxall, J., Wolfaardt, A., Gales, R., Phillips, R.
Recommended citation BirdLife International (2024) Species factsheet: Diomedea exulans . Downloaded from https://datazone.birdlife.org/species/factsheet/wandering-albatross-diomedea-exulans on 24/04/2024. Recommended citation for factsheets for more than one species: BirdLife International (2024) IUCN Red List for birds. Downloaded from https://datazone.birdlife.org on 24/04/2024.
Taxonomic source(s) AERC TAC. 2003. AERC TAC Checklist of bird taxa occurring in Western Palearctic region, 15th Draft. Available at: #http://www.aerc.eu/DOCS/Bird_taxa_of _the_WP15.xls# . Brooke, M. de L. 2004. Albatrosses and Petrels Across the World . Oxford University Press, Oxford. Christidis, L. and Boles, W.E. 2008. Systematics and Taxonomy of Australian Birds . CSIRO Publishing, Collingwood, Australia. del Hoyo, J., Collar, N.J., Christie, D.A., Elliott, A. and Fishpool, L.D.C. 2014. HBW and BirdLife International Illustrated Checklist of the Birds of the World. Volume 1: Non-passerines . Lynx Edicions BirdLife International, Barcelona, Spain and Cambridge, UK. Robertson, C. J. R.; Nunn, G. B. 1998. Towards a new taxonomy for albatrosses. In: Robertson, G.; Gales, R. (ed.), Albatross biology and conservation , pp. 13-19. Surrey Beatty & Sons, Chipping Norton, Australia. SACC. 2005 and updates. A classification of the bird species of South America. Available at: #http://www.museum.lsu.edu/~Remsen/SACCBaseline.htm# .
Related state of the world's birds case studies
- Many albatross species are in alarming slow decline
- Seabirds suffer from eating "junk"
- Tracking devices provide new insights into seabird distribution
- The wildlife of the Tasman Sea is facing a range of threats
ACAP. 2009. ACAP Species Assessment: Wandering Albatross Diomedea exulans . Available at: #http://www.acap.aq/acap-species/download-document/1207-wandering-albatross# .
BirdLife International. 2004. Tracking ocean wanderers: the global distribution of albatrosses and petrels . BirdLife International, Cambridge, U.K.
Carboneras, C. 1992. Diomedeidae (Albatrosses). In: del Hoyo, J.; Elliott, A.; Sargatal, J. (ed.), Handbook of the birds of the world , pp. 198-215. Lynx Edicions, Barcelona, Spain.
Cherel, Y.; Klages, N. 1998. A review of the food of albatrosses. In: Robertson, G.; Gales, G. (ed.), Albatross biology and conservation , pp. 113-136. Surrey Beatty & Sons, Chipping Norton, Australia.
Clokie, L. 2007. A little wanderer. Bee-eater 58(4): 65-66.
Crawford, R. J. M.; Cooper, J.; Dyer, B. M.; Greyling, M.; Klages, N. T. W.; Ryan, P. G.; Petersen, S.; Underhill, L. G.; Upfold, L.; Wilkinson, W.; de Villiers, M.; du Plessis, S.; du Toit, M.; Leshoro, T. M.;…authors continued in notes. 2003. Populations of surface nesting seabirds at Marion Island, 1994/95-2002/03. African Journal of Marine Science 25: 427-440.
Croxall, J. P. and Gales, R. 1998. Assessment of the conservation status of albatrosses. In: Robertson, G. and Gales, R. (eds), Albatross biology and conservation , pp. 46-65. Surrey Beatty & Sons, Chipping Norton, Australia.
de la Mare, W. K.; Kerry, K. R. 1994. Population dynamics of the Wandering Albatross ( Diomedea exulans ) on Macquarie Island and the effects of mortality from longline fishing. Polar Biology 14: 231-241.
Delord, K.; Besson, D.; Barbraud, C.; Weimerskirch, H. 2008. Population trends in a community of large Procellariforms of Indian Ocean: potential effects of environment and fisheries interactions. Biological Conservation 141(7): 1840-1856.
Dilley, B. J., Schoombie, S., Schoombie, J., Ryan, P. G. 2016. Scalping’of albatross fledglings by introduced mice spreads rapidly at Marion Island. Antarctic Science 28(02): 73-80.
Froy, H., Lewis, S., Catry, P., Bishop, C.M., Forster, I.P., Fukuda, A., Higuchi, H., Phalan, B., Xavier, J.C., Nussey, D.H., Phillips, R.A. 2015. Age-related variation in foraging behaviour in the wandering albatross at South Georgia: no evidence for senescence. PloS one 10(1): p.e0116415.
Gales, R. 1998. Albatross populations: status and threats. In: Robertson, G.; Gales, R. (ed.), Albatross biology and conservation , pp. 20-45. Surrey Beatty & Sons, Chipping Norton, Australia.
Gales, R.; Brothers, N.; Reid, T. 1998. Seabird mortality in the Japanese tuna longline fishery around Australia, 1988-1995. Biological Conservation 86: 37-56.
Jiménez S., Phillips, R.A., Brazeiro, A., Defeo, O., Domingo, A. 2014. Bycatch of great albatrosses in pelagic longline fisheries in the southwest Atlantic: Contributing factors and implications for management. Biological Conservation 171: 9-20.
Jiménez, S., A. Domingo, A. Brazeiro, O. Defeo, A. G. Wood, H. Froy, J. C. Xavier, Phillips, R. A. 2015. Sex‐related variation in the vulnerability of wandering albatrosses to pelagic longline fleets. Animal conservation 19(3): 281–295.
Jiménez, S., Domingo, A., Brazeiro, A, Defeo, O., Wood, A.G., Froy, H., Xavier, J.C., Phillips, R.A. 2016. Sex-related variation in the vulnerability of wandering albatrosses to pelagic longline fleets. Animal Conservation 19: 281-295.
Jones, M.G.W., Ryan, P.G. 2010. Evidence of mouse attacks on albatross chicks on sub-Antarctic Marion Island. Antarctic Science 22: 39-42.
Nel, D. C.; Nel, J. L. 1999. Marine debris and fishing gear associated with seabirds at sub-antarctic Marion Island, 1996/97 and 1997/98: in relation to longline fishing activity. CCAMLR Science 6: 85-96.
Nel, D. C.; Ryan, P. G.; Cooper, J. 2002. Population dynamics of Wandering Albatrosses Diomedea exulans at sub-Antarctic Marion Island: longline fishing and environmental influences .
Nel, D. C.; Ryan, P. G.; Crawford, R. J. M.; Cooper, J.; Huyser, O. 2002. Population trends of albatrosses and petrels at sub-Antarctic Marion Island. Polar Biology 25: 81-89.
Nel, D. C.; Ryan, P. G.; Nel, J. L.; Klages, N. T. W.; Wilson, R. P.; Robertson, G. 2002. Foraging interactions of wandering albatrosses Diomedea exulans breeding on Marion Island with longline fisheries in the southern Indian Ocean. Ibis 144: E141-E154.
Nel, D. C.;Taylor, F.; Ryan, P. G.; Cooper, J. 2003. Population dynamics of the Wandering Albatross Diomedea exulans at Marion Island: longline fishing and environmental influences. African Journal of Marine Science 25(1): 503-517.
Pardo, D.; Forcada, J.; Wood, A.G.; Tuck, G.N.; Ireland, L.; Pradel, R.; Croxall, J.P.; Phillips, R.A. 2017. Additive effects of climate and fisheries drive ongoing declines in multiple albatross species. Proceedings of the National Academy of Sciences 114(50): E10829-E10837.
Phillips, R. A.; Ridley, C.; Reid, K.; Pugh, P. J. A.; Tuck, G. N.; Harrison, N. 2010. Ingestion of fishing gear and entanglements of seabirds: monitoring and implications for management. Biological Conservation 143: 501-512.
Phillips, R.A., Gales, R., Baker, G.B., Double, M.C., Favero, M., Quintana, F., Tasker, M.L., Weimerskirch, H., Uhart, M., Wolfaardt, A. 2016. The conservation status and priorities for albatrosses and large petrels. Biological Conservation 201: 169-183.
Pinaud, D.; Weimerskirch, H. 2007. At-sea distribution and scale-dependent foraging behaviour of petrels and albatrosses: a comparative study. Journal of Animal Ecology 76: 9-19.
Poncet, S.; Robertson, G.; Phillips, R. A.; Lawton, K.; Phalan, B.; Trathan, P. N.; Croxall, J. P. 2006. Status and distribution of Wandering, Black-browed and Grey-headed Albatrosses breeding at South Georgia. Polar Biology 29: 772-781.
Quin, B. 2008. Trends in threatened species: Wandering Albatross. Wingspan 18(4 Suppl): 36.
Rolland, V.; Weimerskirch, H.; Barbraud, C. 2010. Relative influence of fisheries and climate on the demography of four albatross species. Global Change Biology 16(7): 1910-1922.
Ryan, P. G.; Cooper, J.; Dyer, B. M.; Underhill, L. G.; Crawford, R. J. M.; Bester, M. N. 2003. Counts of surface-nesting seabirds breeding at Prince Edward Island, Summer 2001/02. African Journal of Marine Science 25(1): 441-451.
Springer, K. 2016. Methodology and challenges of a complex multi-species eradication in the sub- Antarctic and immediate effects of invasive species removal. New Zealand Journal of Ecology 40(2): 273-278.
Tancell C.,Sutherland, W.J., Phillips R.A. 2016. Marine spatial planning for the conservation of albatrosses and large petrels breeding at South Georgia. Biological Conservation 198: 165-176.
Terauds, A.; Gales, R.; Baker, G. B.; Alderman, R. 2006. Population and survival trends of wandering Albatrosses ( Diomedea exulans ) breeding on Macquarie Island. Emu 106(3): 211-218.
Weimerskirch, H., Cherel, Y., Delord, K., Jaeger, A., Patrick, S. C., Riotte-Lambert, L. 2014. Lifetime foraging patterns of the wandering albatross: Life on the move! Journal of Experimental Marine Biology and Ecology 450: 68-78.
Weimerskirch, H., Delord, K., Guitteaud, A., Phillips, R. A., Pinet, P. 2015. Extreme variation in migration strategies between and within wandering albatross populations during their sabbatical year, and their fitness consequences. Scientific reports 5.
Weimerskirch, H.; Akesson, S.; Pinaud, D. 2006. Postnatal dispersal of Wandering Albatrosses Diomedea exulans : implications for the conservation of the species. Journal of Avian Biology 37: 23-28.
Weimerskirch, H.; Brothers, N.; Jouventin, P. 1997. Population dynamics of Wandering Albatross Diomedea exulans and Amsterdam Albatross D. amsterdamensis in the Indian Ocean and their relationships with long-line fisheries: conservation implications. Biological Conservation 79: 257-270.
Weimerskirch, H.; Inchausti, P.; Guinet, C.; Barbraud, C. 2003. Trends in bird and seal populations as indicators of a system shift in the Southern Ocean. Antarctic Science 15: 249-256.
Weimerskirch, H.; Jouventin, P. 1998. Changes in population sizes and demographic parameters of six albatross species breeding on the French sub-antarctic islands. In: Robertson, G.; Gales, R. (ed.), Albatross biology and conservation , pp. 84-91. Surrey Beatty and Sons, Chipping Norton, Australia.
Weimerskirch. 1998. Foraging strategies of Indian Ocean albatrosses and their relationship with fisheries. In: Robertson, G.; Gales, R. (ed.), Albatross biology and conservation , pp. 137-167. Surrey Beatty & Sons, Sydney.
Xavier, J. C.; Trathan, P. N.; Croxall, J. P.; Wood, A. G.; Podesta, G.; Rodhouse, P. G. 2004. Foraging ecology and interactions with fisheries of Wandering Albatrosses ( Diomeda exulans ) breeding at South Georgia. Fisheries Oceanography 13: 324-344.
Additional information is available on the distribution of the Wandering Albatross from the Global Procellariiform Tracking Database (http://www.seabirdtracking.org)
Australian Govt - Action Plan for Australian Birds 2000 - Recovery Outline
Search for photos and videos, and hear sounds of this species from the Macaulay Library
IUCN Red List evaluators Westrip, J.
This information is based upon, and updates, the information published in BirdLife International (2000) Threatened birds of the world. Barcelona and Cambridge, UK: Lynx Edicions and BirdLife International, BirdLife International (2004) Threatened birds of the world 2004 CD-ROM and BirdLife International (2008) Threatened birds of the world 2008 CD-ROM. These sources provide the information for species accounts for the birds on the IUCN Red List.
To provide new information to update this factsheet or to correct any errors, please email BirdLife
To contribute to discussions on the evaluation of the IUCN Red List status of Globally Threatened Birds, please visit BirdLife's Globally Threatened Bird Forums .

Wandering Albatross
Diomedea exulans.
The snowy albatross, also known as the white-winged albatross or goonie, is a majestic seabird belonging to the Diomedeidae family. It is recognized for its impressive wingspan, which is the largest of any living bird, and its predominantly white plumage that becomes whiter with age. The snowy albatross is distinguished by its large pink bill and feet, and the males exhibit whiter wings than females.
Identification Tips
Adult snowy albatrosses have white bodies contrasted with black and white wings. The wings of males are predominantly white, with only the tips and trailing edges presenting as black. This species is the whitest within its complex, with others showing more brown and black on the wings and body. A salt gland above their nasal passage helps them excrete excess salt due to their oceanic diet.
The snowy albatross boasts a wingspan that can exceed 3.5 meters (11 feet), with an average span of around 3.1 meters (10 feet 2 inches). Body length ranges from 107 to 135 cm (3 feet 6 inches to 4 feet 5 inches), with females being slightly smaller than males. Adults typically weigh between 5.9 to 12.7 kg (13 to 28 lb).
Distribution and Habitat
This bird has a circumpolar range in the Southern Ocean and breeds on islands such as South Georgia, Crozet, Kerguelen, Prince Edward, and Macquarie. It is also seen feeding year-round off the coast of New Zealand and is known for its extensive flights, sometimes circumnavigating the Southern Ocean three times in a year.
The snowy albatross is a far-ranging bird, spending most of its life in flight and landing only to breed and feed. It is capable of gliding for hours without flapping its wings, thanks to its large wingspan.
Song & Calls
During courtship, snowy albatrosses engage in a variety of displays, including spreading their wings, head-waving, bill-rapping, and producing a range of vocalizations from screams and whistles to grunts and bill clapping.
Snowy albatrosses are monogamous, often mating for life, and breed biennially. They lay a single white egg with a few spots in a large grassy nest. Incubation takes about 11 weeks, with both parents sharing the responsibility. The chicks are nurtured by both parents, who take turns foraging for food.
Similar Species
The snowy albatross is part of the wandering albatross species complex, which includes the Tristan albatross and the Antipodean albatross. It can be distinguished from its relatives by its whiter plumage and larger size.
Diet and Feeding
These birds feed on cephalopods, small fish, and crustaceans, often foraging further out in the open ocean than other albatross species. They are known to follow ships and can make shallow dives to capture their prey.
Conservation Status
The IUCN lists the snowy albatross as vulnerable. Threats include longline fishing and pollution. Conservation measures have been implemented in some regions to reduce bycatch and protect their breeding grounds.
.css-1cn5y0j{border-radius:0.25rem;font-size:0.875rem;line-height:1.25rem;font-weight:650;letter-spacing:0em;--tw-text-opacity:1;color:rgb(45 49 66 / var(--tw-text-opacity));font-style:normal;font-weight:650;margin-left:2rem;margin-right:2rem;margin-bottom:1.5rem;font-size:1.875rem;line-height:2.25rem;}@media (min-width: 768px){.css-1cn5y0j{margin-left:3rem;margin-right:3rem;}} Wandering Albatrosses on Birda
More albatrosses, amsterdam albatross, antipodean albatross, tristan albatross, southern royal albatross, northern royal albatross, short-tailed albatross, laysan albatross, waved albatross, black-footed albatross, sooty albatross, light-mantled albatross, buller's albatross, indian yellow-nosed albatross, shy albatross, atlantic yellow-nosed albatross, grey-headed albatross, chatham albatross, campbell albatross, black-browed albatross, salvin's albatross, your birdwatching journey like never before, connect with nature in minutes, discover the joy of birding, play your part in saving nature.

What Our Birders Say
Such a great app, awesome app, fantastic app - love it, great bird recording, learning birding with birda, helped me to identify more birds, awesome birding community, great app for bird lovers, makes you want to spot birds more.

You appear to be using an old browser Please ensure you update your browser to be able to experience our site properly.

Albatross (Wandering)
The wandering albatross has the largest wingspan of any bird and is perhaps the most magnificent of all twelve species of albatross.

Family: Diomedeidae
Species: Diomedea exulans
IUCN Red List Status: Vulnerable
Distribution: The southern oceans and its small islands. Between Antarctica and the Tropic of Capricorn.
Habitat: Oceans and remote islands.
Description: Goose-sized with long, narrow wings. Black and white plumage. Long, hooked bill; large webbed feet.
Size: Length: - 1.1 - 1.35m. Wingspan:- max. 3.6m. Weight:- 8 - 12kg; female lighter.
Life-span: Up to 80 years
Food: Mainly squid, octopus, cuttlefish and crustaceans.
The wandering albatross has the largest wingspan of any bird and is perhaps the most magnificent of all twelve species of albatross. It is aptly named as it is a great traveller, covering enormous distances, gliding effortlessly on updraughts. It sometimes spends several months in the air, without ever touching land.
Flying and Feeding

The albatross usually feeds far out at sea, alone or in groups. It swoops down to land on the surface and catches its main prey - octopus, squid and cuttlefish - with its large bill, which can be as much as 18cm in length. Sometimes shallow dives are made to catch fish and other creatures below the surface. Albatrosses seem to like refuse from ships too, flopping down into the water and sometimes following a ship for days, waiting for scraps to be thrown overboard.

The albatross is a very long-lived bird but it does not start breeding until it is at least seven years old. The breeding grounds are usually on the top of cliffs where the birds can take off easily in the prevailing winds.
The birds gather in large numbers and the males and females perform elaborate and spectacular courtship displays. The two birds of a pair dance awkwardly around each other, bowing and clattering their bills, with the wings outstretched. At the end of the performance they point their bills to the sky and scream loudly.
At the beginning of the breeding season, which lasts from November until July, several males may be seen dancing around one female. Once a bird has found a suitable mate, which may take a few years, they remain together until one of them dies.
A large, untidy nest is built by both birds, using soil and vegetation to make a cup-shaped mound about 1 metre across and 30cm high. A single egg is laid, white with red spots, and the parents share the incubation, the male doing most of the sitting. The pair usually change over every two to three weeks and lose quite a lot of body weight during each shift. The chick hatches after about 78 days, which includes three days for the chick to break out of the shell.
The parents brood their chick for a short time and it is fed daily for the first 20 days with regurgitated squid, etc. Then the parents leave their offspring alone while they go out to sea and return every 10 days or so to feed it with huge meals. At this stage, the chick may be vulnerable to predators such as skuas, who will eat both eggs and chicks if left unguarded. The large, fluffy white chick continues to sit in its nest and is fed throughout the whole of the severe southern winter, until the following summer - a period of nearly nine months. As a result, the parents can only breed every other year. Eventually the young albatross launches itself into the wind and glides away over the ocean. It may circle the globe many times before returning to the breeding ground to look for a mate.
The Wandering Albatross and Humans
One of the biggest threats to the wandering albatross is 'long line' fishing. Longline fishing is a method used to catch more expensive kinds of tuna. This method involves putting out fishing lines up to 100km long from which there are as many as one thousand shorter lines attached with baited hooks. This still indiscriminately kills untargetted marine life such as turtles (of which 6 out of 7 species are considered threatened) which are tempted by the jelly fish appearance of the bait. Abatrosses and other seabirds can also get caught on the hooks. According to a study in 2011, 300,000 albatrosses are killed yearly by long line fishing. 1 For information see our factsheet Over Fishing. Another threat to the future of the albatross is plastic, oil and chemical pollution of the sea. There may be more competition for food too if fishing increases in the southern oceans.
Sailors gave the albatross its name. The name is taken from a Portuguese word "alcatraz" originally meaning any large seabird. Over the years many less complimentary names have been given to albatrosses, all of them suggesting stupidity. These names include "mollymawk", (from the dutch word meaning "stupid gull") and "gooney"(derived from the old English "gooney"used to describe a stupid person).
This reputation for stupidity probably resulted from the fact that the albatross is very clumsy on land. It waddles awkwardly, often tripping over its own feet! Landing can be difficult too; quite often a bird crash lands into the breeding colony, sometimes turning several somersaults!
Sailors also used to regard the albatross as a harbinger of wind and storms, possibly because it has difficulty in flying during very calm weather. They also thought an albatross was a reincarnation of a sailor washed overboard and it was thought very unlucky to kill one. See the poem 'The Rime of the Ancient Mariner' by Samuel Taylor Coleridge about a story of the sailor who killed an albatross.
During the latter part of the nineteenth century however, most species of albatross were sought after for the fashion trade, and thousands were killed for their feathers. These were used by the millinery trade for decorating hats - sometimes whole wings were used for this purpose. The feathers were also used for stuffing mattresses and pillows, though it was called "swans' down" at the time. Fortunately, the fashion for wearing birds' feathers died out before the albatrosses became too seriously threatened. In 2020, the ACAP launched World Albatross Day as an opportunity to spread the message of conservation to the wider public.
1. BBC News 'Seabirds such as albatrosses killed by longline fishing' by Victoria Gill
Albatross (Wandering) Image by: Leo

The Young People's Trust for the Environment is a charity which aims to encourage young people's understanding of the environment and the need for sustainability.
- Online: ypte.org.uk
- Email: [email protected]
- Phone: 01935 315025
Please donate £5 to help YPTE to continue its work of inspiring young people to look after our world.
Advertisement
Variation among colonies in breeding success and population trajectories of wandering albatrosses Diomedea exulans at South Georgia
- Open access
- Published: 04 January 2021
- Volume 44 , pages 221–227, ( 2021 )
Cite this article
You have full access to this open access article

- Carola Rackete 1 , 4 ,
- Sally Poncet 2 ,
- Stephanie D. Good 3 ,
- Richard A. Phillips 4 ,
- Ken Passfield 2 &
- Philip Trathan ORCID: orcid.org/0000-0001-6673-9930 4
2384 Accesses
5 Citations
4 Altmetric
Explore all metrics
The wandering albatross, Diomedea exulans, is a globally threatened species breeding at a number of sites within the Southern Ocean. Across the South Georgia archipelago, there are differences in population trends even at closely located colonies. Between 1999 and 2018 the largest colony, at Bird Island, declined at 3.01% per annum, while in the Bay of Isles, the decline was 1.44% per annum. Using mean demographic rates from a 31-year study at Bird Island and an 11-year study of breeding success at Prion Island in the Bay of Isles in a VORTEX model, we show that differences in breeding success do not fully explain observed differences in population trends. Other potential contributing factors are differential use of foraging areas, with possible knock-on effects on adult body condition, provisioning rate and breeding success, or on bycatch rates of adults or immatures.
Avoid common mistakes on your manuscript.
Introduction
Understanding variation in demography and ecology of seabirds is important in terms of testing life-history theory and for assessing population dynamics, particularly with regard to conservation management of threatened species (Hunt and He 2019 ).
The wandering albatross Diomedea exulans is listed as Vulnerable (BirdLife International 2018 ) by the International Union for Conservation of Nature (IUCN), with the population decline attributed largely to incidental mortality (bycatch) in longline fisheries (Rolland et al. 2010 ; Pardo et al. 2017 ). The impacts of fisheries are severe because the wandering albatross has an extreme life history even in contrast to other seabirds, characterised by a long life span under natural circumstances, biannual breeding if successful, and high breeding success, all of which influence population dynamics (Weimerskirch and Jouventin 1987 ; Pardo et al. 2017 ).
Wandering albatrosses are well monitored globally, and breeding populations in the southwest Indian Ocean at Kerguelen, Crozet and the Prince Edward Islands have been recovering from previous fisheries-related declines since the mid-1980s, a situation mainly attributed to changes in the degree of overlap with vessels (Weimerskirch et al. 2018 ). Moreover, variation between colonies in population trends has been documented on the Crozet Archipelago (Weimerskirch et al. 2018 ), where tracking data have shown differences in the use of foraging areas between colonies (Weimerskirch et al. 1993 ) which may expose birds to different fisheries and mortality risks. In contrast, wandering albatrosses at South Georgia (South Atlantic) have shown a continued population decline since the 1960s and so have been identified as one of nine global High Priority populations for conservation by the Agreement on the Conservation of Albatrosses and Petrels (ACAP) (ACAP 2017 ). At South Georgia, the last archipelago-wide census of wandering albatrosses (Fig. 1 ) carried out in summer 2014/15 showing an overall decline of 1.76% breeding pairs per annum since the previous census in 2004/05, with declines varying between colonies (Poncet et al. 2017 ). At the largest colony, Bird Island, a longitudinal study of demographic rates between 1972 and 2012 determined that low adult survival was the main driver of decline. Further, that low juvenile survival became increasingly important over time, resulting in reduced recruitment which was not balanced by a slight increase in breeding success (Pardo et al. 2017 ).
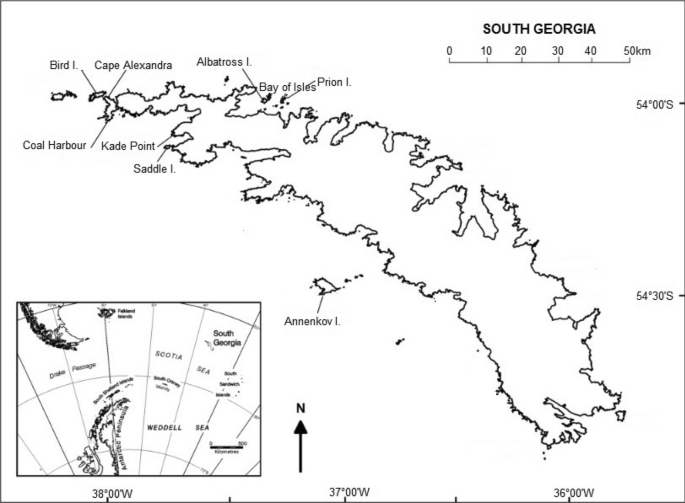
adapted from Poncet et al. ( 2006 )
Locations of sizeable breeding sites (> 15 pairs) of wandering albatross on South Georgia with inset map of South Georgia and the Scotia Sea,
Although there have been many studies of variation in demographic parameters of seabirds at single colonies (e.g. Mauck et al. 2004 ; Oro et al. 2010 ; Pardo et al. 2017 ), there has been less exploration of variation between colonies, generally because of a lack of suitable data (but see, e.g. Frederiksen et al. 2007 ; Sandvik et al. 2012 ; Descamps et al. 2017 ). However, at South Georgia, annual monitoring of breeding numbers and success has been carried out at the Bay of Isles in addition to Bird Island.
Here, we compare population trajectories and breeding success between these areas, separated by approximately 50 km. We model the population dynamics with the aim of improving understanding of inter-colony variation in population trends at South Georgia. We hypothesize that a difference in breeding success is the main driver of the observed disparity in population trends, and discuss the results in the context of other potential contributing factors.
Materials and methods
Study sites and data collection.
The South Georgia Archipelago holds 17.64% of the global wandering albatross breeding population (Phillips et al. 2016 ), with the last census in 2014/5 giving an estimate of 1278 breeding pairs in total (Poncet et al. 2017 ); hereafter, breeding seasons will be referred to as the year in which the chicks fledge, in this case 2015. Bird Island (54°00′S, 38°03′W) held 60.41% of the breeding pairs at South Georgia while the Bay of Isles colonies represented 16.36% (Poncet et al. 2017 ), of which Albatross Island (54°01′S, 37°20′W) and Prion Island (54°01′S, 37°15′W) together held 80–85% of the total. In addition to the census in 2014/15, we also use breeding pair counts from 1984 and 2004, including from five other breeding sites at South Georgia that held > 15 pairs in 2004: Annenkov Island, Saddle Island, Cape Alexandra, Kade Point and Coal Harbour (Poncet et al. 2006 , 2017 ).
Annual monitoring of breeding pairs at Prion Island and Albatross Island began in 1999, and of breeding success at Prion Island in 2007. At Bird Island, annual monitoring of demographic parameters, including breeding success began in 1980 (Pardo et al. 2017 ). The number of breeding pairs was estabilished by counting incubating adults between the end of December and early January, after the peak of egg laying. Counts of breeding pairs at Albatross Island and Prion Island are pooled and referred to as “Bay of Isles “ as there was no difference in trends between these two sites (results not shown). Breeding success was calculated as the proportion of eggs that resulted in a well-grown chick. On Prion Island, breeding success was estimated by counting chicks shortly before fledging, between the middle of October and early November, while breeding success at Bird Island was based on chick survival until 1 November. However, < 1% of chicks at Bird Island die during October (British Antarctic Survey, unpublished data), and therefore, no correction factor was applied. We used adult and juvenile survival, return and breeding probability from the long-term mark-recapture study carried out at Bird Island according to Pardo et al. ( 2017 ).

Statistical analyses
Statistical tests were carried out in R version 3.5.0 (R CORE team 2018 ). We compared linear models of breeding pair counts at the Bay of Isles and Bird Island between 1999 and 2018 using an analysis of variance (ANOVA).
To test for breakpoints in population trends over the study period, we fitted segmented piecewise regression models for each site using the R package “segmented“ (Muggeo 2008 ) and tested for significant differences in the slopes of the segments using a Davies Test. We compared trends at Albatross and Prion, and Bird Island, with those at the other sites (Annenkov Island, Saddle Island, Cape Alexandra, Kade Point and Coal Harbour). Breeding success at Bird Island was compared to Prion Island by Student’s t test, using data from 2007 to 2018.
Population trends were modelled for Prion Island and Bird Island using the Population Viability Analysis (PVA) software VORTEX v10 (Lacy and Pollack 2014 ). PVA models provide a means of understanding the influence of differences in demographic rates on the growth or decline of populations (Hamilton and Moller 1995 ). The VORTEX program simulates the effects of deterministic forces as well as stochastic events using Monte-Carlo methods (Lacy et al. 2018 ). To obtain key demographic parameters to populate our model, we first used GetData software to extract values for adult survival (AS), juvenile survival (JS) and percent females breeding (FB) (return probability * breeding probability) from estimated vital rates for wandering albatross (Fig. 1 ) in Pardo et al. 2017 . We then ran bootstrapping (1000 replicates) from the sample of values for years 1981 to 2012 using “boot” function in R (Table 1 ) . Mean and 95% Confidence Intervals were calculated from the distributions. Age at first breeding was set at 10 following Pardo et al. ( 2017 ), as this was the age that gave the best model fit.
We applied the VORTEX sensitivity test function to randomly sample the values from the bootstrapped distribution of each parameter (adult mortality, juvenile mortality and percent breeding females) for the population models. The model was run 1000 times each for Bird Island (breeding success 72%) and Prion Island (breeding success 83%). The main output variable tracked was the population growth rate (r). We applied the Shapiro–Wilk test to the r values from each model and determined that the data were normally distributed. We then applied a Welch 2 sample t test to determine if there was a significant difference in the mean values from r modelled from each population.
Annual fluctuations in birth and death rates due to environmental variation were included for each parameter, assuming a standard deviation of 10%, which is similar to observed variability in Pardo et al. ( 2017 ) and produced stable model outputs. All values are given as means ± standard deviation (SD).
Between 1999 and 2018, the number of breeding pairs of wandering albatrosses at Albatross Island and Prion Island declined from 220 to 167 (−24.09% overall; equivalent to −1.44% per annum) (Fig. 2 ). Over the same period, the number of breeding pairs at Bird Island declined from 1182 to 661 (−44.08% overall; equivalent to −3.01% per annum). The linear models indicated that these trends differed significantly between the two regions (ANOVA, F 1 = 34.41, p < 0.0001). The decline of breeding pairs at Bird Island showed a single breakpoint in 2006 (± 1.3 years, R 2 = 0.91, p < 0.0001), whereas the model for Albatross and Prion indicated a single breakpoint in 2002 (± 1.0 year, R 2 = 0.61, p = 0.01). For Bird Island, the slopes of the two segments (−43.97 before and −11.67 after the breakpoint) differed significantly (Davies Test, ( p = 0.04) indicating a more rapid decline before 2006, whereas there was no significant difference between the slopes in the model for Albatross and Prion islands (Davies Test, p = 0.80).
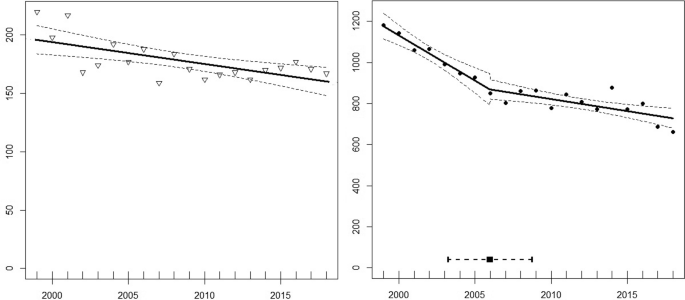
Changes in numbers of breeding pairs of wandering albatrosses at Bay of Isles (left) and Bird Island (right) between 1999 and 2018. Linear regressions displayed incl. 95% confidence intervals
Based on the archipelago-wide censuses, the number of breeding pairs in the Bay of Isles decreased by −27.37% between 1984 and 2015, whereas numbers at all other sites with > 15 breeding pairs declined more rapidly (ranging from −39.60% at Cape Alexandra to −76.61% decline at Kade Point). The only site where the decline was less than at Albatross and Prion was the small colony at Coal Harbour (Table 2 ).
Breeding success was significantly higher at Prion Island (83.27 ± 3.64%; n = 11) than at Bird Island (72.00 ± 5.27%; n = 11) between 2007 and 2018 (Student’s t test, t 18 = −5.84, p = < 0.0001.
In the VORTEX output from the population model (Table 3 ), we found a significant difference in modelled mean exponential growth rate (r) between the two populations (Welch 2 sample t test, t 1999.9 = −21.742, p < 0.0001). This supports the hypothesis that differences in breeding success partly explain the contrasting growth rate between the populations. However, the estimated annual population trends according to the model input parameters would be −1.37% and −0.79% for Bird Island and the Bay of Isles, respectively, and hence, other factors must also be contributing to the steeper observed declines.
Based on analyses of long-term monitoring data at South Georgia, we report significant differences in population trajectories and breeding success between wandering albatrosses in the Bay of Isles and Bird Island. The annual decline in breeding numbers at the Bay of Isles was approximately half that at Bird Island (−1.44% vs −3.01% per annum). This is consistent with the variability in population trends between 1984 and 2015 observed among wandering albatross colonies at South Georgia (Table 2 ). Similarly large differences also exist between colonies of other albatross species at South Georgia (Poncet et al. 2017 ), and in wandering albatrosses at the Crozet Archipelago where most have increased since 1982 with the exception of Ile de l’Est (Weimerskirch et al. 2018 ). Based on our VORTEX population models, differences in breeding success could partly explain the differences in population growth rate, despite variance in the parameters for adult mortality, juvenile mortality and proportion of females breeding. Our model results did not fully match the observed rate of decline in breeding pairs, however, which suggests either that the 11-year dataset on breeding success at Prion Island may not be representative of earlier years or birds on Albatross Island, or that there are additional factors that also influence demographic rates.
One possible explanation for the difference in population trajectories, though very unlikely, is dispersal from Bird Island to the Bay of Isles. Wandering albatrosses generally show very high site fidelity, although they do change their nest site on average once in their lifetime, usually when they change partner (Gauthier et al. 2010 ). During the 2019 survey at Albatross and Prion islands, all breeding birds present at nests—half the breeding population—were checked for leg rings. Only two ringed birds were found, one originating from Bird Island and the other from Possession Island (Crozet Archipelago). Given that all chicks that fledge from Bird Island, and all breeding adults have been ringed for the last 35 + years, rates of emigration to the Bay of Isles colonies are clearly very low. Checking all breeding birds for rings during subsequent censuses would improve data on dispersal between colonies.
The reason for the higher breeding success on Prion Island remains unclear. Extreme weather can affect breeding success in other seabirds, including black-browed albatrosses Thalassarche melanophris (Descamps et al. 2015 ; Cleeland et al. 2020 ). Human disturbance could also be a factor Carey ( 2009 ), as regular nest visits are made only at Bird Island and elsewhere are known to result in elevated heart rate of wandering albatrosses and potentially higher energy expenditure for 2 to 3 h after exposure (Weimerskirch et al. 2002 ). However, productivity at Bird Island is comparable or higher than at other colonies of great albatrosses Diomedea spp. worldwide (Cuthbert et al. 2004 ; Rolland et al. 2010 ; Jones et al. 2017 ). There is no indication that the weather is unusually severe or researcher disturbance is a particular problem at Bird Island. It is plausible that environmental conditions at the Bay of Isles are more benign, e.g. more sheltered from predominant westerly winds, than typical for great albatross colonies, but there are no meteorological data to confirm this hypothesis.
Another possibility is that the difference in breeding success, and indeed in other demographic parameters (adult and juvenile survival, breeding propensity) reflects the consequences of between-colony variation in foraging areas during the breeding or nonbreeding seasons. Tracking of wandering albatrosses at the Crozet Archipelago during the nonbreeding season indicated that part of the population is sedentary, their foraging areas overlapping extensively in breeding and sabbatical years, and a proportion of the population, mainly females, breed annually (Weimerskirch et al. 2015 ). Differences in foraging areas between breeding sites in the same island group or region have also been found in several other seabird species. The mean growth rate of black-legged kittiwakes Rissa tridactyla differs between colonies, related to variation in environmental conditions such as sea surface temperature or in prey abundance in foraging areas, which may affect adult body condition, trip duration or provisioning rate (Frederiksen et al. 2005 ). In Scopoli’s shearwaters Calonectris diomedea , differences in foraging areas led to spatial differences in overlap with fisheries, influencing bycatch rates and hence survival, with long-term impacts on population growth rate (Genovart et al. 2018 ).
Overlap of wandering albatrosses from Bird Island with diverse demersal and pelagic fishing fleets varies extensively both seasonally and among years (Jiménez et al. 2016 ; Clay et al. 2019 ). It is quite possible that wandering albatrosses from other breeding sites at South Georgia might be exposed to different fleets and hence bycatch risks, possibly with additional knock-on consequences for population age structure; this could affect age of first breeding, breeding propensity or breeding success (Froy et al. 2013 ).
The differences in population growth rate among sites could also be influenced by a density-dependent reduction in recruitment age, observed not only in wandering albatrosses at South Georgia, but also in other seabird species elsewhere, including black-legged kittiwakes and great skuas Stercorarius skua (Croxall 1990 ; Coulson 2011 ; Furness et al. 2015 ). In wandering albatrosses, there was also a density-dependent reduction in recruitment rate in the southern Indian Ocean, and a clear negative relationship between-colony size and juvenile survival (Fay et al. 2015 ). As the age structure of the birds breeding at the Bay of Isles is unknown, it is not possible to determine whether disparities in juvenile survival or recruitment age are contributing to the difference in growth trajectory from Bird Island.
In conclusion, differences in breeding success between Bird Island and the Bay of Isles colonies only partly explain the divergent population trends. Collection of tracking and demographic data from the Bay of Isles would allow testing of other hypotheses. Tracking data would also inform the need for further marine conservation measures for the South Georgia population.
Agreement on the conservation of albatrosses and petrels (2017) Report of the populations and conservation status working group. https://acap.aq/advisory-committee/ac10/ac10-meeting-documents/3126-ac10-doc-11-pacswg-report/file . Accessed 15 Aug 2020
BirdLife International (2018) Diomedea exulans . The IUCN red list of threatened species. https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22698305A132640680.en . Accessed 06 Oct 2020
Carey JM (2009) The effects of investigator disturbance on procellariiform seabirds: a review. NZ J of Zool 36:367–377
Article Google Scholar
Clay TC, Small C, Tuck GN, Pardo D, Carneiro APB, Wood AG, Croxall JP, Crossin GT, Phillips RA (2019) A comprehensive large-scale assessment of fisheries bycatch risk to threatened seabird populations. J Appl Ecol 56:1882–1893
Cleeland J, Pardo D, Raymond B, Terauds A, Alderman R, McMahon C, Phillips RA, LeaHindell MA (2020) Introduced species and extreme weather as key drivers of reproductive output in three sympatric albatrosses. Sci Rep 10:E8199
Coulson J (2011) The kittiwake. T and AD Poyser, London
Google Scholar
Croxall JP, Rothery P, Pickering SPC, Prince PA (1990) Reproductive performance, recruitment and survival of wandering albatrosses Diomedea exulans at bird island, south Georgia. J Anim Ecol 59:775–796
Cuthbert R, Sommer E, Ryan P, Cooper J, Hilton G (2004) Demography and conservation of the tristan albatross Diomedea [exulans] dabbenena . Biol Conserv 117:471–481
Descamps S, Anker-Nilssen T, Barrett RT, Irons DB, Merkel F, Robertson GJ, Yoccoz NG, Mallory ML, Montevecchi WA, Boertmann D (2017) Circumpolar dynamics of a marine top-predator track ocean warming rates. Glob Chang Biol 23:3770–3780
Descamps S, Tarroux A, Varpe O, Yoccoz NG, Tveraa T, Lorentsen SH (2015) Demographic effects of extreme weather events: snow storms, breeding success, and population growth rate in a long-lived antarctic seabird. Ecol Evol 5:314–325
Fay R, Weimerskirch H, Delord K, Barbraud C (2015) Population density and climate shape early-life survival and recruitment in a long-lived pelagic seabird. J Anim Ecol 84:1423–1433
Frederiksen M, Edwards M, Mavor RA, Wanless S (2007) Regional and annual variation in black-legged kittiwake breeding productivity is related to sea surface temperature. Mar Ecol Prog Ser 350:137–143
Frederiksen M, Harris MP, Wanless S (2005) Inter-population variation in demographic parameters: a neglected subject? Oikos 111:209–214
Froy H, Philips RA, Wood AG, Nussey DH, Lewis S (2013) Age-related variation in reproductive traits in the wandering albatross: evidence for terminal improvement following senescence. Ecol Lett 16:642–649
Furness RW (2015) Density dependence in seabirds: great skuas Stercorarius skua start to breed at a younger age when conditions are better. Ringing Migr 30:43–50
Gauthier G, Milot E, Weimerskirch H (2010) Small scale dispersal and survival in a long-lived seabird, the wandering albatross. J Anim Ecol 79:879–887
PubMed Google Scholar
Genovart M, Becares J, Igual JM, Martinez-Abrain A, Escandell R, Sanchez A, Rodriguez B, Arcos JM, Oro D (2018) Differential adult survival at close seabird colonies: the importance of spatial foraging segregation and bycatch risk during the breeding season. Glob Chang Biol 41:1290–1279
Hamilton S, Moller H (1995) Can PVA models using computer packages offer useful conservation advice? Sooty shearwaters Puffinus griseus in New Zealand as a case study. Biol Conserv 73:107–117
Hunt LG, He X (2019) Seabird population dynamics. In: Cochran JK, Bokuniewicz HJ, Yager PL (eds) Encyclopedia of ocean sciences, 3rd edn. Academic Press, New York, pp 76–79
Chapter Google Scholar
Jiménez S, Domingo A, Brazeiro A, Defeo O, Wood AG, Froy H, Xavier JC, Phillips RA (2016) Sex-related variation in the vulnerability of wandering albatrosses to pelagic longline fleets. Anim Conserv 19:281–295
Jones MGW, Dilley BJ, Hagens QA, Louw H, Mertz EM, Vissel P, Ryan PG (2017) Wandering albatross Diomedea exulans breeding phenology at Marion Island. Polar Biol 40:1139–1148
Lacy RC, Miller PS, Traylor-Holzer K (2018) Vortex 10 user’s manual. 1 June 2018 update. IUCN SSC conservation breeding specialist group, Gland, and Chicago Zoological Society, Apple Valley, Minnesota
Lacy RC, Pollak JP (2014) Vortex: a stochastic simulation of the extinction process. Version 10.0. Chicago Zoological Society, Brookfield
Mauck RA, Huntington CE, Grubb TC (2004) Age-specific reproductive success: evidence for the selection hypothesis. Evolution 58:880–885
Article CAS Google Scholar
Muggeo VMR (2008) Segmented: an R package to fit regression models with broken-line relationships. R News 8:20–25
Oro D, Torres R, Rodríguez C, Drummond H (2010) Climatic influence on demographic parameters of a tropical seabird varies with age and sex. Ecology 91:1205–1214
Pardo D, Forcada J, Wood AG, Tuck GN, Ireland L, Pradel R, Croxall JR, Phillips RA (2017) Additive effects of climate and fisheries drive ongoing declines in multiple albatross species. Proc Natl Acad Sci USA 114:E10829–E10837
Phillips RA, Gales R, Baker GB, Double MC, Favero M, Quintana F, Tasker ML, Weimerskirch H, Uhart M, Wolfaardt A (2016) The conservation status and priorities for albatrosses and large petrels. Biol Conserv 201:169–183
Poncet S, Robertson G, Philips RA, Lawton K, Phalan B, Trathan PN, Croxall JP (2006) Status and distribution of wandering, black-browed and grey-headed albatrosses breeding at South Georgia. Polar Biol 29:772–781
Poncet S, Wolfaardt AC, Black A, Browning S, Lawton K, Lee J, Passfield K, Strange G, Phillips RA (2017) Recent trends in numbers of wandering, black-browed and grey-headed albatrosses breeding at South Georgia. Polar Biol 40:1347–1358
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rolland V, Weimerskirch H, Barbraud C (2010) Relative influence of fisheries and climate on the demography of four albatross species. Glob Chang Biol 16:1910–1922
Sandvik H, Erikstad KE, Sather BE (2012) Climate affects seabird population dynamics both via reproduction and adult survival. Mar Ecol Prog Ser 454:273–284
Weimerskirch H, Delord K, Barbraud C, Le Bouard F, Ryan PG, Fretwell P, Marteau C (2018) Status and trends of albatrosses in the French southern territories, western Indian ocean. Polar Biol 41:1963–1972
Weimerskirch H, Delord K, Guitteaud A, Phillips RA, Pinet P (2015) Extreme variation in migration strategies between and within wandering albatross populations during their sabbatical year, and their fitness consequences. Sci Rep 5:8853
Weimerskirch H, Jouventin P (1987) Population dynamics of the wandering albatross, Diomedea exulans , of the crozet islands: causes and consequences of the population decline. Oikos 49:315–322
Weimerskirch H, Salamolard M, Sarrazin F, Jouventin P (1993) Foraging strategy of wandering albatrosses through the breeding season: a study using satellite telemetry. Auk 110:325–342
Weimerskirch H, Shaffer SA, Mabille G, Martin J, Boutard O, Rouanet JL (2002) Heart rate and energy expenditure of incubating wandering albatrosses: basal levels, natural variation, and the effects of human disturbance. J Exp Biol 205:475–483
Download references
Acknowledgements
We thank two referees, Rachael Herman and Tegan Carpenter-Kling, as well as the editor, Dieter Piepenburg, for their helpful comments which greatly improved our manuscript. The study was conducted using funding and support from the Government of South Georgia and the Sandwich Islands, South Georgia Surveys and British Antarctic Survey. We are very grateful to the many field workers who assisted with monitoring at the Bay of Isles and Bird Island. This study represents a contribution to the Ecosystems component of the British Antarctic Survey Polar Science for Planet Earth Programme, funded by The Natural Environment Research Council.
Author information
Authors and affiliations.
Biosciences, Edge Hill University, St Helen‘s Road, Ormskirk, L39 4QP, UK
Carola Rackete
South Georgia Surveys, Falkland Islands, PO Box 538, Stanley, FIQQ 1ZZ, UK
Sally Poncet & Ken Passfield
Centre for Ecology & Conservation, University of Exeter, Cornwall campus, Penryn, TR10 9EF, UK
Stephanie D. Good
British Antarctic Survey, Natural Environment Research Council, High Cross, Madingley Road, Cambridge, CB3 0ET, UK
Carola Rackete, Richard A. Phillips & Philip Trathan
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Philip Trathan .
Ethics declarations
Conflict of interest.
The authors have declared that no conflicts of interest exist.
Ethical approval
The study was carried out under science permits approved by the Government of South Georgia and the South Sandwich Islands (GSGSSI).
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Rackete, C., Poncet, S., Good, S.D. et al. Variation among colonies in breeding success and population trajectories of wandering albatrosses Diomedea exulans at South Georgia. Polar Biol 44 , 221–227 (2021). https://doi.org/10.1007/s00300-020-02780-6
Download citation
Received : 11 November 2019
Revised : 24 November 2020
Accepted : 01 December 2020
Published : 04 January 2021
Issue Date : January 2021
DOI : https://doi.org/10.1007/s00300-020-02780-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Wandering albatross
- Breeding success survival
- Inter-colony variation
- Find a journal
- Publish with us
- Track your research

- Frogs in USA
- Bats in USA
- Lizards in USA
- Turtles in USA
- Hawks, Eagles, and Falcons in USA
- Birds in USA
- Woodpeckers in USA
- Hummingbirds in USA
- Owls in USA
- Hummingbird
- Desert Birds
- Colorful Birds
- Fastest Birds
- Birds of Prey
- Dangerous Birds
- Birds That Lay Blue Eggs
- Birds Around the World
- Birds That Sing at Night
- Birds by Color
- Crested Birds
- Alpine Birds
- Smartest Birds
- Herbivorous Birds
- Antarctic Birds
- Arctic Birds
- Poisonous Birds
- Longest Living Birds
- Birds That Mate For Life
- Long-Legged Birds
- Long-tailed Birds
- Diving Birds
- Birds That Eat Mosquitoes
- Fish-eating Birds
- Mountain Birds
- Small Birds
- Whistling Birds
- Nocturnal Birds
- Grasshopper
- South American
- North American
- Sonoran Desert
- Live in Lakes
- Deciduous Forest
- Temperate Forest
- Small Animals
- Hybrid Animals
- Rare Animals
- Monogamous Animals
- Animals that are Carnivorous
- Amazon Rainforest
- Death Valley
- Galápagos Islands
- Animals with Horns
- Animals with Antlers
- Camouflage Animals
- Ice Age Animals
- Animals that Migrate
- Animals with Big Eyes
- Endangered Animals
- Animals that are Omnivorous
- Animals You Can See On a Safari
- Animals Living in the Mariana Trench
- Animals with Long Necks
- Ugly Animals
- Smartest Animals
- Flying Animals
- Dumbest Animals
- Biggest Animals in the World
- Animals that Hibernate
- Fastest Animals in the World
- Hoofed Animals
- Animals that are Herbivorous
- Fluffy Animals
- Extinct Animals
- Melanistic Animals
- Longest Living Animals
- Animals That Mate For Life
- Ruminant Animals
- Scary Animals
- Poisonous Animals
- Colorful Animals
- Asexual Animals
- Animals that Burrow
- Fat Animals
- Dangerous Animals
- Slow Animals
- Nocturnal Animals
- Strong Animals
- Gay Animals
- Weird Animals
- Black Birds in Florida
- Beautiful Animals
- Animals That Lay Eggs (Oviparous Animals)
- Animals Living in Death Valley
- Yellowstone National Park
- Domestic Animals
- Land Animals
- Animals That Kill the Most Humans
Wandering Albatross
Table of Contents
Scientific Classification
Table of content.

Physical Description
Size : They measure at around 3 ft 6 in to 4 ft 5 in (1.07-1.35 m).
Weight : Adult wandering albatrosses typically weigh between 13 and 28 lbs (5.9-12.7 kg).
Color : The plumage for juveniles is chocolate brown which becomes whiter with age. The wings in adults are white with black around the tips while the female’s wings have more black on them. The bill and feet are pink.
Sexual Dimorphism : Males are a little bit larger than females.
Wingspan : They have the largest wingspan among birds , measuring at around 8 ft 3 in to 11 ft 6 in (2.51-3.5 m).
The two recognized subspecies of the wandering albatross are D. e. exulans (nominate subspecies) and the D. e. gibsoni (also known as Gibson’s albatross).
Distribution
The breeding range for the wandering albatross includes South Georgia Island, Crozet Islands, Prince Edward Islands, Kerguelen Islands, and Macquarie Islands. It also feeds around the Kaikoura Peninsula on New Zealand’s South Island east coast.
They inhabit subantarctic islands with tussock grass, sedges, shrubs, mosses and peat soils. They nest on ridges, plateaus, valleys, and plains.

Wandering Albatross Pictures

Wandering Albatross Images
- These birds spend most of their lives in the air, traveling long distances.
- They live in small groups during their forages in the sea.
- They become rather social during the breeding season.
- They are territorial towards members of the same sex during the breeding season and defend their nesting area with vocalizations.
Wandering albatrosses eat fish, squids, and crustaceans.
Mating & Reproduction
These birds mate for life and mate every other year. Males reach the breeding grounds before females and locate the same nesting sites they had used the previous season, although they may also choose to build new ones. Females arrive after males. The breeding season usually occurs between December and March. The female lays one egg per breeding season which is then incubated for 74-85 days. Both parents take part in incubation.
The hatchling stays in its parents’ care for up to 9 months of age, after which they achieve independence. They reach sexual maturity by the time they are 9 years old.

Wandering Albatross Chick

Wandering Albatross Size
Wandering albatrosses can live for up to 50 years.
Sounds & Communication
These birds communicate by croaking, bill-clapping, bill-touching, trumpeting, and pointing towards the sky with their bills.
Adaptations
- The large wings of the wandering albatross help them fly for vast distances over several hours without flapping. For every meter of drop in altitude, they can travel 22 meters in distance.
- The salt gland at the nasal passage helps them desalinate their bodies of the excess salt they come in contact with because of their oceanic lifestyle.
- They can dive up to a meter into the ocean to catch their prey. They, however, prefer to catch the fish from the surface of the ocean.

The Wandering Albatross

Wandering Albatross Flying
Adult wandering albatrosses have no predators. Eggs, hatchlings, and juveniles, on the other hand, are preyed upon by sheathbills and skuas. In addition to these two, several introduced animals like goats, pigs, rats, mice, and cats also eat the chicks and eggs.
IUCN Conservation Status
The International Union for Conservation of Nature lists the wandering albatross under their ‘Vulnerable’ category.
Interesting Facts
- The wandering albatross is the biggest bird in its genera and one the largest in the world.
- One individual lived to be 60 years old in New Zealand. She was named ‘Grandma.’
- Another banded individual was recorded to have traveled 3,730 miles in just 12 days.

Wandering Albatross Wingspan

Wandering Albatross Bird
- http://www.coolantarctica.com/Antarctica%20fact%20file/wildlife/wandering-albatross.php https://oceanwide-expeditions.com/to-do/wildlife/wandering-albatross https://beautyofbirds.com/wandering-albatrosses/ http://animaldiversity.org/accounts/Diomedea_exulans/#ff4ee5a1ac2a7a07a049350b7c9b6fbc https://www.britannica.com/animal/albatross#ref243427 http://www.iucnredlist.org/details/22698305/0
Related Articles

Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Recent Wallpapers

- Invertebrates
Subscribe our newsletter
Follow us on:.
- Privacy Policy
- Animal Habitats
- Animal Memes
© 2024 ( Animal Spot ). All rights reserved. Reproduction in whole or in part without permission is prohibited.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Wiley-Blackwell Online Open

Linking demographic processes and foraging ecology in wandering albatross—Conservation implications
Associated data.
This synthesis does not use new data.
- Population dynamics and foraging ecology are two fields of the population ecology that are generally studied separately. Yet, foraging determines allocation processes and therefore demography. Studies on wandering albatrosses Diomedea exulans over the past 50 years have contributed to better understand the links between population dynamics and foraging ecology. This article reviews how these two facets of population ecology have been combined to better understand ecological processes, but also have contributed fundamentally for the conservation of this long‐lived threatened species.
- Wandering albatross research has combined a 50‐year long‐term study of marked individuals with two decades of tracking studies that have been initiated on this species, favoured by its large size and tameness.
- At all stages of their life history, the body mass of individuals plays a central role in allocation processes, in particular in influencing adult and juvenile survival, decisions to recruit into the population or to invest into provisioning the offspring or into maintenance.
- Strong age‐related variations in demographic parameters are observed and are linked to age‐related differences in foraging distribution and efficiency. Marked sex‐specific differences in foraging distribution, foraging efficiency and changes in mass over lifetime are directly related to the strong sex‐specific investment in breeding and survival trajectories of the two sexes, with body mass playing a pivotal role especially in males.
- Long‐term study has allowed determining the sex‐specific and age‐specific demographic causes of population decline, and the tracking studies have been able to derive where and how these impacts occur, in particular the role of long‐line fisheries.
1. INTRODUCTION
Changes in population sizes results from demographic processes, and the associated evolutionary processes, take place over long periods of time, especially in long‐lived animals. Thus, long‐term individual‐based studies are necessary to address fundamental questions about these processes (Clutton‐Brock & Sheldon, 2010 ). In particular, long‐term studies allow measurements on an annual basis of demographic parameters at the individual level to understand the reasons for variation in population size. But the demographic traits themselves result from complex allocation processes (Stearns, 1992 ), trade‐offs between reproduction and survival, that depend entirely on the foraging performance of individuals (Figure 1 ) and thus the resource acquisition (VanNoordwijk & DeJong, 1986 ). The ability of an individual to extract resources from the environment determines the amount of energy available to an individual to be expended in fitness‐related activities, such as self‐maintenance and reproduction, and the relative level of between‐individual variation in resource acquisition determines whether we can observe the trade‐offs (VanNoordwijk & DeJong, 1986 ). Foraging efficiency is known to be a major determinant of individual fitness (Stephens, Brown, & Ydenberg, 2007 ). Thus, foraging and the allocation of resources during reproduction should be considered under the conceptual framework of life‐history theory (Stearns, 1992 ), and foraging effort could be regarded as the result of an allocation decision (Boggs, 1992 ). In this context, evidence is accumulating about the role of the animal physiological state, for example body condition, in these allocation decisions (McNamara & Houston, 1996 ), underlining the interest of recording information on the condition or mass of individuals in long‐term studies (Ozgul et al., 2010 ). Being able to couple long‐term demographic studies, mass and foraging behaviour at the individual level make it possible to investigate the links between foraging and survival and reproduction, and ultimately the links between the environment and the demography of populations (Figure 1 ).

Conceptual framework showing the links between climate and demography through foraging and allocation processes
Long‐term studies started in the late 1940s and 1950s on several species of birds, such as great tits, kittiwakes or fulmars, and have been the catalysts for many new long‐term studies in the following decades (Clutton‐Brock & Sheldon, 2010 ). Increasingly, they have played a central role in research in ecology. In the earlier years of long‐term studies, studying foraging behaviour in the wild was not an easy task and difficult to perform at the individual level. Foraging studies were mainly possible in controlled conditions or in the wild were based on observations of non‐marked individuals. It is only since the late 1970s that VHF (very high frequency) telemetry allowed the tracking of individuals in their environment, and in the late 1980s that satellite telemetry emerged as a tracking method (Jouventin & Weimerskirch, 1990 ), available first on large animals, and progressively with the miniaturization of electronics to smaller and smaller animals. Today, not only it is possible to track remotely and accurately animals in their environment, but also to record many other parameters such as activity, heart rate through methods grouped as bio‐logging (Ropert‐Coudert & Wilson, 2005 ) and thus to estimate foraging effort. Applying these methodologies to animals from long‐term studies whose age, pedigree or lifetime reproductive success is recorded has opened the possibility to address questions on the links between foraging and demographic traits.
The wandering albatross Diomedea exulans has been the animal model in an effort to link demographic traits and foraging behaviour. In this synthesis, I summarize the past 50 years of wandering albatross research on the Crozet Islands that was conducted on two parallel fronts of research that have converged over the past 20 years, demography and foraging ecology. The aims of this review are to (1) briefly describe wandering albatross biology, (2) provide a historical overview of the wandering albatross research from late 1950s, (3) examine how foraging studies have been able to apprehend the age‐related and sex‐specific demography and (4) illustrate how the combination of these two fields of ecological research have been critical for the conservation of seabirds.
2. SHORT HISTORY OF WANDERING ALBATROSS RESEARCH
On the Crozet Islands, south‐western Indian Ocean, studies on the wandering albatross started in 1959 with the banding of most breeding birds on one of the large island of the archipelago, Possession Island (52°E, 46°S). Members of the expedition led by Captain Tilman on sailing boat Mischief banded 200 albatrosses with bands provided by the California Wildlife Society. At the same time, Lance Tickell was carrying out a major study on the breeding biology of the species at South Georgia (Tickell, 1968 ). At Crozet, Jean‐Louis Mougin, who worked with George Dunnet on his starting long‐term study of fulmars, and Jean Prevost from the Paris National Museum set up a demographic study from 1965 on the model of the fulmar mark–recapture protocol, using as a basis the 1959 bandings. Since then, every year wandering albatrosses have been banded as fledglings or adults, and their status (visitor, non‐breeding, breeding etc.….), breeding success and mate identity recorded, and the nest located. The long‐term monitoring programme was taken over from the early 1980s by CNRS researchers and then managed by the CNRS Chizé laboratory as part of a programme funded by the French Polar Institute led by Pierre Jouventin and later myself. With the continuous monitoring programme since 1965, today each wandering albatross from Possession Islands is identified by a metal band, and its age, as well as its pedigree is known. Mass and measurements are taken every year on large sample sizes of adults and fledglings. In addition, since 10 years, we have been studying personalities in our population, measured as a gradient between shy and bold birds reacting to human approach (Patrick, Charmantier, & Weimerskirch, 2013 ). A total of 14,920 individuals were banded for the Possession Island population, with more than 53,647 capture–recapture histories recorded over 56 years.
Because of their large size, tameness and easy access to their colonies on Crozet, wandering albatrosses have been the animal model on which many new bio‐logging techniques have been experimented first. In 1989, the first successful satellite tracking of a bird was carried out on wandering albatrosses from Crozet (Jouventin & Weimerskirch, 1990 ), and studies were continued on an annual basis for years with Argos transmitters. In late 1990s—early 2000s, wandering albatrosses were the first species on which GPS (Weimerskirch, Bonadonna et al., 2002 ) or geolocators measuring light level (Weimerskirch & Wilson, 2000 ) were tested. Geolocators, albeit providing low accuracy locations, allow the tracking over years of large number of individuals because of their low cost and extremely light weight (1–2 g). As subject of many new advances in foraging studies, such as prey capture recording (Weimerskirch & Wilson, 1992 ; Wilson, Cooper, & Plötz, 1992 ), heart rate recording (Weimerskirch, Guionnet, Martin, Shaffer, & Costa, 2000 ; Weimerskirch, Shaffer et al., 2002 ) or radar detectors (Weimerskirch, Filippi, Collet, Waugh, & Patrick, 2017 ), the species has become an animal model for which long‐term demographic studies could be combined with tracking studies, with more than 1,000 individual deployments on the species at Possession Island only.
3. WANDERING ALBATROSS BIOLOGY AND POPULATION DYNAMICS
The wandering albatross is a very large seabird (8–12 kg, up to 3.5 m in wingspan) breeding on sub‐Antarctic islands of the Southern Ocean (Photograph (Photograph1). 1 ). It is a very long‐lived animal living more than 60 years of age. The species is sexually dimorphic, males being 20% larger and heavier than females, males weigh 8–12 kg, compared to 7–9 kg in females. The breeding cycle lasts an entire year with nest building starting in December, laying of the single egg in early January. The single large egg is incubated alternatively by the male and female for bouts of 10 days on average (Weimerskirch, 1995 ), and hatching occurs in mid‐March after an incubation period of 80 days (Tickell, 1968 ). After 1 month of brooding by both parents alternatively, the chick is left alone on the nest and fed infrequently by both parents so that growth is extremely slow, attaining a peak weight in September, when parents progressively decrease the number of visits to the chick, and then leave it alone on the nest (Weimerskirch & Lys, 2000 ). In November, the chick has lost 20%–50% of its peak weight and is almost abandoned by its parents. Chicks fledge in November, after 8 months of parental care, one of the longest periods of parental care in birds. They leave the nest independently from their parents, spend several years at sea continuously before visiting their birth place at the age of 3–7 years, and breeding at an average age of 10 years (range 6–15)(Weimerskirch, Brothers, & Jouventin, 1997 ). After the 1‐year‐long breeding season, parents generally take a sabbatical year when they stay permanently at sea before returning the next breeding season (Tickell, 1968 ), although some individuals, especially females, are able to breed again the next year (Barbraud & Weimerskirch 2012 ).

A pair of adult wandering albatrosses on their nest, with a juvenile individual practising flight in the back ground (Photograph H. Weimerskirch) [Colour figure can be viewed at http://wileyonlinelibrary.com ]
Wandering albatrosses are well known for using a dynamics soaring flight whereby they use the wind as an energy source (Pennycuick 1982 ), and studies measuring heart rate have shown that when in flight birds have metabolic rates close to resting (Weimerskirch, Guionnet et al., 2000 ). The wind is therefore a major determinant of the foraging efficiency (Figure 1 ). During breeding, birds cover extensive distances to search for prey, mainly squids (Cherel, Xavier, de Grissac, Trouvé, & Weimerskirch, 2017 ), that are dispersed, and found on average every 64 km (Weimerskirch, Gault, & Cherel, 2005 ; Weimerskirch, Pinaud, Pawlowski, & Bost, 2007 ). The search strategy is to cover extensive distances at low costs while searching for prey dispersed over the huge surface of ocean.
When the season ends with the fledging of the chick, parents disperse to sea for a sabbatical year, staying permanently at sea. Satellite tracking and geolocators show that the behaviour is very variable between individuals, from purely sedentary behaviour where birds remain in the western Indian Ocean in a radius of 3–4,000 km around the nesting grounds, to migratory behaviour (Weimerskirch, Delord, Guitteaud, Phillips, & Pinet, 2015 ). Migratory birds either migrate back and forth between Crozet and various sectors off Australia, using the same sector every second year or do a single, double or triple circumpolar navigation, always with an eastward movement following the westerlies, and staging in one, two or three oceanic sectors off eastern New Zealand, Chili or Argentina (Weimerskirch et al., 2015 ). These specific sectors are used every second year by the same individual, throughout its lifetime (Weimerskirch, Jouventin, Mougin, Stahl, & VanBeveren, 1985 ; Weimerskirch & Wilson, 2000 ). Moult occurs in these sectors, and the extent of moult of the flight feathers depends on the length of the sabbatical period (Weimerskirch, 1991 ). Males renew more feathers than females. First time breeders have less new feathers than other breeders, suggesting that moult may be a significant constraint in the life history of the species.
4. WANDERING ALBATROSS SEX‐ AND AGE‐RELATED DEMOGRAPHY AND FORAGING ECOLOGY, AND THE ROLE OF MASS
Over the past 50 years, important changes occurred in the demography of the Crozet wandering albatross population (Figure 2 ). After a period of stability in the 1960s, the number of pairs breeding every year crashed abruptly in the 1970s, and increased from the mid‐1980s to peak at the end of the 1990s, then declined slightly (Figure 2 ). Over the entire period breeding success increased to attain high values of 80% of eggs producing fledglings leaving the colony. Annual juvenile survival increased until the mid‐1980s and then declined afterwards, while adult survival was high through the period, apart for low values in the 1970s and in 2005. The mass of breeding males and females increased by almost one kilogram over the past 25 years (Figure 2 ). All these changes have been the results of several factors affecting the males and females differently and find their roots in the strong age‐ and sex‐specific differences in the foraging ecology of the different life stages of the population.

Changes over the study period in the population size, breeding success, adult and juvenile annual survival, and in the mass of breeding males and females
Foraging behaviour, the set of processes by which organisms acquire energy and nutrients is considered to play a key role in shaping patterns of age‐specific reproduction in the wild (Forslund & Pärt, 1995 ). Age, sex and foraging ability interact in shaping ageing patterns in natural conditions. Therefore, we are in a position to examine how the interplay between condition, foraging abilities and demography operates throughout the lifetime of males and females, in particular age‐related changes in mass, breeding success and survival (Figure 3 ). In wandering albatrosses, age strongly affects survival, reproductive performance, mass and foraging performance (Figure 3 ), and age affects males and females differently.

Changes over age in adult survival, mass, breeding success and latitudinal distribution at sea of males and females wandering albatross
4.1. Early life and recruitment
The early life of animals is generally a poorly known period, especially the juvenile phase when young animals disperse and are generally affected by a high mortality. Yet, in long‐lived species, younger age classes represent up to half of the total population and variations in vital rates of younger age classes are likely to have a high influence on the population dynamics and the rate of evolutionary change in long‐lived species (Cam & Aubry, 2011 ; Saether et al., 2013 ). In addition, there is increasing evidence that conditions experienced in early life may have long‐term individual fitness consequences with important demographic and evolutionary effects (Gaillard, Festa‐Bianchet, Yoccoz, Loison, & Toïgo, 2000 ). After fledging, juvenile wandering albatrosses disperse widely to the north of the species range, in subtropical waters (Weimerskirch, Akesson, & Pinaud, 2006 ). Previous studies in mammals and birds suggested that most of the mortality between fledging and recruitment occurs during the first months of life (Gaillard et al., 2000 ). This pattern has been linked to progressive improvement of foraging skills in early life (Marchetti & Price, 1989 ). In the wandering albatross, learning of basic foraging skills allowing immediate survival takes place within a few months after fledging. Foraging movement capacities become similar to those of adults within a few months after fledging (Riotte‐Lambert & Weimerskirch, 2013 ). Individuals are also able during this short period to use efficiently wind conditions and detect environmental gradients (De Grissac, Bartumeus, Cox, & Weimerskirch, 2017 ). Juvenile survival over the first 2 years of life is 0.64, which is one of the highest values of early‐life survival estimated for a bird species, but still lower than annual adult survival (Figure 3 ). Males have a higher mortality rate at this early stage than females. Male wandering albatrosses which are structurally larger and heavier than females could be more sensitive to starvation during this critical step of independence due to their higher food requirements for self‐maintenance. Juvenile male survival is higher for individuals in better condition at peak mass during growth (Cornioley, Jenouvrier, Börger, Weimerskirch, & Ozgul, 2017 ). Males of larger structural size at fledging are those that better survive, whereas females in better body condition survive better (Weimerskirch, Barbraud, & Lys, 2000 ). Early‐life survival was also highly variable across years although the sensitivity of young birds to environmental variability decreased with age (Fay, Weimerskirch, Delord, & Barbraud, 2015 ). In addition, early‐life survival was more affected by natal environmental conditions (i.e., those that the parents encountered during their foraging trips to provision the chicks) than by climatic conditions encountered during the first year of life at sea when young animals disperse over large oceanic sectors (Fay et al., 2015 ). This high susceptibility of natal condition is also observed in other long‐lived species (Gaillard et al., 2000 ; Reid, Bignal, Bignal, McCracken, & Monaghan, 2003 ).
After the two‐first years at sea, the sex difference in survival is reversed (Fay et al., 2015 ), and males have a higher survival than females (Figure 3 ). Immature birds show sex‐specific distributions at sea with male wandering albatrosses from Possession Island moving more to the east of the southern Indian Ocean than females (Weimerskirch et al., 2014 ). Thus, environmental conditions experienced by immature individuals could be different between sexes and females may experience less favourable climatic and trophic conditions than males. This period of immaturity is a period when mass increases progressively (Figure 3 ) and when individuals settle in their future habitat used during sabbatical years when adults. There, they have to moult, and in immatures the extent of moult is related to body mass, in males only (Weimerskirch, 1991 ), suggesting strong energetic constraints. But at the same time, immatures aged 3–7 years are also visiting the breeding grounds for the first time and then visit regularly the colony: it is the period when young birds are performing their spectacular displays (Photograph (Photograph2) 2 ) used during pair formation. At this time, they alternate short stay on land of displaying in search of a partner with foraging trips at sea similar to those of breeders (Riotte‐Lambert & Weimerskirch, 2013 ). This period of transition is probably when immature individuals progressively acquire the foraging skills necessary to be an efficient breeder operating as a central place forager from the future colony.

A pair of young wandering albatross in the spectacular “ecstatic display” used during pair formation (Photograph Aurélien Prudor) [Colour figure can be viewed at http://wileyonlinelibrary.com ]
4.2. Recruitment
This transition phase between first return on land and first reproduction lasts several years, a long period critical for attaining sufficient foraging skills. In the wandering albatross, the onset of breeding depends entirely on mass (Box 1 ) and is not dependent on age per se. Indeed, wandering albatrosses are sexually mature at age 6 years (Hector, Pickering, Croxall, & Follett, 1990 ), and some breed at this age in our population, but most individuals recruit several years later. Recruitment depends mainly on the ability of individuals to reach a mass threshold from which they can breed (Weimerskirch, 1992 ). This threshold is around 8 kg for females and 9.4 kg for males and is attained after several years of immaturity. The ability to attain this minimum mass threshold depends on the foraging efficiency during the phase of transition where birds have to be central place foragers and learn how to forage not only for themselves, but also to store additional energy that will be expended in reproduction. Age at first reproduction is a key demographic parameter that is probably under high selective pressure, but is surprisingly highly variable in the wandering albatross, from 6 to 15 years, and is negatively related to both reproductive and survival adult performances, suggesting that individual quality is an important factor to explain variations in the age at first reproduction (Fay, Barbraud, Delord, & Weimerskirch, 2016 ).
Summary of how different body mass during the life‐history stages of the wandering albatross affect vital rates

4.3. Adult stage
When they have attained adulthood, wandering albatrosses have high survival rates, similar in males and females. When breeding, the incubation period is when most failures occur. At this time wind speed increases bird ground speed, which in turn reduces trip duration. Wandering albatrosses forage until their energy requirements is fulfilled, but trip duration does not affect mass gain and lighter individuals gained more mass, suggesting that wandering albatrosses adjust energy requirement to their body condition (Cornioley, Börger, Ozgul, & Weimerskirch, 2016 ). The mass of males increased breeding success and their survival increased with increasing mass (Cornioley et al., 2017 ). During the long chick‐rearing period during winter, to provision the chick, wandering albatrosses use a twofold strategy whereby they alternate (1) short trips close to the colony (at hundreds of kilometres) to provide food regularly to the chick, but during these trips where birds do not use optimally the wind conditions, they lose mass; with (2) long trips at thousands of kilometres when adults feed mainly for themselves with a high efficiency using wind conditions optimally, but at the expense of reducing feeding frequency to the chick (Weimerskirch, Barbraud et al., 2000 ). This strategy is the result of a trade‐off between self‐feeding (survival) and chick provisioning (reproduction) and is under the control of the body mass of the parent (Weimerskirch, Barbraud et al., 2000 ) (Figure 1 , Box 1 ). When adult mass is good, above a threshold value, the adult provisions the chick, but loses condition until mass falls below the threshold than starts a long trip to restore its condition (Weimerskirch, 1999 ). The mass is again of utmost importance for decisions regarding breeding investment, as it was for the recruitment into the population and for decisions to breed or not at the start of every breeding season (Weimerskirch, 1992 ; Box 1 ). When they provision their chick, the mass of males affects the mass of sons, but not of daughters (Cornioley et al., 2017 ) giving evidence of higher investment of fathers in sons, but not in daughters with increasing father mass. This strategy is in line with the current theory stating that long‐lived seabirds should adjust their reproductive performance to both their own body conditions and the need of their offspring (Erikstad, Fauchald, Tveraa, & Steen, 1998 ). Thus, both parents provide more food to sons than to daughters and parents adjust meal size to male chick needs (Weimerskirch, Barbraud et al., 2000 ). As a consequence, two life‐history outcomes, longevity and lifetime reproductive output, were higher for males that were heavy as chicks compared to initially lighter ones (Cornioley et al., 2017 ). These results suggest that a higher investment in sons by fathers can increase their inclusive fitness, which is not the case for daughters. Again these results highlight the sex‐specific differences in the effect of body mass on the life history of this monogamous species with bi‐parental care (Cornioley et al., 2017 ).
When the breeding season ends, both parents disperse in the Southern Ocean to take a sabbatical year, remaining either in the Southern Indian Ocean, or migrating in various sites in the Southern Ocean. However, a small proportion of individuals is able to breed immediately after a successful breeding (Barbraud & Weimerskirch, 2012 ). Males are more likely to have a long range migratory behaviour, compared to females that are mainly sedentary. This difference in migratory behaviour has strong implications for the fitness of individuals, as almost exclusively females are able to breed annually (Weimerskirch et al., 2015 ). As partners are extremely faithful during their lifetime, with very low divorce rate (Jouventin, Lequette, & Dobson, 1999 ), when a female breed during two consecutive seasons and her mate takes a sabbatical year, she changes temporarily of partner (Weimerskirch et al., 2015 ).
4.4. Senescence
Wandering albatrosses have demographic characteristics that make them interesting models for the study of ageing. Being very long‐lived, they can attain more than 60 years, we have in our population individuals aged “more than” 57 years, because they were banded as adults of unknown age 50 years ago. We will need a further 10 or 20 years of monitoring to be able to determine the maximum age they can attain, probably 60–70 years. The ageing pattern especially appearance of senescence nicely falls within the primate continuum of ageing, between chimpanzee and human (Bronikowski et al., 2011 ), with the same tendencies for males to have shorter life spans and higher mortality at old ages than females (Pardo, Barbraud, & Weimerskirch, 2013 ), a sex ratio at birth biased systematically towards males (Weimerskirch, Lallemand, & Martin, 2005 ), suggesting that mortality patterns in long‐lived species are shaped by local selective forces rather than phylogeny (Forslund & Pärt, 1995 ). Of course, they differ from primates and from other long‐lived marine animals such as killer whale (Croft et al., 2016 ) where menopause occurs in that female albatrosses reproduce throughout their life until old ages. Why are males suffering a higher mortality at old ages than females? In terms of foraging, breeding males progressively shift their foraging zone to the south up to remote Antarctic waters, whereas young and middle‐aged males never foraged south of the Polar Front (Lecomte et al., 2010 ; Weimerskirch et al., 2014 ). Old males travelled a greater distance but were less active at the sea surface, and returned from sea with elevated levels of stress hormone (corticosterone), suggesting a low foraging efficiency (Lecomte et al., 2010 ). In parallel, the mass of old males progressively decreases after 30 years of age (Figure 3 ). Foraging efficiency of males appears to play a central role in shaping ageing patterns in natural conditions in wandering albatrosses, whereas females do not show a similar deleterious effect of ageing.
Personality is heritable (Patrick et al., 2013 ) and affected breeding success differently between males and females at old ages. Bold males had a higher reproductive success in later life than shy males, demonstrating a slower rate of senescence, whereas no such effects were found in females (Patrick & Weimerskirch, 2015 ). This is in accordance with the observation that during old ages bolder males gain more mass during foraging trips, suggesting they are able to replenish resources essential for successful reproduction. Bolder birds also make longer foraging trips as they get older. Such interaction between boldness and age did not occur at younger ages, nor for determining the age at first reproduction, indicating that these results are not driven by differences in early life. Thus, trade‐offs between current and future reproduction for bolder individuals may be exhibited in albatross but only during later life when reproductive success is more variable and has a stronger impact on survival (Patrick & Weimerskirch, 2015 ).
Thus, throughout their lifetime, our studies on wandering albatrosses have shown that foraging efficiency and the regulation of mass are the major factor regulating the trade‐off between breeding and survival (Figure 1 ). The mass‐dependent demography is likely to strongly influence the population dynamics, possibly by providing a delayed response in population size to environmental changes, generating autocorrelation in population fluctuations. Thus, to properly describe the population dynamics of such species, it would be important to develop in the future structured population models that include not only age but also body mass as a stage.
Many demographic parameters are influenced by environmental conditions at various stages of the life cycle of the wandering albatrosses. In particular, wind conditions play a central role for the foraging efficiency of albatrosses (Pennycuick, 1982 ; Weimerskirch, Guionnet et al., 2000 ) and it is no surprise that environmental conditions strongly affect demography through foraging and allocation processes (Figure 1 ). Climate change has affected wind in the Southern Ocean, with westerly winds having increased in intensity and moved pole ward (Thompson & Solomon, 2002 ; Weimerskirch, Louzao, de Grissac, & Delord, 2012 ). As a consequence, over the past 20 years, the foraging range of wandering albatrosses has shifted to the south in conjunction with these changes in wind pattern (Péron et al., 2010 ), while their rates of travel and flight speeds had increased (Weimerskirch et al., 2012 ). Consequently, the duration of foraging trips has decreased, breeding success has improved, and birds have increased in mass by more than one kilogram. These positive consequences of climate change may be temporary if patterns of wind in the southern westerlies follow predicted climate change scenarios suggesting a further shift towards south of strong wind area. Again these results based on long‐term records stress the importance of foraging performance as the key link between environmental changes and population processes.
5. DEMOGRAPHY AND FORAGING ECOLOGY AS TOOLS FOR CONSERVATION
Understanding the patterns and processes that generate changes in population size over time is essential for the conservation of species and their conservation status such as for IUCN listing. Yet, this is often not sufficient to understand the ultimate causes of a decline in survival or breeding success which is the source of the decline. Long‐term studies of animals can not only help us to answer important questions in ecology, but also play a key role in conservation. The case of the wandering albatross monitoring programme on Crozet is a striking example. Long‐term monitoring of the Crozet population detected as soon as the late 1980s that wandering albatrosses had declined severely over the past 20 years (Weimerskirch & Jouventin, 1987 ), similarly to the South Georgia population (Croxall, 1979 ). However, whereas at South Georgia, only population censuses were considered, and the causes of the decline were not known, the study at Crozet indicated that the decline of the population was due to a decrease in female adult survival. The decline was likely due to fishing operation in the range of females that favoured warmer waters than males (Weimerskirch & Jouventin, 1987 ). From this first study suggesting—possibly long lining as a potential threat because hooks were founds in the colony at Crozet, the first dedicated observations on board Japanese long liners confirmed that long lining for tuna was killing large numbers of albatrosses and petrels (Brothers, 1991 ). The Crozet study indicated that mortality probably occurred during breeding, but tuna long lining was located at more than 1,000 km from Crozet, a distance that was not considered at this time to be within the foraging range for a breeding seabird. In 1989, the first tracks of wandering albatrosses proved that breeding albatrosses had foraging range of more than 2,000 km (Jouventin & Weimerskirch, 1990 ), and following studies at Crozet showed that mainly breeding females from Crozet were able to reach the fishing zones of tuna long liners in subtropical waters while breeding (Weimerskirch, Salamolard, Sarrazin, & Jouventin, 1993 ). The first study combining tracks of foraging albatrosses with detailed distribution year after year of Japanese long liners in the Southern Ocean showed that the Crozet population overlapped with the Japanese long‐line fishery and that the development of this fishery coincided with the decline of wandering albatrosses at Crozet, but also in other populations of the species (Weimerskirch et al., 1997 ). After these first results, many studies have been carried out throughout the Southern Ocean and have shown that the long‐line fisheries constitute the major threat for albatross and petrels world‐wide (Croxall et al., 2012 ), and conservation programmes have been developed to reduce by catch, with good success in several fisheries, although the problem remains for other fisheries operating in international waters. Thus, the Crozet long‐term monitoring programme, and the associated tracking of birds from the same population, was crucial for detecting the cause of the decline of wandering albatrosses and the origin of the problem, long‐line fisheries operating at thousands of kilometres from the colonies. The shift towards the south of females due to changes in wind regime related to climate change could reduce in the future the interactions between wandering albatrosses and tuna long‐line fisheries (Weimerskirch et al., 2012 ).
Today fisheries are spread to the north of the range of wandering albatrosses, and the use of Radar detectors fitted on birds with GPS indicates that nearly 80% of individuals from Crozet encounter boats during their foraging trips (Weimerskirch et al., 2017 ). Such a widespread and almost systematic attendance to boats suggests that the foraging behaviour of wandering albatrosses has been modified over the past 50 years when fisheries have spread in the Southern Ocean. Thus, not only long‐line fisheries have increased mortality rates of all categories of the population, but the presence of boats has induced changes in the foraging routines of individuals (Collet, Patrick, & Weimerskirch, 2017 ). Historic analyses of albatross–fisheries interactions suggest that birds may not have modified their foraging zones but rather fisheries occupy the original foraging zone of the species (Weimerskirch, 1998 ). It will be important in the future to understand why wandering albatrosses are attracted since historical time by boats, and measure the benefits of attending boats to balance them with costs.
6. CONCLUSIONS
During the recent years, there is an increasing number of studies aiming at understanding the links between foraging and demography, especially linking habitat selection to demography (Matthiopoulos et al., 2015 ), and more rarely integrating body condition in the link between habitat selection and demography, for example (McLoughlin et al., 2007 ). The Crozet wandering albatross population is probably the one of the few wild population where demography, allocation and foraging behaviour have been studies simultaneously, with a few case studies in terrestrial habitats (Clutton‐Brock, Guinness, & Albon, 1982 ; Grant & Grant, 2002 ). In addition, an important by‐product of the combined studies is that the results had unexpected important applications in terms of conservation, as it is the study that allowed to detect the conservation problem of albatrosses linked to fisheries. This result alone stresses the importance of long‐term studies as sentinels of the long‐term changes occurring in the environment.
The long‐term study of the Crozet wandering albatross population has been successful in tackling several questions that have not been addressed similarly so far in other animal systems. They mainly concern the links between foraging, mass (allocation) and demographic parameters, with strong interactions between sex and age. In the wandering albatross, sexes differ in size, and mass affects multiple interrelated physiological, behavioural and ecological processes. The larger size of males confers them higher travel speed in windier conditions and higher fasting abilities but also higher energetic requirements. These advantages may be operating during some stages of their lifetime, at adulthood, and favour, for example, the migratory behaviour of males or their preference for windier southern conditions, but may be disadvantageous at others, that is during the juvenile and old age stages as suggested by the higher mortality rates at these stages. This example illustrates the complexity of interactions occurring across a life‐history strategy that has evolved over thousands of years.
DATA ACCESSIBILITY
Acknowledgements.
I thank all my colleagues, students and postdocs who have been involved in the wandering albatross studies over the past 40 years when I started to work on the species, and in particular Pierre Jouventin, Yves Cherel, Christophe Barbraud, Samantha Patrick, Remy Fay and Tina Cornioley. I thank all the fieldworkers who carried out every year since 1965 the mark recaptures and monitoring programme on Crozet and Dominique Besson and later Karine Delord for invaluable help with data management. The long‐term demographic study at Crozet was supported continuously throughout the last 50 years by Terres Australes and Antarctiques Françaises and later by the French Polar Institute IPEV (programme no. 109). The Ethics Committee of IPEV and Comité de l'Environnement Polaire approved the field procedures. The synthesis is a contribution to the Program EARLYLIFE funded by a European Research Council Advanced Grant under the European Community's Seven Framework Program FP7/2007–2013 (grant agreement no. ERC‐2012‐ADG_20120314 to H.W.). I thank N.G. Yoccoz, an anonymous referee and the editor for helpful comments and suggestions on the manuscript.
Weimerskirch H. Linking demographic processes and foraging ecology in wandering albatross—Conservation implications . J Anim Ecol . 2018; 87 :945–955. https://doi.org/10.1111/1365-2656.12817 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Barbraud, C. , & Weimerskirch, H. (2012). Estimating survival and reproduction in a quasi‐biennially breeding seabird with uncertain and unobservable states . Journal of ornithology , 152 , S605–S615. https://doi.org/10.1007/s10336-011-0686-1 [ Google Scholar ]
- Boggs, C. L. (1992). Resource allocation: Exploring connections between foraging and life history . Functional Ecology , 6 , 508–518. https://doi.org/10.2307/2390047 [ Google Scholar ]
- Bronikowski, A. M. , Altmann, J. , Brockman, D. K. , Cords, M. , Fedigan, L. M. , Pusey, A. , … Alberts, S. C. (2011). Aging in the natural world: Comparative data reveal similar mortality patterns across primates . Science , 331 , 1325–1328. https://doi.org/10.1126/science.1201571 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brothers, N. (1991). Albatross mortality and associated bait loss in the Japanese longline fishery in the Southern Ocean . Biological Conservation , 55 , 255–268. https://doi.org/10.1016/0006-3207(91)90031-4 [ Google Scholar ]
- Cam, E. , & Aubry, L. (2011). Early development, recruitment and life history trajectory in long‐lived birds . Journal of Ornithology , 152 , 187–201. https://doi.org/10.1007/s10336-011-0707-0 [ Google Scholar ]
- Cherel, Y. , Xavier, J. C. , de Grissac, S. , Trouvé, C. , & Weimerskirch, H. (2017). Feeding ecology, isotopic niche, and ingestion of fishery‐related items of the wandering albatross Diomedea exulans at Kerguelen and Crozet Islands . Marine Ecology Progress Series , 565 , 197–215. https://doi.org/10.3354/meps11994 [ Google Scholar ]
- Clutton‐Brock, T. H. , Guinness, . E. , & Albon, S. D. (1982). Red deer: Behavior and ecology of two sexes . Chicago, IL: University of Chicago Press. [ Google Scholar ]
- Clutton‐Brock, T. , & Sheldon, B. C. (2010). Individuals and populations: The role of long‐term, individual‐based studies of animals in ecology and evolutionary biology . Trends in Ecology & Evolution , 25 , 562–573. https://doi.org/10.1016/j.tree.2010.08.002 [ PubMed ] [ Google Scholar ]
- Collet, J. , Patrick, S. C. , & Weimerskirch, H. (2017). Behavioral response to encounter of fishing boats in wandering albatrosses . Ecology and Evolution , 7 , 3335–3347. https://doi.org/10.1002/ece3.2677 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cornioley, T. , Börger, L. , Ozgul, A. , & Weimerskirch, H. (2016). Impact of changing wind conditions on foraging and incubation success in male and female wandering albatrosses . Journal of Animal Ecology , 85 , 1318–1327. https://doi.org/10.1111/1365-2656.12552 [ PubMed ] [ Google Scholar ]
- Cornioley, T. , Jenouvrier, S. , Börger, L. , Weimerskirch, H. , & Ozgul, A. (2017). Fathers matter: Male body mass affects life‐history traits in a size dimorphic seabird . Proceedings of the Royal Society of London. Series B: Biological Sciences , 284 , pii: 20170397 https://doi.org/10.1098/rspb.2017.0397 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Croft, D. P. , Johnstone, R. A. , Ellis, S. , Nattrass, S. , Franks, D. W. , Brent, N. J. N. , … Cant, M. A. (2016). Reproductive conflict and the evolution of menopause in killer whales . Current Biology , 27 , 298–304. [ PubMed ] [ Google Scholar ]
- Croxall, J. P. (1979). Distribution and population changes in the Wandering albatross Diomedea exulans at South Georgia . Ardea , 67 , 15–21. [ Google Scholar ]
- Croxall, J. P. , Butchart, S. H. , Lascelles, B. , Stattersfield, A. J. , Sullivan, B. , Symes, A. , & Taylor, P. (2012). Seabird conservation status, threats and priority actions: A global assessment . Bird Conservation International , 22 , 1–34. https://doi.org/10.1017/S0959270912000020 [ Google Scholar ]
- De Grissac, S. , Bartumeus, F. , Cox, S. , & Weimerskirch, H. (2017). Early life foraging: Behavioural responses of newly fledged albatrosses to environmental conditions . Ecology and Evolution , 7 , 6766–6778. https://doi.org/10.1002/ece3.3210 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Erikstad, K. E. , Fauchald, P. , Tveraa, T. , & Steen, H. (1998). On the cost of reproduction in long‐lived birds: The influence of environmental variability . Ecology , 79 , 1781–1788. https://doi.org/10.1890/0012-9658(1998)079[1781:OTCORI]2.0.CO;2 [ Google Scholar ]
- Fay, R. , Barbraud, C. , Delord, K. , & Weimerskirch, H. (2016). Variation in the age of first reproduction: Different strategies or individual quality? Ecology , 97 , 1842–1851. https://doi.org/10.1890/15-1485.1 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fay, R. , Weimerskirch, H. , Delord, K. , & Barbraud, C. (2015). Population density and climate shape early‐life survival and recruitment in a long‐lived pelagic seabird . Journal of Animal Ecology , 84 , 1423–1433. https://doi.org/10.1111/1365-2656.12390 [ PubMed ] [ Google Scholar ]
- Forslund, P. , & Pärt, T. (1995). Age and reproduction in birds hypotheses and tests . Tree , 10 , 374–378. [ PubMed ] [ Google Scholar ]
- Gaillard, J.‐M. , Festa‐Bianchet, M. , Yoccoz, N. G. , Loison, A. , & Toïgo, C. (2000). Temporal variation in fitness components and population dynamics of large herbivores . Annual Review of Ecology and Systematics , 31 , 367–393. https://doi.org/10.1146/annurev.ecolsys.31.1.367 [ Google Scholar ]
- Grant, P. R. , & Grant, B. R. (2002). Unpredictable evolution in a 30‐year study of Darwin's finches . Science , 296 , 707–711. https://doi.org/10.1126/science.1070315 [ PubMed ] [ Google Scholar ]
- Hector, J. A. L. , Pickering, S. P. C. , Croxall, J. P. , & Follett, B. K. (1990). The endocrine basis of deferred sexual maturity in the wandering albatross, Diomedea exulans L . Functional Ecology , 4 , 59–66. https://doi.org/10.2307/2389653 [ Google Scholar ]
- Jouventin, P. , Lequette, B. , & Dobson, F. S. (1999). Age‐related mate choice in the wandering albatross . Animal Behaviour , 57 , 1099–1106. https://doi.org/10.1006/anbe.1999.1083 [ PubMed ] [ Google Scholar ]
- Jouventin, P. , & Weimerskirch, H. (1990). Satellite tracking of Wandering albatrosses . Nature , 343 , 746–748. https://doi.org/10.1038/343746a0 [ Google Scholar ]
- Lecomte, V. , Sorci, G. , Cornet, S. , Jaeger, A. , Faivre, B. , Arnoux, E. , … Weimerskirch, H. (2010). Patterns of aging in the long‐lived wandering albatross . Proceedings of the National Academy of Sciences of the USA , 107 , 6370 https://doi.org/10.1073/pnas.0911181107 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Marchetti, K. , & Price, T. (1989). Differences in the foraging of juvenile and adult birds: The importance of developmental constraints . Biological Reviews , 64 , 51–70. https://doi.org/10.1111/j.1469-185X.1989.tb00638.x [ Google Scholar ]
- Matthiopoulos, J. , Fieberg, J. , Aarts, G. , Beyer, H. L. , Morales, J. M. , & Haydon, D. T. (2015). Establishing the link between habitat selection and animal population dynamics . Ecological Monographs , 85 , 413–436. https://doi.org/10.1890/14-2244.1 [ Google Scholar ]
- McLoughlin, P. D. , Gaillard, J. M. , Boyce, M. S. , Bonenfant, C. , Messier, F. , Duncan, P. , … Klein, F. (2007). Lifetime reproductive success and composition of the home range in a large herbivore . Ecology , 88 , 3192–3201. https://doi.org/10.1890/06-1974.1 [ PubMed ] [ Google Scholar ]
- McNamara, J. M. , & Houston, A. I. (1996). State‐dependent life histories . Nature , 380 , 215–221. https://doi.org/10.1038/380215a0 [ PubMed ] [ Google Scholar ]
- Ozgul, A. , Childs, D. , Oli, M. , Armitage, K. , Blumstein, D. , Olson, L. , … Coulson, T. (2010). Coupled dynamics of body mass and population growth in response to environmental change . Nature , 466 , 482–485. https://doi.org/10.1038/nature09210 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pardo, D. , Barbraud, C. , & Weimerskirch, H. (2013). Females better face senescence in the wandering albatross . Oecologia , 173 , 1283–1294. https://doi.org/10.1007/s00442-013-2704-x [ PubMed ] [ Google Scholar ]
- Patrick, S. C. , Charmantier, A. , & Weimerskirch, H. (2013). Differences in boldness are repeatable and heritable in a long‐lived marine predator . Ecology and Evolution , 3 , 4291–4299. https://doi.org/10.1002/ece3.748 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Patrick, S. C. , & Weimerskirch, H. (2015). Senescence rates and late adulthood reproductive success are strongly influenced by personality in a long‐lived seabird . Proceedings of the Royal Society of London B: Biological Sciences , 282 , 20141649 https://doi.org/10.1098/rspb.2014.1649 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pennycuick, C. J. (1982). The flight of petrels and albatrosses (Procellariiformes), observed in South Georgia and its vicinity . Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences , 300 , 75–106. https://doi.org/10.1098/rstb.1982.0158 [ Google Scholar ]
- Péron, C. , Authier, M. , Barbraud, C. , Delord, K. , Besson, D. , & Weimerskirch, H. (2010). Interdecadal changes in at‐sea distribution and abundance of subantarctic seabirds along a latitudinal gradient in the Southern Indian Ocean . Global Change Biology , 16 , 1895–1909. https://doi.org/10.1111/j.1365-2486.2010.02169.x [ Google Scholar ]
- Reid, J. M. , Bignal, E. M. , Bignal, S. , McCracken, D. I. , & Monaghan, P. (2003). Environmental variability, life‐history covariation and cohort effects in the red‐billed chough Pyrrhocorax pyrrhocorax . Journal of Animal Ecology , 72 , 36–46. https://doi.org/10.1046/j.1365-2656.2003.00673.x [ Google Scholar ]
- Riotte‐Lambert, L. , & Weimerskirch, H. (2013). Do naïve juvenile seabirds forage differently from adults? Proceedings of the Royal Society of London. Series B: Biological Sciences , 280 , 20131434 https://doi.org/10.1098/rspb.2013.1434 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ropert‐Coudert, Y. , & Wilson, R. P. (2005). Trends and perspectives in animal‐attached remote sensing . Frontiers in Ecology and the Environment , 3 , 437–444. https://doi.org/10.1890/1540-9295(2005)003[0437:TAPIAR]2.0.CO;2 [ Google Scholar ]
- Saether, B.‐E. , Coulson, T. , Grotan, V. , Engen, S. , Altwegg, R. , Armitage, K. B. , … Dobson, F. S. (2013). How life history influences population dynamics in fluctuating environments . The American Naturalist , 182 , 743–759. https://doi.org/10.1086/673497 [ PubMed ] [ Google Scholar ]
- Stearns, S. C. (1992). The evolution of life histories . Oxford, UK: Oxford University Press. [ Google Scholar ]
- Stephens, D. W. , Brown, J. S. , & Ydenberg, R. C. (2007). Foraging: Behavior and ecology . Chicago, IL: University of Chicago Press; https://doi.org/10.7208/chicago/9780226772653.001.0001 [ Google Scholar ]
- Thompson, D. , & Solomon, S. (2002). Interpretation of recent Southern Hemisphere climate change . Science , 296 , 895 https://doi.org/10.1126/science.1069270 [ PubMed ] [ Google Scholar ]
- Tickell, W. L. N. (1968). The biology of the great albatrosses, Diomedea exulans and Diomedea epomophora . Antarctic Research Series , 12 , 1–55. [ Google Scholar ]
- VanNoordwijk, A. J. , & DeJong, G. (1986). Acquisition and allocation of resources: Their influence on variation in life history tactics . The American Naturalist , 128 , 137–142. https://doi.org/10.1086/284547 [ Google Scholar ]
- Weimerskirch, H. (1991). Sex‐specific differences in molt strategy in relation to breeding in the wandering albatross . Condor , 93 , 731–737. https://doi.org/10.2307/1368205 [ Google Scholar ]
- Weimerskirch, H. (1992). Reproductive effort in long‐lived birds: Age‐specific patterns of condition, reproduction and survival in the wandering albatross . Oikos , 63 , 464–473. https://doi.org/10.2307/3545162 [ Google Scholar ]
- Weimerskirch, H. (1995). Regulation of foraging trips and incubation routine in male and female wandering albatrosses . Oecologia , 102 , 37–43. https://doi.org/10.1007/BF00333308 [ PubMed ] [ Google Scholar ]
- Weimerskirch, H. (1998). Foraging strategies of southern albatrosses and their relationship with fisheries In Robertson G. & Gales R. (Eds), Albatross biology and conservation (pp. 168–179). Sydney, Australia: Surrey, Beatty & Sons. [ Google Scholar ]
- Weimerskirch, H. (1999). The role of body condition in breeding and foraging decisions in albatrosses and petrels . Proc. 22 Int. Ornithol. Congr , pp. 1178–1189.
- Weimerskirch, H. , Akesson, S. , & Pinaud, D. (2006). Postnatal dispersal of wandering albatrosses Diomedea exulans : Implication for the conservation of the species . Journal of Avian Biology , 37 , 23–28. https://doi.org/10.1111/j.2006.0908-8857.03675.x [ Google Scholar ]
- Weimerskirch, H. , Barbraud, C. , & Lys, P. (2000). Sex differences in parental investment and chick growth in wandering albatrosses: Fitness consequences . Ecology , 81 , 309–318. https://doi.org/10.1890/0012-9658(2000)081[0309:SDIPIA]2.0.CO;2 [ Google Scholar ]
- Weimerskirch, H. , Bonadonna, F. , Bailleul, F. , Mabille, G. , Dell'Omo, G. , & Lipp, H. P. (2002). GPS tracking of foraging albatrosses . Science , 295 , 1259 https://doi.org/10.1126/science.1068034 [ PubMed ] [ Google Scholar ]
- Weimerskirch, H. , Brothers, N. , & Jouventin, P. (1997). Population dynamics of wandering albatross Diomedea exulans and Amsterdam albatross D. amsterdamensis in the Indian Ocean and their relationships with long‐line fisheries: Conservation implications . Biological Conservation , 79 , 257–270. https://doi.org/10.1016/S0006-3207(96)00084-5 [ Google Scholar ]
- Weimerskirch, H. , Cherel, Y. , Delord, K. , Jaeger, A. , Patrick, S. C. , & Riotte‐Lambert, L. (2014). Lifetime foraging patterns of the wandering albatross: Life on the move! . Journal of Experimental Marine Biology and Ecology , 450 , 68–78. https://doi.org/10.1016/j.jembe.2013.10.021 [ Google Scholar ]
- Weimerskirch, H. , Delord, K. , Guitteaud, A. , Phillips, R. A. , & Pinet, P. (2015). Extreme variation in migration strategies between and within wandering albatross populations during their sabbatical year, and their fitness consequences . Scientific Reports , 5 , Art. no. 8853 https://doi.org/10.1038/srep08853 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Weimerskirch, H. , Filippi, D. P. , Collet, J. , Waugh, S. M. , & Patrick, S. C. (2017). Use of radar detectors to track attendance of albatrosses at fishing vessels . Conservation Biology , 32 , 240–245. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Weimerskirch, H. , Gault, A. , & Cherel, Y. (2005). Prey distribution and patchiness: Factors affecting the foraging success and efficiency of wandering albatrosses . Ecology , 86 , 2611–2622. https://doi.org/10.1890/04-1866 [ Google Scholar ]
- Weimerskirch, H. , Guionnet, T. , Martin, J. , Shaffer, S. A. , & Costa, D. (2000). Fast and fuel efficient? Optimal use of wind by flying albatrosses . Proceedings of the Royal Society of London. Series B: Biological Sciences , B267 , 1869–1874. https://doi.org/10.1098/rspb.2000.1223 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Weimerskirch, H. , & Jouventin, P. (1987). Population dynamics of the Wandering albatross, Diomedea exulans , of the Crozet Islands: Causes and consequences of the population decline . Oikos , 49 , 315–322. https://doi.org/10.2307/3565767 [ Google Scholar ]
- Weimerskirch, H. , Jouventin, P. , Mougin, J. C. , Stahl, J. C. , & VanBeveren, M. (1985). Banding recoveries and the dispersal of seabirds breeding in French Austral and Antarctic territories . Emu , 85 , 22–33. https://doi.org/10.1071/MU9850022 [ Google Scholar ]
- Weimerskirch, H. , Lallemand, J. , & Martin, J. (2005). Population sex ratio variation in a monogamous long‐lived bird, the wandering albatross . Journal of Animal Ecology , 74 , 285–291. https://doi.org/10.1111/j.1365-2656.2005.00922.x [ Google Scholar ]
- Weimerskirch, H. , Louzao, M. , de Grissac, S. , & Delord, K. (2012). Changes in wind pattern alter albatross distribution and life‐history traits . Science , 335 , 211–214. https://doi.org/10.1126/science.1210270 [ PubMed ] [ Google Scholar ]
- Weimerskirch, H. , & Lys, P. (2000). Seasonal changes in the provisioning behaviour and mass of male and female wandering albatrosses in relation to the growth of their chick . Polar Biology , 23 , 733–744. https://doi.org/10.1007/s003000000144 [ Google Scholar ]
- Weimerskirch, H. , Pinaud, D. , Pawlowski, F. , & Bost, C. (2007). Does prey capture induce area‐restricted search? A fine‐scale study using GPS in a marine predator, the wandering albatross . The American Naturalist , 170 , 734–743. [ PubMed ] [ Google Scholar ]
- Weimerskirch, H. , Salamolard, M. , Sarrazin, F. , & Jouventin, P. (1993). Foraging strategy of wandering albatrosses through the breeding season: A study using satellite telemetry . The Auk , 110 , 325–342. [ Google Scholar ]
- Weimerskirch, H. , Shaffer, S. A. , Mabille, G. , Martin, J. , Boutard, O. , & Rouanet, J. L. (2002). Heart rate and energy expenditure of incubating wandering albatrosses: Basal levels, natural variation, and the effects of human disturbance . The Journal of Experimental Biology , 205 , 475–483. [ PubMed ] [ Google Scholar ]
- Weimerskirch, H. , & Wilson, R. P. (1992). When do wandering albatrosses Diomedea exulans forage? Marine Ecology Progress Series , 86 , 297–300. https://doi.org/10.3354/meps086297 [ Google Scholar ]
- Weimerskirch, H. , & Wilson, R. P. (2000). Oceanic respite for wandering albatrosses . Nature , 406 , 955–956. https://doi.org/10.1038/35023068 [ PubMed ] [ Google Scholar ]
- Wilson, R. P. , Cooper, J. , & Plötz, J. (1992). Can we determine when marine endotherms feed? A case study with seabirds . Journal of Experimental Biology , 167 , 267–275. [ Google Scholar ]
You will be redirected to IUCN Accounts to input your credentials. After log in you will be redirected back to this site.
Rest assured your personal data resides with IUCN and IUCN only. For more information please review our Data policy.

- General search
- Publications
- Resolutions & Recommendations
You are here
The wandering albatross.
- Jameson, W.
Call number:
- Biota-Fa-Bi-087
Albatrosses
Introduction.
All but seven of the world’s 22 species of albatrosses are threatened with extinction. The main threats are incidental mortality (bycatch*) in commercial fisheries. British Antarctic Survey science and technology underpins international efforts to conserve these charismatic birds.
Why are albatross populations declining?
Each year, tens of thousands of albatrosses are drowned as they scavenge behind fishing vessels. Both trawling and longlining – where fishing vessels set lines containing thousands of baited hooks – attract seabirds looking for food. Many albatrosses are dragged to their death as they swallow these baited hooks. Others are injured or killed in collisions with trawler cable. Plastic waste ingested at sea, past introductions of non-native, invasive species such as rats, mice and cats onto breeding islands, and disease pose additional hazards.
How serious is the problem?
Fifteen of the world’s 22 albatross species are threatened with extinction, and six are ‘Near-threatened’ according to the World Conservation Union (IUCN). Two species – the waved and Tristan albatross – are critically endangered. However, several of the populations that are declining most rapidly breed on the UK Overseas Territories in the South Atlantic. On the subantarctic islands of South Georgia, for example, three species of albatrosses monitored by British Antarctic Survey (BAS) are declining at between 2% and 4% a year, and are included among less than ten global “Priority Populations” by the Agreement on the Conservation of Albatrosses and Petrels (ACAP).
How do we know?
Since the early 1960s, BAS scientists have monitored albatross populations at Bird Island, South Georgia – home to some of the largest, and best studied, albatrosses in the world. During this time a range of innovative techniques have been developed to understand breeding and foraging ecology. The return rates of ringed birds give scientists an indication of breeding frequency and survival rates, while tiny tracking devices reveal the vast areas of ocean covered by these birds in search of food. Pioneering satellite tracking studies by BAS scientists in the early 1990s gave the first real evidence that some albatrosses spend substantial amounts of time foraging behind commercial fishing vessels. Since then, BAS has accumulated a wealth of tracking data, used recently in one of the most comprehensive studies to date of fisheries overlap and bycatch risk across the Southern Ocean.
Why are albatross populations so vulnerable?
As well as being the largest of all seabirds, albatrosses are also the longest lived, some surviving for more than 60 years. They take many years to reach sexual maturity, not breeding until they are around 10 years old. Although most breed annually, nine species – including the wandering albatross – lay only one egg every two years, and it takes the best part of a year for a young albatross to leave the nest. Because chick production is so slow, even small increases in death rates among adults will cause populations to decline.
What action is being taken?
The international Agreement on the Conservation of Albatrosses and Petrels (ACAP) came into force in 2004 and is coordinating efforts to address the conservation crisis faced by these birds. Argentina, Australia, Brazil, Chile, Ecuador, France, New Zealand, Norway, Peru, South Africa, Spain, the UK and Uruguay have ratified the treaty, thereby agreeing to take specific actions to reduce bycatch and remove introduced species from nesting islands. The Albatross Task Force, part of the BirdLife International Global Marine Programme engages directly engages with fisheries operators and crew to try to reduce bycatch rates. BAS has very close links with both ACAP and BirdLife International, frequently providing data and advice to help develop effective conservation management.
But what are fishing companies doing about it?
Many commercial fishing companies have introduced measures to reduce seabird bycatch rates. On longliners, these include: fitting streamer lines, which flap behind boats and deter birds from trying to feed on baited hooks; weighting hooks so they sink quickly beyond the reach of birds; setting baits underwater; setting lines at night, when albatrosses are not feeding; keeping waste bait and offal on board; and introducing closed seasons for fishing.
Are these measures making a difference?
Yes, to some extent. For example, the Government of South Georgia and South Sandwich Islands applied the scientific evidence and advice provided by BAS scientists to introduce mandatory mitigation measures for its commercial fishery. During the late 1990s, 6,000 seabirds were killed each year by fishing vessels around South Georgia, but the subsequent introduction of mitigation measures has successfully reduced seabird bycatch to negligible levels since 2006. Efforts by the Albatross Task Force have led to substantial reductions in seabird bycatch rates off southern Africa.
What more needs to be done?
Even though albatrosses may be safe around South Georgia, the birds range so far in search of food (sometimes over 1,000 miles a day) that they will inevitably encounter fisheries that do not currently use mitigation measures. As a result, South Atlantic albatross populations are still falling. The challenge now is to persuade other national and international bodies responsible for managing fisheries within the nonbreeding areas of these birds to introduce and enforce mitigation measures.
- Albatrosses belong to a group of birds known as Procellariiformes, or ‘tubenoses’. Tubes on their beaks allow them get rid of excess salt, so they never need to drink fresh water.
- Three of the world’s 22 albatross species live in the North Pacific and there is a tropical species that breeds on the Galapagos Islands. No albatrosses breed in the North Atlantic.
- BirdLife International estimates that longlining kills around 100,000 albatrosses each year.
- Albatrosses cover vast distances when foraging. When breeding, wandering albatrosses range from sub-tropical to Antarctic waters on trips of up to 10,000km in 10-20 days. In the non-breeding season, many species (including wandering and grey-headed albatrosses from South Georgia) migrate long distances, some travelling right around Antarctica.
- With wing spans of up to 3.5m, albatrosses have the longest wings of all birds. They are also amongst the longest lived.
*Bycatch is the term used to describe the non-target species such as seabirds, turtles, fish and marine mammals caught or killed in fishing gear.

IMAGES
VIDEO
COMMENTS
Established in 1964, the IUCN Red List of Threatened Species has evolved to become the world's most comprehensive information source on the global conservation status of animal, fungi and plant species.
List Partnership: IUCN (including the Species Survival Commission), Birdlife, Conservation International, ... The Wandering Albatross, Diomedea exulans, is classified as Vulnerable on the IUCN Red List of Threatened SpeciesTM. It is one of the largest birds in the world with a wingspan of 2.5 - 3.35 meters. They spend a large amount of time ...
Length. 107-135. cm inch. Wingspan. 2.5-3.5. m ft. Described as "The bird which made the breeze to blow" the wingspan of a Wandering albatross ( Diomedea exulans) is the longest of any bird. It lives up to its name when it takes fishing trips that last 10-20 days and can cover 10,000 km while using hardly more energy than when sitting on its nest.
The wandering albatross (Diomedea exulans) is the world's largest flying bird, with a wingspan reaching an incredible 3.5 metres.These birds are oceanic nomads: they spend most of their 60 years ...
Identification. The adult Wandering Albatross appears entirely white from a distance. Close up, the fine black wavy lines on the breast, neck and upper back become visible. The bill can vary in colour, but is normally yellowish-pink. The white tail is occasionally tipped with black and the back of the wing changes from black to white with age.
There is some disagreement over how many subspecies of wandering albatross (Diomedea exulans) there are, and whether they should be considered separate species. ... IUCN, 2006. "2006 IUCN Red List of Threatened Species" (On-line). Accessed November 06, 2006 at http ...
The IUCN Red List of Threatened Species™ ISSN 2307-8235 (online) IUCN 2008: T22698305A110676747 Scope: Global Language: English Diomedea exulans, Wandering Albatross Amended version Assessment by: BirdLife International View on www.iucnredlist.org Citation: BirdLife International. 2017. Diomedea exulans. The IUCN Red List of Threatened Species
Longline fishing is likely to be the main cause of decline in this species, causing reductions in adult survival and juvenile recruitment, and this threat is on-going. Population size: 20100 mature individuals. Population trend: decreasing. Extent of occurrence (breeding/resident): 128,000,000 km 2. Country endemic: no.
Longline fishing is likely to be the main cause of decline in this species, causing reductions in adult survival and juvenile recruitment, and this threat is on-going. Population size: 20100 mature individuals. Population trend: decreasing. Extent of occurrence (breeding/resident): 128,000,000 km 2. Country endemic: no.
Behaviour Diomedea exulans is a biennial breeding species, although about 30% of successful and 35% of failed breeders (on average) defer breeding beyond the expected year. Adults return to colonies in November, and eggs are laid over a period of 5 weeks during December and January. Most chicks hatch in March and fledge in December.
The snowy albatross is part of the wandering albatross species complex, which includes the Tristan albatross and the Antipodean albatross. It can be distinguished from its relatives by its whiter plumage and larger size. ... The IUCN lists the snowy albatross as vulnerable. Threats include longline fishing and pollution.
IUCN Red List Status: Vulnerable. Distribution: The southern oceans and its small islands. Between Antarctica and the Tropic of Capricorn. ... The wandering albatross has the largest wingspan of any bird and is perhaps the most magnificent of all twelve species of albatross. It is aptly named as it is a great traveller, covering enormous ...
Wandering Albatross have the largest wingspan of any living bird species. They are true ocean wanderers, and adult birds can circumnavigate the whole Southern Ocean within one year during their migration. Listed as Vulnerable by the IUCN Red List, tracking data from this species has helped to determine where and when they are at most risk from ...
IUCN Red List Status: Vulnerable. Distribution: The southern oceans and its small islands. Between Antarctica and the Tropic of Capricorn. ... The wandering albatross has the largest wingspan of any bird and is perhaps the most magnificent of all twelve species of albatross. It is aptly named as it is a great traveller, covering enormous ...
Nineteen (66%) are listed as threatened by IUCN, and 11 (38%) are declining. Most have extensive at-sea distributions, and the greatest threat is incidental mortality (bycatch) in industrial pelagic or demersal longline, trawl or artisanal fisheries, often in both national and international waters. ... Wandering albatross: 35: B:
The wandering albatross, Diomedea exulans, is a globally threatened species breeding at a number of sites within the Southern Ocean. Across the South Georgia archipelago, there are differences in population trends even at closely located colonies. Between 1999 and 2018 the largest colony, at Bird Island, declined at 3.01% per annum, while in the Bay of Isles, the decline was 1.44% per annum ...
IUCN Conservation Status. The International Union for Conservation of Nature lists the wandering albatross under their 'Vulnerable' category. Interesting Facts. The wandering albatross is the biggest bird in its genera and one the largest in the world. One individual lived to be 60 years old in New Zealand. She was named 'Grandma.'
The wandering albatross is a large seabird of the family Diomedeidae. It is also called the snowy albatross or white-winged albatross. It is the largest member of the genus Diomedea (the great albatrosses) and has the largest wingspan of any living bird. It can have a wing-span of up to 11.5 feet (3.50 m). The IUCN says it is a vulnerable species.
The wandering albatross is a very large seabird (8-12 kg, up to 3.5 m in wingspan) breeding on sub‐Antarctic islands of the Southern Ocean ... in population size over time is essential for the conservation of species and their conservation status such as for IUCN listing. Yet, this is often not sufficient to understand the ultimate causes ...
Rest assured your personal data resides with IUCN and IUCN only. For more information please review our Data policy. IUCN Library System. Main menu. General search; Publications; Resolutions & Recommendations; You are here. Home. The wandering albatross . Complete Title: The wandering albatross. Non IUCN Publication. Author(s): Jameson, W ...
(IUCN 2012a) and in South Africa (Taylor et al. in press), based on an overall decline of more than 30% in the last three generations, spanning 70 years. The Wandering Albatross is listed in Appendix II of the Convention for the Conservation of Migratory Species of Wild Animals (CMS), in Annex 1 of the Agreement on the Conservation
Albatrosses cover vast distances when foraging. When breeding, wandering albatrosses range from sub-tropical to Antarctic waters on trips of up to 10,000km in 10-20 days. In the non-breeding season, many species (including wandering and grey-headed albatrosses from South Georgia) migrate long distances, some travelling right around Antarctica.